Abstract
Background
Venous thromboembolism (VTE) is a major patient safety issue. The PSF-sponsored Venous Thromboembolism Prevention Study (VTEPS) examined whether post-operative enoxaparin prevents symptomatic VTE in plastic surgery patients.
Methods
VTEPS eligibility criteria included age ≥18, general anesthesia, and post-operative hospital admission. In 2009, four sites uniformly adopted a clinical protocol. Patients with Caprini score ≥3 received post-operative enoxaparin prophylaxis starting 6–8 hours after surgery and continuing for the duration of their inpatient stay. VTEPS historic control patients had an operation between 2006 and 2008 but received no chemoprophylaxis for 60 days after surgery. The primary study outcome was symptomatic 60-day VTE. Stratified analyses were performed. Multivariable logistic regression controlled for baseline risk and other identified confounders.
Results
3334 patients (1876 controls and 1458 enoxaparin patients) were included. Notable risk reduction was present in patients with Caprini >8 (8.54% vs. 4.07%, p=0.182) and Caprini 7–8 (2.55% vs. 1.15%, p=0.230) who received post-operative enoxaparin. Logistic regression was limited to highest risk patients (Caprini ≥7) and demonstrated that length of stay (LOS) ≥4 days (adjusted odds ratio (OR) 4.63, p=0.007) and Caprini score >8 (OR 2.71, p=0.027) were independent predictors of VTE. When controlling for LOS and Caprini score, receipt of post-operative enoxaparin was protective against VTE (OR 0.39, p=0.042).
Conclusions
In high-risk plastic surgery patients, post-operative enoxaparin prophylaxis is protective against 60-day VTE when controlling for baseline risk and LOS. Hospitalization ≥4 days is an independent risk factor for VTE.
Clinical Question
Risk
Level of Evidence
III (retrospective cohort study)
Keywords: venous thromboembolism, deep venous thrombosis, pulmonary embolus, plastic surgery, reconstructive surgery, patient safety, never event, VTEPS
INTRODUCTION
Venous thromboembolism (VTE) encompasses deep venous thrombosis (DVT) and pulmonary embolus (PE) and is a major source of morbidity and mortality among hospitalized patients. Symptomatic PE has a 10% mortality rate within the first hour. Among survivors of PE, many develop right ventricular dysfunction or chronic pulmonary hypertension 1. Untreated, proximal DVT carries a 90-day PE risk of 50%. Additionally, DVT is associated with a localized inflammatory process that can permanently damage venous valves, resulting in venous reflux. This phenomenon, known as the post-thrombotic syndrome (PTS), occurs in at least 10% of patients and causes a chronically swollen, infection-prone extremity that inhibits ambulation 1. Development of PTS is the major driver of poor quality of life after DVT2.
VTE has been identified as a major patient safety issue in surgical patients. In 2008, then-Surgeon General Steven K. Galson issued a “Call to Action” for DVT and PE. This document stressed the importance of ongoing efforts to promote VTE awareness, risk-stratification, and prevention 3, 4. Concomitantly, several manuscripts were published that demonstrated VTE risk among plastic surgery patients was higher than previously thought 5–8. VTE was thus identified as a major patient safety issue among plastic surgery patients. In response to growing concerns among the ASPS membership, the Plastic Surgery Foundation’s Research Oversight Committee identified VTE risk stratification and prevention as its top patient safety research priority in 2008 9.
The VTEPS study was funded in 2008 and was designed to address several critical questions in plastic surgery patients. Questions examined appropriate VTE risk assessment as well as effectiveness and safety of post-operative chemoprophylaxis. The VTEPS Network has previously demonstrated that the Caprini Risk Assessment Model (RAM) 10 can risk-stratify plastic surgery patients for 60-day VTE events 11. This manuscript addresses the effectiveness of post-operative enoxaparin, a low-molecular weight heparin (LMWH), for prevention of 60-day, symptomatic VTE events among adult plastic surgery patients. The safety profile of postoperative enoxaparin will be discussed in a separate manuscript.
METHODS
Study inclusion and exclusion criteria
In 2008, VTEPS was funded by the Plastic Surgery Foundation. The VTEPS Network consisted of four tertiary care hospitals, including the University of Pittsburgh (Pittsburgh, PA), the University of Texas-Southwestern (Dallas, Texas), Regions Hospital (St. Paul, MN), and the University of Michigan (Ann Arbor, MI). Over a six-month period after funding, VTEPS Network members refined and mutually agreed upon the study’s clinical protocol. The protocol was based on an extensive review of the surgical literature and was designed to reflect evidence-based “best practice” for VTE prophylaxis. Studies from the general surgery and surgical subspecialty literature were extrapolated to the plastic surgery patient population where appropriate. Between March 2009 and September 2009, the study protocol was implemented at each site. Data acquisition concluded on December 31, 2010. All data were acquired retrospectively.
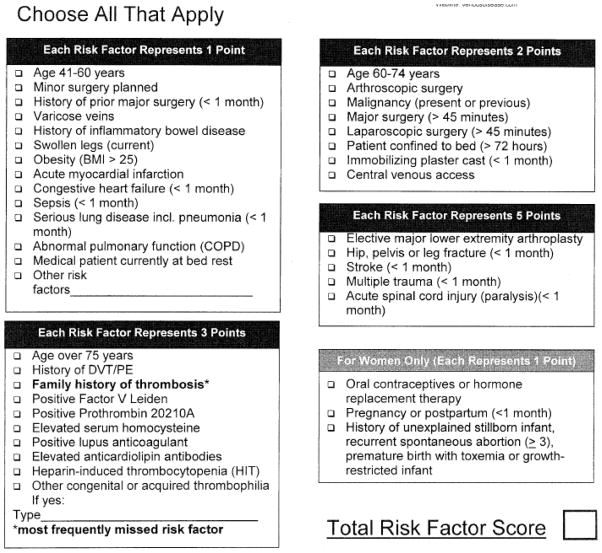
Each VTEPS site implemented an identical clinical protocol to risk-stratify and subsequently provide post-operative chemoprophylaxis to adult (age ≥18) plastic surgery patients. Pre-operative risk stratification was performed using the Caprini RAM (Figure 1) 10. Eligibility requirements for the clinical protocol included adult patients at moderate to high risk for VTE (Caprini score ≥3), operation under general anesthesia, and post-operative admission to the hospital for at least an overnight stay. Eligible patients received a standard VTE chemoprophylaxis regimen of post-operative enoxaparin (40mg subcutaneous once daily or 30mg subcutaneous twice daily for patients with body mass index >40). Subcutaneous enoxaparin administration was initiated 6–8 hours after surgery and continued for the duration of inpatient stay. Timing of medication initiation and duration of enoxaparin prophylaxis was confirmed using inpatient pharmacy records.
Figure 1.
The Caprini Risk Assessment Model. Reprinted from reference 10, with permission.
Patients who received any pre-operative heparin product were excluded. Patients who received any non-aspirin anti-coagulant medication after surgery (including but not limited to intravenous heparin, subcutaneous unfractionated heparin, non-enoxaparin low-molecular weight heparins, or coumadin) were excluded, except when these medications were used to treat a newly diagnosed VTE. Patients who received a single bolus of intravenous heparin during microsurgical procedures were not excluded. Patients who received a non-protocol enoxaparin dosage, who had enoxaparin initiated more than 6–8 hours after surgery, or who had gaps in their daily post-operative enoxaparin regimen were excluded. Post-operative aspirin administration was allowable and was tracked as a separate independent variable. Patients who were prescribed post-discharge prophylaxis with any non-aspirin anti-coagulant medication were excluded. Use of peri- and post-operative sequential compression devices was the standard of care at all four VTEPS sites.
Initial in-progress review of VTEPS data indicated that the majority of lower extremity trauma reconstruction patients had multiple operations, including debridement and/or bony fixation, prior to plastic surgery consultation. The vast majority of these patients received prophylactic-dose anti-coagulation prior to definitive reconstruction by plastic surgery. Receipt of pre-operative anticoagulation would represent a notable confounder for our clinical question. To avoid confounding, all patients who had lower extremity reconstruction after acute traumatic injury were excluded from VTEPS.
At each VTEPS site, historic control patients were identified using medical record review for cases performed between 2006 and 2008. Historic control eligibility criteria were identical to patients in the clinical protocol group with one exception. Control patients did not receive unfractionated heparin, low-molecular weight heparin, coumadin, or other means of prophylactic or therapeutic anti-coagulation for 60 days after surgery. This included the patient’s inpatient stay and post-discharge course. Receipt of aspirin did not exclude patients from being historic controls.
Independent variables
Medical record review was performed by physician-led teams at each VTEPS site. Prior to chart review, each team leader was required to participate in a standardized training session. Training was administered by VTEPS study coordinators and included focused educational sessions on VTEPS eligibility criteria and outcomes of interest, the Caprini RAM, and proper use of the web-based data collection system (see below). Retrospective chart review was performed for all patients to identify VTE risk factors per the 2005 version of the Caprini RAM. An aggregate Caprini score which reflected risk factors present before (e.g. age, body mass index, medical comorbidities, or personal/family history of VTE) and during (e.g. total operative time or insertion of central venous line) hospitalization was generated. We collected several additional independent variables that were not included in the Caprini score. These included year procedure was performed, VTEPS site, patient gender, whether multiple operations were performed during the initial hospitalization, surgical procedure type and location, enoxaparin administration per protocol, administration of aspirin, and length of hospitalization.
Dependent variables
Dependent variables included symptomatic deep venous thrombosis or symptomatic pulmonary embolus. All DVT or PE events required confirmation using an objective image method, such as venous duplex ultrasound, venography, ventilation-perfusion scan, or computed tomography. Autopsy-proven DVT or PE were considered as post-operative events only if the pathologist’s report indicated that VTE was the cause of or a major contributor to death. Medical record review was performed for 60 days after surgery to identify DVT or PE events. Patients whose medical records lacked 60 days of followup were excluded. A composite VTE variable, encompassing patients with either DVT or PE, was created.
Web-based data collection
The American Society of Plastic Surgery launched the “Tracking Operations and Outcomes for Plastic Surgeons” (TOPS) in 2002 to provide a HIPAA-compliant, secure, and confidential data repository 12. The existing TOPS platform was modified for VTEPS’ purposes. Sites were provided with individualized login and password information. Upload of de-identified data to the modified TOPS site was performed by physician-led teams at each VTEPS site. De-identified data were stored on a secure data server and was provided to study personnel for analysis upon request.
Statistical analysis
The Stata11 statistical package (StataCorp LP, College Station, Texas) was used to perform all statistical analyses. Bivariate statistics were generated using the two-tailed student’s t-test, chi-squared test, Fisher’s exact test, or the Wilcoxon Rank-Sum test as appropriate. Descriptive statistics which examined DVT, PE, and VTE incidence were generated and were stratified by various risk factors. Patients were stratified by Caprini score at accepted and published levels (Caprini scores of 3–4, 5–6, 7–8, and >8) 11, 13, 14. Caprini score was treated as an ordinal variable that provided an estimate of baseline VTE risk 11. Risk-stratified analyses were performed, including simple stratified analyses and multivariable logistic regression. To avoid co-linearity, variables utilized in Caprini score generation were not used as independent variables in the logistic regression model. A value of p<0.05 was considered significant.
Expected risk reduction and sample size calculation
Our pilot data included 634 adult plastic surgery patients with Caprini score ≥3 who received no chemoprophylaxis after surgery. The 60-day incidence of symptomatic VTE among these patients was 2.52%. Prior research 15, 16 supports a 50% reduction in symptomatic VTE using postoperative, inpatient low-molecular weight heparin chemoprophylaxis. Thus, we hypothesized that our postoperative enoxaparin chemoprophylaxis protocol would decrease the incidence of symptomatic VTE from 2.52% to 1.26%.
Sample size calculation was performed for the primary study endpoint, specifically an expected reduction in symptomatic VTE from 2.52% to 1.26%. Our assumptions included alpha equal to 0.05, beta equal to 0.20, power of 0.80, and n1:n2 of 1:1. With these assumptions, the VTEPS study would have 80% power to detect the expected difference if 1988 patients were included in each cohort. Our initial study design included 1988 patients in each of the historic control and intervention groups (approximately 500 patients per study cohort per study site).
Prior to initiating this study, each VTEPS site received Institutional Review Board approval.
RESULTS
Complete data were present for 3,334 patients who met eligibility criteria. This included 1,876 historic control patients and 1,458 intervention patients. When compared to historic controls, intervention patients had significantly increased age, higher body mass index, longer operative time, longer length of hospitalization, and higher Caprini score (Table 1).
Table 1.
Demographics comparing historic control and intervention groups.
| Historic controls N=1876 |
Post-op enoxaparin N=1458 |
p value | |
|---|---|---|---|
| Age, mean (years) | 48.7 | 50.3 | 0.002 |
| BMI, mean | 29.0 | 30.0 | <0.001 |
| BMI ≥30, % | 34.2% | 41.2% | <0.001 |
| Female gender, % | 63.2 | 68.7 | 0.001 |
| Caprini score, median | 4 | 5 | <0.001 |
| Mean operative time, hours | 3.1 | 3.8 | <0.001 |
| Multiple operations during hospitalization, % | 13.1% | 13.4% | 0.780 |
| Post-operative aspirin use, % | 8.6% | 7.8% | 0.357 |
| Length of stay, mean (days) | 3.1 | 3.8 | <0.001 |
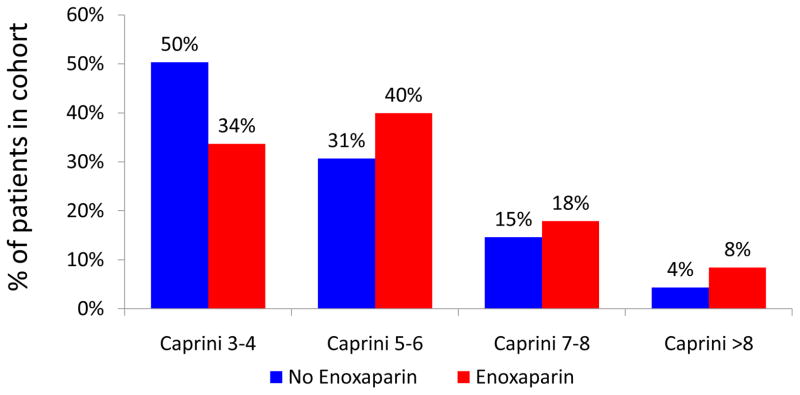
We have previously shown that increased Caprini score correlates with increased 60-day VTE events in a non-linear fashion 11. Stratified analysis was performed to examine the composition of historic control and intervention cohorts. The intervention cohort consisted of a notably higher-risk patient population (Figure 2).
Figure 2.
Composition of cohorts stratified by Caprini score
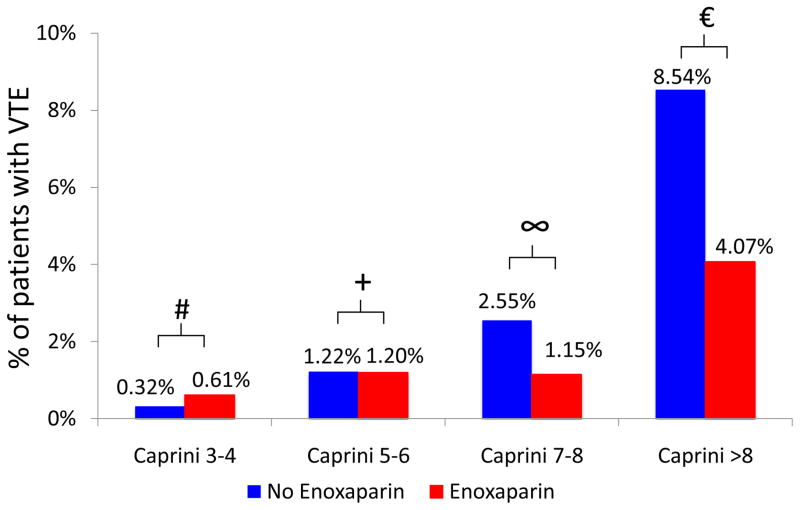
Stratified analysis demonstrated that VTE risk reduction was most apparent among high and highest risk patients (those with Caprini score ≥7) who received post-operative enoxaparin (Figure 3). Minimal risk reduction was seen in the Caprini 3–4 and Caprini 5–6 groups. When post-operative enoxaparin was provided, notable risk reduction was present for patients with Caprini score of 7–8 (2.55% vs. 1.15%, p=0.230) and Caprini score>8 (8.54% vs. 4.07%, p=0.182). In patients with Caprini score 7–8 and >8, the observed absolute risk reductions of 1.40% and 4.47% correspond to a number needed to treat of 71.4 and 22.4, respectively, to prevent one VTE event.
Figure 3.
Rates of VTE stratified by Caprini score and receipt of post-operative enoxaparin.
# p=0.414, + p=0.982, ∞ p=0.230, € p=0.182
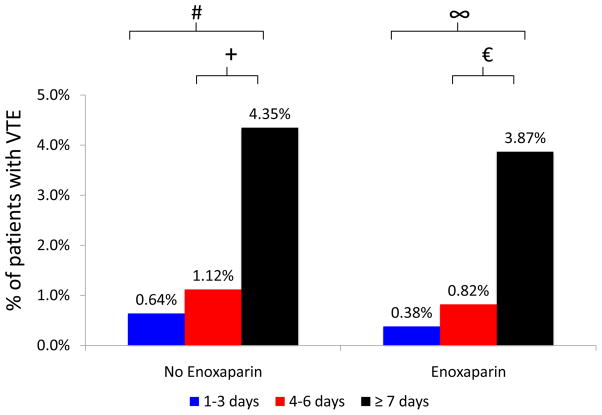
Length of stay is a marker of illness severity and is not included in the Caprini score. Longer lengths of stay were associated with increased rates of VTE in bivariate analysis. Among historic controls, patients who stayed ≥7 days (4.35% vs. 0.64%, p<0.001) and patients who stayed 4–6 days (1.12% vs. 0.64%, p=0.411) were more likely to have post-operative VTE when compared to those who stayed for 1–3 days. Among intervention patients, those who stayed ≥7 days (3.87% vs. 0.38%, p<0.001) and those who stayed 4–6 days (0.82% vs. 0.38%, p=0.336) were more likely to experience post-operative VTE when compared to patients with length of stay 1–3 days (Figure 4).
Figure 4.
Rates of VTE stratified by length of stay and receipt of post-operative enoxaparin.
# p<0.001, + p=0.411, ∞ p<0.001, p=0.336
Logistic regression analysis was limited to the high and highest risk patient subgroups (Caprini score ≥7). VTE was the dependent variable of interest. Independent variables included length of stay (dichotomized to length of stay ≥4 or <4 days), stratified Caprini score, and receipt of post-operative enoxaparin. Logistic regression demonstrated that length of stay ≥4 days (adjusted odds ratio (OR) 4.63, p=0.007) and Caprini score >8 (adjusted OR 2.71, p=0.027) were each independent predictors of VTE. When controlling for length of stay and Caprini score, receipt of post-operative enoxaparin was protective against VTE (adjusted OR 0.39, p=0.042) (Table 2).
Table 2.
Adjusted odds for VTE from a multivariable logistic regression model.
| Adjusted odds ratio (95% confidence interval) | p value | |
|---|---|---|
|
| ||
| Length of stay (days) | ||
| 1–3 days | Reference | ---- |
| ≥4 days | 4.63 (1.52–14.17) | 0.007 |
|
| ||
| Caprini score | ||
| Caprini 7–8 | Reference | ---- |
| Caprini >8 | 2.71 (1.12–6.52) | 0.027 |
|
| ||
| Group | ||
| Historic control | Reference | ---- |
| Post-operative enoxaparin | 0.39 (0.16–0.97) | 0.042 |
For both control and intervention groups, there were no significant differences in reported rates of VTE by site. Frequencies of individual Caprini score risk factors in patients with and without VTE are provided in Table 3. Frequencies of VTE stratified by procedure type are shown in Table 4.
Table 3.
Frequency of individual Caprini RAM risk factors in patients with and without postoperative VTE.
| Risk Factor | No VTE (N=3,292) | Yes VTE (N=42) | p value |
|---|---|---|---|
|
| |||
| ONE POINT RISK FACTORS | |||
| Age 41–59 | 54.3% (1789) | 45.2% (19) | 0.239 |
| Minor surgery planned | 5.0% (164) | 11.9% (5) | 0.042 |
| Major surgery within 30 days | 13.2% (434) | 42.9% (18) | <0.001 |
| Varicose veins | 1.0% (32) | 0 (0) | 0.521 |
| History of IBD | 0.7% (23) | 0(0) | 0.587 |
| Swollen legs (current) | 3.2% (106) | 1.3% (2) | 0.575 |
| BMI>25 | 73.7% (2426) | 88.1% (37) | 0.035 |
| Acute myocardial infarction <3 months | 0.1% (2) | 7.1% (3) | <0.001 |
| Congestive heart failure <1 month | 0.6% (21) | 7.1% (3) | <0.001 |
| Sepsis <1 month | 0.5% (15) | 0 (0) | 0.661 |
| Serious lung disease (inc. pneumonia) <1 month | 0.5% (15) | 0 (0) | 0.661 |
| Chronic obstructive pulmonary disease | 2.1% (68) | 9.5% (4) | 0.001 |
|
| |||
| TWO POINT RISK FACTORS | |||
| Age 60–74 years | 16.1% (531) | 6.2% (11) | 0.079 |
| Arthroscopic surgery | 0.2% (5) | 0 (0) | 0.800 |
| Malignancy (present or previous) | 37.2% (1224) | 31.0% (13) | 0.406 |
| Major surgery >45 minutes | 94.3% (3105) | 90.5% (38) | 0.287 |
| Laparoscopic surgery >45 minutes | 0.2% (6) | 0 (0) | 0.782 |
| Central venous access | 8.7% (286) | 31.0% (13) | <0.001 |
|
| |||
| THREE POINT RISK FACTORS | |||
| Age ≥75 | 4.5% (149) | 7.1% (3) | 0.419 |
| History of DVT/PE | 3.3% (109) | 11.9% (5) | 0.002 |
| Family history of DVT/PE | 1.0% (34) | 2.4% (1) | 0.394 |
| Positive Factor V Leiden | 0.2% (8) | 0 (0) | 0.749 |
| Positive Prothrombin 20210A | 0.03% (1) | 0 (0) | 0.910 |
| Positive Lupus anticoagulant | 0.1% (3) | 0 (0) | 0.845 |
| Heparin induced thrombocytopenia | 0.1% (3) | 0 (0) | 0.845 |
| Elevated serum homocysteine | 0 (0) | 0 (0) | ---- |
| Elevated anticardiolipin antibodies | 0 (0) | 0 (0) | ---- |
| Other congenital or inherited thrombophilia | 0.2% (7) | 0 (0) | 0.765 |
| Polycythemia vera | 0.1% (3) | 0 (0) | 0.845 |
|
| |||
| FIVE POINT RISK FACTORS | |||
| Elective major lower extremity arthroplasty | 0.6% (18) | 0 (0) | 0.631 |
| Hip, pelvis, or leg fracture <1 month | 0.4% (13) | 0 (0) | 0.683 |
| Stroke <1 month | 0.03% (1) | 0 (0) | 0.910 |
| Multiple trauma <1 month | 2.2% (73) | 7.1% (3) | 0.034 |
| Acute spinal cord injury or paralysis <1 month | 0.1% (3) | 0 (0) | 0.845 |
| Females Only | No VTE (N=2168) | Yes VTE (N=20) | p value |
|---|---|---|---|
|
| |||
| ONE POINT RISK FACTORS | |||
| Oral contraceptives | 7.1% (154) | 10.0% (2) | 0.616 |
| Pregnancy or postpartum (<1 month) | 0.2% (4) | 0 (0) | ---- |
| History of unexplained stillborn infant recurrent spontaneous abortion (≥3), premature birth with toxemia or growth-restricted infant | 0.3% (6) | 0 (0) | ---- |
Table 4.
Rate of VTE stratified by procedure type.
| Procedure Type | Number of patients | Rate of VTE (N) |
|---|---|---|
| Upper extremity reconstruction | 494 | 1.21% (6 patients) |
| Post-mastectomy breast reconstruction (implant or autologous tissue) | 846 | 0.71% (6 patients) |
| Breast reduction | 302 | 0.66% (2 patients) |
| Cosmetic breast surgery | 39 | 0 |
| Body contouring (non post- bariatric) | 153 | 0 |
| Body contouring (post- bariatric) | 229 | 0 |
| Non-trauma lower extremity reconstruction | 263 | 0.76% (2 patients) |
| Head and neck reconstruction | 421 | 1.66% (7 patients) |
| Chest/abdominal wall/back reconstruction | 301 | 3.99% (12 patients) |
| Burn reconstruction | 31 | 3.23% (1 patient) |
| Decubitus ulcers (debridement or reconstruction) | 232 | 2.16% (5 patients) |
| Facial cosmetic surgery | 70 | 0 |
| Microsurgery/free tissue transfer | 218 | 2.29% (5 patients) |
| Genitourinary reconstruction | 58 | 1.72% (1 patient) |
DISCUSSION
We report the results of the VTEPS study, a multi-center, retrospective cohort study that examined whether post-operative enoxaparin decreases symptomatic, 60-day VTE events in adult plastic and reconstructive surgery patients. Our results indicate that several factors are independently associated with VTE. These include elevated Caprini score and length of stay ≥4 days. Additionally, when controlling for Caprini score and length of stay, receipt of postoperative enoxaparin was protective against 60-day VTE events in high-risk patients (patients with Caprini score ≥7).
As Kent and Hayward note, risk should be considered at the individual level, not at the aggregate trial or population level 17. Summary results from a study reflect only the arithmetic mean, which can be misleading when the population consists of largely of low-risk patients. Additionally, different risk/benefit ratios may exist for patients at variable levels of baseline risk. Multivariable risk-stratified analysis is thus preferred to identify clinically important subgroups that may receive improved benefit or excess harm from an intervention 17–19. Risk-stratified analyses were presented throughout this manuscript.
Between 1% and 7% of surgeons have personally experienced a VTE-related patient death after high-risk plastic surgery 20–22. Plastic surgeon’s self-reported practice patterns indicate a disparity between clinical understanding and clinical practice. The majority of surgeons can identify patients at high risk for postoperative VTE. However, examination of their self-reported practice patterns indicates that a substantial proportion of surgeons (more than 50%) provide inadequate levels of VTE prophylaxis for high-risk patients 20, 22. Additionally, surgeons recognize modifiable VTE risk factors (such as oral contraceptive use) but may fail to modify those factors prior to surgery 23.
“Never event” is a poor descriptor for VTE, as it implies that all events are potentially preventable 24. Breakthrough VTE events routinely occur in the face of rigorous protocols and gold-standard prophylaxis, as has been reported in the plastic surgery 25, 26, orthopaedic surgery 27–29 and general surgery 30–32 literature. We observed multiple breakthrough events in the VTEPS enoxaparin group, although the distinct causes of these events remains unclear. Unrecognized hypercoagulability has been identified as a major contributor to VTE risk 33–36. VTEPS data supports that prior personal history of VTE is an important risk factor as well (Table 3).
VTE represents a financial burden for patients and payers. The mean cost of hospitalization for an index DVT event is over $20,000 37. Previous work has shown that enoxaparin is a cost-effective method of VTE prevention 38–40. In July 2010, the US Food and Drug Administration approved production of enoxaparin in generic form, which should result in substantially decreased costs to patients 41.
For a complete overview of VTE in plastic surgery, we refer readers to two excellent reviews that have recently been published by Miszkiewicz and colleagues 42 and Venturi and colleagues 43. These reviews built upon the foundation of several outstanding reviews and consensus statements published previously 44–46.
Limitations
Figure 2 demonstrates that our intervention group consisted of a patient population at higher baseline risk for VTE. This may have been due to two factors. First, an increasing proportion of plastic and reconstructive surgery is being performed in the outpatient setting. Young, healthy patients are preferentially selected for day-case surgery. This may account for the decreased proportion of low-risk patients in our more recent cohort. Additionally, this finding may be explained by a surgeon-level bias in provision of post-operative chemoprophylaxis. In the period of time from which our historic controls were collected (2006–2008), some surgeons may have identified high-risk patients and provided them with chemoprophylaxis. By definition, these patients were not eligible for inclusion in the historic control cohort. Given that a selection bias was clearly present between our two cohorts, a risk-stratified analysis was most appropriate.
Table 4 reports the observed rates of VTE stratified by procedure type. Due to a paucity of outcome events in each subgroup, we cannot provide a subgroup analysis of rates of VTE stratified by both procedure type and receipt of enoxaparin.
We believe that length of stay is an important marker of illness severity (e.g. sicker patients have longer hospitalizations) and, as a result, have incorporated this variable into our multivariable risk model. However, given our study protocol, length of stay could also be viewed as a marker of duration of intervention (e.g. post-operative enoxaparin was provided for the duration of inpatient stay). We have attempted to control for these factors by using length of stay as an independent variable in a logistic regression model. The model results demonstrate that length of stay ≥4 days is an independent risk factor for VTE. Additionally, when controlling for length of stay, receipt of post-operative enoxaparin is protective against VTE (OR 0.39, p=0.042).
A recent review article on VTE in plastic surgery patients, co-written by leaders from plastic and vascular surgery, recommends that patients with ongoing VTE risk factors receive 7 days of post-operative chemoprophylaxis. Additionally, they recommend that cancer patients receive 28 days of post-operative chemoprophylaxis 43. These recommendations are not based on data from the plastic surgery literature; they are extrapolated from randomized-controlled trials conducted in abdominal and pelvic cancer patients 30–32. The optimal duration of chemoprophylaxis in plastic surgery patients remains unknown. Future trials should randomize plastic surgery patients at equal baseline risk to different durations of chemoprophylaxis to examine this important issue.
Studies published after the VTEPS protocol was designed and implemented indicated that VTE risk may remain elevated for up to 90 days after surgery 47. As VTEPS followup was limited to 60 days after surgery, late VTE events may not be included in our data. Similarly, screening studies have shown that high-risk plastic surgery patients have rates of asymptomatic VTE between 3.4% and 16.7% 48, 49. These rates are similar to rates of asymptomatic VTE reported in other high-risk populations 27–32, 50–54. VTEPS reports the 60-day rate of symptomatic VTE, which likely underestimates the true rate of VTE after plastic and reconstructive surgery.
CONCLUSION
In high-risk plastic surgery patients (Caprini score ≥7), receipt of post-operative, prophylactic dose enoxaparin is protective against 60-day VTE events when controlling for baseline risk and length of stay. Length of stay ≥4 days is also an independent risk factor for VTE. Optimal duration of prophylaxis remains an important topic for further research.
Footnotes
FINANCIAL DISCLOSURE AND PRODUCTS PAGE
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Dr. Pannucci receives salary support from the NIH T32 grant program (T32 GM-08616).
Meeting disclosure:
Portions of this work were presented at the 2011 American Association of Plastic Surgeons meeting (Boca Raton, Florida) and will be presented at the 2011 Plastic Surgery Research Council (Louisville, Kentucky).
Contributor Information
Christopher J. Pannucci, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
George Dreszer, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Christine Fisher Wachtman, Division of Plastic and Reconstructive Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Steven H. Bailey, Department of Plastic Surgery, University of Texas-Southwestern, Dallas, Texas.
Pamela R. Portschy, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Jennifer B. Hamill, JBH Consulting, Shohola, Pennsylvania.
Keith M. Hume, American Society of Plastic Surgeons, Arlington Heights, Illinois.
Ronald E. Hoxworth, Department of Plastic Surgery, University of Texas-Southwestern, Dallas, Texas.
J. Peter Rubin, Division of Plastic and Reconstructive Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Loree K. Kalliainen, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Andrea L. Pusic, Plastic and Reconstructive Surgery Service, Memorial Sloan Kettering Cancer Center, New York, New York.
Edwin G. Wilkins, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
References
- 1.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 3.Wakefield TW, McLafferty RB, Lohr JM, et al. Call to action to prevent venous thromboembolism. J Vasc Surg. 2009;49:1620–1623. doi: 10.1016/j.jvs.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 4.The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and PulmonaryEmbolism. [Accessed March 3, 2011]; http://www.surgeongeneral.gov/library/calls/index.html.
- 5.Seruya M, Venturi ML, Iorio ML, Davison SP. Efficacy and safety of venous thromboembolism prophylaxis in highest risk plastic surgery patients. Plast Reconstr Surg. 2008;122:1701–1708. doi: 10.1097/PRS.0b013e31818dbffd. [DOI] [PubMed] [Google Scholar]
- 6.Pannucci CJ, Chang EY, Wilkins EG. Venous thromboembolic disease in autogenous breast reconstruction. Ann Plast Surg. 2009;63:34–38. doi: 10.1097/SAP.0b013e318188bedf. [DOI] [PubMed] [Google Scholar]
- 7.Keyes GR, Singer R, Iverson RE, et al. Mortality in outpatient surgery. Plast Reconstr Surg. 2008;122:245–50. doi: 10.1097/PRS.0b013e31817747fd. discussion 251–3. [DOI] [PubMed] [Google Scholar]
- 8.Hatef DA, Kenkel JM, Nguyen MQ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122:269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 9.Hume Keith. ASPS Vice President of Research and Development, personal communication. Dec, 2010.
- 10.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Pannucci CJ, Bailey SH, Dreszer G, et al. Validation of the caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212:105–112. doi: 10.1016/j.jamcollsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [accessed March 3, 2011];Tracking Outcomes and Operations for Plastic Surgeons summary. http://www.plasticsurgery.org/Medical_Professionals/TOPS.html.
- 13.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA, Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181b7fca6. [DOI] [PubMed] [Google Scholar]
- 14.Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3–10. doi: 10.1016/j.amjsurg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Liao EC, Taghinia AH, Nguyen LP, Yueh JH, May JW, Jr, Orgill DP. Incidence of hematoma complication with heparin venous thrombosis prophylaxis after TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;121:1101–1107. doi: 10.1097/01.prs.0000302454.43201.83. [DOI] [PubMed] [Google Scholar]
- 16.Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 17.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: A risk-modelling study. european carotid surgery trialists’ collaborative group. Lancet. 1999;353:2105–2110. doi: 10.1016/s0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Sackett DL, Cook DJ. Users’ guides to the medical literature. II. how to use an article about therapy or prevention. B. what were the results and will they help me in caring for my patients? evidence-based medicine working group. JAMA. 1994;271:59–63. doi: 10.1001/jama.271.1.59. [DOI] [PubMed] [Google Scholar]
- 20.Clavijo-Alvarez JA, Pannucci CJ, Oppenheimer AJ, Wilkins EG, Rubin JP. Prevention of venous thromboembolism in body contouring surgery: A national survey of 596 ASPS surgeons. Ann Plast Surg. 2011;66:228–232. doi: 10.1097/SAP.0b013e3181e35c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broughton G, 2nd, Rios JL, Rohrich RJ, Brown SA. Deep venous thrombosis prophylaxis practice and treatment strategies among plastic surgeons: Survey results. Plast Reconstr Surg. 2007;119:157–174. doi: 10.1097/01.prs.0000240810.52392.51. [DOI] [PubMed] [Google Scholar]
- 22.Pannucci CJ, Oppenheimer AJ, Wilkins EG. Practice patterns in venous thromboembolism prophylaxis: A survey of 606 reconstructive breast surgeons. Ann Plast Surg. 2010;64:732–737. doi: 10.1097/SAP.0b013e3181ba57a0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RL, Hemington-Gorse SJ, Dhital SK. Do cosmetic surgeons consider estrogen-containing drugs to be of significant risk in the development of thromboembolism? Aesthetic Plast Surg. 2008;32:743–747. doi: 10.1007/s00266-008-9156-4. [DOI] [PubMed] [Google Scholar]
- 24.Lembitz A, Clarke TJ. Clarifying “never events and introducing “always events”. Patient Saf Surg. 2009;3:26. doi: 10.1186/1754-9493-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaine V, McCarthy C, Kaplan K, et al. Venous thromboembolism following microsurgical breast reconstruction: An objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. 2010 doi: 10.1097/PRS.0b013e318208d025. [DOI] [PubMed] [Google Scholar]
- 26.Murphy RX, Jr, Peterson EA, Adkinson JM, Reed JF., 3rd Plastic surgeon compliance with national safety initiatives: Clinical outcomes and “never events”. Plast Reconstr Surg. 2010;126:653–656. doi: 10.1097/PRS.0b013e3181de1929. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 28.Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 29.Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 30.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen MS. Preventing thromboembolic complications in cancer patients after surgery: A role for prolonged thromboprophylaxis. Cancer Treat Rev. 2002;28:141–144. doi: 10.1016/s0305-7372(02)00043-9. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: A multicenter randomized open-label study. J Thromb Haemost. 2006;4:2384–2390. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 33.Serletti JM. Discussion. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124:496–499. doi: 10.1097/PRS.0b013e3181adcfab. [DOI] [PubMed] [Google Scholar]
- 34.Baba-Ahmed M, Le Gal G, Couturand F, et al. High frequency of factor V Leiden in surgical patients with symptomatic venous thromboembolism despite prophylaxis. Thromb Haemost. 2007;97:171–5. [PubMed] [Google Scholar]
- 35.Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124:490–495. doi: 10.1097/PRS.0b013e3181adcf35. [DOI] [PubMed] [Google Scholar]
- 36.Friedman T, O’Brien Coon D, Michaels VJ, et al. Hereditary coagulopathies: Practical diagnosis and management for the plastic surgeon. Plast Reconstr Surg. 2010;125:1544–552. doi: 10.1097/PRS.0b013e3181d51344. [DOI] [PubMed] [Google Scholar]
- 37.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653–1661. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 38.Shorr AF, Ramage AS. Enoxaparin for thromboprophylaxis after major trauma: Potential cost implications. Crit Care Med. 2001;29:1659–1665. doi: 10.1097/00003246-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Schadlich PK, Kentsch M, Weber M, et al. Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients: Modelling study from the hospital perspective in germany. Pharmacoeconomics. 2006;24:571–591. doi: 10.2165/00019053-200624060-00005. [DOI] [PubMed] [Google Scholar]
- 40.Wilbur K, Lynd L, Sadatsafavi M. Low-molecular-weight heparin versus unfractionated heparin for prophylaxis of venous thromboembolism in medicine patients: A pharmacoeconomic analysis. Clin Appl Thromb Hemost. 2010 doi: 10.1177/1076029610376935. [DOI] [PubMed] [Google Scholar]
- 41. [accessed March 3, 2011];US Food and Drug Administration press release. 2010 Jul 23; Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm220092.htm.
- 42.Miszkiewicz K, Perreault I, Landes G, et al. Venous thromboembolism in plastic surgery: Incidence, current practice and recommendations. J Plast Reconstr Aesthet Surg. 2009;62:580–588. doi: 10.1016/j.bjps.2008.11.109. [DOI] [PubMed] [Google Scholar]
- 43.Venturi ML, Davison SP, Caprini JA. Prevention of venous thromboembolism in the plastic surgery patient: Current guidelines and recommendations. Aesthet Surg J. 2009;29:421–428. doi: 10.1016/j.asj.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 44.McDevitt NB. Deep vein thrombosis prophylaxis. american society of plastic and reconstructive surgeons. Plast Reconstr Surg. 1999;104:1923–1928. doi: 10.1097/00006534-199911000-00052. [DOI] [PubMed] [Google Scholar]
- 45.Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114:43E–51E. doi: 10.1097/01.prs.0000131276.48992.ee. [DOI] [PubMed] [Google Scholar]
- 46.Young VL, Watson ME. Continuing medical education article—patient safety: the need for venous thromboembolism (VTE) prophylaxis in plastic surgery. Aesth Surg J. 2006;26:157–175. doi: 10.1016/j.asj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: Prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim EK, Eom JS, Ahn SH, Son BH, Lee TJ. The efficacy of prophylactic low-molecular-weight heparin to prevent pulmonary thromboembolism in immediate breast reconstruction using the TRAM flap. Plast Reconstr Surg. 2009;123:9–12. doi: 10.1097/PRS.0b013e3181904be7. [DOI] [PubMed] [Google Scholar]
- 49.Lemaine V, McCarthy C, Kaplan K, et al. Venous thromboembolism following microsurgical breast reconstruction: An objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. 2010 doi: 10.1097/PRS.0b013e318208d025. [DOI] [PubMed] [Google Scholar]
- 50.Joynt GM, Kew J, Gomersall CD, Leung VY, Liu EK. Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000;117:178–183. doi: 10.1378/chest.117.1.178. [DOI] [PubMed] [Google Scholar]
- 51.Lapidus L, de Bri E, Ponzer S, Elvin A, Noren A, Rosfors S. High sensitivity with color duplex sonography in thrombosis screening after ankle fracture surgery. J Thromb Haemost. 2006;4:807–812. doi: 10.1111/j.1538-7836.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 52.Sugimoto Y, Ito Y, Tomioka M, et al. Deep venous thrombosis in patients with acute cervical spinal cord injury in a japanese population: Assessment with doppler ultrasonography. J Orthop Sci. 2009;14:374–376. doi: 10.1007/s00776-009-1342-y. [DOI] [PubMed] [Google Scholar]
- 53.Wahl WL, Brandt MM, Ahrns KS, et al. Venous thrombosis incidence in burn patients: Preliminary results of a prospective study. J Burn Care Rehabil. 2002;23:97–102. doi: 10.1097/00004630-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Wibbenmeyer LA, Hoballah JJ, Amelon MJ, et al. The prevalence of venous thromboembolism of the lower extremity among thermally injured patients determined by duplex sonography. J Trauma. 2003;55:1162–1167. doi: 10.1097/01.TA.0000057149.42968.1D. [DOI] [PubMed] [Google Scholar]