Abstract
Clinical reports of limited and treatable cancer metastases, a disease state that exists in a transitional zone between localized and widespread systemic disease, were noted on occasion historically and are now termed oligometastasis. The ramification of a diagnosis of oligometastasis is a change in treatment paradigm, i.e. if the primary cancer site (if still present) is controlled, or resected, and the metastatic sites are ablated (surgically or with radiation), a prolonged disease-free interval, and perhaps even cure, may be achieved. Contemporary molecular diagnostics are edging closer to being able to determine where an individual metastatic deposit is within the continuum of malignancy. Preclinical models are on the outset of laying the groundwork for understanding the oligometastatic state. Meanwhile, in the clinic, patients are increasingly being designated as having oligometastatic disease and being treated owing to improved diagnostic imaging, novel treatment options with the potential to provide either direct or bridging therapy, and progressively broad definitions of oligometastasis.
Keywords: metastasis, therapy, tumor, spectrum theory, diaspora
INTRODUCTION
Hellman first proposed the theory of oligometastases in 1995 as a sequel to the spectrum theory of cancer metastasis. Hellman hypothesized that the process of cancer metastases occurred along a continuum, from locally confined cancer to widely metastatic disease. Although the phenomenon of limited and treatable cancer metastases had been noted historically, Hellman and Weichselbaum proposed the term oligometastases, suggesting that in some patients with a limited number of clinically detectable metastatic tumors, the extent of disease exists in a transitional state between localized and widespread systemic disease. In this model local control (LC) of oligometastases would have the potential to yield improved systemic control, going against the dogma that control of oligometastatic disease would not have a therapeutic benefit since it represents a clinical manifestation of a few detectable lesions in the setting of widespread occult disease. Oligometastasis continues to be defined as a state of metastatic disease that is limited in total disease burden, usually by number of clinically evident or radiographic sites (either 1–3 or 1–5), and that is not rapidly spreading to more sites. The clinical implication of oligometastasis suggests that if the primary site (if still present) is controlled, or resected, and the metastatic sites are ablated (surgically or with radiation), there will be a prolonged disease-free interval, and perhaps even cure. As the understanding of the mechanisms underlying cancer metastasis have evolved, possible mechanisms for the oligometastatic state must be explained and examined within that context.
Biological basis for oligometastasis
Theories of metastasis
Metastasis is the cause of most cancer-related deaths [1]. In 1889, Stephen Paget [2] theorized that circulating tumor cells would “seed” to an amenable “soil”, suggesting that metastasis was not a matter of chance. Five years later, [3, 4], in 1894, Halstead theorized that cancer was an orderly disease that progressed in a contiguous manner, by direct extension from the primary tumor through the lymphatics, to the lymph nodes, and then to distant sites. Halstead proposed that breast cancer metastasis was a progressive, anatomical process of contiguous seeding; his hypothesis supported the use of radical surgery and radiotherapy. Radical en bloc surgery, radical hysterectomy, and primary and regional irradiation for several tumor sites were all based on Halstead's theory of tumor spread [4]. James Ewing, in 1928, complemented the Paget and Halstead theories to propose that cancer cells grow at a particular site because they are directed by the direction of blood flow and lymphatics. [5]
The ‘systemic’ theory of metastasis, first suggested by Keynes [6] and further developed by Fisher [7], held that clinically apparent cancer was a systemic disease, and that small tumors were an early manifestation of systemic disease [8]. In this theory, nodal involvement was not part of an orderly contiguous extension but rather a marker of distant metastases. According to this theory, local control would not impact survival.
In contrast to the ‘Halsted’ theory and the ‘systemic’ theory, the ‘spectrum’ theory of cancer metastases, first described for breast cancer metastases in 1994, held that disease stage at the time of initial disease presentation fell into a spectrum ranging from indolent disease to widely metastatic, with the degree of clonal evolution determining the ability of the tumor to metastasize [9]. The spectrum theory was refined just one year later to describe the limited metastasis of any solid tumor and the term ‘oligometastasis’ was coined.
The spectrum theory conceptualized the entire range of metastatic competence, analogous to a diapason, which is the entire range of an instrument. To that end, the social sciences concept of a diaspora has recently been utilized to inform biologic understanding and therapeutic paradigms of cancer metastasis [10]. A diaspora refers to the scattering or movement of a population from its original homeland. In the case of systemic metastases, the diaspora resembles an imperial colonization in which the populations spread widely and eventually conquer the new host lands (aggressive cancer clones to multiple organs). Oligometastases resemble trading post diasporas, representing a limited number of outposts with limited growth potential (less aggressive cancer clones to few organs). (Table 1, Figure 1). Systemic versus oligometastatic diasporas may be dependent on the types of mutations present in the cancer cells (quality of the diaspora migrants), the quality of the original tumor site (factors in the homeland that cause the population to migrate), and the quality of the new hostland (factors that allow immigrants to establish and flourish) (Table 2).
Table 1. A comparison of migrants, diaspora, and the spectrum of cancer metastases.
| Social Demography | Cancer Demography | ||
|---|---|---|---|
| Imperial Diaspora | Trading Post Diaspora | Trading Post Diaspora → Oligometastasis | Imperial Diaspora → Cancer metastasis |
| Large populations from a single homeland | Small population from a single homeland | Migrated from primary cancer in passive manner | Dispersed from a primary cancer in an active manner |
| Settle multiple countries in aggressive manner | Settle in few countries while avoiding upsetting host country | Mild hypoxia and unlimited nutrients; Home niche conditions do not cause evolutionary clonal pressure | Hypoxia and lack of nutrients cause pressure to leave primary; Evolving home niche conditions cause undifferentiated, aggressive clones. |
| Host country may or may not be receptive | Host country may or may not be receptive | Target organ may or may not be receptive | Target organ may or may not be receptive |
| Group maintains collective memory of their homeland and culture | Group maintains collective memory of their homeland and culture | Pathologists can identify where a cancer cell originated | Pathologists can identify where a cancer cell originated |
| Often assimilate the new homeland | Survive as distinct communities | Few distinct metastases | Multiple metastases as distinct masses |
| Relationship with host country is uneasy and degenerates over time | Relationship with host country may be uneasy but is maintained over time | Immune system may not see a threat | Immune system tries to destroy the cancer cells |
| Tied to the homeland by exchange of resources | Tied to the homeland by exchange of resources | Limited need for outside resources from homeland; fewer cells trafficking | Multiple cell-type trafficking, trafficking of resources/info |
Table adapted from Pienta et al. Clin Can Research, 2013 [10]
Figure 1. Oligometastatic disease versus systemic disease.
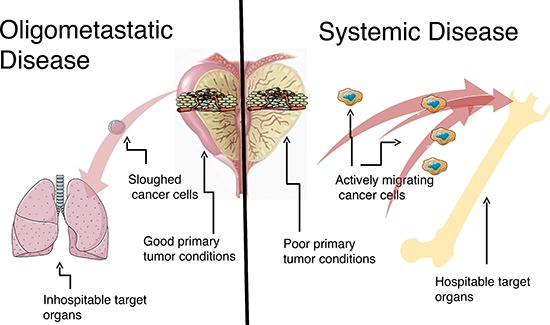
(left) Oligometastatic disease. Metastatic growth potential is limited. This could be (a) secondary to due to environmental conditions in the primary tumor forestalling evolutionary clonal pressure, (b) cancer cells that slough out of the primary tumor that do not have the properties necessary to survive the circulation and invade into target organ sites, and/or (c) the cancer cells land in inhospitable target organs. (right) Systemic disease. Widespread metastatic growth potential is unlimited. This could be (a) secondary to due to environmental conditions in the primary tumor creating many undifferentiated, aggressive clones, (b) cancer cells that actively migrate out of the primary tumor that have the properties necessary to survive the circulation and invade into target organ sites, and/or (c) the cancer cells land in hospitable target organs.
Table 2. Factors that define the rate and success or failure of oligometastases and systemic metastases.
| Cancer dispersion | Quality of primary microenvironment [ΔQO][Δt] | Fitness of migrant cancer cells (EFnΔt) + (MFnΔt) + (SFnΔt) | Quality of metastatic sites (H1QnΔt)) + (H2QnΔt))… |
| Oligometastasis | ΔQO = little change | [(EFnΔt) > (MFnΔt) (SFnΔt)] [TR] | [(H1Qn)) + (H2Qn)] = low |
| Widespread metastasis | ΔQO = decreasing | [(EFnΔt) < (MFnΔt) (SFnΔt)] [TR] | [(H1Qn)) + (H2Qn)) = high |
| Primary microenvironment quality: ΔQO = the changing quality (ΔQ) of the primary cancer site over time (Δt). The quality of the primary microenvironment is dependent on multiple factors, including pH, oxygenation, amount of nutrients, interaction with supporting host cells, and the quality of the immune response. Primary nonlethal, epithelial cancer cells in a highly vascularized environment with rich nutrients are presumed to be less likely to evolve to a more aggressive clone by undergoing an epithelial to mesenchymal transition (EMT) and leave the primary over time. As the quality of the environment decreases, it is likely that the generation of lethal, mesenchymal clones increases. | |||
| Fitness of migrant cancer cells: EFnΔt = the number (n) and fitness (F) of passively shed epithelial cancer cells (E) over time (Δt). This represents the likelihood that a cancer cell passively shed into the circulation will survive transport to a target organ. MFnΔt = the number (n) and fitness (F) of actively emigrant cancer cells (M) over time (Δt). SFnΔt = number (n) and fitness (F) of cancer stem cells (S) over time. It is likely that the fitness of a passively shed cell is less than a migrating mesenchymal cell or stem cell that actively exits the primary tumor through the lymphatics, nerves, or circulation. Fitness depends on many variables, including EMT state, stemness, ability to secrete MMPs, ability to avoid anoikis, etc. | |||
| Quality of metastatic sites: HQn = the quality (Q) of the target organ or host-land sites (H1, H2…). Migrating cancer cells will land in multiple sites (n) within different target organs in order to immigrate. Success depends on the quality of the soil of each of these microenvironments as well as the host immune response. | |||
Table adapted from Pienta et al. Clin Can Research, 2013, [10]
Biology of systemic and oligometastases
It is now widely accepted that there are discrete steps in tumor metastasis. Initially there is a loss in cellular adhesion, followed by increased motility > invasiveness of the primary tumor > entry into and survival in the circulation > entry into new organs > eventual colonization of these organs [11, 12]. Shortfalls at any stage of this metastatic progression could result in phenotypes of limited metastatic potential [13]. Gupta et al. described specific tumorigenic genes – initiation, progression, and virulence genes – that fulfilled specific roles in the metastatic cascade. Initiation genes afford a selective advantage to primary tumor cells to enter circulation. Progression genes fulfill rate-limiting steps for colonization. Virulence genes provide a selective advantage in cells to colonize a secondary site(s) [14]. As Weichselbaum and Hellman noted, this paradigm suggests “that there may be primary tumor cells with a limited capability in one or more of the necessary biological requirements for metastasis; thus proposing a possible biologic explanation of the origin of oligometastases” [15].
In 2000 and updated in 2011, Hanahan and Weinberg proposed the, widely accepted, ‘hallmarks of cancer’ [16]. The original cancer hallmarks consisted of six transformations in cellular physiology that allow cells to survive, proliferate and disseminate, and together support carcinogenesis. The update posited that underlying the original hallmarks were two ‘enabling hallmarks’: ‘genome instability and mutation’ and ‘tumor-promoting inflammation’. Additionally, the update proposed two new ‘emerging hallmarks’: ‘deregulation of cellular energetics’ and ‘avoidance of immune destruction’ [17]. The exact sequence in which the transformations occur, and thus the appearance of the ‘hallmarks’ (self-sufficiency in growth signals ↔ insensitivity to anti-growth signals ↔ tissue evasion and metastasis ↔ limitless replicative potential ↔ sustained angiogenesis ↔ evading apoptosis) can vary throughout the course of progression. Despite the ordering, collectively, the hallmarks can terminate into a cancer. However, the ordering and the degree of specific hallmarks, may potentially allow for a subsequent oligometastatic state. For example, cancer cells that lack the hallmarks to actively metastasize still may be able to slough into the circulation and inefficiently establish metastases. Other cells may be less efficient at proliferation, establishing slow growing metastases. Warburg (1956) suggested that “We may have cells which indeed look like cancer cells but are still energetically insufficient… such cells which are clinically not cancer cells, have lately been found not only in the prostate, but also in the lungs, kidney, and stomach of elderly persons such cells have been referred to as “sleeping cancer cells” [18].
Preclinical models of oligometastasis
Traditional clinicopathologic factors are inadequate when attempting to define the potential underlying biology of oligometastases. Several investigations have demonstrated the marked genetic and epigenetic heterogeneity present in metastatic cancer sites within the same patient [19–22]. These studies demonstrate that cancer cells at different sites within a patient can have varied malignant potential [23–25]. Preclinical models of tumors with varying degrees of metastatic potential, including low metastatic potential exist. Using a cell line derived from B16F1 melanoma, Fidler et al. [26] found that variant metastatic cells pre-exist in a heterogeneous primary tumor as opposed to originating through adaption during metastasis from an otherwise homogenous primary tumor. This finding was advanced in later work that showed the KHT sarcoma line demonstrated similar heterogeneity whether grown in vitro or in vivo, which suggested that clonal variation seen in vitro derived from heterogeneity present in the primary tumor [27]. As an extension of this work in the KHT sarcoma line, it was demonstrated that effective metastatic variants developed at a high rate with low frequency, as opposed to the more frequent and stable subpopulations of metastatic variants [28]. In comparing B16 cell lines, Cillo et al. showed that the more highly metastatic, and less genetically stable cell line, generated increased metastatic variants corresponding to increased chemotherapy resistance [29]. Numerous in vitro studies and analyses of animal models have indicated that cells isolated from metastases differ greatly—both genetically and phenotypically—from cells isolated from their parental primary tumors [30]. The preclinical models point toward variation in individual tumor cells' metastatic potential, which supports the concept of oligometastases [15].
Given that stochastic models have been used to predict biologic phenomena, a Bayesian model has been proposed to predict the chance of occult metastases in the presence of detectable oligometastases [31]. Using the size and number of metastases, the proposed model inferred, (1) that the probability of occult metastases may increase substantially with minor increases in metastatic potential and (2) that extended disease-free periods were predictive of a substantial decrease in additional occult disease. Although compelling, such models are in their infancy and as yet remain in pre-clinical testing where the host, tumor and experimental factors are controlled. [31]
Clinical evidence of oligometastasis
Evidence for the evolution of the oligometastatic phenotype comes from various clinical and pre-clinical sources [26, 32–34]. Recent studies of the molecular biology of renal cell cancer metastasis have implied biologic differences between less and more aggressive metastases, as well as between fewer and multiple metastases. In order to better determine which patients presenting with localized RCC harbor an aggressive tumor and may not benefit from surgery, Kosari et al. using gene expression profiling, found gene expression alterations associated with an aggressive tumor and metastatic potential in the primary tumor [35]. From a cohort of 20 resected pulmonary metastases taken from 18 patients, Wuttig et al demonstrated the predictive potential of identified gene signatures, when comparing disease-free intervals (DFI) and number of metastases, both of which are predictive of prognosis in metastatic RCC (mRCC). There were 306 differentially expressed genes in comparing DFI ≥5 years and DFI ≤9 months, and 135 differentially expressed genes in comparing multiple metastases (≥16) and few metastases (≤8). [36]
In colorectal cancer, there is growing evidence that liver-limited disease is a distinct biological cohort that may benefit from aggressive management. While only a minority of patients are technically resectable, approximately 40% of patients with resected liver limited disease are alive 5 years after diagnosis compared with less than 1% for those with disseminated disease. [37] There is genetic evidence that patients undergoing hepatic resection for metastatic cancer had a different disease than those who did not [37]. For example, it was noted that BRAF V600E mutant tumors, which were typically associated with aggressive biology, rarely came to liver resection [38, 39]. In addition, novel chromosomal aberrations have been identified that are associated with intra- and extra-hepatic recurrence after liver resection [40].
MicroRNAs, small non-coding RNA known to regulate tumor proliferation and apoptosis, are frequently dysregulated in cancer and metastasis [41–43]. MicroRNA profiling has shown a possible method to distinguish patients with oligometastases from those with polymetastatic disease. Examples of pro-metastatic microRNAs include microRNA-10b (upregulated in primary breast tumors that had metastasized), microRNA-21 (correlated with advanced stage, incidence of metastases, and poor outcomes in breast and pancreatic tumors), and microRNA-373/520c (increased expression in breast metastases) [43]. MicroRNA-210, a known transcriptional target of the HIF-1α signaling pathway, was elevated in sera from patients with metastatic castrate-resistant prostate cancer, as compared to controls, and was correlated with treatment response as assessed by change in PSA [41]. Lussiter et al. found microRNA-200c was associated with polymetastatic progression in a oligometastatic cell line, derived from patients treated with high-dose radiotherapy, tested in a xenograft model [44]. The investigators then stratified patients with resected pulmonary oligometastases into subgroups, based on high-risk versus low-risk of further metastatic progression. Differential microRNA expression patterns were identified between these two groups (high rate of progression (n = 16 prioritized microRNAs) and low rate of progression (n = 32 prioritized microRNAs) and, in an independent dataset, the expression patterns were associated with risk of progression and decreased overall survival. [8] Most recently, Uppal et al. identified three microRNAs overexpressed in clinical metastasis samples from patients with limited metastatic disease. MicroRNA-127–5p, microRNA-544a, and microRNA-655–3p were shown to limit, but not fully inhibit, metastasis in a model of breast cancer lung colonization [45].
Controversies surrounding the treatment of oligometastatic disease
Hellman theorized that, 1) whereas some tumors were destined to remain localized, 2) other tumors, as they increased in size, acquired an increasingly greater metastatic phenotype, suggesting at an early stage these tumors seeded distant sites with clones that had not reached full metastatic potential, and finally, 3) that some tumors already had occult distant dissemination at the time they are diagnosed. He also proposed that metastatic potential was not only directed by the tumor phenotype, but that it was also influenced by the tumor's location, venous drainage, and host factors [4, 46]. Based on Hellman's theory, later scientists further theorized that tumors in the oligometastatic disease state were tumors early in their evolution of metastatic progression; therefore they produced metastases that were limited in number and location. These data support the presumption of a temporal evolution with an intermediate stage of limited metastatic capacity, where oligometastatic tumors may not have acquired the broad array of genetic changes required to develop widespread metastases [14, 47, 48].
The clinical implication of the oligometastatic state is that locally ablative therapies, given with the intent of targeting sites of clinically evident metastatic disease could result in long-term survival or cure [15, 49]. Treatment of oligometastatic disease may also result in decreased overall tumor burden, decreasing morbidity and increasing survival. These arguments are opposed by the concept that clinical metastases are evidence of systemic disease and locally directed treatment will not alter the natural history of the disease course within a patient. In this scenario, only systemic therapy may be beneficial. Indeed, oligometastatic treatment paradigms are controversial (due to the limited data available) [4, 34, 50, 51]. Without randomized studies, it is impossible to know if treatment of oligometastatic disease helps the patient. In addition, oligometastatic disease may represent indolent disease that does not require potentially toxic treatments [4, 34, 50, 51].
Patients are increasingly being diagnosed with oligometastatic disease due to the advent of sensitive imaging technologies as well as effective therapies that are allowing patients to live longer with the diagnosis of cancer [34, 52]. In addition, the fact that novel treatment options with acceptable safety profiles, such as stereotactic radiation, cryoablation, and minimally invasive surgery, are available to treat limited metastases, has led to a renewed interest in treating oligometastatic disease. Treatment of oligometastatic disease not only has the potential to prevent further evolution of genetically unstable clones and metastatic spread, it may improve overall disease control and delay more toxic systemic treatment [13, 34, 53, 54]. Finally, the definition of oligometastases had gradually evolved (Table 3), which further inflates the increasing population of patients diagnosed with oligometastasis. In the absence of data to guide decisions, treatment of oligometastatic disease may be seen as a quality-of-life oriented approach, choosing personalized treatments with a reasonable risk to benefit ratio and taking into account the patient's own attitude in guiding them toward more or less, intensive therapy [50].
Table 3. Definitions of Oligometastasis.
| Terms | Definition | Reference |
|---|---|---|
| Oligometastasis | “…metastases (from tumors early in the chain of progression) limited in number and location because the facility for metastatic growth has not been fully developed and the site for growth is restricted…” | [46] |
| Oligometastatic disease | Solitary or few detectable metastatic lesions that are usually confined to a single organ | [50] |
| Oligometastases | Due to limited metastatic competence and does not occur following otherwise successful systemic treatment. New metastases in this situation, albeit even limited, is likely to have more extensive malignant capabilities that were somehow spared from eradication by therapeutic means, or from the development of resistant clones | [15] |
| Induced oligometastases | Occurs when widespread micrometastatic disease is mostly eradicated by systemic chemotherapy but drug resistant clones are left behind, or tumor foci is located in a site not accessed by chemotherapy | [4] |
| Oligorecurrence | Limited metastases in the presence of a controlled primary lesion | [195] |
| Sync-oligometastases | ≤5 metastatic or recurrent lesions in the presence of active primary lesions | [196] |
| Synchronous oligometastasis | Oligometastatic disease is detected at the time of diagnosis of the primary tumor, therefore there is an active primary tumor | [196] |
| Metachronous oligometastasis | Development of oligometastatic disease after treatment of the primary tumor; interval for classification of metachronous versus synchronous is not standardized; between Controlled primary lesion except for concomitant primary and distant recurrence | [196] |
| Oligoprogression | Progression of a limited number of metastatic deposits, while remaining metastases are controlled with systemic therapy | [197] |
| Oligometastasis (specific to prostate cancer) | Rising PSA following primary therapy, with oligometastasis on imaging, in whom local treatment (surgical metastasectomy (usually LN dissection), or SBRT for bony mets or LN recurrence) is required to defer initiation of ADT | [54] |
| Oligometastasis (specific to prostate cancer) | Castrate resistant prostate cancer with a rising PSA and oligometastasis on imaging, in whom local treatment (surgical metastasectomy (usually LN dissection), or SBRT for bony mets or LN recurrence) may allow deferral of ADT | [54] |
Abbreviations: LN = lymph node; SBRT = stereotactic body radiation therapy; mets = metastases; ADT = androgen deprivation therapy; PSA = prostate-specific antigen
Therapeutic options for oligometastases
Radiation therapy
While much of the literature supporting the oligometastatic states is within the surgical literature, there is an increasing body of literature describing the use of stereotactic body radiotherapy (SBRT) and stereotactic radiosurgery (SRS), in addition to conventional fractionated radiotherapy techniques [51]. SBRT is a noninvasive method of delivering high doses of radiation to ablate a target lesion while sparing the neighboring normal tissue, thus reducing long-term effects of radiation on the non-malignant tissues. The radiation is delivered from many beams originating from multiple directions that converge on the target site. [55] Through improved targeting and management of tumor motion, SBRT may improve tumor control and reduce treatment-related toxicity, as compared to conventional fractionated RT. Improved radiation targeting allows for higher-dose, hypofractionated, more efficient treatment regimens that can be delivered within narrow margins sparring adjacent organs. [56] ‘Hypofractionation’ is the delivery of large doses of radiation over a shorter time period as compared to conventional radiation fraction sizes. Therapy can generally be completed in 1–5 sessions, as compared to conventional radiation therapy that is delivered in smaller doses 5 days/week over ≥ 6 weeks. [55]
SBRT can be used to manage oligometastatic disease presentations that would be associated with added morbidity if managed by surgery, such as deep-seated or osseous lesions. Patients who are poor surgical candidates may often be treated with SBRT, given that it is noninvasive and has a modest morbidity profile. Whereas surgery, when used to manage oligometastatic disease, tends to be seen as the gold standard as it allows for pathologic evaluation and assessment of the surgical margins. In addition, lesions greater than 7–8 cm and those difficult to target with SBRT, are better left for surgical management. [55]
Although traditionally radiation therapy was thought to be immunosuppressive, there is increasing pre-clinical and clinical evidence that high dose, hypofractionated radiation –SBRT-may reverse antitumor immunity via CD8+ T-cells and cellular stress signals. [55] Although uncommon, the abscopal effect (regression in tumors distant to the targeted field of radiation) is an example of recovery of anti-tumor immunity following RT [57]. While the exact mechanism is unknown, it has been proposed that the radiation effect may result in the release of anti-tumor proliferative antigens and cytokines. Matzinger [58] theorized a ‘danger model’, which suggested that the immune system is stimulated by injured tissues rather than ‘non-self’, therefore damaged tissues in irradiated sites may stimulate the immune system. Lee et al. [59]demonstrated in a murine model that hypofractionated, high dose RT may possibly reverse T-cell unresponsiveness to primary and metastatic tumors. More specifically, considering that local RT to a tumor may modify its microenvironment by producing inflammatory cytokines that may increase its immune responsiveness, Lugade et al. demonstrated that IFN-γ (a cytokine), following RT, promotes T cell function [60]. It is thought that a tumor has ongoing crosstalk with the immune system-from one end of a spectrum where the immune system eliminates the tumor, to tumor-immune system equilibrium (a subclinical tumor), until selective pressure from the immune system stimulates the evolution of tumor cells resistant to the immune system (clinically detectable). A way to think of how RT may promote immune recovery is that the irradiated tumor is converted into a ‘vaccine’ that promotes tumor specific T cells to bestow immune memory against non-treated tumors. [57]. Thus, an emerging advantage of SBRT is the possibility that it may be exploited to benefit the immune system.
Surgery
Historically, most of the surgical data in regards to oligometastatic disease is centered on hepatic resection. [37]. Perioperative mortality related to hepatic resection has decreased from 20% (before 1980) to 1%. Recent improvement in overall survival, following hepatectomy, are likely from improvements in patient selection (shift in definition of resectability to new criteria based on whether a macroscopic and microscopic complete resection of the liver lesion as well as complete resection of any extrahepatic disease), surgical technique, and more effective adjuvant therapy. The use of portal vein embolization and of neoadjuvant chemotherapy have also expanded the population of patients who are eligible for resection. [61] In a recent series of patients having undergone hepatic resection, despite progression on chemotherapy, the 5-yr survival was 53% [51].
Secondary resection remains a worthwhile therapeutic goal; patients brought to resection by systemic therapy enjoy comparable long-term survival to patients who had resectable disease at the time of presentation, and far superior to those receiving palliative systemic chemotherapy [62]. 10–20% of patients that develop colorectal liver metastases present with, or are converted by systemic treatment to, an oligometastatic state defined as metastatic lesions that are limited in number and involving only a single organ. This type of disease is potentially amenable to local therapeutic modalities, of which hepatic resection is the most effective. [51]
Cryotherapy
Cryotherapy has been utilized in multiple settings for the ablation of metastatic disease [63, 64]. In the largest series published to date, Littrup and colleagues treated a total of 251 oligometastatic tumors from multiple primary cancers in 126 patients [65]. Sites of treatment included retroperitoneal, superficial, intraperitoneal, bone, and head and neck; average diameter of tumors was 3.4 cm. At 11 months average follow-up (range, 0–82 months), a 10% total recurrence rate (26 of 251) was noted; three occurred within the ablation zone, for a local progression rate of 1.2%. The average time to recurrence was 4.9 months, and, at 21 months, the initial ablation zone had reduced in volume by 93% [65].
Treatment of oligometastases in different cancers
Anecdotal reports of tumors with limited metastases, having undergone treatment to the metastases and had long-term response date back to the 1930's [66]. Several studies, with a variety of endpoints and meeting different levels of evidence criteria, have been performed in a variety of cancers to treat oligometastatic disease. For each clinical study reviewed, we considered the definition of oligometastasis that was used, the therapy given, and sample size. In order to provide an ordinal categorization to assess the strength of the study designs and of the study endpoints, we used the National Cancer Institute Levels of Evidence for Adult and Pediatric Cancer Treatment Studies ranking system to assess each study [67]. In this system, the strength of the studies in descending order is: randomized controlled trials (double-blinded): Level 1i > randomized controlled trials (non-blinded): Level 1ii > non-randomized controlled clinical trials: Level 2 > case series (population-based, consecutive series): Level 3i > case series (consecutive cases (not population-based)): Level 3ii > case series (nonconsecutive cases): Level 3iii. The system ranks the study endpoints in descending order of strength as follows: total mortality: Level A > cause-specific mortality: Level B > quality of life: Level C > event-free survival: Level Di > disease-free survival: Level Dii > progression-free survival: Level Diii > tumor response rate: Level Div.
Breast cancer
Metastatic breast cancer (MBC) at diagnosis constitutes 3.5–7% of all new breast cancers [68], while oligometastases comprises 1–3% of the total MCB population [50]. Traditionally MBC patients were managed primarily with systemic modalities, with limited local treatments given only for palliative intent.
We searched PubMed using the terms ‘breast oligometastases’, ‘breast oligometastasis’, and ‘oligometastatic breast cancer’; results were as follows, n = 20, n = 5, and n = 51, respectively. We omitted reviews (including special features), studies including mixed primary tumors, case reports, studies not in the English language, studies not focused on oligometastatic breast cancer, and a survey. There were seven clinical studies remaining; oligometastases held six definitions and each study used a different therapy (Table 4).
Table 4. Oligometastatic breast cancer.
| 1st Author, year [Ref] | Strength of evidence (study design /endpoint) | Prospective (P) or Retrospective (R) | Sample size | Definition-Oligometastases | Therapy | Endpoint | Conclusion |
|---|---|---|---|---|---|---|---|
| Kobayashi, 2012 [71] | 3ii /A | R | 75 | 1–2organs with met lesions, ≤5 lesions/organ, ≤5cm lesion diameter | +/− CT, then +/− local therapy + CT | 10yr OS-59.2%; 20yr OS 34.1% | Prognosis of OMBC superior to that of MBC |
| Bojko, 2004 [69] | 3iii / A | P | 48 | 1 organ with 1-few met lesions | Surgery or RT + CT, then peripheral-blood-stem-cell transplant | MOS-42.2 mths | Combined modality therapy safe in OMBC; promising relapse-free survival |
| Milano, 2009 [70] | 3iii / A | P | 40 | ≤5 met lesions | Curative-intent SBRT | 4yr OS-59%; MOS- NR | SBRT may yield prolonged survival + perhaps cure in select OMBC |
| Mimoto, 2014 [72] | 3iii /A | R | 14 | 1–2 organs with met lesions, ≤5 lesions/organ, ≤5cm lesion diameter | Surgery | 10yr OS-59.2%, 20yr OS-34.1%; CD44+/CD24–/low tumor cells in 9% OMBC versus 73% non-OMBC | In OMBC, low levels of cancer-initiating cells may be associated with better prognosis |
| Vander Walde, 2012 [73] | 3iii /A | R | 12 | ≤3 sites | CT, then peripheral stem cell rescue | 3-yr OS- 73% | Therapy was safe |
| Nieto, 2002 [75] | 3iii /A | R | 60 | Low tumor burden, w met lesion could be either excised en bloc before HDC, or encompassed w a single RT field w curative intent. | CT | MOS- 80 mths; 5-yr OS 62% | Possibly re-evaluate current tenet that early detection MBC is of no benefit |
| Bourgier, 2010 [74] | 3iii /D | R | 159 | 1 met site | RT versus RT + surgery | 3yr OS- RT- 39% versus RT+ surg-57%; equivalent when adjusted for prognostic factors | In sub-analysis, OMBC had better metastatic PFS as compared to patients with >1 met site |
Abbreviations: Met(s) = metastasis (es); CT = chemotherapy; yr = year; OS = overall survival; OMBC = oligometastatic breast cancer; MBC = metastatic breast cancer; RT = radiation therapy; MOS = median overall survival; NR = not reached; mths = months; SBRT = stereotactic body radiation therapy; HDC = high dose chemotherapy; PFS = progression free survival
The evidence for treatment of oligometastases in breast cancer is weak based on the study designs. There were two prospective studies [69, 70], however they were both nonconsecutive case series (Level 3iii) as were the remaining studies, with the exception of one consecutive case series (Level 3ii) [71]. There was a conglomerate of therapies given for patients diagnosed with oligometastases using various definitions. The fact that overall survival endpoints were reported in all studies was helpful (Level A, n = 7 studies) [69–75], however, they were not always significant — stronger study designs may have shown whether the survival endpoints were significant. Overall survival (OS) was reported at various intervals making comparisons between studies difficult: median (42.2 months) [69], 20-yr (34%) [71, 72], 5-yr (62%) [75], 4-yr (59%) [70] and 3-yr (73% [73] and 39–57%) [74]).
Lung cancer
Non-small cell lung cancer (NSCLC) is the leading cause of death worldwide with > 50% of patients having metastatic disease at diagnosis [76, 77]. The primary treatment for most patients with metastatic NSCLC is palliative chemotherapy (CT), which results in median survivals of 8–11 months [78]. However, multiple studies have demonstrated a subset of long-term survivors with OMLC [76, 77]. Additionally, curative outcomes were documented in patients with treated adrenal metastases [79].
We searched PubMed using the terms ‘lung oligometastasis’, ‘lung oligometastases’, and ‘oligometastatic lung cancer’; results were as follows, n = 14, n = 90, n = 123, respectively. We omitted reviews, mixed primary tumors, studies not focused on oligometastatic lung cancer, case reports, editorials, studies not in the English language, surveys, commentaries, cost analyses, studies including less than 10 patients, and studies we were unable to retrieve. There were twenty clinical studies remaining (Table 5); oligometastasis held 17 different definitions and each study had a different treatment paradigm.
Table 5. Oligometastatic lung cancer.
| 1st Author, Year [Ref] | Strength of evidence-based on study design / endpoint | Prospective (P) or Retrospective (R) | Sample size | Definition-Oligometastases | Therapy | Endpoint | Conclusion |
|---|---|---|---|---|---|---|---|
| DeRuysscher, 2012 [81] | 2 /A | P | 39 | <5 synchronous mets | Local trt to mets | MOS-13.5 mths. 3yr OS- 17.5% | Subgroup with synchronous OM may benefit from radical trt |
| Collen, 2014 [80] | 2 /A | P | 26 | ≤5 met lesions | SBRT to primary and all mets | MOS-23 mths. 1yr OS- 67% | SBRT acceptable option and results in acceptable PFS |
| Khan, 2006 [83] | 3i /A | R | 23 | 1–2 sites | CT + local-regional therapy | MOS- 20 mths | Subset of pats may benefit from aggressive local, regional, and systemic treatment |
| Nieder, 2014 [82] | 3i /A | R | 23 | maximum of 3 metastases to 1 organ | ‘Active therapy’, irrespective of specific treatment received | MOS- 11.7 mths for OM and 5.6 mths for advanced mets | Prospective studies for this population are warranted |
| Guerra 2012 [85] | 3ii/A | R | 78 | <5 mets at diagnosis | Definitive CRT to primary + mets | 3yr OS-25% | Tumor volume, KPS, + at least 63Gy to primary tumor are associated with improved OS in OM NSCLC |
| Ashworth, 2014 [84] | 3ii /A | R | 757 | Hx of curative trt to primary and w 1–5 mets treated w surgery, RT or XRT | Controlled primary tumor and locally ablative treatments to all mets | MOS-26 mths, 5yr OS- 29.4%; | Significant OS differences in OM according to type of metastatic presentation and N status |
| Collaud, 2012 [86] | 3iii /A | R | 29 | Synchronous single organ met | Lung resection and local trt to mets | 1yr OS- 65%, 5yr OS- 36%; MOS- 20.5 mths | Multimodality trt including lung resection should be considered in select pats |
| Congedo, 2012 [87] | 3iii /A | R | 53 | Resected primary with 1–2 met lesions considered to be resectable | Trt with curative intent | 5yr OS- 24%, MOS- 19 mths | Surgical trt for selected patients is feasible and safe |
| Hasselle 2012 [88] | 3iii/A | R | 25 | ≤5 mets | Hypofractionated image-guided RT (HIGRT) | MOS- 22.7 mths; 18mth OS- 52.9% | HIGRT for OM NSCLC provides durable control in ≤2 lesions |
| Ashworth, 2013 [76] | 3iii /A | R | 2176 | 1–5 mets | Surgery, SART or SRS | 5yr OS-8.3–86%, MOS- range- 5.9–52 mths | Survival times for OM were highly variable, however long-term survivors do exist. |
| Griffioen, 2013 [89] | 3iii /A | R | 61 | 1–3 synchronous mets | Radical trt (Surgery or RT) to primary and mets | MOS- 13.5 mths; 2yr OS- 38% | Radical trt to selected pats can result in favorable 2yr survival |
| Yano 2013 [90] | 3iii/Diii | R | 13 | Completely resected NSCLC, with post-op recurrence, excluding secondary lung site. 1–3 distant mets, not brain only | Resection or RT of mets versus CT of mets | Median PFS resection/RT-20 mths; Median PFS for CT was 5 and 15 mths, respectively | Local therapy is a choice for 1st line treatment in post-op OM recurrence |
| Yu 2013 [91] | 3iii/A | R | 18 | EGFR-mutant lung cancer previously treated with erlotinib or gefitinib, then progression on EGFR TKI therapy, (<5 sites disease) | RT, RFA, or resection of a site of progressive disease | MOS from local therapy was 41 mths | EGFR-mutant lung cancers q acquired resistance to EGFR TKI therapy are amenable to local therapy to treat OM disease when used in conjunction with continued EGFR inhibition |
| Endo, 2014 [98] | 3iii/A | P | 20 | single-organ met, or single-organ metachronous met s/p resect path T1–2N0–1 lung cancer | Resection primary tumor and mets | 5yr OS-44.7% | Resection of primary tumor and mets had outcomes comparable to stage II patients |
| Gray, 2014 [92] | 3iii /A | R | 66 | 1–4 synchronous brain mets | Aggressive thoracic therapy (ATT) Surgery or CRT versus no-ATT | MOS-26.4 mths for ATT versus 10.5 mths no-ATT | Aggressive management of thoracic disease in OM NSCLC associated with improved survival |
| Cheufou, 2014 [93] | 3iii /A | R | 37 | Synchronous single brain met | Resection cerebral mets and primary tumor | 2yr OS- 24% | No increased risk of complication or mortality; median survival encouraging |
| Parikh, 2014 [94] | 3iii /A | R | 186 | ≤5 synchronous distant met lesions | Definitive primary therapy | MOS-17 mths for OM versus 14 mths for advanced disease; Among OM, MOS- 19 mths for definitive therapy versus 16 mths for no definitive therapy | Definitive therapy to primary tumor may provide survival benefit |
| Sheu, 2014 [95] | 3iii/A | R | 90 | ≤3 synchronous mets | CT, then Surgery or RT before disease progression. Then +/−comprehensive local therapy (CLT) | MOS- 22.3 mths; 1yr OS- 75% | CLT associated with improved OS and PFS with matched analysis using propensity score's |
| Tonnies, 2014 [96] | 3iii/A | R | 99 | Solitary hematogenous metastasis within 3 mths of primary resection | Primary NSCLC curatively resected; then metastasectomy | 5yr OS- 38% | Metastasectomy for synchronous OM NSCLC can be performed in selected patients |
| Ouyang, 2014 [97] | 3iii/A | R | 95 | Not defined | 3DRT + CT | 3yr OS-15.8% | Radiation dose ≥63Gy and having bone only mets associated with better OS; aggressive thoracic radiation may play a role in improving OS |
Abbreviations: Met(s) = metastasis(es); Trt = treatment; MOS = median overall survival; OM = oligometastases; SBRT = stereotactic body radiation therapy; mths = months; PFS = progression free survival; CT = chemotherapy; CRT = chemoradiation therapy; SART = Stereotactic ablative radiation therapy; KPS = Karnofsky performance status; OS = overall survival; NSCLC = non-small cell lung cancer; Hx = history; RT = radiation therapy; XRT = external radiation therapy; N = node; EGFR = epidermal growth factor receptor; TKI = tyrosine kinase inhibitor; RFA = radio-frequency ablation
The evidence for treatment of oligometastases in lung cancer was weak-to-moderate based on the study designs. There were two nonrandomized controlled clinical trials (Level 2) [80, 81], however the sample sizes were small (n = 26 and n = 39, respectively). There was one large study (n = 2176), although it was retrospective and a nonconsecutive case series (Level 3iii) [76]. Of the remaining 17 studies, two were population-based consecutive case series (Level 3i) [82, 83], two were non population–based consecutive case series (Level 3ii) [84, 85], and twelve were nonconsecutive case series (Level 3iii) [76, 86–97], one of which was prospective [98]. Therapies given varied not only among the studies but within most of them as well, for patients with diversely defined oligometastases. Overall survival endpoints (level A) were reported in all but one study [90], however stronger study designs would have shown whether the survival endpoints were significant. OS was reported as MOS (11.7 [82], 17 [94], 20 [83], 26.4 [92], and 41months [91] and at four intervals in the remaining studies: 5-year (29.4% [84], 8.3–86% [76], 44.7% [98], 38% [96], 36% [86], and 24% [87]), 3-year (17.5% [81], 25% [85], and 15.8% [97]), 2-year (24% [93]and 38% [89]), 18-month (52.9%) [88] and 1-year (67% [80]and 75% [95]).
Melanoma
Approximately 30% of patients with melanoma will develop metastases. The 5-yr survival of stage IV melanoma is about 5%.
We searched PubMed using the terms ‘melanoma oligometastasis’, ‘melanoma oligometastases’ and ‘oligometastatic melanoma’; results were as follows, n = 5, n = 5, and n = 15, respectively. We omitted reviews, studies including mixed primary tumors, and studies not focused on oligometastatic melanoma. An additional search through the bibliographies of the review papers allowed us to retrieve one additional clinical study for review, therefore we reviewed two clinical studies (Table 6). The two studies differed in their therapies, which included resection [99], and SRS followed by +/− immunotherapy [100], and definitions of oligometastases.
Table 6. Oligometastatic melanoma.
| 1st Author, Year [Ref]b | Strength of evidence-based on study design / endpoint | Prospective (P) or Retrospective (R) | Sample size | Definition-Oligo metastases | Therapy | Endpoint | Conclusion |
|---|---|---|---|---|---|---|---|
| Essner, 2004 [99] | 3i /A | R | 877 | 1 met | Curative surgery | 5yr OS- 29 mths if mets 1 site, 16 mths if mets 2–3 sites, 14 mths if met ≥4 sites. 5yr OS- 17% disease-free if distant mets in <36 mths, 30% if >36 mths | Pats with limited mets should be considered for curative resection |
| Knisely, 2012 [100] | 3iii /A | R | 77 | Brain mets treated with SRS | SRS to brain mets, then 35% of group received ipilimumab | MOS- 21.3 mths in ipilimumab group versus 4.9 mths in no-ipilimumab group. 2yr OS- 47% in ipilimumab group and 19.7% in no-ipilimumab group | Survival of patients with melanoma and brain mets managed with ipilimumab + SRS can exceed expected 4–6 mths |
Abbreviations: Met(s) = metastasis(es); OS = overall survival; SRS = stereotactic radiosurgery; MOS = median overall survival
The evidence for treatment of oligometastases in melanoma is weak based the two, retrospective studies conducted. Essner et al. was a population-based consecutive case series (Level 3i), which included a large sample size (n = 877), and determined factors prognostic for increased survival [99]. The second study was a nonconsecutive case series (Level 3iii) with a more moderate sample size [100]. The two studies differed in their therapies, definitions of oligometastases, and interval endpoint of OS. Although OS was reported in both studies (Level A), it was reported in different intervals; 2-year (19.7–47%) [100] and 5-year (17–30%) [99].
Colorectal cancer
Colorectal cancer is the fourth most common cancer diagnosis in the world (around 1.2 million diagnoses each year), and accounts for the second highest number of deaths [37]. Nearly one-fourth of patients with newly diagnosed colorectal cancer (CRC) will present with synchronous liver metastases [101, 102]. Hepatic resection is considered a standard treatment option for metastatic CRC and can result in 10-year survival rates of 20–26%, and potential cure [103–109].
We searched PubMed using the terms ‘colorectal cancer oligometastasis’, ‘colorectal cancer oligometastases’, and ‘oligometastatic colorectal cancer’; results were as follows, n = 5, n = 24, and n = 28, respectively. We omitted reviews, studies including mixed primary tumors, studies not focused on oligometastatic colorectal cancer, case reports, perspectives, pre-clinical reports, and earlier reports if later reports for the same study were available. There were nine studies remaining (Table 7); oligometastasis held 9 different definitions and each study had a different treatment paradigm.
Table 7. Oligometastatic colorectal cancer.
| 1st Author, Year [Ref] | Strength of evidence- based on study design / endpoint | Prospective (P) / Retrospective (R) | Sample size | Definition- Oligo metastases | Therapy | Endpoint | Conclusion |
|---|---|---|---|---|---|---|---|
| Engels, 2012 [104] | 2 /A | P | 24 | ≤ 5 mets | Resected primary tumor, and inoperable mets treated with helical tomotherapy | 1yr OS- 78% | Helical tomotherapy is an attractive for consolidation of inoperable OM disease after effective chemo |
| Dellas, 2012 [110] | 2/ D | P | 9 | 1–3 mets or local recurrence plus max. 2 mets | CT + 3D-CRT to all met lesions | 3/9 pats survived 3.5–4.4 yrs; DLT not documented | 3D-CRT to mets feasible in addition to standard CT |
| Van den Begin, 2014 [113] | 3ii/A | R | 47 | Resected primary + ≤5 mets, liver, lung, LNs | SBRT | 1yr OS- 53% | Nature + location of local recurrences demonstrated need for breathing management and dose >75Gy. |
| Filippi, 2014 [114] | 3ii/A | R | 40 | Resected primary + 1–5 lung mets, max diameter <5cm | 3D conformational RT or image guided volumetric modulated arc therapy | 5yr OS- 39% | Suggests stereotactic ablative RT is safe + efficacious in CRC with lung oligometastases |
| Kang, 2010 [117] | 3iii /A | R | 59 | 1–4 met lesions confined to 1 organ, largest <7cm, progressive after CT | Met lesions progressed after chemo, then treated SBRT | 5yr OS- 29% | Patients generally fare well after SBRT |
| Salah, 2012 [112] | 3iii /A | R | 927 | Underwent lung metastasectomy | Lung metastasectomy | 5yr OS- 54.3%. Prognostic risk groups good-, intermediate-, and high-, had 5yr OS- 68%, 46%, 26% | More studies need to investigate if surgery offers advantage over CT in the poor-risk group |
| Bae, 2012 [116] | 3iii/A | R | 41 | Met lesions confined to 1 organ | 3 fractions SBRT | 5yr OS- 38% | SBRT results comparable with Surgery |
| Salah, 2013 [115] | 3iii /A | R | 148 | Repeat resection of pulmonary mets | Repeat resection of lung met | 5yr OS- 52% for 1 lung met resection, 58% 2 lung met resection. >2 lung mets + ≥3 cm risk factors for decreased survival | In selected patients, repeated pulmonary resection offers good survival outcome |
| Comito, 2014 [111] | 3iii/A | P | 82 | 1–3 inoperable mets in 1 organ (Liver or lung) | SBRT | 3yr OS- 43% | SBRT safe + feasible treatment for mets not amenable to resection |
Abbreviations: Met(s) = metastasis(es); OS = overall survival; CT = chemotherapy; CRT = chemoradiation therapy; DLT = dose-limiting toxicity; SBRT = stereotactic body radiation therapy; LNs = lymph nodes; RT = radiation therapy; CRC = colorectal cancer
The evidence for treatment of oligometastases in colorectal cancer is weak-to-moderate based on the study designs. There were two prospective, nonrandomized controlled clinical trials (Level 2) studies [104, 110], however both with small sample sizes (n = 24 and n = 9, respectively) and one prospective nonconsecutive case series (Level 3iii) study with a moderate sample size (n = 82) [111]. Salah et al. conducted a pooled analysis of 8 studies with a large sample size (n = 927) and reported 5-yr survival, however it was retrospective and a nonconsecutive case series (Level 3iii) [112]. The remaining five studies were (non population-based consecutive case series (Level 3ii) [113, 114] and Level 3iii [115–117]. Overall survival endpoints (Level A) were reported in all but one study [110], however stronger study designs would have shown whether the survival endpoints were significant. OS was reported at three intervals: 5-year (52–58%) [115], 54.3% [112], 39% [114], 38% [116] and 29%) [117], 3-year (43%) [111], and 1-year (78%) [104] and 53%) [113].
Sarcoma
Soft tissue sarcomas are relatively rare with about 10, 000 cases diagnosed each year in the United States. If diagnosed early, the prognosis is excellent, however, in some subtypes (of which there are greater than 50), up to 50% of patients will develop distant metastases and will have a 5-year survival of below 10%. The median overall survival for patients with advanced and metastatic soft tissue sarcoma is about 12 months [118].
We searched PubMed using the terms ‘sarcoma oligometastasis’, ‘sarcoma oligometastases’, and ‘oligometastatic sarcoma’; results were as follows, n = 3, n = 3, and n = 13, respectively. We omitted reviews, studies including mixed primary tumors, studies not focused on oligometastatic sarcoma, and case reports. There were two clinical studies remaining (Table 8); both studies used different definitions of oligometastasis and a different treatment paradigm.
Table 8. Oligometastatic sarcoma.
| 1st Author, Year [Ref] | Strength of evidence-based on study design / endpoint | Prospective (P) or Retrospective (R) | Sample size | Definition-Oligometastases | Therapy | Endpoint | Conclusion |
|---|---|---|---|---|---|---|---|
| Falk, 2014 [119] | 3ii /A | R | 281 | 1–5 lesions, any site | 164/281 pats received local treatment (surgery, radiofrequency ablation, or RT | MOS- was 45.3 for local treatment group and was12.6 mths for non-local treatment group | Local ablative treatment seemed to improve OS. Surgery yielded most relevant results, alternative approaches were promising |
| Rhomberg, 2008 [120] | 3iii/Diii | R | 16 | <7 distant mets | Received CT + RT +/− surgery; | Median survival until 1st distant mets- 17 mths in OM sarcoma versus 9 months in control group | Razoxane, vindesine + RT feasible in early met soft tissue sarcoma; inhibits development of remote mets in most patients |
Abbreviations: RT = radiation therapy; MOS = median overall survival; OS = overall survival; mets = metastases; CT = chemotherapy; OM = oligometastases
The evidence for oligometastases in sarcoma was weak based on the two retrospective studies available for review. Both studies were nonconsecutive case series (Level 3iii). Falk et al. included a moderate sample size (n = 281) and MOS was reported (45.3%) (Level A) [119]. The second study was small (n = 16) and the endpoint was time to progression (17–months) (Level Diii) [120].
Renal cell carcinoma
Approximately 25–30% of patients diagnosed with renal cell carcinoma have metastatic disease at initial presentation. Approximately 1/3 with clinically localized primary tumor at diagnosis will eventually develop metastatic disease [121]. Historically patients with metastatic RCC (mRCC) have a poor prognosis, with 5-yr survival rates of ≤ 10%, however prolonged survival has been noted in those with solitary or oligometastatic disease amenable to resection. RCC is often considered resistant to cytotoxic chemoconventional RT and cytokine-based immunotherapy. The lung is the most common site of metastases in mRCC; the second most common is bone. SABR is being suggested as a potential new therapeutic option.
The standard of care in mRCC is systemic therapy; however, in patients with solitary or limited metastases, aggressive local therapies may potentially prolong survival. The literature suggests a survival benefit with surgical metastasectomy, with a reported 5-year survival as high as 45% in those who achieve complete resection. [55]
We searched PubMed using the terms ‘renal cell carcinoma oligometastasis’, ‘renal cell carcinoma oligometastases’, and ‘oligometastatic renal cell carcinoma’; results were as follows, n = 1, n = 9, and n = 3, respectively. We omitted reviews, studies including mixed primary tumors, and studies not focused on oligometastatic renal cell carcinoma. There were three clinical studies remaining (Table 9); each study used a different definition of oligometastases and a different treatment paradigm.
Table 9. Oligometastatic Renal Cell Carcinoma.
| 1st Author, Year [Ref] | Strength of evidence-based on study design / endpoints | Prospective (P) or Retrospective (R) | Sample size | Definition-Oligometastases | Therapies | Endpoints | Conclusion |
|---|---|---|---|---|---|---|---|
| Mickisch, 2001 [125] | 1ii /A | P | 85 | N/A- patients identified as having metastatic RCC | Surgery + interferon OR interferon only | TTP (5 versus 3 mths) + MOS (17 versus 7 mths) in Surgery + interferon versus interferon only | Radical nephrectomy before interferon-based immunotherapy may delay TTP and improve survival in mRCC |
| Flanigan, 2001 [126] | 1ii /A | P | 241 | N/A- patients identified as having metastatic RCC | Surgery followed by interferon OR interferon alone | Surgery followed by interferon MOS- 11.1 mths versus interferon alone MOS-8.1 mths | Nephrectomy followed by interferon had longer survival |
| Bang, 2012 [124] | 3iii/A | R | 27 | Localized soft tissue mass <7cm + ≤5 lesions in 1 organ | Cryoablation | 5yr OS- 27% | Multisite cryoablation of OM RCC associated with low morbidity and low recurrence with apparent increased OS |
| Ranck, 2013 [122] | 3ii/A | R | 18 | Limited metastatic disease | SBRT: 3 fractions or 10 fractions | 2yr OS- 85% | SBRT produces promising lesion control with minimal toxicity |
| Thibault, 2014 [123] | 3iii/A | R | 13 | <5 spinal mets | SBRT | 1yr OS- 83.9% in OM RCC (n = 13) versus 52.5% in non-OM RCC (n = 24) | Multivariate analysis identified OM RCC as a prognostic factor for survival. OM RCC may benefit the most from aggressive local therapy |
Abbreviations: TTP = time to progression; mRCC = metastatic renal cell carcinoma; MOS = median overall survival; OM = oligometastatic; OS = overall survival; SBRT = stereotactic body radiation therapy; mets = metastases
The evidence for treatment of oligometastases in renal cell carcinoma is weak based on the study designs. There were three retrospective studies with smaller sample sizes; a non population-based consecutive case series (Level 3ii) [122] and two nonconsecutive case series (Level 3iii) [123, 124]. Overall survival endpoints (Level A) were reported in at three intervals: 5-year (27%) [124], 2-year (86%) [122], and 1-year (83.9%) [123].
Additional searching through the bibliographies of other selected papers allowed us to retrieve two clinical studies that investigated the effects of removal of the primary tumor in patients with metastatic renal cell carcinoma (mRCC) (Table 9) [125, 126]. The evidence for removal of the primary tumor in mRCC was strong; both studies were nonblinded randomized controlled clinical trials (Level 1ii), had moderate sample sizes, and reported median OS (17 versus 7 months, [125] and 11.1 versus 8.1 months [126]) (Level A). The therapies given in both studies were similar: nephrectomy + interferon versus interferon only [125] and nephrectomy was followed by interferon versus interferon only [126]. The evidence for oligometastases in RCC was not assessed in these studies. However, they were important studies owing to strong design, with comparable treatments and endpoints, and most importantly they demonstrated that removal of the primary tumor in metastatic disease increased survival.
Prostate cancer
There is a body of evidence, contrary to historical clinical practice, that treating men with metastatic prostate cancer to the lymph nodes at the time of diagnosis with surgery or radiation therapy results in increased long-term survival (Table 10), [127–131] and Table 11 [101, 132]. Historically, the ideal patient to be cured by radical prostatectomy (RP) was one with organ-confined cancer. At that time, the morbidity of RP was substantial; therefore the surgery was generally only offered to those where the probability of cure was high. More recent data, however, show that RP may provide a survival benefit-albeit not a cure — to men with metastatic prostate cancer. Engel et al. [101] conducted a retrospective study (n = 938) and found that in men with prostate cancer and positive lymph nodes, treated with +/− RP, that the 5- and 10-yr survival rates and the prostate cancer specific survival rates, were better in men who had undergone RP. Cadeddu et al [132] conducted a retrospective study (n = 38) and found that in men with prostate cancer and positive lymph nodes, treated with LND +/− RP, that the 5- and 10-yr prostate cancer specific survival was better in men who had undergone LND + RP. Ost et al (2014) conducted a systemic review of the literature of metastasis-directed therapy of regional and distant recurrences after curative treatment for PCa (prostate cancer). They found that salvage LND and RT appear to be safe in treatments for OM PCa recurrence. [133] Culp et al. [134] studied the impact of survival of definitive treatment of the prostate in men diagnosed with metastatic PCa. Using the SEER database, he reviewed 8185 men treated with NSR (no surgery no radiation), brachytherapy, or RT. The 5-year OS and disease specific survival (DSS) was significantly higher in men with metastases having undergone RT.
Table 10. Prostate cancer (staged T1c-T3b [198]).
| 1st Author, Year [Ref] | Study population | Strength of evidence-based on study design / endpoint | Prospective (P) / Retrospective (R) | Sample size | Therapy | Endpoints | Conclusion |
|---|---|---|---|---|---|---|---|
| Widmark (2009) [127] | Locally advanced | 1ii /A | P | 875 | Primary: HT +/− RT | 10yr PCa specific mortality in ET + RT group and the ET alone group was 11.9% and 23.9%. 10yr PSA recurrence higher in the ET alone group (74.7% vs. 29.5% | Addition of RT to ET, halved 10-yr PCa-specific mortality and decreased overall mortality in locally advanced PCa |
| Vickers (2012) [128] | T1–T2 | 1ii /B | P | 695 | Primary: RP | RP beneficial to Gleason 8, or Gleason 7, stage 2 | Younger men w more aggressive disease had larger reduction in risk of PCa death |
| Zelefsky (2010) [129] | Clinically localized: T1c-T3b | 3iii /B | R | 2380 | Primary: RP or RT to prostate | 8-year probability of freedom from met progression was 97% for RP and 93% for EBRT | RP with higher risk disease, had lower risk of met progression and PCa specific death |
| Pierorazio (2013)[130] | Up to clinically localized | 3iii /B | R | 842 | Primary + LNs: RP+PLND | PSA ≥20 and perineural invasion at biopsy increased likelihood of unfavorable, high-grade disease. | High-Gleason PCa not uniformly associated with poor outcomes after RP, but unfavorable (pT3b/N1) disease fared poorly |
| Shao (2014) [131] | Localized | 3iii /B | R | 916 | Primary: RP or RT | RP had longer PCaSS as compared to primary RT | Results add to the growing evidence that controlling the primary site may be important in patient with met cancer |
Abbreviations: HT = hormone therapy; RT = radiation therapy; PCa = prostate cancer; ET = endocrine therapy; RP = radical prostatectomy; EBRT = external beam radiation therapy; LNs = lymph nodes; PLND = positive lymph node dissection; PCaSS = prostate cancer specific survival; met = metastatic
Table 11. Oligometastatic prostate cancer.
| 1st Author, Year [Ref] | Strength of evidence-based on study design / endpoints | Prospective (P) / Retrospective (R) | Sample size | Definition-Oligometa stases | Therapy | Endpoints | Conclusion |
|---|---|---|---|---|---|---|---|
| James, 2014 [137] | 1ii /A | P | 917 | N/A – newly diagnosed M1 | LT ADT | FFS- 11 mths. 2yr FFS- 29%. MOS- 42 mth. 2yr OS- 72%. | Survival disappointing in M1 disease started only on LT ADT, despite active treatments available at first ADT failure. Spend most of their time in CR relapse. |
| Singh, 2004 [149] | 3iii/A | R | 30 | ≤5 met lesions | External RT | 5- and 10yr OS- 73% and 36% in OM, as compared to 45% and 18% in those with > 5 met lesions | Findings suggest early detection and aggressive treatment is worth testing to improve long-term survival |
| Engel, 2010 [101] | 3iii /A | R | 938 | +LNs | +/− RP | 5yr- and 10yr OS- 84% and 64% with completed RP, and 60% and 28%, with aborted RP. PCa-specific survival at 5- and 10-yrs- 95% and 86%, with completed RP and was 70% and 40%, with aborted RP | Abandonment of RP in men with positive LNs may not be appropriate |
| Tabata, 2012 [148] | 3iii/A | R | 35 | <6 bone mets on bone scan, each site less than 50% the size of a vertebral body | RT | 3yr OS- 77%; 14/16 (87%) of pats who had pain were improved 1 mth after RT; median duration of pain control 12 month | RT for bone OM in PCa was effective for long-term pain relief |
| Schick, 2013 [141] | 3iii /A | R | 50 | 1–4 mets, synchronous or metachronous | Mets- ADT and HDRT | 3yr biochemical relapse-free survival (bRFS), clinical failure-free survival, and OS- 54.5, 58.6, and 92% | OM may be treated w short ADT and HDRT to the met regions. High dose improves bRFS. May prolong failure-free interval between 2 consecutive ADT courses. |
| Ponti, 2014 [143] | 3iii/A | R | 16 | Distant relapse in a limited number of regions, ≤5 mets | SBRT +/− HT | Local control, biochemical PFS, OS, toxicity. OS at 29 mths 95% Distant relapse in a limited number of regions, ≤5 mets | SBRT safe, effective, minimally invasive in limited LN recurrence in OMPCa |
| Jereczek-Fossa, 2014 [147] | 3iii/A | R | 69 | Single abdominal LN recurrence | SBRT | 3-yr in-field PFS, PFS, OS- 64%, 11.7%, and 50% | SBRT is feasible for single abdominal LN recurrence, offering excellent in-field tumor control. |
| Cadeddu, 1997 [132] | 3iii /B | R | 38 | +LN: pelvic lymph adenopathy | PLND +/− RP | PCa-specific survival at 5- and 10-yrs- (93% and 56% in the PLND/RP group and 58% and 34% in the PLND group | RP, as compared to conservative therapy, may prolong survival |
| Ahmed, 2013 [145] | 3iii/B | R | 17 | ≤5 met lesions | SBRT | Local control-100% at 6mo; cancer specific survival (CSS)-6- and 12mo-100%; freedom from distant progression (FFDP)- 6- and 12mo- 74%, 40% | Excellent LC with SBRT for OM PCa; over 50% patients achieved undetectable PSA after SBRT |
| Ost, 2014 [142] | 3iii /B | R | 80 | Metachronous mets | Mets- ADT, AS, or MDT | Median PCSS- 6.6 yrs. | Longer PSA DT, involvement of nodes or axial skeleton and lower # mets assoc w improved PCSS. |
| Decaestecker, 2014 [144] | 3iii/B | R | 50 | ≤3 metachronous asymptomatic mets | SBRT (2 RT schedules used) +/− HT | Median PFS- 19mo; median ADT-FS- 25 month; 2-, 5yr PCSS-96%, 90% | Repeated SBRT for OM PCa postpones palliative ADT |
| Berkovic, 2013 [146] | 3iii/Di | R | 24 | Biochemical recurrence after curative treatment to primary (RP, RT, or both), then ≤3 synchronous asymptomatic mets | SBRT | Androgen deprivation therapy-free survival (ADT-FS)- 1-, 2yr-82%, 54%; clinical progression free survival- 1-, 2yr- 72% and 42% | Repeated salvage SBRT feasible, well tolerated, and defers palliative ADT with a median 38mo in OMPca |
| Ost, 2014 [133] | 3iii/Diii | R | 450 | Metachronous mets with controlled primary, + underwent MDT for recurrent PCa | RT or LND | About 50% PFS at 1–3 yrs post-MDT | MDT promising approach for OM PCa recurrence but low level of evidence |
Abbreviations: M1 = distant metastases; LT ADT = long-term androgen deprivation therapy; FFS = failure-free survival; OS = overall survival; CR = castrate resistance; met = metastasis; RT = radiation therapy; OM = oligometastasis; LNs = lymph nodes; RP = radical prostatectomy; PCa = prostate cancer; HDRT = high dose radiation therapy; ADT = androgen deprivation therapy; SBRT = stereotactic body radiation therapy; HT = hormone therapy; PFS = progression free survival; LN = lymph node; OM PCa = oligometastatic prostate cancer; PLND = positive lymph node dissection; LC = local control; PSA = prostate specific antigen; PCSS = prostate cancer specific survival; AS = active surveillance; MDT = metastasis directed therapy; DT = doubling time; ADT-FS = androgen deprivation free survival; LND = lymph node dissection; PFS = progression free survival
Zapatero et al., reported on a study of men with intermediate and high risk localized prostate cancer (n = 362), treated with RT + long term ADT versus RT + short term ADT, and found that long term ADT was superior in 5-year biochemical disease free survival, metastasis free survival, and OS [135].
The standard of care treatment for metastatic prostate cancer is androgen deprivation therapy (ADT) [136]. Men with newly diagnosed metastatic prostate cancer, entered into the control arm of the Systemic Therapy Multi-Arm Randomized Controlled Trial (STAMPEDE) and treated with ≥ 2 years of ADT alone, had a 2-year OS of 72% [137]. The response rate for primary hormonal therapy for men with metastatic prostate cancer exceeds 80% and the median duration of response is approximately 18–24 months [138]. In men with prostate cancer with limited metastases, radical prostatectomy may be advantageous in that the primary tumor and its ability to continuously metastasize, to secrete tumor promoting growth factors and immunosuppressive cytokines, and to generate bulk-related morbidity, is removed. Heidenreich et al. recently evaluated survival outcomes following radical prostatectomy (RP) in men with low volume metastatic prostate cancer. RP led to improved progression-free survival, time to castrate resistance and overall survival, as compared to a cohort treated with androgen deprivation therapy alone. [139] Moreover, Abdollah et al. found that in men with pN1 prostate cancer, treated with RP and extended lymph node dissection, adding adjuvant radiotherapy improved cancer-specific mortality [140].
We searched PubMed using the terms ‘prostate cancer oligometastasis’, ‘prostate cancer oligometastases’, and ‘oligometastatic prostate cancer’; results were as follows, n = 3, n = 19, and n = 22, respectively. We omitted reviews, studies including mixed primary tumors, studies not focused on oligometastatic prostate cancer, case reports, editorials/commentaries, studies that we were not able retrieve, and studies with no results reported. There were ten clinical studies remaining (Table 11); oligometastasis was defined differently in each study and there were 7 different treatment paradigms.
The evidence for treatment of oligometastases in prostate cancer is weak based on the study designs being all nonconsecutive case series (Level 3iii) [101, 132, 133, 141–149], although Ost el al. included a large sample (450 men) [133]. Overall survival endpoints (Level A) were reported in seven studies: 10-year (36% [149] and 64% [101]), 3-year (50% [147], 77% [148], and 92% [141]), 29-month (95%) [143], and 2-year (72%) [137].
Currently there are two studies, not yet completed, for which we are anxiously awaiting the results. Decaestecker et al. are conducting the first randomized, phase 2 trial on men with an oligometastatic recurrence to assess the impact of metastases-directed therapy - versus active surveillance - on the start of palliative ADT [150]. Attard et al., as part of the ongoing STAMPEDE trial, describes the addition of a new treatment arm (enzalutamide, abiraterone and prednisone with ADT) for men with newly diagnosed M1 disease [151].
Current trials of oligometastatic disease
We reviewed clinicaltrials.gov to gauge the ongoing studies for oligometastatic disease. Multiple trials are underway to determine if the treatment of oligometastatic disease is beneficial in cancer as reported on clinicaltrials.gov [152] (see Table 12).
Table 12. Current studies for oligometastatic disease (ClinicalTrials.gov (CTG) as of 2/1/2015).
| CTG NCT# / site | Condition | Name | Purpose | Algorithm | Primary Outcome/Endpoint |
|---|---|---|---|---|---|
| 01859221 University Florida [162] |
Prostate oligometastases | Radiotherapy for OMPC | Phase II study to evaluate outcomes of patients treated with Stereotactic radiation therapy for OMPC | RT | Improved PFS over historic controls. |
| 01777802 Mayo Clinic [163] |
Prostate oligometastases | Monitoring Anti-Prostate Cancer Immunity Following SBRT | Determine if SBRT conditions solid tumors to be favorable to the initiation of robust antitumoral immune responses | Observation following SBRT | Induction of anti-prostate cancer immunity |
| 02020070 MSKCC [170] |
Prostate oligometastases | Ipilimumab, degarelix, + RP in castrate sensitive PC or ipilimumab + degarelix in biochemical recurrent castrate sensitive PC after RP | Assess safety + efficacy of combining HT + immunotherapy in non-castrate resistant PC. Cohort 1: ipilimumab + degarelix pre- and post- RP in newly diagnose OM castrate-sensitive disease. Cohort 2: post definitive local therapy with RP, but with biochemical recurrence | IT, LHRH antagonist + surgery -OR- IT, LHRH antagonist | Undetectable PSA at 12- and 20-mths with non-castrate testosterone. |
| 02264379 Technische U Dresden [165] |
Prostate oligometastases | Percutaneous high-dose RT in OMPC | Evaluate outcomes of patients treated with high-dose radiation using either hypofractionated or normofractionated RT; to establish efficacy + safety | HDRT Hypofraction -Or- Normofraction | Toxicity |
| 01558427 UHosp, Ghent [169] |
Prostate oligometastases | Salvage treatment of active clinical surveillance for OMPC: PhII RCT | To determine if salvage treatment of OMPC with either surgery or RT might postpone the start of ADT | Surgery -Or- RT, Which delays ADT? | ADT-free survival |
| 02192788 Hospital Provincial de Castellon [164] |
Prostate oligometastases | PhII study SBRT as treatment for OMPC | Evaluate effect SBRT for OMPC, regardless of basal treatment received | SBRT | # patients without progression of PC treated by SBRT |
| 02274779 Institut cancerologie de l'Ouest [166] |
Prostate oligometastases | PHII trial salvage RT + HT in OM pelvic node relapses of PC | Assess BC or clinical relapse-free survival at 2yrs of PC with 1–5 OM treated with concomitant HDCRT + HT | RT + HT | BC or clinical relapse-free survival at 2yrs |
| 00544830 Institute CoHMCNC [167] |
Prostate oligometastases | IMRT in treating patients undergoing ADT for mPC | Assess how well IMRT works in pats undergoing ADT for mPC | HT + RT | Time to PSA relapse |
| 00544830 City of Hope Medical Center [168] |
Prostate oligometastases | IMRT in Patients Undergoing ADT for Metastatic Prostate Cancer | Evaluate intensity-modulated radiation therapy works in treating patients undergoing androgen deprivation therapy for metastatic prostate cancer | ADT + IMRT | Time to PSA relapse |
| 01728779 Sidney Kimmel Comprehensive Cancer center [180] |
Oligometastases to lung, liver, bone | Stereotactic Body Radiation With Nelfinavir for Oligometastases | Evaluate efficacy radiosensitizer nelfinavir used concurrently with SBRT | Nelfinavir + SBRT | PFS at 6months |
| 01345539 U Pittsburgh [181] |
Oligometastatic disease | Radiosurgery for OM Disease at Initial Presentation | Evaluate feasibility of radiosurgery for all metastatic sites in OM | SRS | Feasibility SRS/SBRT in OM at initial presentation |
| 02076477 Sichuan Cancer Hospital + Research Institute [158] |
OM stage IV NSCLC | The Optimal Intervention Time of Radiotherapy for OM Stage IV NSCLC | Evaluates optimal time for RT for OM stage IV lung cancer | RT | Short-term effects (response rate using RECIST) |
| 01796288 Wu Jieping Medical Foundation [159] |
OM NSCLC | Radiotherapy in Oligometastatic Non-squamous NSCLC With Clinical Benefits From 2nd Line Erlotinib | Evaluate RT in combination with erlotinib in OM NSCLC | Erlotinib +/− RT | PFS |
| 01345552 >U Pittsburgh [182] |
Recurrent OM disease | Radiosurgery for Patients Recurrent OM Disease | Evaluate feasibility of SRS in recurrent OM disease | SRS | Being able to complete accrual to study |
| 01565837 Comprehensive Cancer Centers Nebraska [177] |
OM Melanoma | Concurrent Ipilimumab and Stereotactic Ablative Radiation Therapy (SART) for OM Unresectable Melanoma | Evaluate if SART + ipilimumab will improve survival in OM melanoma | Ipilimumab + SART | Safety and tolerability |
| 02316002 U Pennsylvania [160] |
OM NSCLC | Phase II Study of Pembrolizumab After Curative Intent Treatment for OM NSCLC | Evaluate how well pembrolizumab works in previously treated OM NSCLC | Pembrolizumab | PFS |
| 01646034 Netherlands Cancer Institute [171] |
OM Breast cancer | High Dose CT in OM Homologous Recombination Deficient Breast Cancer | Studies effect of high-dose alkylating CT versus standard CT in OM breast cancer with recombination deficiency | Carboplatin, thiotepa, and cyclophosphamide versus docetaxel, doxorubicin, cyclophosphamide, carboplatin, paclitaxel, gemcitabine | Event free survival |
| 02228356 Universitair Zeikenhuis Brussel [183] |
Neoplasm metastasis | Non-interventional Observational Study of SBRT for OM Cancer | Investigate if respiration control and improved technique improve local control | SBRT | 1-year local control |
| 01725165 M.D. Anderson Cancer Center [161] |
OM Lung cancer | OM Disease | Learn if surgery or radiation after CT help control NSCLC | +/− local consolidation therapy | PFS |
| 02303366 Peter MacCallum Cancer Center, Australia [172] |
OM breast cancer | Pilot Study SABR for OM Breast Neoplasia in Combination With the Anti-PD-1 Antibody MK-3475 | Assess safety + feasibility of MK-3475 + SABR for definitive treatment of OM breast cancer | SABR + MK-3475 | # of patients who complete treatment; safety |
| 01759238 Universitatsklinikum Hamburg-Eppendorf [193] |
OM CRC metastases | Chemoradiotherapy for Patients With Oligometastatic Colorectal Cancer | Evaluate role of CRT with capecitabine and bevacizumab in OM patients who are neither progressive nor resectable after CT | Capecitabine, bevacizumab and RT | PFS |
| 01282450 Maastricht Radiation Oncology [157] |
Stage IV OM NSCLC | Concurrent and Non-concurrent CRT or RT Alone for OM Stage IV NSCLC | Aim is to improve long term survival in OM NSCLC | RT | OS Note: Study completed |
| 02086721 Maastricht Radiation Oncology [188] |
Solid tumors | Assess Toxicity of Immunocytokine L19-IL2 After SABR OM Solid Tumour | Hypothesis is that the immunocytokine L19-IL2 and RT will synergize to improve OS in OM solid tumors | L19-IL2 + RT | Toxicity |
| 01965223 Trans-Tasman Radiation Oncolog Group [189] |
Cancer metastases to lung | Randomized Phase II Study SABR Metastases to the Lung | Determine safety of SABR versus SRS for OM to lung | Multi-fraction SABR and single fraction SABR | Toxicity |
| 02107755 Ohio State U Comprehensive Cancer Center [178] |
Metastatic melanoma | Stereotactic RT and Ipilimumab in Metastatic Melanoma | Determine if stereotactic radiosurgery + ipilimumab kills more tumor cells by causing additional melanoma antigens to be presented to immune system, | Ipilimumab + stereotactic radiosurgery | PFS |
| 01446744 Lawson Health Research institute [190] |
Metastatic tumors | SABR for Comprehensive Treatment of OM Tumors | Compare SABR with CT and conventional RT to assess impact on OS and Q of L | SABR versus palliative RT | OS |
| 02264886 Washington U School of Medicine [191] |
Central thorax cancer, liver cancer, or non-liver abdominal cancer | Adaptive MRI-Guided SBRT for Unresectable Primary or OM Central Thorax and Abdominal Malignancies | Assess feasibility of RT using MRI-guided adaptive technique (day by day re-planning while patient is receiving treatment) | MRI guided SBRT | Feasibility of MRI-guided SBRT (treatment can be delivered in <80 minutes for >75% of patients) |
| 01761929 Princess Margaret Hospital, Canada [184] |
OM solid tumors | 5 Fraction SBRT for OM Regimen, for Extra-Cranial OM | Monitor side effects and outcomes from higher doses of RT, while limiting dose to normal tissues (using a 5 day schedule) | RT in 5 fractions | 1 year PFS at index site |
| 02170181 U of Texas SW Medical Center [185] |
Cancer and receiving RT | Prospective Clinical Registry for OM Disease, Consolidation Therapy, Debulking Prior to CT, or Re-Irradiation | Determine trends in patterns of care and outcomes for refinement and justification of this treatment | SBRT | Patterns of care |
| 02089100 Gustave Roussy, Cancer Campus, Grand Paris [173] |
Breast cancer | Trial of Superiority of SBRT in Breast Cancer | Prospectively study role of metastases SBRT with curative intent in de novo OM disease | SBRT versus placebo | PFS |
| 01898962 Rocky Mountain Cancer Centers [186] |
Stage IV or recurrent carcinoma or sarcoma | Definitive Therapy for OM Solid Malignancies | Aggressive treatment to clinically active sites of disease (alone or + systemic therapy) may improve survival | Definitive local treatment (surgery, RT, radioembolization) | OS and disease-specific survival |
| 00463060 Mount Sinai School of Medicine [187] |
Metastatic cancer | Sutent and Radiation as Treatment for Limited Extent Metastatic Cancer | Combine sutent and RT to determine safety and best method of combining the treatments | Sutent + RT | Survival |
| 02231775 M.D. Anderson Cancer Center [179] |
Stage IV melanoma | Combi-Neo Study for Stage IV Melanoma | Compare dabrafenib + trametinib before surgery versus surgery alone | Surgery +/−dabrafenib +/−, trametinib | 1-year relapse-free survival |
| 01781741 Roswell Park cancer Institute [154] |
Stage III-IV NSCLC | SBRT After Surgery in Stage III-IV Non-small Cell Lung Cancer | Evaluate SBRT post lymphadenectomy | Therapeutic lymphadenectomy, SBRT | Toxicity |
| 00776100 North Central Cancer Treatment Group/NCI [156] |
Stage IV NSCLC | RT or Observation After CT in Stage IV NSCLC | Randomized Phase II trial studying how well RT works compared to CT in Stage IV NSCLC | RT versus observation | OS Note: Study completed |
| 00182793 City of Hope Medical Center/NCI [174] |
Breast cancer | Combination CT +/− Trastuzumab Followed By an Autologous Stem Cell Transplant (SCT) and RT in Stage III or IV Breast Cancer | Evaluate combination CT +/− trastuzumab, then autologous SCT and RT | CT+/− Trastuzumab, then SCT + RT | TTP, OS, 5-year response rate, feasibility Note: study completed |
| 01941654 Chinese University of Hong Kong [153] |
NSCLC activating EGFR mutation | ATOM_local Ablative Therapy | Efficacy of local ablative therapy in NSCLC with activating EGFR mutation with active OM disease after 1st line TKI EGFR | Local ablative RT | PFS at 1-year |
| 01185639 Comprehensive Cancer Center Wake Forest U [155] |
NSCLC | SBRT in Metastatic NSCLC | Evaluate feasibility, safety, and efficacy of SBRT after 4cycles 1st line CT | SBRT | PFS |
| 01763970 Sidney Kimmel Comprehensive Cancer Center [194] |
Pediatric sarcoma | SBRT for Pediatric Sarcomas | Evaluate efficacy of SBRT (5 fractions) in pediatric sarcoma | SBRT | SBRT efficacy |
| 01875666 UNC Lineberger Comprehensive Cancer Center [175] |
Breast neoplasm | Defining the HER2 Positive (+) Breast Cancer Kinome Response to Trastuzumab (T), Pertuzumab (P), Combination Trastuzumab +Pertuzumab (T+P), or Combination Trastuzumab + Lapatinib (T+L) | Evaluate kinome response in Stage I-IV HER2+ scheduled to undergo definitive therapy for OM disease | Randomized to T, P, T+P, or T+L | Difference in kinome activation pre- and post-treatment |
| 01706432 U of Chicago / NCI [176] |
Stage IV breast cancer | Hypofractionated IGRT in Stage IV Breast Cancer | Studies hypofractionated IGRT in stage IV breast cancer | Hypofractionated RT | Feasibility of correlating number of circulating tumor cells with TTP in metastatic breast cancer |
| 01347333 St. John's Mercy Research Institute, St. Louis [192] |
Liver metastases, hepatocellular carcinoma, intrahepatic cholangiocarcinoma | SBRT for Liver Tumors | Evaluate local control rate of SBRT to liver tumors | SBRT | Local tumor recurrence rate |
Abbreviations: OMPC = oligometastatic prostate cancer; RT = radiation therapy; PFS = progression free survival; SBRT = stereotactic body radiation therapy; RP = retropublic prostatectomy; PC = prostate cancer; HT = hormone therapy; OM = oligometastatic; IT = immunotherapy; LHRH = luteinizing hormone-releasing hormone; PSA = prostate specific antigen; HD = high dose; RCT = randomized controlled trial; ADT = androgen deprivation therapy; BC = biochemical; IMRT = image guided radiation therapy; PFS = progression free survival; SRS = stereotactic radiosurgery; CRT = chemoradiation therapy; RECIST = response evaluation criteria in solid tumors; NSCLC = non-small cell lung cancer; CT = chemotherapy; OS = overall survival; SABR = stereotactic ablative radiation therapy; MRI = magnetic resonance imaging; SCT = stem cell transplant; TTP = time to progression; ATOM = ablative therapy oligometastases; EGFR = epidermal growth factor receptor; Q of L = Quality of Life
Nine studies are in progress for oligometastatic NSCLC, with therapies ranging from SBRT [153–155], to RT [156, 157], to chemoradiation therapy [158], to targeted therapy +/− RT [159], to immunotherapy [160], to any local therapy [161]. For men with oligometastatic prostate cancer, nine trials are underway investigating a variety of therapies including single agent RT [162–165], RT in combination with HT [166, 168], salvage treatment (surgical or RT) of metastases [169], and combination of HT + immunotherapy [170]. For women with oligometastatic breast cancer, six studies are currently open investigating several therapies including various chemotherapy regimens [171], SABR + antibodies [172], SBRT [173], chemotherapy + antibodies + RT [174], targeted therapies [175] and RT [176]. Three studies are studying oligometastatic melanoma, two are investigating immunotherapy + SABT/SRS [177, 178] and the third study is investigating chemotherapy with and without surgery [179]. There are thirteen studies enrolling patients with oligometastases without a specific primary tumor [180–192], employing a variety of treatment options. Finally, there is one study each for oligometastatic colorectal cancer using chemoradiation [193] and for oligometastatic pediatric sarcoma using SBRT [194].
CONCLUSIONS
Hellman proposed that tumors early in the chain of progression may give rise to metastases limited in number and location due to an undeveloped metastatic competence of the cancer “seeds” as well as the restrictive nature of the host tissue at some metastatic sites. The rationales of discrete steps of metastases [11, 12] and the hallmarks of cancer [16, 17] have both provided a biologic framework to support the oligometastatic state. Molecular diagnostics have not yet arrived to the point to determine where an individual metastatic deposit is within the continuum of malignancy [32–44, 45], preclinical models are beginning to lay the groundwork of the oligometastatic state [19–31].
While Hellman posited that oligometastasis may be underrecognized due to limitations in imaging diagnostics, imaging has advanced to such a degree that we are now in a yin-yang position such that, 1) imaging often likely demonstrates very early findings not likely to manifest into disease and, 2) imaging demonstrates very early disease that is destined to become widespread, which cannot be distinguished from oligometastases.
Hellman described potential cure as an attractive upshot of treating an oligometastatic state, but prolonged survival has been added as a potential benefit. There is an increasing shift toward individualized, multidisciplinary management of OM disease [34] because it is difficult to conduct randomized controlled trials in oligometastatic patients due to the variety of presentations. While other trial designs may be more realistic, such as N of 1 studies, tissue-based approaches, propensity matched analyses and adaptive design methods [55], Hellman originally endorsed that the ability to identify the oligometastatic state would profit from careful review of past experience, i.e. re-analysis of clinical data found in archival materials to arbitrate when, during the evolution of individual tumors, the oligometastatic state can be found.
Acknowledgments
This work is financially supported by the NCI (grant no. U54CA143803, CA163124, CA093900 and CA143055 to K.J. Pienta).
REFERENCES
- 1.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nature reviews Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. Distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 3.Halsted WS. The results of radical operations for the cure of carcinoma of the breast. Annals of Surgery. 1907;46:1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tait CR, Waterworth A, Loncaster J, Horgan K, Dodwell D. The oligometastatic state in breast cancer: hypothesis or reality. Breast. 2005;14:87–93. doi: 10.1016/j.breast.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Ewing J. MetastasisNeoplastics. Philadelphia: Saunders; 1928. pp. 77–89. [Google Scholar]
- 6.Keynes SGL. Carcinoma of the breast. Post-Graduate Committee FoM, Dalhousie University. In: Halifax, editor. The Nova Scotia Medical Bulletin. St. Bartholomew's Hospital; London: 1956. pp. 162–169. [Google Scholar]
- 7.Fisher B. Laboratory and clinical research in breast cancer—a personal adventure: the David A. Karnofsky memorial lecture. Cancer research. 1980;40:3863–3874. [PubMed] [Google Scholar]
- 8.Lussier YA, Khodarev NN, Regan K, Corbin K, Li H, Ganai S, Khan SA, Gnerlich JL, Darga TE, Fan H, Karpenko O, Paty PB, Posner MC, Chmura SJ, Hellman S, Ferguson MK, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PloS one. 2012;7:e50141. doi: 10.1371/journal.pone.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 10.Pienta KJ, Robertson BA, Coffey DS, Taichman RS. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:5849–5855. doi: 10.1158/1078-0432.CCR-13-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature reviews Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 13.Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:1384–1390. doi: 10.1200/JCO.2012.45.9651. [DOI] [PubMed] [Google Scholar]
- 14.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Weichselbaum RR, Hellman S. Oligometastases revisited. Nature reviews Clinical oncology. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 19.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA, Kim R, Rubin MA, Pienta KJ. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer research. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 20.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 21.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 22.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nature genetics. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 23.Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA, Adejola N, Gurel M, Hicks J, Meeker AK, Halushka MK, Simons JW, Isaacs WB, De Marzo AM, Nelson WG, Yegnasubramanian S. Tracking the clonal origin of lethal prostate cancer. The Journal of clinical investigation. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake JM, Graham NA, Lee JK, Stoyanova T, Faltermeier CM, Sud S, Titz B, Huang J, Pienta KJ, Graeber TG, Witte ON. Metastatic castration-resistant prostate cancer reveals intrapatient similarity and interpatient heterogeneity of therapeutic kinase targets. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4762–4769. doi: 10.1073/pnas.1319948110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 27.Chambers AF, Hill RP, Ling V. Tumor heterogeneity and stability of the metastatic phenotype of mouse KHT sarcoma cells. Cancer research. 1981;41:1368–1372. [PubMed] [Google Scholar]
- 28.Harris JF, Chambers AF, Hill RP, Ling V. Metastatic variants are generated spontaneously at a high rate in mouse KHT tumor. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:5547–5551. doi: 10.1073/pnas.79.18.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cillo C, Dick JE, Ling V, Hill RP. Generation of drug-resistant variants in metastatic B16 mouse melanoma cell lines. Cancer research. 1987;47:2604–2608. [PubMed] [Google Scholar]
- 30.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nature reviews Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 31.Kendal WS. Oligometastasis as a predictor for occult disease. Mathematical biosciences. 2014;251:1–10. doi: 10.1016/j.mbs.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World journal of gastroenterology: WJG. 2001;7:630–636. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shindo-Okada N, Takeuchi K, Nagamachi Y. Establishment of cell lines with high- and low-metastatic potential from PC-14 human lung adenocarcinoma. Japanese journal of cancer research: Gann. 2001;92:174–183. doi: 10.1111/j.1349-7006.2001.tb01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rastogi S, Gulia S, Bajpai J, Ghosh J, Gupta S. Oligometastatic breast cancer: A mini review. Indian journal of medical and paediatric oncology: official journal of Indian Society of Medical & Paediatric Oncology. 2014;35:203–206. doi: 10.4103/0971-5851.142035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosari F, Parker AS, Kube DM, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Vasmatzis G. Clear cell renal cell carcinoma: gene expression analyses identify a potential signature for tumor aggressiveness. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:5128–5139. doi: 10.1158/1078-0432.CCR-05-0073. [DOI] [PubMed] [Google Scholar]
- 36.Wuttig D, Baier B, Fuessel S, Meinhardt M, Herr A, Hoefling C, Toma M, Grimm MO, Meye A, Rolle A, Wirth MP. Gene signatures of pulmonary metastases of renal cell carcinoma reflect the disease-free interval and the number of metastases per patient. International journal of cancer Journal international du cancer. 2009;125:474–482. doi: 10.1002/ijc.24353. [DOI] [PubMed] [Google Scholar]
- 37.Jones RP, Stattner S, Sutton P, Dunne DF, McWhirter D, Fenwick SW, Malik HZ, Poston GJ. Controversies in the oncosurgical management of liver limited stage IV colorectal cancer. Surgical oncology. 2014;23:53–60. doi: 10.1016/j.suronc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Vakiani E, Janakiraman M, Shen R, Sinha R, Zeng Z, Shia J, Cercek A, Kemeny N, D'Angelica M, Viale A, Heguy A, Paty P, Chan TA, Saltz LB, Weiser M, Solit DB. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:2956–2962. doi: 10.1200/JCO.2011.38.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stremitzer S, Stift J, Gruenberger B, Tamandl D, Aschacher T, Wolf B, Wrba F, Gruenberger T. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. The British journal of surgery. 2012;99:1575–1582. doi: 10.1002/bjs.8909. [DOI] [PubMed] [Google Scholar]
- 40.Bruin SC, de Ronde JJ, Wiering B, Braaf LM, de Wilt JH, Vincent AD, van Velthuysen ML, Ruers TJ, Wessels LF LJ. Selection of patients for hepatic surgery of colorectal cancer liver metastasis based on genomic aberrations. Annals of surgical oncology. 2013;20:S560–569. doi: 10.1245/s10434-013-2985-7. [DOI] [PubMed] [Google Scholar]
- 41.Cheng HH, Mitchell PS, Kroh EM, Dowell AE, Chery L, Siddiqui J, Nelson PS, Vessella RL, Knudsen BS, Chinnaiyan AM, Pienta KJ, Morrissey C, Tewari M. Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PloS one. 2013;8:e69239. doi: 10.1371/journal.pone.0069239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang F, Wu G, Yang K. Oligometastasis and oligo-recurrence: more than a mirage. Radiation oncology. 2014;9:230. doi: 10.1186/s13014-014-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valastyan S, Weinberg RA. MicroRNAs: Crucial multi-tasking components in the complex circuitry of tumor metastasis. Cell cycle. 2009;8:3506–3512. doi: 10.4161/cc.8.21.9802. [DOI] [PubMed] [Google Scholar]
- 44.Lussier YA, Xing HR, Salama JK, Khodarev NN, Huang Y, Zhang Q, Khan SA, Yang X, Hasselle MD, Darga TE, Malik R, Fan H, Perakis S, Filippo M, Corbin K, Lee Y, et al. MicroRNA expression characterizes oligometastasis(es) PloS one. 2011;6:e28650. doi: 10.1371/journal.pone.0028650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uppal A, Wightman SC, Mallon S, Oshima G, Pitroda SP, Zhang Q, Huang X, Darga TE, Huang L, Andrade J, Liu H, Ferguson MK, Greene GL, Posner MC, Hellman S, Khodarev NN, et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget. 2015 doi: 10.18632/oncotarget.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellman S, Weichselbaum RR. Oligometastases. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 47.de Vin T, Engels B, Gevaert T, Storme G, De Ridder M. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25:467–471. doi: 10.1093/annonc/mdt537. [DOI] [PubMed] [Google Scholar]
- 48.Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, Roizman B, Bergh J, Pawitan Y, van de Vijver MJ, Minn AJ. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macdermed DM, Weichselbaum RR, Salama JK. A rationale for the targeted treatment of oligometastases with radiotherapy. Journal of surgical oncology. 2008;98:202–206. doi: 10.1002/jso.21102. [DOI] [PubMed] [Google Scholar]
- 50.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? Journal of the National Cancer Institute. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uppal A, Weichselbaum RR, Posner MC. Optimizing management of colorectal hepatic metastases: now and in the future. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2014;40:1033–1035. doi: 10.1016/j.ejso.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Yao HH, Hong M, Corcoran NM, Siva S, Foroudi F. Advances in local and ablative treatment of oligometastasis in prostate cancer. Asia-Pacific journal of clinical oncology. 2014;10:308–321. doi: 10.1111/ajco.12256. [DOI] [PubMed] [Google Scholar]
- 53.Moreno A, Albiach C, Soria R, Vidal V, Gomez R, Antequera M. Oligometastases in prostate cancer: restaging stage IV cancers and new radiotherapy options. Radiation oncology. 2014;9:258. [Google Scholar]
- 54.Reeves F, Murphy D, Evans C, Bowden P, Costello A. Targeted local therapy in oligometastatic prostate cancer: a promising potential opportunity after failed primary treatment. BJU international. 2014 doi: 10.1111/bju.12957. [DOI] [PubMed] [Google Scholar]
- 55.Loh J, Davis ID, Martin JM, Siva S. Extracranial oligometastatic renal cell carcinoma: current management and future directions. Future oncology. 2014;10:761–774. doi: 10.2217/fon.14.40. [DOI] [PubMed] [Google Scholar]
- 56.Bhattasali O, Chen LN, Tong M, Lei S, Collins BT, Krishnan P, Kalhorn C, Lynch JH, Suy S, Dritschilo A, Dawson NA, Collins SP. Rationale for stereotactic body radiation therapy in treating patients with oligometastatic hormone-naive prostate cancer. Frontiers in oncology. 2013;3:293. doi: 10.3389/fonc.2013.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. Journal of the National Cancer Institute. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 59.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. Journal of immunology. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 61.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 62.Lam VW, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Annals of surgical oncology. 2012;19:1292–1301. doi: 10.1245/s10434-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 63.Deschamps F, Farouil G, Ternes N, Gaudin A, Hakime A, Tselikas L, Teriitehau C, Baudin E, Auperin A, de Baere T. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? European radiology. 2014;24:1971–1980. doi: 10.1007/s00330-014-3202-1. [DOI] [PubMed] [Google Scholar]
- 64.Solomon LA, Munkarah AR, Vorugu VR, Deppe G, Adam B, Malone JM, Jr, Littrup PJ. Image-guided percutaneous cryotherapy for the management of gynecologic cancer metastases. Gynecologic oncology. 2008;111:202–207. doi: 10.1016/j.ygyno.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Littrup PJ, Bang HJ, Currier BP, Goodrich DJ, Aoun HD, Heilbrun LK, Adam BA. Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes. Journal of vascular and interventional radiology: JVIR. 2013;24:1817–1825. doi: 10.1016/j.jvir.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Dongen JA, van Slooten EA. The surgical treatment of pulmonary metastases. Cancer treatment reviews. 1978;5:29–48. doi: 10.1016/s0305-7372(78)80004-8. [DOI] [PubMed] [Google Scholar]
- 67.Institute NC. PDQ(R) Levels of Evidence for Adult and Pediatric Cancer Treatment Studies. Bethesda, MD: National Cancer Institute; [PubMed] [Google Scholar]
- 68.Camacho LH, Kurzrock R, Cheung A, Barber DF, Gupta S, Madoff DC, Wallace MJ, Kim EE, Curley SA, Hortobagyi GN, Mavligit G. Pilot study of regional, hepatic intra-arterial paclitaxel in patients with breast carcinoma metastatic to the liver. Cancer. 2007;109:2190–2196. doi: 10.1002/cncr.22672. [DOI] [PubMed] [Google Scholar]
- 69.Bojko P, Welt A, Schleucher R, Borquez D, Scheulen ME, Vanhoefer U, Poettgen C, Stuschke M, Broelsch CE, Stamatis G, Wilke H, Seeber S, Harstrick A. High-dose chemotherapy with autologous stem cell transplantation in patients with oligometastatic breast cancer. Bone marrow transplantation. 2004;34:637–643. doi: 10.1038/sj.bmt.1704613. [DOI] [PubMed] [Google Scholar]
- 70.Milano MT, Zhang H, Metcalfe SK, Muhs AG, Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast cancer research and treatment. 2009;115:601–608. doi: 10.1007/s10549-008-0157-4. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi T, Ichiba T, Sakuyama T, Arakawa Y, Nagasaki E, Aiba K, Nogi H, Kawase K, Takeyama H, Toriumi Y, Uchida K, Kobayashi M, Kanehira C, Suzuki M, Ando N, Natori K, et al. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast cancer. 2012;19:218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 72.Mimoto R, Kobayashi T, Imawari Y, Kamio M, Kato K, Nogi H, Toriumi Y, Hirooka S, Uchida K, Takeyama H. Clinical relevance and low tumor-initiating properties of oligometastatic breast cancer in pulmonary metastasectomy. Breast cancer research and treatment. 2014;147:317–324. doi: 10.1007/s10549-014-3111-7. [DOI] [PubMed] [Google Scholar]
- 73.VanderWalde A, Ye W, Frankel P, Asuncion D, Leong L, Luu T, Morgan R, Twardowski P, Koczywas M, Pezner R, Paz IB, Margolin K, Wong J, Doroshow JH, Forman S, Shibata S, et al. Long-term survival after high-dose chemotherapy followed by peripheral stem cell rescue for high-risk, locally advanced/inflammatory, and metastatic breast cancer. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18:1273–1280. doi: 10.1016/j.bbmt.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bourgier C, Khodari W, Vataire AL, Pessoa EL, Dunant A, Delaloge S, Uzan C, Balleyguier C, Mathieu MC, Marsiglia H, Arriagada R. Breast radiotherapy as part of loco-regional treatments in stage IV breast cancer patients with oligometastatic disease. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2010;96:199–203. doi: 10.1016/j.radonc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 75.Nieto Y, Nawaz S, Jones RB, Shpall EJ, Cagnoni PJ, McSweeney PA, Baron A, Razook C, Matthes S, Bearman SI. Prognostic model for relapse after high-dose chemotherapy with autologous stem-cell transplantation for stage IV oligometastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20:707–718. doi: 10.1200/JCO.2002.20.3.707. [DOI] [PubMed] [Google Scholar]
- 76.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 77.Lanuti M, Sequist LV, Sharma A, Mino-Kenudson M. Case records of the Massachusetts General Hospital. Case 31–2011. A 55-year-old man with oligometastatic lung cancer. The New England journal of medicine. 2011;365:1426–1435. doi: 10.1056/NEJMcpc1013930. [DOI] [PubMed] [Google Scholar]
- 78.Grossi F, Kubota K, Cappuzzo F, de Marinis F, Gridelli C, Aita M, Douillard JY. Future scenarios for the treatment of advanced non-small cell lung cancer: focus on taxane-containing regimens. The oncologist. 2010;15:1102–1112. doi: 10.1634/theoncologist.2010-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strong VE, D'Angelica M, Tang L, Prete F, Gonen M, Coit D, Touijer KA, Fong Y, Brennan MF. Laparoscopic adrenalectomy for isolated adrenal metastasis. Annals of surgical oncology. 2007;14:3392–3400. doi: 10.1245/s10434-007-9520-7. [DOI] [PubMed] [Google Scholar]
- 80.Collen C, Christian N, Schallier D, Meysman M, Duchateau M, Storme G, De Ridder M. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25:1954–1959. doi: 10.1093/annonc/mdu370. [DOI] [PubMed] [Google Scholar]
- 81.De Ruysscher D, Wanders R, van Baardwijk A, Dingemans AM, Reymen B, Houben R, Bootsma G, Pitz C, van Eijsden L, Geraedts W, Baumert BG, Lambin P. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450) Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2012;7:1547–1555. doi: 10.1097/JTO.0b013e318262caf6. [DOI] [PubMed] [Google Scholar]
- 82.Nieder C, Tollali T, Reigstad A, Pawinski A, Haukland E, Dalhaug A. Oligometastatic non-small cell lung cancer: a significant entity outside of specialized cancer centers? Medical principles and practice: international journal of the Kuwait University, Health Science Centre. 2014;23:526–531. doi: 10.1159/000365634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan AJ, Mehta PS, Zusag TW, Bonomi PD, Penfield Faber L, Shott S, Abrams RA. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC) Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2006;81:163–167. doi: 10.1016/j.radonc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, Congedo MT, Gomez DR, Wright GM, Melloni G, Milano MT, Sole CV, De Pas TM, Carter DL, Warner AJ, Rodrigues GB. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clinical lung cancer. 2014;15:346–355. doi: 10.1016/j.cllc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 85.Lopez Guerra JL, Gomez D, Zhuang Y, Hong DS, Heymach JV, Swisher SG, Lin SH, Komaki R, Cox JD, Liao Z. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. International journal of radiation oncology, biology, physics. 2012;84:e61–67. doi: 10.1016/j.ijrobp.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collaud S, Stahel R, Inci I, Hillinger S, Schneiter D, Kestenholz P, Weder W. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung cancer. 2012;78:234–238. doi: 10.1016/j.lungcan.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 87.Congedo MT, Cesario A, Lococo F, De Waure C, Apolone G, Meacci E, Cavuto S, Granone P. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. The Journal of thoracic and cardiovascular surgery. 2012;144:444–452. doi: 10.1016/j.jtcvs.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 88.Hasselle MD, Haraf DJ, Rusthoven KE, Golden DW, Salgia R, Villaflor VM, Shah N, Hoffman PC, Chmura SJ, Connell PP, Vokes EE, Weichselbaum RR, Salama JK. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2012;7:376–381. doi: 10.1097/JTO.0b013e31824166a5. [DOI] [PubMed] [Google Scholar]
- 89.Griffioen GH, Toguri D, Dahele M, Warner A, de Haan PF, Rodrigues GB, Slotman BJ, Yaremko BP, Senan S, Palma DA. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung cancer. 2013;82:95–102. doi: 10.1016/j.lungcan.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 90.Yano T, Okamoto T, Haro A, Fukuyama S, Yoshida T, Kohno M, Maehara Y. Local treatment of oligometastatic recurrence in patients with resected non-small cell lung cancer. Lung cancer. 2013;82:431–435. doi: 10.1016/j.lungcan.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, Pietanza MC, Azzoli CG, Rizvi NA, Krug LM, Miller VA, Kris MG, Riely GJ. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gray PJ, Mak RH, Yeap BY, Cryer SK, Pinnell NE, Christianson LW, Sher DJ, Arvold ND, Baldini EH, Chen AB, Kozono DE, Swanson SJ, Jackman DM, Alexander BM. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung cancer. 2014;85:239–244. doi: 10.1016/j.lungcan.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheufou DH, Welter S, Chalvatzoulis E, Christof D, Theegarten D, Stamatis G. Surgery of primary lung cancer with oligometastatic m1b synchronous single brain metastasis: analysis of 37 cases. The Thoracic and cardiovascular surgeon. 2014;62:612–615. doi: 10.1055/s-0034-1377060. [DOI] [PubMed] [Google Scholar]
- 94.Parikh RB, Cronin AM, Kozono DE, Oxnard GR, Mak RH, Jackman DM, Lo PC, Baldini EH, Johnson BE, Chen AB. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2014;89:880–887. doi: 10.1016/j.ijrobp.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Sheu T, Heymach JV, Swisher SG, Rao G, Weinberg JS, Mehran R, McAleer MF, Liao Z, Aloia TA, Gomez DR. Propensity score-matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. International journal of radiation oncology, biology, physics. 2014;90:850–857. doi: 10.1016/j.ijrobp.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 96.Tonnies M, Pfannschmidt J, Bauer TT, Kollmeier J, Tonnies S, Kaiser D. Metastasectomy for synchronous solitary non-small cell lung cancer metastases. The Annals of thoracic surgery. 2014;98:249–256. doi: 10.1016/j.athoracsur.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 97.Ouyang WW, Su SF, Ma Z, Hu YX, Lu B, Li QS, Geng YC, Li HQ. Prognosis of non-small cell lung cancer patients with bone oligometastases treated concurrently with thoracic three-dimensional radiotherapy and chemotherapy. Radiation oncology. 2014;9:147. doi: 10.1186/1748-717X-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Endo C, Hasumi T, Matsumura Y, Sato N, Deguchi H, Oizumi H, Sagawa M, Tsushima T, Takahashi S, Shibuya J, Hirose M, Kondo T. A prospective study of surgical procedures for patients with oligometastatic non-small cell lung cancer. The Annals of thoracic surgery. 2014;98:258–264. doi: 10.1016/j.athoracsur.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 99.Essner R, Lee JH, Wanek LA, Itakura H, Morton DL. Contemporary surgical treatment of advanced-stage melanoma. Archives of surgery. 2004;139:961–966. doi: 10.1001/archsurg.139.9.961. discussion 966–967. [DOI] [PubMed] [Google Scholar]
- 100.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. Journal of neurosurgery. 2012;117:227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Engel J, Bastian PJ, Baur H, Beer V, Chaussy C, Gschwend JE, Oberneder R, Rothenberger KH, Stief CG, Holzel D. Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. European urology. 2010;57:754–761. doi: 10.1016/j.eururo.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 102.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 103.Timmerman RD, Bizekis CS, Pass HI, Fong Y, Dupuy DE, Dawson LA, Lu D. Local surgical, ablative, and radiation treatment of metastases. CA: a cancer journal for clinicians. 2009;59:145–170. doi: 10.3322/caac.20013. [DOI] [PubMed] [Google Scholar]
- 104.Engels B, Gevaert T, Everaert H, De Coninck P, Sermeus A, Christian N, Storme G, Verellen D, De Ridder M. Phase II study of helical tomotherapy in the multidisciplinary treatment of oligometastatic colorectal cancer. Radiation oncology. 2012;7:34. doi: 10.1186/1748-717X-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 106.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 107.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D'Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 110.Dellas K, Reese T, Richter M, Arnold D, Dunst J. Concurrent chemoradiation of metastases with capecitabine and oxaliplatin and 3D-CRT in patients with oligometastatic colorectal cancer: results of a phase I study. Radiation oncology. 2012;7:83. doi: 10.1186/1748-717X-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Comito T, Cozzi L, Clerici E, Campisi MC, Liardo RL, Navarria P, Ascolese A, Tozzi A, Iftode C, De Rose F, Villa E, Personeni N, Rimassa L, Santoro A, Fogliata A, Mancosu P, et al. Stereotactic Ablative Radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC cancer. 2014;14:619. doi: 10.1186/1471-2407-14-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salah S, Watanabe K, Welter S, Park JS, Park JW, Zabaleta J, Ardissone F, Kim J, Riquet M, Nojiri K, Gisabella M, Kim SY, Tanaka K, Al-Haj Ali B. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2012;23:2649–2655. doi: 10.1093/annonc/mds100. [DOI] [PubMed] [Google Scholar]
- 113.Van den Begin R, Engels B, Gevaert T, Duchateau M, Tournel K, Verellen D, Storme G, De Ridder M. Impact of inadequate respiratory motion management in SBRT for oligometastatic colorectal cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014;113:235–239. doi: 10.1016/j.radonc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 114.Filippi AR, Badellino S, Ceccarelli M, Guarneri A, Franco P, Monagheddu C, Spadi R, Ragona R, Racca P, Ricardi U. Stereotactic Ablative Radiation Therapy as First Local Therapy for Lung Oligometastases From Colorectal Cancer: A Single-Institution Cohort Study. International journal of radiation oncology, biology, physics. 2014 doi: 10.1016/j.ijrobp.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 115.Salah S, Watanabe K, Park JS, Addasi A, Park JW, Zabaleta J, Ardissone F, Kim J, Riquet M, Nojiri K, Gisabella M, Kim SY, Tanaka K. Repeated resection of colorectal cancer pulmonary oligometastases: pooled analysis and prognostic assessment. Annals of surgical oncology. 2013;20:1955–1961. doi: 10.1245/s10434-012-2860-y. [DOI] [PubMed] [Google Scholar]
- 116.Bae SH, Kim MS, Cho CK, Kang JK, Kang HJ, Kim YH, Shin US, Moon SM, Lee DH. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. Journal of surgical oncology. 2012;106:138–143. doi: 10.1002/jso.23058. [DOI] [PubMed] [Google Scholar]
- 117.Kang JK, Kim MS, Kim JH, Yoo SY, Cho CK, Yang KM, Yoo HJ, Seo YS, Lee DH, Kang HJ, Kim YH, Shin US. Oligometastases confined one organ from colorectal cancer treated by SBRT. Clinical & experimental metastasis. 2010;27:273–278. doi: 10.1007/s10585-010-9325-0. [DOI] [PubMed] [Google Scholar]
- 118.Hohenberger P, Kasper B, Ahrar K. Surgical management and minimally invasive approaches for the treatment of metastatic sarcoma. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2013:457–464. doi: 10.14694/EdBook_AM.2013.33.457. [DOI] [PubMed] [Google Scholar]
- 119.Falk AT, Moureau-Zabotto L, Ouali M, Penel N, Italiano A, Bay JO, Olivier T, Sunyach MP, Boudou-Roquette P, Salas S, Le Maignan C, Ducassou A, Isambert N, Kalbacher E, Pan C, Saada E, et al. Effect on survival of local ablative treatment of metastases from sarcomas: a study of the French sarcoma group. Clinical oncology. 2015;27:48–55. doi: 10.1016/j.clon.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 120.Rhomberg W, Eiter H, Schmid F, Saely CH. Combined vindesine and razoxane shows antimetastatic activity in advanced soft tissue sarcomas. Clinical & experimental metastasis. 2008;25:75–80. doi: 10.1007/s10585-007-9103-9. [DOI] [PubMed] [Google Scholar]
- 121.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. The New England journal of medicine. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 122.Ranck MC, Golden DW, Corbin KS, Hasselle MD, Liauw SL, Stadler WM, Hahn OM, Weichselbaum RR, Salama JK. Stereotactic body radiotherapy for the treatment of oligometastatic renal cell carcinoma. American journal of clinical oncology. 2013;36:589–595. doi: 10.1097/COC.0b013e31825d52b2. [DOI] [PubMed] [Google Scholar]
- 123.Thibault I, Al-Omair A, Masucci GL, Masson-Cote L, Lochray F, Korol R, Cheng L, Xu W, Yee A, Fehlings MG, Bjarnason GA, Sahgal A. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. Journal of neurosurgery Spine. 2014;21:711–718. doi: 10.3171/2014.7.SPINE13895. [DOI] [PubMed] [Google Scholar]
- 124.Bang HJ, Littrup PJ, Goodrich DJ, Currier BP, Aoun HD, Heilbrun LK, Vaishampayan U, Adam B, Goodman AC. Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation. Journal of vascular and interventional radiology: JVIR. 2012;23:770–777. doi: 10.1016/j.jvir.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon- alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 126.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Jr, Munshi N, Crawford ED. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. The New England journal of medicine. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 127.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, Lund JA, Tasdemir I, Hoyer M, Wiklund F, Fossa SD. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 128.Vickers A, Bennette C, Steineck G, Adami HO, Johansson JE, Bill-Axelson A, Palmgren J, Garmo H, Holmberg L. Individualized estimation of the benefit of radical prostatectomy from the Scandinavian Prostate Cancer Group randomized trial. European urology. 2012;62:204–209. doi: 10.1016/j.eururo.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y, Vickers A, Scardino PT. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:1508–1513. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pierorazio PM, Lin BM, Mullins JK, Hyndman ME, Schaeffer EM, Han M, Partin AW, Pavlovich CP. Preoperative characteristics of men with unfavorable high-Gleason prostate cancer at radical prostatectomy. Urologic oncology. 2013;31:589–594. doi: 10.1016/j.urolonc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 131.Shao YH, Kim S, Moore DF, Shih W, Lin Y, Stein M, Kim IY, Lu-Yao GL. Cancer-specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population-based, propensity score-matched analysis. European urology. 2014;65:693–700. doi: 10.1016/j.eururo.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cadeddu JA, Partin AW, Epstein JI, Walsh PC. Stage D1 (T1-3, N1-3, M0) prostate cancer: a case-controlled comparison of conservative treatment versus radical prostatectomy. Urology. 1997;50:251–255. doi: 10.1016/S0090-4295(97)00186-6. [DOI] [PubMed] [Google Scholar]
- 133.Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, Roach M, 3rd, Briganti A. Metastasis-directed Therapy of Regional and Distant Recurrences After Curative Treatment of Prostate Cancer: A Systematic Review of the Literature. European urology. 2014 doi: 10.1016/j.eururo.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 134.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. European urology. 2014;65:1058–1066. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 135.Zapatero A, Guerrero A, Maldonado J, Alvarez A, San Segundo C.G, Rodriguez M.C, Macias V, Pedro-Olive A, Casas F, Boladeras A, de Vidales C.M, de la Torre M.V, Calvo F.A. Randomized Phase III Trial of Adjuvant Androgen Deprivation in Combination with High-dose Conformal Radiotherapy in Intermediate and High Risk Localized Prostate Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:52. [Google Scholar]
- 136.McKay RR, Choueiri TK, Taplin ME. Rationale for and review of neoadjuvant therapy prior to radical prostatectomy for patients with high-risk prostate cancer. Drugs. 2013;73:1417–1430. doi: 10.1007/s40265-013-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, Hetherington J, Hoskin PJ, Jones RJ, Laing R, Lester JF, McLaren D, Parker CC, Parmar MK, Ritchie AW, Russell JM, et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019) European urology. 2014 doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 138.Maximum androgen blockade in advanced prostate cancer. an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet. 2000;355:1491–1498. [PubMed] [Google Scholar]
- 139.Heidenreich A, Pfister D, Brehmer B, Porres D. [Cytoreductive radical prostatectomy for prostate cancer with minimal osseous metastases: Results of a first feasibility and case control study] Der Urologe Ausg A. 2015;54:14–21. doi: 10.1007/s00120-014-3697-8. [DOI] [PubMed] [Google Scholar]
- 140.Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, Vizziello D, Sun M, Karakiewicz PI, Menon M, Montorsi F, Briganti A. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:3939–3947. doi: 10.1200/JCO.2013.54.7893. [DOI] [PubMed] [Google Scholar]
- 141.Schick U, Jorcano S, Nouet P, Rouzaud M, Vees H, Zilli T, Ratib O, Weber DC, Miralbell R. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta oncologica. 2013;52:1622–1628. doi: 10.3109/0284186X.2013.764010. [DOI] [PubMed] [Google Scholar]
- 142.Ost P, Decaestecker K, Lambert B, Fonteyne V, Delrue L, Lumen N, Ameye F, De Meerleer G. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. The Prostate. 2014;74:297–305. doi: 10.1002/pros.22750. [DOI] [PubMed] [Google Scholar]
- 143.Ponti E, Ingrosso G, Carosi A, Di Murro L, Lancia A, Pietrasanta F, Santoni R. Salvage Stereotactic Body Radiotherapy for Patients With Prostate Cancer With Isolated Lymph Node Metastasis: A Single-Center Experience. Clinical genitourinary cancer. 2014 doi: 10.1016/j.clgc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 144.Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, De Vos F, Huysse W, Hautekiet A, Maes G, Ost P. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiation oncology. 2014;9:135. doi: 10.1186/1748-717X-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Frontiers in oncology. 2012;2:215. doi: 10.3389/fonc.2012.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, Decaestecker K, Villeirs G, Vuye P, Ost P. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clinical genitourinary cancer. 2013;11:27–32. doi: 10.1016/j.clgc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 147.Jereczek-Fossa BA, Piperno G, Ronchi S, Catalano G, Fodor C, Cambria R, Fossati Ing P, Gherardi F, Alterio D, Zerini D, Garibaldi C, Baroni G, De Cobelli O, Orecchia R. Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. American journal of clinical oncology. 2014;37:227–233. doi: 10.1097/COC.0b013e3182610878. [DOI] [PubMed] [Google Scholar]
- 148.Tabata K, Niibe Y, Satoh T, Tsumura H, Ikeda M, Minamida S, Fujita T, Ishii D, Iwamura M, Hayakawa K, Baba S. Radiotherapy for oligometastases and oligo-recurrence of bone in prostate cancer. Pulmonary medicine. 2012;2012:541656. doi: 10.1155/2012/541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh D, Yi WS, Brasacchio RA, Muhs AG, Smudzin T, Williams JP, Messing E, Okunieff P. Is there a favorable subset of patients with prostate cancer who develop oligometastases? International journal of radiation oncology, biology, physics. 2004;58:3–10. doi: 10.1016/s0360-3016(03)01442-1. [DOI] [PubMed] [Google Scholar]
- 150.Decaestecker K, De Meerleer G, Ameye F, Fonteyne V, Lambert B, Joniau S, Delrue L, Billiet I, Duthoy W, Junius S, Huysse W, Lumen N, Ost P. Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC cancer. 2014;14:671. doi: 10.1186/1471-2407-14-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Attard G, Sydes MR, Mason MD, Clarke NW, Aebersold D, de Bono JS, Dearnaley DP, Parker CC, Ritchie AW, Russell JM, Thalmann G, Cassoly E, Millman R, Matheson D, Schiavone F, Spears MR, et al. Combining Enzalutamide with Abiraterone, Prednisone, and Androgen Deprivation Therapy in the STAMPEDE Trial. European urology. 2014 doi: 10.1016/j.eururo.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 152.Health USNIo. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. [Google Scholar]
- 153.Hospital CUoHKPYNE. ATOM_local Ablative Therapy. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. [Google Scholar]
- 154.Institute RPCINC. Stereotactic Body Radiation Therapy After Surgery in Treating Patients With Stage III-IV Non-small Cell Lung Cancer. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. [Google Scholar]
- 155.University CCCoWF. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Stereotactic Body Radiation Therapy (SBRT) in Metastatic Non-small Cell Lung Cancer. [Google Scholar]
- 156.Institute NCCTGNC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Radiation Therapy or Observation After Chemotherapy in Treating Patients With Stage IV Non-Small Cell Lung Cancer. [Google Scholar]
- 157.Oncology MR. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Concurrent and Non-concurrent Chemo-radiotherapy or Radiotherapy Alone for Patients With Oligo-metastatic Stage IV Non-small Cell Lung Cancer (NSCLC) [Google Scholar]
- 158.Institute SCHaR. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. The Optimal Intervention Time of Radiotherapy for Oligometastatic Stage IV Non-small Cell Lung Cancer(NSCLC) (OITROLC) [Google Scholar]
- 159.Foundation WJM. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. The Value of Radiotherapy in the Oligometastatic Non-squamous Non-small Cell Lung Cancer With Clinical Benefits From Erlotinib as Second-line Treatment (ROLE) [Google Scholar]
- 160.Pennsylvania ACCotUo. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Phase II Study of Pembrolizumab After Curative Intent Treatment for Oligometastatic Non-Small Cell Lung Cancer. [Google Scholar]
- 161.Center MDAC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Oligometastatic Disease. [Google Scholar]
- 162.Florida Uo. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Radiotherapy for Oligometastatic Prostate Cancer. [Google Scholar]
- 163.Clinic M. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Monitoring Anti-Prostate Cancer Immunity Following Stereotactic Body Radiotherapy (SBRT) [Google Scholar]
- 164.Castellon GdICeORHPd. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Phase II Study of SBRT as Treatment for Oligometastases in Prostate Cancer. [Google Scholar]
- 165.Dresden TU. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Percutaneous High-dose Radiotherapy in Patients With Oligometastases of Prostate Carcinoma (Oli-P) [Google Scholar]
- 166.l'Ouest ICd. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Salvage Radiotherapy Combined With Hormonotherapy in Oligometastatic Pelvic Node Relapses of Prostate Cancer (OLIGOPELVIS) [Google Scholar]
- 167.Institute CoHMCNC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Intensity-Modulated Radiation Therapy in Treating Patients Undergoing Androgen Deprivation Therapy for Metastatic Prostate Cancer. [Google Scholar]
- 168.Center CoHM. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Intensity-Modulated Radiation Therapy in Treating Patients Undergoing Androgen Deprivation Therapy for Metastatic Prostate Cancer. [Google Scholar]
- 169.University Hospital G. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Non-systemic Treatment for Patients With Low-volume Metastatic Prostate Cancer. [Google Scholar]
- 170.Center MS-KC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Combining Ipilimumab, Degarelix, and Radical Prostatectomy in Men With Newly Diagnosed Metastatic Castration Sensitive Prostate Cancer or Ipilimumab and Degarelix in Men With Biochemically Recurrent Castration Sensitive Prostate Cancer After Radical Prostatectomy. [Google Scholar]
- 171.Institute TNC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. High Dose Chemotherapy in Oligo-metastatic Homologous Recombination Deficient Breast Cancer. [Google Scholar]
- 172.Peter MacCallum Cancer Centre A. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. A Pilot Study of Stereotactic Ablation for Oligometastatic Breast Neoplasia in Combination With the Anti-PD-1 Antibody MK-3475. (BOSTON II) [Google Scholar]
- 173.Gustave Roussy CC Grand Paris. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Trial of Superiority of Stereotactic Body Radiation Therapy in Patients With Breast Cancer (STEREO-SEIN) [Google Scholar]
- 174.Institute CoHMCNC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Combination Chemotherapy With or Without Trastuzumab Followed By an Autologous Stem Cell Transplant and Radiation Therapy in Treating Patients With Stage III or Stage IV Breast Cancer. [Google Scholar]
- 175.Center ULCC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Defining the HER2 Positive (+) Breast Cancer Kinome Response to Trastuzumab, Pertuzumab, Combination Trastuzumab +Pertuzumab, or Combination Trastuzumab + Lapatinib. [Google Scholar]
- 176.Institute UoCNC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Hypofractionated Image Guided Radiation Therapy in Treating Patients With Stage IV Breast Cancer. [Google Scholar]
- 177.Nevada CCCo. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Concurrent Ipilimumab and Stereotactic Ablative Radiation Therapy (SART) for Oligometastatic But Unresectable Melanoma. [Google Scholar]
- 178.Center OSUCC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Stereotactic Radiation Therapy and Ipilimumab in Treating Patients With Metastatic Melanoma. [Google Scholar]
- 179.Center MDAC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Combi-Neo Study for Stage IV Melanoma. [Google Scholar]
- 180.Center SKCC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Stereotactic Body Radiation With Nelfinavir for Oligometastases. [Google Scholar]
- 181.Dwight Heron UoP. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Radiosurgery for Patients With Oligometastatic Disease at Initial Presentation. [Google Scholar]
- 182.Dwight Heron UoP. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Radiosurgery for Patients Recurrent Oligometastatic Disease. [Google Scholar]
- 183.Brussel UZ. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Non-interventional Observational Study of Stereotactic Body Radiotherapy (SBRT) for Oligometastatic Cancer. [Google Scholar]
- 184.University Health Network TPMH, Canada. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. 5 Fraction Stereotactic Body Radiation Therapy for Oligometastases Regimen, for Extra-Cranial Oligometastases. [Google Scholar]
- 185.Center UoTSM. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Prospective Clinical Registry for Oligometastic Disease, Consolidation Therapy, Debulking Prior to Chemotherapy, or Re-Irradiation. [Google Scholar]
- 186.Centers RMC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Definitive Therapy for Oligometastatic Solid Malignancies. [Google Scholar]
- 187.Medicine MSSo. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Sutent and Radiation as Treatment for Limited Extent Metastatic Cancer. [Google Scholar]
- 188.Oncology MR. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Assessment of Toxicity of the Immunocytokine L19-IL2 Administered Directly After the Course of Stereotactic Ablative Body Radiotherapy in Patients Suffering From Oligometastatic Solid Tumour. [Google Scholar]
- 189.(TROG) T-TROG. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. A Randomized Phase II Study of Stereotactic Ablative Body Radiotherapy for Metastases to the Lung, (TROG 13.01 SAFRON II) [Google Scholar]
- 190.Lawson Health Research Institute; London Regional Cancer Program CVUoA. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET) [Google Scholar]
- 191.Medicine WUSo. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Adaptive MRI-Guided SBRT for Unresectable Primary or Oligometastatic Central Thorax and Abdominal Malignancies. [Google Scholar]
- 192.Stereotactic Body Radiotherapy for Liver Tumors. Bethesda (MD): National Library of Medicine (US); 2000–. St. John's Mercy Research Institute SLICgI. [Google Scholar]
- 193.Hamburg-Eppendorf U. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Chemoradiotherapy for Patients With Oligometastatic Colorectal Cancer (OLGA) [Google Scholar]
- 194.Center SKCC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–. Stereotactic Radiation Therapy for Pediatric Sarcomas. [Google Scholar]
- 195.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Japanese journal of clinical oncology. 2010;40:107–111. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Niibe Y, Chang JY. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulmonary medicine. 2012;2012:261096. doi: 10.1155/2012/261096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R, Weichselbaum R. The oligometastatic state - separating truth from wishful thinking. Nature reviews Clinical oncology. 2014;11:549–557. doi: 10.1038/nrclinonc.2014.96. [DOI] [PubMed] [Google Scholar]
- 198.Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D'Amico AV, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Kawachi MH, Kuettel M, Lee RJ, Macvicar GR, Malcolm AW, et al. Prostate cancer, version 1.2014. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11:1471–1479. doi: 10.6004/jnccn.2013.0174. [DOI] [PubMed] [Google Scholar]


