Abstract
Background
Obesity and obstructive sleep apnea syndrome (OSA) are highly prevalent and frequently overlapping conditions in children that lead to systemic inflammation, the latter being implicated in the various end-organ morbidities associated with these conditions.
Aim
To examine the effects of adenotonsillectomy (T&A) on plasma levels of inflammatory markers in obese children with polysomnographically diagnosed OSA who were prospectively recruited from the community.
Methods
Obese children prospectively diagnosed with OSA, underwent T&A and a second overnight polysomnogram (PSG) after surgery. Plasma fasting morning samples obtained after each of the 2 PSG were assayed for multiple inflammatory and metabolic markers including interleukin-6 (IL-6), IL-18, plasminogen activator inhibitor-1 (PAI-1), monocyte chemoattractant protein-1 (MCP-1), matrix metalloproteinase- (MMP-9), adiponectin, apelin C, leptin and osteocrin.
Results
Out of 122 potential candidates, 100 obese children with OSA completed the study with only 1/3 exhibiting normalization of their PSG after T&A (i.e., AHI≤1/hrTST). However, overall significant decreases in MCP-1, PAI-1, MMP-9, IL-18 and IL-6, and increases in adropin and osteocrin plasma concentrations occurred after T&A. Several of the T&A responsive biomarkers exhibited excellent sensitivity and moderate specificity to predict residual OSA (i.e., AHI≥/hrTST).
Conclusions
A defined subset of systemic inflammatory and metabolic biomarkers is reversibly altered in the context of OSA among community-based obese children, further reinforcing the concept on the interactive pro-inflammatory effects of sleep disorders such as OSA and obesity contributing to downstream end-organ morbidities.
Keywords: sleep apnea, children, obesity, inflammation, cytokines, biomarkers
Introduction
Obstructive Sleep Apnea (OSA) is a very frequent condition in obese children that has been associated with a heightened risk for end-organ morbidities affecting the central nervous system (CNS), as well as promoting the emergence of cardiovascular and metabolic deficits in the context of pediatric obesity (1). The mechanisms underlying the increased risk for adverse consequences in obese children with OSA have been putatively ascribed to activation of systemic inflammatory pathways (2-5). Indeed, increased plasma levels of inflammatory mediators, such as high–sensitivity C-reactive protein, tumor necrosis factor α, IL-6 and interferon γ have been repeatedly documented (6,7). However, most of the published studies have relied on either the cross-sectional exploration of potential associations between sleep perturbations that characterize OSA and inflammatory markers, or alternatively have explored the effect of adenotonsillectomy (T&A) in clinical referral cohorts rather than community-based populations (2,8,9).
In the present study, we hypothesized that a cluster of inflammatory biomarkers previously identified as being affected by pediatric OSA (10, 11) may be responsive to treatment and enable identification of obese children with residual OSA after T&A. To this effect, we took advantage of a multicenter prospective pediatric clinical trial (12), to investigate the effect of T&A on several plasma inflammatory biomarkers in obese children from the community who were prospectively diagnosed with OSA and underwent surgical removal of enlarged tonsils and adenoids.
Subjects and Methods
124 obese children (ages 4-15 years) who were initially recruited from the community through their general pediatrician offices and were polysomnographically diagnosed with OSA (44 from Spain and 80 from Chicago) were referred for T&A, and invited to return for a follow-up PSG and morning fasting blood draw within 6 months of their surgical intervention. The study was approved by the human subject committee of each of the participating centers (University of Chicago IRB Protocol #: 10-708-A-AM017 and Comité Ético de Investigación Clínica del Área de Salud de Burgos y Soria protocol number 603), and is in accordance with the STROBE statement. The study was registered at ClinicalTrials.gov under NCT01322763. Informed consent was obtained from each legal caretaker, and assent was obtained from children if they were 12 years old or older. Data were coded to mask the identity, and any other personal information for all the subjects, thereby ensuring confidentiality. Samples and de-identified data from Spanish subjects included in this study were provided by the Basque Biobank for research OEHUN (www.biobancovasco.org), and were processed following standard operating procedures with appropriate approvals from the Ethical and Scientific Committees.
Anthropometric Measures and Overnight Polysomnography (PSG)
In the evening of the PSG tsting, assessments of height in meters, weight in kilograms, and BMI z score were performed. In addition, neck circumference was measured at the level of the cricopharyngeal membrane, and both waist and abdominal circumferences were measured with a measuring tape. Obesity was defined as a BMI >95% for age and gender (5, 12). PSG tests were conducted in a sleep laboratory under standardized conditions both during the initial phase aiming at determining the presence of OSA and within 6 months after T&A to assess the outcomes of the surgical procedure. The studies were scored, after removal of movement and technical artifacts, according to the standard criteria defined by the American Academy of Sleep Medicine (AASM) (13). Briefly, obstructive sleep apnea was defined as cessation of airflow with continued chest wall and abdominal movements for the duration of at least of two breaths. Hypopnea was defined as a decrease in nasal flow greater than 50%, corresponding to at least 4% decrease in the oxygen saturation (SpO2) as measured by pulse oximetry and/or terminated by a 3-second EEG arousal (14). The obstructive Respiratory Disturbance Index (oRDI) was calculated from the number of respiratory-effort related arousals and the number of apneas and hypopneas per hour of TST. To accommodate all the studies from both cohorts, both an apnea-hypopnea index (AHI)≥5/hrTST and a oRDI ≥3/hr of TST were considered to indicate the presence of OSA either in the initial PSG or after T&A (residual OSA) in accordance with the clinical practice guidelines in Spain and the USA (1, 15). In addition, we also assessed for the presence of AHI>1/hrTST as evidence of residual OSA after T&A (1). Furthermore, nadir and mean SpO2, as well total sleep time during which SpO2 below 90% or end-tidal CO2> 50 mmHg occurred, were recorded. Oxygen desaturation index (ODI) was defined as the number of desaturation events ≥ 4% per hour of TST.
Inflammatory Mediator Assays
Plasma was separated from the whole blood morning samples drawn from each child and stored in -80°C until assay. Commercially available ELISA kits specific for each cytokine were used to measure levels of interleukin (IL) -6, IL-18, monocyte chemoattractant protein (MCP)-1, adiponectin, matrix metalloproteinase (MMP)-9, apelin C, leptin (all individual kits from RayBiotech, Inc., Norcross, GA, USA), adropin (Peninsula laboratories LLC, San Carlos, CA, USA), osteocrin (MyBioSource, San Diego, CA, USA), and plasminogen activator inhibitor (PAI)-1 (Assaypro LLC, St. Charles, MO, USA). Assays were performed according to manufacturers’ recommendations.
Statistical analysis
Descriptive data for continuous variables are presented as means ± standard deviation (SD) and for categorical variables as percentages or ratios. The normal distribution of the data was ascertained with the Kolmogorov-Smirnov test, and natural logarithmic transformations were applied to linearize the data as needed. Analyses for comparisons between clinical and laboratory values in pre- and post T&A conditions were performed using paired Student’s t-tests. Group comparisons were conducted using one-way ANOVA followed by Bonferroni correction for multiple comparisons. Pearson’s correlation was used to compare between the marker levels and clinical parameters. Multivariate linear regression analysis was applied to assess relationships of significantly different markers between the pre- and post-treatment conditions. In addition, receiver operator curves were constructed for individual changes in each of the significantly modified plasma biomarkers after T&A using previously published approaches (16). Simply, a cut-off criterion was initially identified using ROC analyses for each of the bioassay analytes, and then each individual subject was scrutinized as to whether they fulfilled the cut-off values for 1, 2, 3, etc… of the analytes. Then, a ROC analysis for those fulfilling 1, 2, >2 cut-offs of the previously identified significantly predictive analytes was performed to determine the predictive performance of the combinatorial approach using MedCalc software (http://download.cnet.com/MedCalc/3000-2053_4-10079096.html). Statistical significance was assumed at two-tailed p<0.05. Statistical analyses were performed using SPSS software (version 21.0; SPPS Inc., Chicago, Ill.).
Results
Demographic data
Of the 124 obese children from the community (ages 4-15 years) who were initially diagnosed with OSA and treated with surgical adenotonsillectomy,100 children completed their follow-up PSG and blood sample, and their demographic characteristics are described in Table 1. Of note, 23 of 44 initially evaluated children originated from Spain and the 77 from the 80 children with OSA were from Chicago. The time elapsed between surgical T&A and the repeat PSG was 184.4 ± 23.7 days (range: 147-253 days). There were no significant differences between those who completed the study and those who did not, as far as their demographic or PSG characteristics (Tables 1 and 2). The major reasons for non-completion were repeated no-shows to scheduled appointments (n=17), lack of willingness to return for a PSG (n=6) or inability to contact the family (n=1).
Table 1.
Antropometric measures in 100 obese children with OSA before and after adenotonsillectomy (T&A) and in 24 children with OSA who did not return for follow-up after T&A.
| Pre-T&A | Post-T&A | p value | Pre-T&A Lost to F/U |
|
|---|---|---|---|---|
| Age (years) | 10.7 ± 2.4 | 11.4 ± 2.8 | 10.5± 2.8 | |
|
| ||||
| Gender (Male / Female) | 54/46 | 13/11 | ||
|
| ||||
| Height (m) | 1.47 ± 0.14 | 1.53 ± 0.17 | <0.01 | 1.49± 0.23 |
|
| ||||
| Weight (Kg) | 63.2 ± 17.6 | 66.9 ± 20.1 | <0.01 | 64.3±18.9 |
|
| ||||
| BMI | 26.8 ± 4.1 | 27.7 ± 4.6 | <0.05 | 26.9±5.3 |
|
| ||||
| BMI % | 96.7 ± 0.6 | 98.1 ± 0.7 | <0.05 | 96.8±0.8 |
|
| ||||
| BMI z score | 1.78±0.23 | 1.83±0.29 | <0.05 | 1.79±0.28 |
|
| ||||
| Neck Circumference (cm) | 33.7 ± 3.8 | 34.3 ± 3.8 | >0.05 | 33.9±4.2 |
|
| ||||
| Waist circumference /Hip circumference | 0.92 ± 0.06 | 0.97 ± 0.07 | >0.05 | 0.93±0.08 |
|
| ||||
Data presented as Mean ± SD
Table 2.
Polysomnographic characteristics in 100 obese children before and following adenotonsillectomy (T&A) and in 24 children with OSA who did not return for follow-up after T&A.
| Pre T&A | Post T&A | p value | Pre-T&A Lost to F/U |
|
|---|---|---|---|---|
| Time in Bed (min) | 486.8 ± 45.2 | 482.1 ± 46.4 | 0.9 | 488.7±65.2 |
|
| ||||
| Total sleep time (min) | 394.6 ± 67.2 | 392.5 ± 65.3 | 0.9 | 392.9±78.7 |
|
| ||||
| Sleep Efficiency % | 79.1 ± 10.2 | 80.3 ± 12.4 | 0.9 | 78.9±14.7 |
|
| ||||
| Arousal Index (/hrTST) | 17.5 ± 10.1 | 12.5 ± 8.1 | <0.001† | 17.8±14.2 |
|
| ||||
| AHI (hrTST) | 17.1± 17.8 | 4.5± 5.3 | <0.0001† | 18.4±23.6 |
|
| ||||
| Respiratory Disturbance Index (/hrTST) | 20.4 ± 18.9 | 7.2 ± 10.5 | <0.001† | 22.6±22.7 |
|
| ||||
| Obstructive RDI (/hrTST) | 19.8 ± 21.3 | 4.1 ± 4.9 | <0.001† | 20.8±23.2 |
|
| ||||
| Baseline SpO2 (%) | 98.3 ± 1.8 | 98.9 ± 1.3 | 0.2 | 98.3±2.1 |
|
| ||||
| Nadir SpO2 (%) | 81.4 ± 9.5 | 88.2 ±6.2 | 0.0001† | 80.9±11.2 |
|
| ||||
| Time SpO2 <90% | 2.8 ± 3.3 | 1.1 ± 2.9 | 0.01† | 2.9±3.7 |
|
| ||||
| Oxygen Desaturation Index (/hrTST) | 7.1 ± 8.2 | 2.2 ± 5.7 | 0.001† | 7.4±9.7 |
|
| ||||
| Peak End-Tidal CO2 (mmHg) | 49.1 ± 7.1 | 46.2 ± 8.3 | 0.001† | 49.0±8.2 |
|
| ||||
| Total Sleep time with End-Tidal CO2>50 mmHg (min) | 36.7 ± 15.6 | 28.9 ± 17.7 | 0.003† | 35.9±17.3 |
|
| ||||
Statistically significant difference
Sleep Studies
PSG findings before and following T&A are summarized in Table 2. As would be anticipated from the literature, significant improvements in respiratory measures during sleep emerged after T&A (Table 2). There were no significant differences in either the total duration of sleep and total time in bed (Table 2). However, a substantial proportion of children had post-operative AHI>1/hrTST (67.0%), AHI>5/hrTST (30.0%), or RDI>3/hrTST (40.0%), indicative of the presence of residual OSA. There were no differences however in either demographic, anthropometric or polysomnographic measures among the obese children with OSA who normalized their PSG findings and those who did not, including weight gain or BMI z score changes after T&A.
Plasma Inflammatory Mediators in Obese Children with OSA before and after T&A
As shown in Table 3, among the inflammatory markers included in the present study, several markers were significantly improved after T&A, namely IL-6 (p<0.0001), IL-18 (p<0.005), PAI-1 (p<0.0001), MCP-1 (p<0.003), MMP-9 (p<0.0001), and adropin (p<0.0001). However, no significant changes emerged in leptin and adiponectin levels for the whole cohort. No differences in inflammatory marker levels emerged between boys and girls regarding the effects of T&A. Furthermore, no significant associations emerged between the changes in lnAHI and lnoRDI and corresponding pre-post T&A changes in plasma biomarkers. However, the clinically-relevant outcome of T&A, i.e., AHI≥5/hrTST vs. AHI<5/hrTST, revealed major differences in several inflammatory markers that essentially further reinforced the changes previously found in the whole cohort (Table 4). Indeed, in addition to significant improvements in IL-6, IL-18, PAI-1, MCP-1, MMP-9, and adropin, statistically significant increases in plasma adiponectin concentrations and reductions in leptin levels occurred in those children with post-T&A AHI<5/hrTST (Table 4). In contrast, for the 30% of the cohort whose post-operative AHI remained ≥5/hrTST, no significant changes emerged for all biomarkers except for leptin levels, which increased, rather than decreased (Table 4).
Table 3.
Plasma inflammatory markers before and after T&A in 100 obese children with OSA.
| Pre T&A | Post T&A | p value | |
|---|---|---|---|
| IL-6 (pg/ml) | 9.2 ± 5.4 [2.1 – 18.3] |
7.1 ± 4.7 [2.0 – 19.7] |
0.0001† |
|
| |||
| PAI-1 (ng/ml) | 5.1 ± 1.7 [1.5 – 11.5] |
3.7 ± 1.6 [0.5 – 5.8] |
0.0001† |
|
| |||
| MCP-1 (pg/ml) | 50.6 ± 15.6 [24.7 – 115.8] |
42.5 ± 17.7 [16.4 – 112.7] |
0.003† |
|
| |||
| MMP-9 (μg/ml) | 1.08 ± 0.69 [0.18 – 5.01] |
0.78 ± 0.42 [0.25 – 2.3] |
0.0001† |
|
| |||
| IL-18 (pg/ml) | 222.2± 130.6 [96.5-654.3] |
201.8± 132.2 [35.0-433.2] |
0.005† |
|
| |||
| Apelin C (ng/ml) | 168.9± 75.5 [38.7-414.4] |
147.6± 79.2 [7.23-422.4] |
0.5 |
|
| |||
| Adropin (ng/ml) | 5.6± 3.8 [1.1-18.6] |
7.5± 3.4 [3.0-16.0] |
0.0001† |
|
| |||
| Leptin (ng/ml) | 21.9±6.3 [3.2-34.2] |
20.8±7.1 [4.0-38.5] |
0.2 |
|
| |||
| Adiponectin (μg/ml) | 39.7±17.8 [10.5-87.7] |
41.5±15.9 [10.2-88.7] |
0.2 |
|
| |||
| Osteocrin (ng/ml) | 19.0±22.0 [0.4-84.9] |
23.1±22.4 [0.3-121.3] |
0.06 |
Data presented as Mean ± SD [range]
Statistically significant difference
Table 4.
Plasma inflammatory markers before and after T&A in obese children with either residual or resolved OSA.
| OSA Resolved (AHI<5/hrTST) | Residual OSA (AHI≥5/hrTST) | |||||
|---|---|---|---|---|---|---|
| N=70 | N=30 | |||||
|
| ||||||
| Pre T&A | Post T&A | p value < | Pre T&A | Post T&A | p value < | |
|
| ||||||
| IL-6 (pg/ml) | 10.2 ± 11.3 [2.4-18.3] |
6.2 ± 3.1 [2.0-12.1] |
0.0001† | 9.5 ± 7.9 [2.0-18.1] |
9.2 ± 6.7 [2.3-19.7] |
0.7 |
| PAI-1 (ng/ml) | 4.3 ± 1.9 [1.6-11.5] |
1.7 ± 1.8 [0.5-4.2] |
0.0001† | 5.2 ± 1.6 [1.5-11.2] |
5.0 ± 1.8 [0.9-5.8] |
0.6 |
| MCP-1 (pg/ml) | 51.4 ± 14.4 [27.6-111.7] |
40.9 ± 17.2 [16.4-89.5] |
0.0001† | 48.3 ± 17.7 [24.7-115.8] |
48.8 ± 18.2 [32.6-112.7] |
0.9 |
| MMP-9 (μg/ml) | 1.13 ± 0.77 [0.20-5.01] |
0.69 ± 0.36 [0.25-1.37] |
0.0001† | 0.94 ± 0.45 [0.18-4.87] |
0.97 ± 0.48 [0.45-2.30] |
0.7 |
| IL-18 (pg/ml) | 197.2± 100.2 [98.9-654.3] |
160.6± 67.2 [35.0-293.7] |
0.0001† | 280.7± 171.2 [96.5-587.8] |
297.9±188.6 [104.7-433.2] |
0.3 |
| Apelin C (ng/ml) | 172.4± 72.2 [45.7-414.4] |
165.8± 77.4 [7.2-287.8] |
0.6 | 161.9± 183.2 [38.7-389.5] |
155.8± 145.6 [56.8-422.4] |
0.6 |
| Adropin (ng/ml) | 5.1± 3.3 [1.1-16.7] |
7.4± 3.2 [3.0-16.0] |
0.0001† | 6.7± 4.6 [1.8-18.6] |
7.6± 3.9 [3.3-15.6] |
0.2 |
| Leptin (ng/ml) | 22.6±6.3 [3.7-34.2] |
19.0±5.7 [4.0-32.7] |
0.01† | 21.7±6.5 [3.2-32.9] |
24.9±9.1 [6.9-38.5] |
0.03† |
| Adiponectin (μg/ml) | 40.6±18.9 [11.6-87.7] |
43.3±17.9 [10.2-69.2] |
0.01† | 38.4±15.4 [10.5-83.4] |
36.3±10.4 [12.7-88.7] |
0.2 |
| Osteocrin (ng/ml) | 16.3±23.0 [0.4-67.3] |
21.2±21.4 [0.3-56.4] |
0.06 | 25.0±18.6 [2.6-84.9] |
27.3±24.2 [2.8-121.3] |
0.6 |
Data presented as Mean ± SD [range]
Statistically significant difference
Based on these findings we examined receiver operator curves for prediction of T&A outcomes (resolved vs. persistent residual OSA using AHI≥5hrTST) initially using single biomarkers, and then clusters of biomarker subsets (Figure 1). Of all the biomarkers, the best performers included MCP-1, MMP-9, PAI-1, and adiponectin. MMP-9 and MCP-1 were essentially interchangeable, such that including them in combinatorial analyses did not yield any improvements in the AUC. Accordingly, MCP-1 and PAI-1 achieved an AUC of 0.809 with a sensitivity of 0.7 and a specificity of 0.9 (p<0.0001; Figure 1). Addition of adiponectin to these 2 markers resulted in improved AUC (0.858; p<0.0001) with higher sensitivity (0.9) but lower specificity (0.63).
Figure 1.
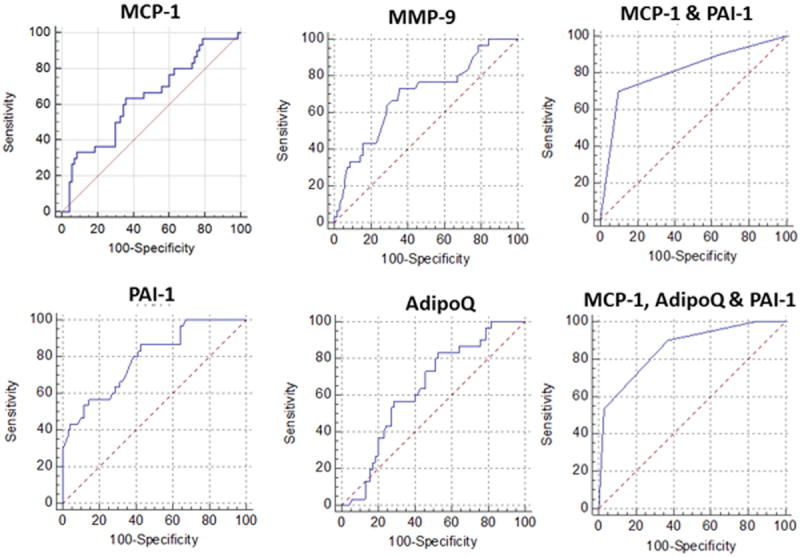
Receiver operator curves using individual plasma metabolic or inflammatory markers defined cut-off values for prediction of residual OSA after T&A in obese children.
Discussion
One of the major findings of the present study is that OSA in obese children amplifies the underlying systemic inflammatory pathways that are a priori activated by obesity alone, as demonstrated by the changes in some of these inflammatory markers following effective resolution of OSA after T&A. Furthermore, we show that effective treatment of OSA, as evidenced by normalization of respiratory disturbances during sleep, is associated with prominent improvements in a subset of systemic inflammatory markers, particularly when compared to children in whom T&A resulted in less severe, yet clinically significant residual OSA at follow-up (i.e., AHI≥5/hrTST). Additionally, our data reinforce the concept that although improvements in the magnitude of respiratory disturbance occur after T&A in obese children with OSA, a very high risk remains that there will be incomplete resolution of sleep disordered breathing in this population, ranging from 30% to 67%, and depending on the PSG-based cut-off value for respiratory disturbance being used (1). Finally, assessment of the overall inflammatory status, as inferred from a subset of such biomarkers not only reliably reflects the improvements in systemic inflammation elicited by T&A treatment of OSA, but may also provide a relatively robust surrogate reporter of OSA resolution vs. the presence of residual OSA.
Before discussing the potential implications of our findings, we will initially focus on the individual inflammatory mediators that significantly changed after T&A. MMP-9 belongs to a large family of zinc-containing enzymes that degrade extracellular matrix (17), and plays important roles in obesity and attendant risk for cardiovascular and metabolic dysfunction (18, 19). Evidence for increased MMP-9 levels has been controversial in obese children, and may reflect genomic variances (20-22). However, studies in both adults and children have indicated increases in MMP-9 levels in the presence of OSA, with the corollary assumption that altered MMP-9 levels may reflect atherogenic risk in these patients (23-25). Our findings are in agreement with those of Kaditis et al who found significant increases in MMP-9 levels in c=obese children with moderate to severe OSA (25), and provide initial cause and effect insights as to the contribution of OSA to MMP-9 circulating levels in obese children, as delineated by the T&A-induced changes leading to effective OSA resolution, and also suggest the potential underlying cardiovascular risk traditionally associated with OSA-induced increases in MMP-9. MCP-1 is a central member of the C-C chemokine superfamily responsible for attracting mononuclear cells to inflammatory sites. MCP-1 increases with obesity in children and lifestyle interventions reduce MCP-1 plasma levels (26-28). Furthermore, MCP-1 elevations have been reported in adult patients with OSA with reductions in MCP-1 levels being reported after continuous positive airway pressure (CPAP) treatment (29, 30). As in adults, effective treatment of OSA in the current obese pediatric cohort resulted in significant reductions in MCP-1 levels, further reinforcing the concept of reduced inflammatory state and metabolic and cardiovascular risk resulting from effective treatment of OSA (31). However, even though adiponectin reductions have been clearly identified in obese patients and associated to inflammatory burden as evidenced by increases in IL-6 and MCP-1 levels (32), here we found that OSA treatment elicited no changes in plasma adiponectin levels despite the decreases in MCP-1 in the total cohort. However, in the subset of children in whom OSA was effectively treated with T&A, significant increases in adiponectin emerged (Table 4). We should remark that our results herein were somewhat anticipated considering the previously reported significant, albeit inconsistent associations between plasma adipokines, namely leptin and adiponectin, and the severity of OSA (33-36). Notably, small yet significant reduction in leptin levels occurred in effectively treated children while increases in leptin emerged in those with residual OSA (Table 4; 37). Although we are unaware of any previous studies examining the effect of OSA treatment on PAI-1 levels in children, PAI-1 has been suggested as a reliable biomarker for metabolic syndrome (38), as well as a robust predictor of vascular complications in children (39). In adults with OSA, conflictive findings have been reported whereby improvements in PAI-1 levels have been inconsistently found following treatment (40-44). Here, we confirm that obese children with OSA have not only increased plasma levels of PAI-1 (5), but that T&A, particularly when effectively normalizing the respiratory disturbance during sleep leads to significant reductions in PAI-1 levels, thereby suggesting previously reported inferences between OSA and vascular dysfunction (45-48). Increased IL-6 concentrations have now been consistently reported in both adults and children with OSA, notwithstanding the attributable genetic variation that modulates such findings (49-51). Here, the usefulness of performing serial IL-6 measurements as a potential biomarker aiming to identify T&A outcomes in obese children with OSA was not confirmed. We should also remark that we have previously reported the increases in adropin levels that accompany effective treatment of OSA in a group of children with OSA, and the unique value of this biomarker in specifically identifying children with endothelial dysfunction (52). Thus, the improvements in circulating adropin concentrations after T&A would suggest the presence of underlying ameliorations in diffuse endothelial injury elicited by interactions between OSA and obesity (53).
The current study assessed T&A outcomes for the first time in a large obese pediatric cohort that was prospectively recruited from the community. As reported above, the changes in systemic inflammatory markers were selective, and also reflected the overall T&A outcomes as far as the magnitude of sleep-disordered breathing after surgery. Our study also emphasizes the relatively less favorable impact of T&A on an obese population with OSA, independently of the respiratory measure cut-off selected as evidence of resolution of OSA. The reduced likelihood for complete resolution of OSA after T&A has now been extensively noted in several retrospective studies, as well as in both non-randomized and randomized prospective trials (54-62). One possible explanation for such relatively unfavorable outcomes could reside in the propensity for accelerated weight gain that occurs after T&A in some obese children (63, 64), but such differences were not present in the present cohort.
In summary, this study provides conclusive evidence for the presence of elevations in selected systemic inflammatory markers among obese children with OSA, and improvements after T&A, particularly when the magnitude of respiratory disturbance expressed as AHI is below 5/hrTST after treatment. Further studies are needed to investigate the role of some of these markers as correlates of T&A outcomes, as well as their significance relative to OSA and obesity-associated end-organ morbidities. In this context, if future studies confirm the validity of these biomarkers in the identification of children with clinically significant residual OSA, their implementation into the clinical setting should pose no major technical problem and could potentially reduce the cost associated with routine post-T&A PSG in all obese children with OSA, as is currently recommended (1).
Acknowledgments
We thank the subjects and their parents for their participation, and the Basque Biobank For Research-OEHUN for their collaboration.
Funded By: LKG and DG are supported by a grant HL-65270 from the National Institutes of Health. RB is supported by American Heart Association grant 13SDG14780079. MLA, JTS and JDC were supported by the Spanish Respiratory Society (SEPAR) and Mutua Madrileña.
Footnotes
Authors’ Contributions: LKG, MLA, JTS and JDC participated in the conceptual framework of the project, performed experiments, analyzed data, and drafted components of the manuscript. EP and AGH performed experiments and assisted with analyses. RB contributed to subject recruitment and critical discussions. LKG, MLA and DG conceptualized the project, provided critical input in all phases of the experiments, analyzed data, drafted the ulterior versions of the manuscript, and are responsible for the financial support of the project and the manuscript content. All authors have reviewed and approved the final version of the manuscript.
ClinicalTrials.gov Identifier: NCT01322763 (url: http://clinicaltrials.gov/ct2/show/NCT013 22763?term=NCT01322763&rank=1)
Conflict of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Leila Kheirandish-Gozal, Email: lgozal@peds.bsd.uchicago.edu.
Alex Gileles-Hillel, Email: agileles@gmail.com.
María Luz Alonso-Álvarez, Email: mlalonsoalv@gmail.com.
Eduard Peris, Email: mayems@gmail.com.
Rakesh Bhattacharjee, Email: rbhattac@peds.bsd.uchicago.edu.
Joaquin Terán-Santos, Email: joaquinteransantos@yahoo.es.
Joaquin Duran-Cantolla, Email: joaquin.durancantolla@gmail.com.
References
- 1.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Gozal D, Sans Capdevila O, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among non-obese and obese pre-pubertal children. Am J Resp Crit Care Med. 2008;177(10):1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9(3):254–259. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalyfa A, Sans Capdevila O, Boazza M, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with obstructive sleep apnea. Sleep Med. 2009;10(1):75–86. doi: 10.1016/j.sleep.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Gileles-Hillel A, Alonso-Álvarez ML, Kheirandish-Gozal L, Peris E, Cordero-Guevara JA, Terán-Santos J, et al. Inflammatory markers and obstructive sleep apnea in obese children: the NANOS study. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/605280. 605280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheirandish-Gozal L, Sans Capdevila O, Tauman R, Gozal D. Plasma C-reactive protein in non-obese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med. 2006;2:301–304. [PMC free article] [PubMed] [Google Scholar]
- 7.Gozal D, Serpero LD, Kheirandish-Gozal L, Sans Capdevila O, Khalyfa A, Tauman R. Sleep measures and morning plasma TNF-α levels in children with sleep-disordered breathing. Sleep. 2010;33(3):319–325. doi: 10.1093/sleep/33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kheirandish-Gozal L, Sans Capdevila O, Kheirandish E, Gozal D. Elevated liver enzymes in children at risk for obstructive sleep apnea. Chest. 2008;133:92–99. doi: 10.1378/chest.07-0773. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Kim J. C-Reactive Protein and obstructive sleep apnea syndrome in children. Frontiers in Bioscience. 2012;4:2410–22. doi: 10.2741/e553. [DOI] [PubMed] [Google Scholar]
- 10.De Luca Canto G, Pachêco-Pereira C, Aydinoz S, Major PW, Flores-Mir C, Gozal D. Biomarkers associated with obstructive sleep apnea: A scoping review. Sleep Med Rev. 2014 Nov 28;23C:28–45. doi: 10.1016/j.smrv.2014.11.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca Canto G, Pachêco-Pereira C, Aydinoz S, Major PW, Flores-Mir C, Gozal D. Diagnostic capability of biological markers in assessment of obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2014 Oct 17; doi: 10.5664/jcsm.4358. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso-Álvarez M, Cordero-Guevara JA, Terán-Santos J, Gonzalez Martinez M, Jurado-Luque MJ, Corral-Peñafiel J, et al. the Spanish Sleep Network. Obstructive sleep apnea in obese community dwelling children: The NANOS study. Sleep. 2014;37(5):943–949. doi: 10.5665/sleep.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Álvarez ML, Canet T, Cubell-Alarco M, Estivill E, Fernández-Julián E, Gozal D, et al. Documento de consenso del síndrome de apneas-hipopneas durante el sueño en niños. Arch Bronconeumol. 2011;47(Supl 5):2–18. doi: 10.1016/S0300-2896(11)70026-6. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17.Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda) 2013;28(6):391–403. doi: 10.1152/physiol.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden G, Song WW. Effects of insulin and free fatty acids on matrix metalloproteinases. Curr Diab Rep. 2008;8(3):239–42. doi: 10.1007/s11892-008-0041-y. [DOI] [PubMed] [Google Scholar]
- 19.Berg G, Schreier L, Miksztowicz V. Circulating and adipose tissue matrix metalloproteinases in cardiometabolic risk environments: pathophysiological aspects. Horm Mol Biol Clin Investig. 2014;17(2):79–87. doi: 10.1515/hmbci-2013-0069. [DOI] [PubMed] [Google Scholar]
- 20.Belo VA, Souza-Costa DC, Lana CM, Caputo FL, Marcaccini AM, Gerlach RF, et al. Assessment of matrix metalloproteinase (MMP)-2, MMP-8, MMP-9, and their inhibitors, the tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in obese children and adolescents. Clin Biochem. 2009;42(10-11):984–90. doi: 10.1016/j.clinbiochem.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Belo VA, Souza-Costa DC, Luizon MR, Lanna CM, Carneiro PC, Izidoro-Toledo TC, et al. Matrix metalloproteinase-9 genetic variations affect MMP-9 levels in obese children. Int J Obes (Lond) 2012;36(1):69–75. doi: 10.1038/ijo.2011.169. [DOI] [PubMed] [Google Scholar]
- 22.Olza J, Gil-Campos M, Leis R, Rupérez AI, Tojo R, Cañete R, et al. Influence of variants in the NPY gene on obesity and metabolic syndrome features in Spanish children. Peptides. 2013;45:22–7. doi: 10.1016/j.peptides.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Chuang LP, Chen NH, Lin SW, Chang YL, Chao IJ, Pang JH. Increased matrix metalloproteinases-9 after sleep in plasma and in monocytes of obstructive sleep apnea patients. Life Sci. 2013;93(5-6):220–5. doi: 10.1016/j.lfs.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Volná J, Kemlink D, Kalousová M, Vávrová J, Majerová V, Mestek O, et al. Biochemical oxidative stress-related markers in patients with obstructive sleep apnea. Med Sci Monit. 2011;17(9):CR491–7. doi: 10.12659/MSM.881935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaditis AG, Alexopoulos EI, Karathanasi A, Ntamagka G, Oikonomidi S, Kiropoulos TS, et al. Adiposity and low-grade systemic inflammation modulate matrix metalloproteinase-9 levels in Greek children with sleep apnea. Pediatr Pulmonol. 2010;45(7):693–9. doi: 10.1002/ppul.21251. [DOI] [PubMed] [Google Scholar]
- 26.Stoppa-Vaucher S, Dirlewanger MA, Meier CA, de Moerloose P, Reber G, Roux-Lombard P, et al. Inflammatory and prothrombotic states in obese children of European descent. Obesity (Silver Spring) 2012;20(8):1662–8. doi: 10.1038/oby.2012.85. [DOI] [PubMed] [Google Scholar]
- 27.Breslin WL, Johnston CA, Strohacker K, Carpenter KC, Davidson TR, Moreno JP, et al. Obese Mexican American children have elevated MCP-1, TNF-α, monocyte concentration, and dyslipidemia. Pediatrics. 2012;129(5):e1180–6. doi: 10.1542/peds.2011-2477. [DOI] [PubMed] [Google Scholar]
- 28.Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60(4):445–52. doi: 10.1016/j.metabol.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol (1985) 2003;94(1):179–84. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 30.Stanke-Labesque F, Pépin JL, de Jouvencel T, Arnaud C, Baguet JP, Petri MH, et al. Leukotriene B4 pathway activation and atherosclerosis in obstructive sleep apnea. J Lipid Res. 2012;53(9):1944–51. doi: 10.1194/jlr.P022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SH, Lee JW, Im JA, Hwang HJ. Monocyte chemoattractant protein-1 is related to metabolic syndrome and homocysteine in subjects without clinically significant atherosclerotic cardiovascular disease. Scand J Clin Lab Invest. 2011;71(1):1–6. doi: 10.3109/00365513.2010.519047. [DOI] [PubMed] [Google Scholar]
- 32.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17(4):332–41. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 33.Tauman R, Serpero LD, Capdevila OS, O’Brien LM, Goldbart AD, Kheirandish-Gozal L, et al. Adipokines in children with sleep disordered breathing. Sleep. 2007;30(4):443–9. doi: 10.1093/sleep/30.4.443. [DOI] [PubMed] [Google Scholar]
- 34.Li AM, Ng C, Ng SK, Chan MM, So HK, Chan I, et al. Adipokines in children with obstructive sleep apnea and the effects of treatment. Chest. 2010;137(3):529–35. doi: 10.1378/chest.09-2153. [DOI] [PubMed] [Google Scholar]
- 35.Kelly A, Dougherty S, Cucchiara A, Marcus CL, Brooks LJ. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep. 2010;33(9):1185–91. doi: 10.1093/sleep/33.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med. 2011;7(3):268–73. doi: 10.5664/JCSM.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smits MM, Woudstra P, Utzschneider KM, Tong J, Gerchman F, Faulenbach M, et al. Adipocytokines as features of the metabolic syndrome determined using confirmatory factor analysis. Ann Epidemiol. 2013;23(7):415–21. doi: 10.1016/j.annepidem.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adly AA, Elbarbary NS, Ismail EA, Hassan SR. Plasminogen activator inhibitor-1 (PAI-1) in children and adolescents with type 1 diabetes mellitus: relation to diabetic microvascular complications and carotid intima media thickness. J Diabetes Complications. 2014;28(3):340–7. doi: 10.1016/j.jdiacomp.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Rångemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18(3):188–94. doi: 10.1093/sleep/18.3.188. [DOI] [PubMed] [Google Scholar]
- 40.Bagai K, Muldowney JA, 3rd, Song Y, Wang L, Bagai J, Artibee KJ, et al. Circadian variability of fibrinolytic markers and endothelial function in patients with obstructive sleep apnea. Sleep. 2014;37(2):359–67. doi: 10.5665/sleep.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Känel R, Natarajan L, Ancoli-Israel S, Mills PJ, Wolfson T, Gamst AC, et al. Effect of continuous positive airway pressure on day/night rhythm of prothrombotic markers in obstructive sleep apnea. Sleep Med. 2013;14(1):58–65. doi: 10.1016/j.sleep.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips CL, McEwen BJ, Morel-Kopp MC, Yee BJ, Sullivan DR, Ward CM, et al. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo-controlled crossover study. Thorax. 2012;67(7):639–44. doi: 10.1136/thoraxjnl-2011-200874. [DOI] [PubMed] [Google Scholar]
- 43.von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Dimsdale JE. Elevated plasminogen activator inhibitor 1 in sleep apnea and its relation to the metabolic syndrome: an investigation in 2 different study samples. Metabolism. 2007;56(7):969–76. doi: 10.1016/j.metabol.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116(20):2307–14. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141(3):682–91. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kheirandish-Gozal L, Etzioni T, Bhattacharjee R, Tan HL, Samiei A, Molero Ramirez H, et al. Obstructive sleep apnea in children is associated with severity-dependent deterioration in overnight endothelial function. Sleep Med. 2013;14(6):526–31. doi: 10.1016/j.sleep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Chan KC, Au CT, Chook P, Lee DL, Lam HS, Wing YK, et al. Endothelial Function in Children with Obstructive Sleep Apnea and the Effects of Adenotonsillectomy. Chest. 2014 Oct 2; doi: 10.1378/chest.14-1307. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Tauman R, O’Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2007 Jun;11(2):77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- 49.Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9(10):1003–12. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaditis AG, Gozal D, Khalyfa A, Kheirandish-Gozal L, Capdevila OS, Gourgoulianis K, et al. Variants in C-reactive protein and IL-6 genes and susceptibility to obstructive sleep apnea in children: a candidate-gene association study in European American and Southeast European populations. Sleep Med. 2014;15(2):228–35. doi: 10.1016/j.sleep.2013.08.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu L, Li Q. The evaluation of adenotonsillectomy on TNF-α and IL-6 levels in obese children with obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2013;77(5):690–4. doi: 10.1016/j.ijporl.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 52.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Molero-Ramirez H, Tan HL, Bandla HP. Circulating adropin concentrations in pediatric obstructive sleep apnea: potential relevance to endothelial function. J Pediatr. 2013;163(4):1122–6. doi: 10.1016/j.jpeds.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr Pulmonol. 2011;46(4):313–23. doi: 10.1002/ppul.21370. [DOI] [PubMed] [Google Scholar]
- 54.Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O’Brien LM, Ivanenko A, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149(6):803–8. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell RB, Kelly J. Adenotonsillectomy for obstructive sleep apnea in obese children. Otolaryngol Head Neck Surg. 2004;131(1):104–8. doi: 10.1016/j.otohns.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 56.Costa DJ, Mitchell R. Adenotonsillectomy for obstructive sleep apnea in obese children: a meta-analysis. Otolaryngol Head Neck Surg. 2009;140(4):455–60. doi: 10.1016/j.otohns.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 57.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–83. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 58.Hsu WC, Kang KT, Weng WC, Lee PL. Impacts of body weight after surgery for obstructive sleep apnea in children. Int J Obes (Lond) 2013;37(4):527–31. doi: 10.1038/ijo.2012.194. [DOI] [PubMed] [Google Scholar]
- 59.Huang YS, Guilleminault C, Lee LA, Lin CH, Hwang FM. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. 2014;37(1):71–6. doi: 10.5665/sleep.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nino G, Gutierrez MJ, Ravindra A, Nino CL, Rodriguez-Martinez CE. Abdominal adiposity correlates with adenotonsillectomy outcome in obese adolescents with severe obstructive sleep apnea. Pulm Med. 2012;2012 doi: 10.1155/2012/351037. 351037. Epub 2012 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhushan B, Sheldon S, Wang E, Schroeder JW., Jr Clinical indicators that predict the presence of moderate to severe obstructive sleep apnea after adenotonsillectomy in children. Am J Otolaryngol. 2014;35(4):487–95. doi: 10.1016/j.amjoto.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Amin R, Anthony L, Somers V, Fenchel M, McConnell K, Jefferies J, et al. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008;177(6):654–9. doi: 10.1164/rccm.200710-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katz ES, Moore RH, Rosen CL, Mitchell RB, Amin R, Arens R, et al. Growth after adenotonsillectomy for obstructive sleep apnea: an RCT. Pediatrics. 2014;134(2):282–9. doi: 10.1542/peds.2014-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]


