Abstract
Frostbite injuries frequently result in devastating ischemic damage to the distal extremities. This ischemia and resultant necrosis have historically been managed expectantly, with amputation of devitalized tissue commonly being the end result after severe injury. Advances in nuclear medicine, interventional radiology, and thrombolytic therapy have contributed to the development of a therapy proving successful in reversing these acute ischemic effects and ameliorating the morbidity of these rare limb-threatening injuries.
Case Report
A previously healthy 16-year-old male presented by ambulance from a referring institution for evaluation of cold exposure/frostbite to both hands. By report the patient had been found by police wandering in the middle of the road with his coat open, his head uncovered, and his hands ungloved. The patient’s feet were protected with insulated snow boots. Heavy moisture and snow to the anterior surface of the patient’s clothing raised concern that he had been unconscious and lain in the snow for a period of time. The police described him as “significantly intoxicated,” and he purportedly admitted to alcohol and marijuana use on the evening of injury. The ambient air temperature on the night of admission ranged between −10.5 to −12.8 degrees Celsius with wind chill factors of −14 to −19 degrees Celsius.1
The patient was transported home by the police and released into the custody of his parents. Upon arriving home his father described the patient’s hands as “cold and red” and re-warming of the hands by soaking them in warmed water was attempted. The temperature of the water used in home therapy and the duration of warm water immersion was not reported. After home warming, the patient’s fingers were noted to swell significantly, with associated darkening of the skin.
At this point the patient was transported by private vehicle to an emergency department (ED) for evaluation. While at the referring facility he had a peripheral intravenous line inserted, and he was given an intravenous dose of cefazolin and divided doses of morphine sulfate and hydromorphone for analgesia. Laboratory studies revealed an ethanol level of 0.19 g/dL, white blood cell count of 27.5 k/mm3, hematocrit 50.3%, international normalized ratio (INR) 1.0, and a random glucose level of 113 mg/dL. After brief evaluation and initial treatment, the patient was transferred by ambulance to tertiary care for plastic surgery evaluation. The time of ground transport was 2 hours.
The patient arrived to the tertiary center ED 4 hours after being found by police. Initial exam revealed a well-nourished, well-developed, 77-kilogram teenage boy who was lethargic but easily aroused and oriented x3. Vital signs included blood pressure 93/54 mm/Hg, respiratory rate 18 per minute, pulse 105 beats per minute, temperature 36.6 degrees Celsius, and pulse oximetry 97% on room air. Primary and secondary surveys of this patient were non-contributory except for the examination of his extremities.
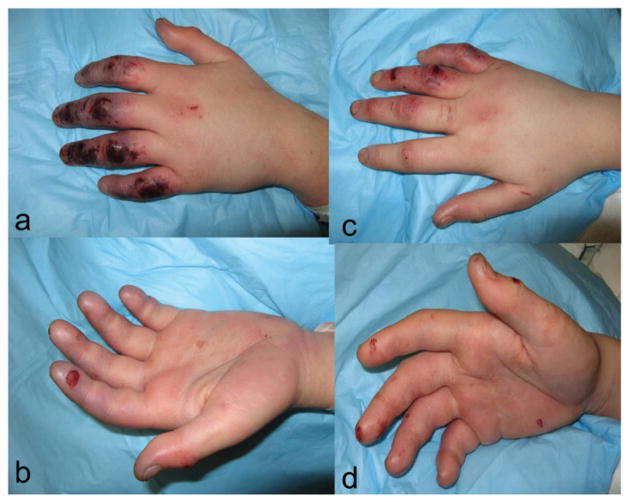
Bilaterally, his hands and fingers demonstrated dorsal abrasions, discoloration, and hemorrhagic blisters. The right hand demonstrated mottling and discoloration of the ring and small fingers distal to the proximal interphalangeal (PIP) joint and of the long finger tip. The ring finger had small hemorrhagic blisters on the dorsal surface. The right long finger had small dorsal abrasions. The right index finger and thumb demonstrated no significant abnormalities on examination.
The left hand was more seriously injured, with all digits affected. The left index, long, ring, and small fingers were mottled and cool, with evidence of hemorrhagic blisters along the dorsal surface. The tip of the left thumb was similarly affected (Figure 1). Doppler pulses to the left index and long finger were not present distal to the PIP joint.
Figure 1.
Appearance of patient’s hands on arrival to our facility. 1a dorsal surface, left hand; 1b palmar surface, left hand; 1c dorsal surface, right hand; 1d palmar surface, right hand.
Abrasions and pain were noted to the patient’s knees bilaterally. Examination of both lower extremities demonstrated no neurovascular compromise and full range of motion. Plain films of the patient’s bilateral knees and hands did not reveal fractures or acute injury. A Breathalyzer test taken 1 hour after arrival was 0.083.
A 1 L bolus of normal saline was infused, and both hands were placed in 40 degrees Celsius warm water baths for 20 minutes. Tetanus prophylaxis was provided, an additional dose of cefazolin was infused, and morphine in divided doses was provided for analgesia. Computerized tomography of the head/cervical spine was performed to identify occult traumatic injury or intracranial hemorrhage. These studies were negative for intracranial hemorrhage, skull fracture, or cervical spine injury. No other interventions were performed in the ED, and the patient was taken emergently to interventional radiology for bilateral upper extremity angiography and evaluation for intra-arterial infusion of thrombolytic therapy.
After informed consent and provision of conscious sedation, bilateral femoral artery sheaths were placed. Initial arteriography revealed standard anatomy of the aortic arch and patency of the bilateral subclavian, axillary, and brachial arteries. Selective angiography of the forearm and hand vessels bilaterally was performed. The radial, anterior interosseus, and posterior interosseus arteries were patent bilaterally. The right ulnar artery was also patent. Hand and finger arteriography demonstrated several areas of impaired arterial microcirculation.
On the right hand initial arteriography revealed impaired distal microcirculation to the thumb, a focal radial digital artery occlusion to the index finger, with diminished tip circulation, diminished tip circulation to the long finger, distal occlusion of both digital arteries of the ring finger with absent tip microcirculation, and near total occlusion of arterial blood supply to the small finger.
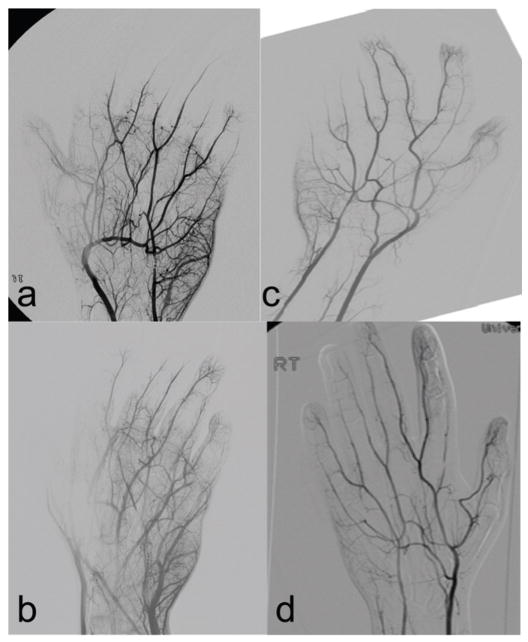
Initial arteriography of the left hand demonstrated ulnar artery spasm and occlusion of the ulnar artery, severe spasm of both digital arteries to the thumb with diminished micro-circulation, occlusion of the digital artery with minimal circulation to the index finger, no microcirculation to the long finger, and occlusion of the ulnar digital arteries with diminished microcirculation to the ring and small finger (Figure 2).
Figure 2.
Angiographic appearance of the patient’s hands before and after thrombolytic therapy. 2a left hand, pre-tPA; 2b left hand, post-tPA; 2c right hand, pre-tPA; 2d right hand, post-tPA.
After initial angiographic exam, 3000 units of heparin were infused into the femoral sheaths, followed by intra-arterial infusion of 50 mcg of nitroglycerine bilaterally via catheters placed in the brachial arteries. Continuous infusions of tissue plasminogen activator (tPA) 0.25 mg and 500 units of heparin per hour were provided to each brachial catheter and femoral sheath, respectively. tPA infusion was begun within 8 hours of the patient being found by police. The patient subsequently was admitted to the intensive care unit for continued wound care and evaluation.
Approximately 8 hours after initiation of tPA therapy, a repeat angiography was performed through the existing arterial catheters that revealed improved circulation in the right hand but continued impairment to the distal micro-circulation of the left hand. The right-sided brachial catheter was removed, but the left remained in place for continued tPA therapy. Twelve hours later a third angiographic exam of the left hand demonstrated improved distal circulation in all digits except for the ring finger. Left-sided tPA infusion was discontinued after this study, and the left brachial catheter and both femoral sheaths were removed. Once hemostasis was obtained at the femoral puncture sites, heparin infusion was re-initiated and continued for an additional 72 hours.
Wound care consisted of daily cleansing and topical silver sulfadiazine dressings to the open areas of both hands. Nutritional support and occupational/physical therapy were also started on hospital day 1. Once the patient was able to perform physical therapy exercises and dressing changes independently, he was discharged home on hospital day 6. Before discharge social workers also completed a brief intervention to address his alcohol and illicit drug use. Outpatient support for substance abuse counseling was offered to the patient and family at this time.
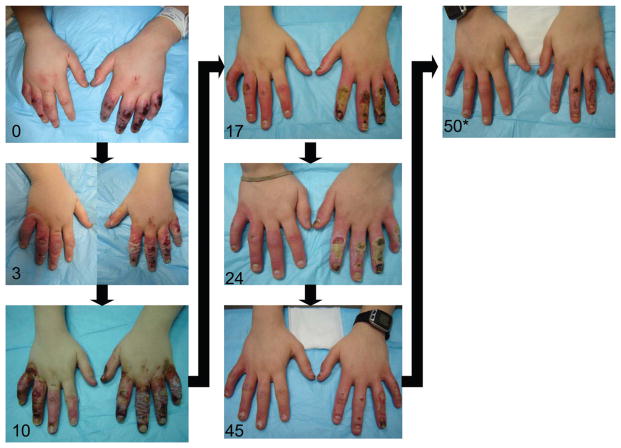
The patient was monitored with weekly outpatient clinic visits to assess wound healing and bilateral hand function. The patient eventually required split thickness skin grafts to the left ring and small finger (total graft size 12.5 cm3). After skin grafts the patient healed without complication and has retained full function and sensation to both hands.
Perfusion images before and after tPA administration are demonstrated in Figure 2. A pictorial progress of the patient’s wounds is provided in Figure 3.
Figure 3.
Time series of patient’s progression toward healing. Days after injury indicated in bottom left corner.
* indicates interval placement of split thickness skin graft to left ring and small finger
Discussion
Frostbite injuries to the hands and feet have historically been associated with high rates of tissue death, amputation, and associated morbidity. The adage, “Frostbite in January, amputate in July,” was an accurate depiction of the common outcome in frostbite injuries.2 An increased understanding of the pathophysiology of tissue ischemia in cold injuries, technological advances in nuclear medicine and interventional radiology, and the development of effective thrombolytic agents have combined to create more effective therapies to treat microvascular thromboses after prolonged or intense cold exposure.
The role of thrombolytic therapy in frostbite injury is based on a greater appreciation of the impact impaired circulation has on initial injury after frostbite. When patients are exposed to prolonged or intense cold stress, two mechanisms of injuries occur.
The first, crystallization caused by freezing of intracellular and extracellular water, is the focus of nearly all prehospital, transport, and ED care. At a tissue temperature of 28° F, ice crystals form in tissues. Intra- and extracellular ice crystals can cause direct damage to cell walls. Standard rewarming procedures will remove ice crystals from the tissue and halt further damage.3,4 Care must be taken not to rewarm frozen tissue before removal from cold exposure because repeated episodes of thawing and refreezing increase morbidity.5
Extravascular ice crystals cause direct damage to cellular structures. Additionally, ice crystals that form within the vascular system represent a second, distinct mechanism of injury. Intravascular ice crystals can produce endothelial injury and inflammation resulting in decreased blood flow, thrombosis, and tissue ischemia. Blood flow disruption after cold injury has been successfully treated with tPA with a marked reduction in amputations to affected hands and feet.5,6
Other pharmacological and surgical approaches to restore blood flow from frostbite have been attempted in both animal and human studies. Investigated modes include heparin, low molecular weight Dextran, papaverine, streptokinase, urokinase, intra-arterial reserpine, intra-arterial prostaglandin E1, aloe vera, hyperbaric oxygen therapy, and sympathectomy.4–16 There were reports of anecdotal success rates with several therapies, but none improved outcomes in controlled studies. Several promising animal models were not advanced to clinical trials.
Two recent studies using tPA have shown consistent and statistically significant decreases in amputation rates after thrombolytic infusion. Twomey et al in 20055 and Bruen et al in 20076 published results of frostbite injuries treated with tPA that demonstrate a marked reduction in amputation of ischemic digits. Both research groups used similar initial rewarming methods, inclusion criteria for consideration for treatment, and used tPA as the thrombolytic agent. The authors differed in choice of the screening imaging study, route and dosage of tPA, and post-thrombolytic management.
Both studies endorse considering thrombolytic therapy for patients with frostbite injuries to the hands or feet that present within 24 hours of injury, those with hemorrhagic blisters on clinical exam, and patients with loss of Doppler pulses in affected digits.5,6
Correspondingly, both approaches use nearly identical exclusion criteria for tPA therapy, including concurrent trauma, hypertension, neurological impairment, recent surgery or hemorrhage, pregnancy, repeated freeze/thaw cycles, and bleeding diasthasis.5,6 Twomey et al also excluded patients with warm ischemia time to affected extremities longer than 6 hours.5
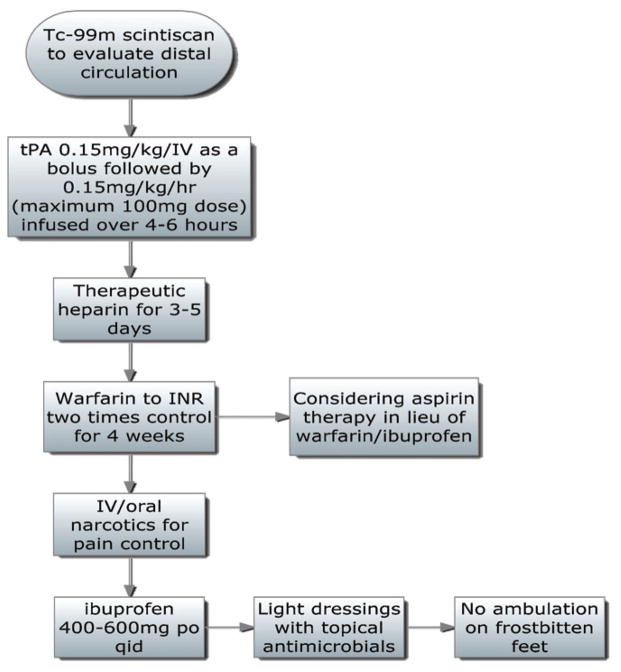
Twomey’s group used technetium (Tc)-99 MDP triple phase bone scans to identify digits at risk. Fourteen patients had received bone scintiscans before initiation of the study and served as a historical control for comparison. In the initial phase of tPA investigation, intra-arterial tPA was infused at a rate of 0.075 mg/kg/hr for 6 hours. These patients were rescanned at 6 hours, and those without improvement received an additional 6-hour infusion of tPA at the same dose.
After observing improvement in patient untreated extremities with this approach, subsequent patients were treated with intravenous doses of tPA with similar effectiveness. Varying dosage schemes were assessed for intravenous use. The authors ultimately endorsed a tPA bolus of 0.15 mg/kg/iv, followed by a 6-hour infusion of 0.15 mg/kg/hr. After completion of the tPA infusion, patients were treated with heparin infusion with the goal of a partial thromboplastin time two times normal. Warfarin therapy was begun 3–5 days after tPA and was continued for 4 weeks. Ibuprofen 400–600 mg was provided orally four times a day, pain was controlled with intravenous and oral narcotics, wounds were treated with topical antimicrobials, and weight bearing on frostbitten feet was prohibited.5
During the study period 19 patients received tPA infusion (6 intra-arterial/13 intravenous) and 174 digits were deemed to be at risk for amputation. After tPA treatment only 33 digits (19% of those initially thought to be at risk) required amputation. Of note the authors found no complications in patients receiving intravenous tPA, while two patients in the intra-arterial arm suffered bleeding complications. The authors concluded that scintiscan accurately predicted amputation level in non-treated patients and flow cut off levels in patients receiving thrombolysis. The authors believe the use of this imaging study, coupled with intravenous tPA infusion, avoided the potential complications of arteriography and arterial infusion.5
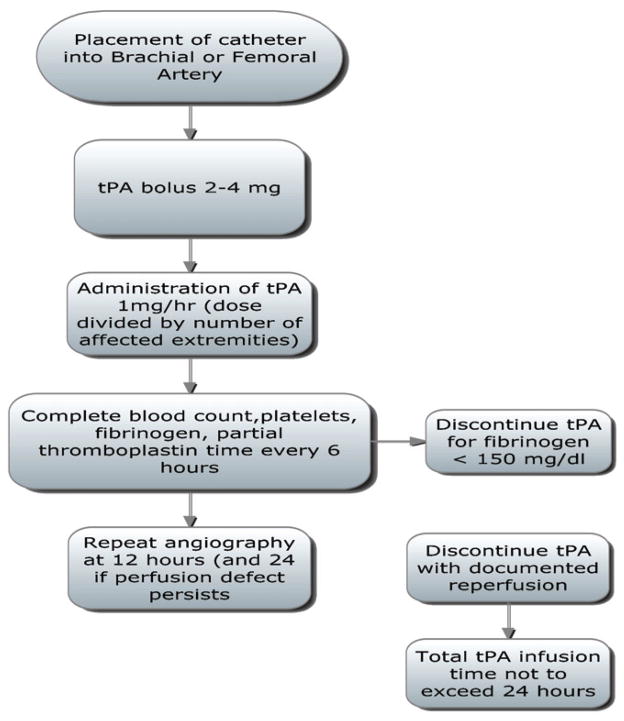
In contrast, Bruen et al performed arteriography to assess distal extremity perfusion in patients (n = 9) presenting with frostbite injury who met study inclusion criteria. Two patients with normal angiography and one patient who presented 48 hours after injury were not included in analysis. Six patients in the study period ultimately received tPA treatment. Twenty-six patients that were admitted with frostbite injuries before initiation of the tPA protocol or who did not meet inclusion criteria served as the control group for comparison.6
Patients with documented perfusion defects on initial arteriography received continuous infusions of tPA. Dosages ranged from a constant infusion of 1.0 mg/hr (dose divided by number of affected extremities) to a tapered dose beginning at 1.0 mg/hr subsequently reduced to 0.25 mg/hr. Heparin was infused at 500 U/hr via the arterial sheath to maintain sheath patency. Repeat angiograms were performed at 8–12 hours to evaluate perfusion. Therapy was discontinued when perfusion was restored with an absolute tPA infusion time not to exceed 48 hours. Actual infusion times in the six treated patients ranged from 8 to 42 hours. Both the study and control group received local wound debridement, topical antimicrobial therapy, and daily range of motion therapy.6
In this study 234 digits in the control group were deemed to be at risk and 97 (41%) ultimately required amputation. In the patients who received tPA, 59 digits were affected, but only six required amputation (10%). Two patients suffered complications during the study period. One patient receiving tPA developed a retroperitoneal hematoma that resolved without the need for surgical intervention. Another patient with an abnormal pyrophosphate scan did not demonstrate perfusion defects on arteriography. While the authors of this study concede that the arterial approach to tPA infusion for frostbite may increase resource needs, they express concern that other screening studies may be overly sensitive when determining distal perfusion defects.6
Both groups have modified their clinical algorithms based on these results and are summarized in Figures 4 and 5.
Figure 4.
University of Utah protocol. Adapted from reference 6.
Figure 5.
Hennepin County Medical Center protocol. Adapted from reference 5.
Transport Considerations
The role of transport crews in managing patients with severe frostbite is multi-faceted. First and foremost prevention education for at-risks groups is essential. Risk factors for frostbite include homelessness, improper clothing, participation in outdoor activities in colder climates, high altitude environmental exposure, psychiatric illness, substance abuse, fatigue, vehicular failure, altered sensation in the extremities (diabetic or other neuropathy), and trauma victims with prolonged exposure.2–5 These risk factors have been catalogued as the “Is” of frostbite: intoxicated, incompetent, infirm, insensate, inducted (increased risk in wartime), inexperienced, and indigent.3 Educational efforts should focus on these at-risk groups to help reduce incidence.
In the case study presented, the response of law enforcement demonstrates the often overlooked need for educating first responders who may encounter this injury. Any delay in care is potentially deleterious. The signs and symptoms of frostbite and need for emergent medical evaluation should be well understood by prehospital providers at every level of care.
A vital educational component is increasing the awareness of emergency care providers regarding the availability of this innovative therapy. In addition to education focused on proper initial patient care and wound management, referring institutions should be aware of the closest tertiary center that can perform thrombolytic therapy and the air and ground transport capabilities available in the region. Special emphasis on the importance of rapid transport while avoiding thaw and refreeze cycles will offer patients the greatest opportunity for a successful outcome.
Standard management of critically injured patients (i.e., the ABCs) should not be overlooked in the attempt to save an extremity. Systemic hypothermia is a dangerous disorder that can be associated with severe metabolic acidosis and may progress to cardiac arrhythmia and sudden death. Patients with severe hypothermia whose core body temperature requires intervention are candidates for neither transport nor the tPA protocol discussed above. Regular assessment of body temperature during transport is imperative to assess for the “afterdrop” phenomenon in which cold blood returned from a recently rewarmed extremity can lower core body temperature.3
During transport, proper wound insulation and analgesia provision are required. Interventions before and during transport should be reserved to those deemed crucial for emergent patient care and safe transfer because of the time-sensitive nature of thrombolytic intervention.
A final consideration required during hospitalization is intervention for alcohol or substance abuse. As noted previously, alcohol and drug abuse are risk factors for this injury, and counseling may be helpful in preventing recidivism.16 The case study outlined above illustrates the dangers of binge alcohol consumption in pediatric patients and the potential life- and limb-threatening consequences of this behavior. Recent literature describes the risks of underage binge drinking and the need for brief alcohol intervention after trauma admission to reduce alcohol use and prevent repeat injury.17
Conclusion
Thrombolytic therapy offers victims with ischemic injury from frostbite the potential for limb and digit salvage if provided within 24 hours of injury. The most advantageous screening study, tPA dose, and route of administration have not been delineated. Early studies demonstrate nearly equal detection, complication rate, and success using both arteriography and scintiscan for diagnosis combined with a variety of tPA-dosing strategies and administration routes. Resource availability may be the determining factor when applying this intervention at a local or regional level. Education is necessary in the prehospital setting to make first responders and referring facilities aware of at-risk populations, initial patient assessment and care, and the role of thrombolytic therapy in the management of severe frostbite injuries. Referral centers should be knowledgeable of tertiary care centers that are able to provide this intervention and the available transport options to prevent delays when transferring patients to definitive care.
Contributor Information
Christopher Wagner, Email: cwag@umich.edu, Trauma program manager/flight nurse specialist for the University of Michigan Health Systems in Ann Arbor, MI.
Christopher J. Pannucci, Practices in the Section of Plastic Surgery, Department of Surgery, at the university.
References
- 1.Historical weather data for Bay City Michigan on January 25, 2009. Available at http://www.wunderground.com/history/airport/KARB/2009/1/24/DailyHistory.html. Cited June 2, 2010.
- 2.Britt LD, Dascombe WH, Rodriguez A. New horizons in management of hypothermia and frostbite. Surg Clin North Am. 1991;71:345–370. doi: 10.1016/s0039-6109(16)45384-3. [DOI] [PubMed] [Google Scholar]
- 3.Jenabzadeh K, Ahrenholz DH. Cold injury. Hand Clinics. 2009;25(4):481–496. doi: 10.1016/j.hcl.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Murphy JV, et al. Frostbite: Pathogenesis and treatment. J Trauma. 2000;48(1):171–178. doi: 10.1097/00005373-200001000-00036. [DOI] [PubMed] [Google Scholar]
- 5.Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350–1355. doi: 10.1097/01.ta.0000195517.50778.2e. [DOI] [PubMed] [Google Scholar]
- 6.Bruen KJ, Ballard JR, Morris SE, et al. Reduction of the incidence of amputation in frostbite with thrombolytic therapy. Arch Surg. 2007;142:546–550. doi: 10.1001/archsurg.142.6.546. [DOI] [PubMed] [Google Scholar]
- 7.Saemi AM, Johnson JM, Morris CS. Treatment of bilateral hand frostbite after using transcatheter arterial thrombolysis after papaverine infusion. Cardiovasc Interventional Radiol. 2009;32:1280–1283. doi: 10.1007/s00270-009-9584-9. [DOI] [PubMed] [Google Scholar]
- 8.Salimi Z, Wolverson MK, Herbold DR, et al. Treatment of frostbite with IV streptokinase. Am J Roentgenol. 1987;149:773–776. doi: 10.2214/ajr.149.4.773. [DOI] [PubMed] [Google Scholar]
- 9.Zdeblick TA, Field GA, Shaffer JW. Treatment of experimental frostbite with urokinase. J Hand Surg. 1988;13A:948–953. doi: 10.1016/0363-5023(88)90278-x. [DOI] [PubMed] [Google Scholar]
- 10.von Heimburg D, Noah EM, Sieckmann UP, Pallua N. Hyperbaric oxygen treatment in deep frostbite of both hands in a boy. Burns. 2001;27:404–408. doi: 10.1016/s0305-4179(00)00132-7. [DOI] [PubMed] [Google Scholar]
- 11.Bouwman DL, Morrison S, Lucas CE, Ledgerwood AM. Early sympathetic blockade for frostbite. Is it of value? J Trauma. 1980;20:744–749. [PubMed] [Google Scholar]
- 12.Pichotka J, Lewis RB. Use of heparin in treatment of experimental frostbite. Proceedings of the Society for Experimental Biology and Medicine. 1949;72:130–136. doi: 10.3181/00379727-72-17354. [DOI] [PubMed] [Google Scholar]
- 13.Kapur BM, Gulati SM, Talwar JR. Low molecular weight dextran in the management of frostbite in monkeys. Indian J Med Res. 1968;56:1675–1681. [PubMed] [Google Scholar]
- 14.Miller MB, Koltai PJ. Treatment of experimental frostbite with pentoxifyline and aloe vera cream. Arch Otolaryngol-Head Neck Surg. 1995;121:678–680. doi: 10.1001/archotol.1995.01890060076015. [DOI] [PubMed] [Google Scholar]
- 15.Snider RL, Porter JM. Treatment of experimental frostbite with intra-arterial sympatethic blocking drugs. Surgery. 1975;77:557–561. [PubMed] [Google Scholar]
- 16.Yeager RA, Campion TW, Kerr JC, et al. Treatment of frostbite with intra-arterial prostaglandin E1. Am J Surg. 1983;49:665–667. [PubMed] [Google Scholar]
- 17.Gentilello LM, Rivara FP, Donovan DM. Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Ann Surg. 1999;230(4):473–480. doi: 10.1097/00000658-199910000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors in high school students. Pediatrics. 2007;119(5):1035–1036. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]