Abstract
Background/Objective
To compare DKA outcomes 6 years before and 6 years after changing rehydration fluids from ½ normal saline to Lactated Ringer's and decreasing the total intended fluid volume administered in the first 24 hours from 3500 mL-m-2-day-1 to ≤ 2500 mL- m-2-day-1 at Texas Children's Hospital (TCH) in response to recommendations by the ESPE, LWPES, and ISPAD in 2004.
Subjects/Methods
A retrospective cohort study was conducted in which 1868 admissions for DKA were identified and reviewed. The cohort was divided into 2 groups: Group A, 1998-2004 and Group B, 2004-2010. Subjects with suspected clinical cerebral edema and adverse outcomes were identified.
Results
Although not statistically significant, there was an equal number (n=3) of adverse outcomes (death or neurological damage) in each group despite more than double the admissions in Group B (1264) compared to Group A (604). Overall, the incidence of suspected clinical cerebral edema was more than double for those admissions in which fluid resuscitation was initiated at an outside hospital versus at TCH (13.6% vs 5.3%, p<0.001).
Conclusions
Decreasing the intended fluid rate during the initial 24 hours to 2500 mL-m-2-day-1 and increasing the IV fluid sodium content did not significantly decrease the incidence of adverse outcomes in children with DKA. However, children transferred from outside hospitals had a higher incidence of suspected clinical cerebral edema. Thus, we need to more readily share our management protocols with the emergency rooms of local referring hospitals to potentially decrease the incidence of suspected clinical cerebral edema and adverse outcomes in children transferred with DKA.
Keywords: diabetic ketoacidosis, cerebral edema, practice guidelines
Introduction
Diabetic ketoacidosis (DKA) has been reported to be the leading cause of overall morbidity and mortality in children and adolescents with type 1 diabetes mellitus.(1) Cerebral edema has been estimated to occur in ∼1% of all DKA episodes and accounts for 50 to 60% of diabetes-related deaths in children.(2) The mortality rate from cerebral edema has been reported to be 20-50% and 15-35% of survivors are left with permanent neurologic deficits.(3, 4) However, a recent retrospective study by White and Dickson reported a cerebral edema rate of 0.5% with an overall morbidity rate of 0.3% and an overall mortality rate of 0.08% due to DKA over a 12 year period at their institution.(5)
In a population based study from the United Kingdom, cerebral edema was 3 times more likely to occur in children with new onset diabetes than patients with the established diagnosis of diabetes.(6) In a multicenter study reporting on 61 children with cerebral edema, severity of acidosis and dehydration (lower pCO2 and higher initial serum urea nitrogen concentrations), younger age, and treatment with bicarbonate were associated with cerebral edema.(2) In addition, complications attributable to brain swelling were more likely to occur among patients whose serum sodium concentrations did not increase as the blood glucose concentration declined.(7) In a report of 4 children presenting to a large children's hospital, Duck et al. initially identified that a common feature of these children with cerebral edema (3 of whom died) was that each received rehydration fluids in excess of 4000 mL -m-2-day-1 in the first 24 hrs.(8) This observation was further reinforced in a subsequent report of 40 patients, 36 of whom developed brain herniation while receiving > 4000 mL-m-2-day-1 and led to the recommended rates of hydration of 3500 mL-m-2-day-1 and not to exceed 4000 mL-m-2-day-1.(9) Finally, hydrating at a rate greater than 50 mL/kg during the first 4 hours has also been associated with an increase in the risk of brain herniation.(10) Despite these reports, there remains a longstanding controversy concerning the optimal fluid regimen for the treatment of pediatric DKA. Much of the debate centers around the causative role of fluid therapy in the development of cerebral edema.(11)
In 2004, an ESPE/LWPES/ISPAD Consensus Statement recommended fluid resuscitation be accomplished with a solution with a tonicity ≥ 0.45% saline and a rate rarely in excess of 1.5 to 2 times the usual daily requirement based on age, weight, or body surface area.(1) In response to this recommendation, a new fluid protocol for DKA management was initiated at Texas Children's Hospital in August of 2004. The replacement IV fluids were changed from ½ normal saline (sodium, 77 mEq/L) to Lactated Ringer's (sodium, 130 mEq/L) and the total fluid volume to be administered in the first 24 hours was decreased from 3500 mL-m-2-day-1 to ≤ 2500 mL-m-2-day-1. During both study periods, the dose of insulin for children with severe DKA was 0.1 unit•kg-1•h-1. In this report, we compare the outcomes for the 6 years before and 6 years after instituting this change in our treatment protocol. We hypothesized that morbidity and mortality will be lower in the 6 years following the change in hydration rate compared to the previous 6 years.
Methods
Study Population
Following approval by the Baylor College of Medicine IRB, we performed a retrospective cohort study of children presenting to Texas Children's Hospital with DKA. All subjects were identified by ICD-9 codes for DKA, poorly controlled diabetes, or cerebral edema from August 1st, 1998 to July 31st, 2004 (Group A) and August 1st 2004 to August 1st, 2010 (Group B), death records when available, and hospitalizations lasting longer than 5 days. DKA was defined by standard criteria: glucose ≥ 200 and HCO3 ≤ 15 or pH ≤ 7.3.(1)
A total of 944 and 1761 hospitalizations were initially identified for Group A and B, respectively. Three hundred forty (340) and 497 hospitalizations in Groups A and B, respectively, were eliminated because of erroneous coding, hospitalizations for reasons other than DKA and/or not meeting the criteria for DKA. Because of changes in the retention or archiving of the medical records, 53 hospitalizations in Group A were identified with inadequate lab data or clinical record information to determine the presence or absence of DKA or suspected clinical cerebral edema; these records were also excluded.
Thus, 1868 episodes of DKA over the 12 years of studies were reviewed either in paper or electronic form. In Group A, there were 604 episodes involving 418 unique patients (1.44 episodes of DKA/patient admitted for documented DKA in the 6 year period). In Group B, there were 1264 hospitalizations involving 942 unique patients (1.34 episodes of DKA/patient admitted for documented DKA over a similar period of time).
Determination of Suspected Clinical Cerebral Edema
Criteria for the bedside evaluation of clinical cerebral edema have been published.(12) Since the signs and symptoms of cerebral edema may be subtle and/or not recognized, we broadened the criteria in an attempt to capture as many patients as possible who may have been at risk for cerebral edema which we labeled as “suspected clinical cerebral edema”. Thus, any indication of altered mental status, GCS ≤ 8, CT scan of the head, treatment with mannitol or 3% saline, or death were recorded as suspected clinical cerebral edema. A single child with long-standing, poorly controlled diabetes presented to the emergency ward in extremis with severe DKA and died shortly after arrival; that child was excluded from the analyses.
Data Collected
Available demographic and clinical data were collected including admission and discharge dates, date of birth, gender, race, preferred language, transfer status from outside hospitals (OSH), new onset diabetes versus established diabetes, type 1 versus type 2 diabetes, and initial lab values (bicarbonate, blood glucose, pH, and basic electrolytes). In particular, we chose to focus on data from the subset of those hospitalizations involving cases of suspected clinical cerebral edema who were at the highest risk for adverse outcomes (Table 3). Furthermore, maximum fluid volume in the first 24 hours and fluid type were collected when available. By protocol (see Table 1), initial volume expansion in Group A was accomplished with 10-20 mL/kg of normal saline (NS) and subsequent rehydration with ½ normal saline at 3500 mL-m-2-day-1 to which 20 mEq/L of potassium acetate and 20 mEq/L of K2PO4 were added. In Group B, initial volume expansion was accomplished with 10-20 mL/kg of Lactated Ringer's and subsequent rehydration was accomplished with Lactated Ringers 2500 mL-m-2-day-1 to which 15 mEq/L of KCl and 30 mEq/L of K2PO4 were added. No estimate of the degree of dehydration is included in the protocol; however, it is at the discretion of the treating physician to change the rehydration rate based on clinical judgment with the guideline not to exceed 4000 mL-m-2-day-1. Moreover, efforts were made to aggressively manage patients with suspected clinical cerebral edema in Group B with further fluid restriction and the early use of mannitol (and/or 3% saline). In 2007 a 2 bag dextrose system (0% and 10% dextrose) was added to the existing protocol to simplify adjustments to the amount of dextrose infused as hyperglycemia resolved but had no bearing on the overall fluid resuscitation rate or composition of the fluids.
Table 3. Comparison of Suspected Clinical Cerebral Edema Cases.
| Group A (n = 58) 1998-2004 |
Group B (n = 105) 2004-2010 |
|
|---|---|---|
| pHa | 7.03 ± 0.14 (53) | 7.02 ± 0.15 (90) |
| Glucose (mg/dL)a | 718 ± 381 (58) | 809 ± 447 (102) |
|
BUN (mg/dL)a % New onset Total Fluids in 24 hours(mL-m-2-day-1)a |
23.4 ± 17.5 (49) | 28.0 ± 17.1 (56) |
| 53% | 50% | |
| 3125 ± 805 (51) | 2736 ± 588 (83)* | |
|
Adverse Outcomesb Number % of Total DKA |
||
| 3 | 3 | |
| 0.50 | 0.24 | |
| % of Suspected Clinical Cerebral Edema Cases with Adverse Outcomes | 5.2 | 2.9 |
n= total cases of suspected clinical cerebral edema
Values in parentheses are the number of observations available for each parameter
Means ± SD
Neurologic impairment or death
p < 0.005
Table 1. Texas Children's Hospital Protocol Specifications.
| Group A 1998-2004 |
Group B 2004-2010 |
|
|---|---|---|
|
Initial Volume Expansion Fluid Type |
10-20 mL/kg of Normal Saline | 10-20 mL/kg of Lactated Ringer's |
| 1/2 Normal Saline(Na 77 mEq/L) | Lactated Ringer's(Na 130 mEq/L) | |
| Recommended Rate for First 24 h | 3500 mL-m-2-day-1 | 2500 mL-m-2-day-1 |
| Potassium Supplement | 20 mEq/L of K acetate and 20 mEq/L of K2PO4 | 15 mEq/L of KCl and 30 mEq/L of K2PO4 |
Statistical Analysis
Normally distributed continuous variables were compared using unpaired t-test. Categorical variables were compared using the chi-square test or Fisher's exact test. Statistical significance was defined as p<0.05. Statistical analysis was performed with StatView software (SAS Institute, Inc., Cary, NC).
Results
None of the criteria including age; sex; % of new onset; length of stay; fraction of children whose initial management occurred at an outside hospital differed between the groups (Table 2). In addition, neither did the use of CT scans, mannitol, or 3% saline differ (data not shown). Most importantly, the overall incidence of suspected clinical cerebral edema based on the number of hospitalizations was no different between the two groups.
Table 2. Group Characteristics.
| Group A (604*) 1998-2004 |
Group B (1264*) 2004-2010 |
|
|---|---|---|
|
Age at admission (y)a % Males/Females % New onset (n) |
11.4 ± 4.7 | 11.9 ± 4.4 |
| 42/58 | 45/55 | |
| 46% (278) | 43% (544) | |
|
Length of stay (days)a % Transfer from OSH (n) % Suspected clinical cerebral edema (n) |
3.5 ± 4.8 | 3.5 ± 3.3 |
| 43% (260) | 40% (506) | |
| 9.6% (58) | 8.3% (105) |
number of DKA admissions OSH = outside hospitals
Means ± SD
Sub-analysis of those patients who had suspected clinical cerebral edema showed that these children were in severe DKA based on their blood pH, glucose, and BUN values with no difference in any of these parameters between Group A and B (Table 3). An equal proportion of the patients from each group were newly diagnosed with diabetes (53 vs 50%). However, the total fluid volume received in the first 24 hours was statistically lower in Group B despite provider discretion to deviate from each protocol. The percent of children receiving mannitol was not different between the 2 groups (46 vs 41%) nor was there any difference in the absolute number of adverse outcomes (neurologic impairment or death) of those with suspected clinical cerebral edema between the two groups. In Group A, 1 child was left with neurological impairment and 2 died. In Group B, 2 children were left with neurological impairment and 1 child died. Although not statistically significant, the percentage of adverse outcomes based on the total number of DKA admissions or admissions with suspected clinical cerebral edema were both lower in Group B (despite nearly twice as many admissions and suspected clinical cerebral edema patients in Group B).
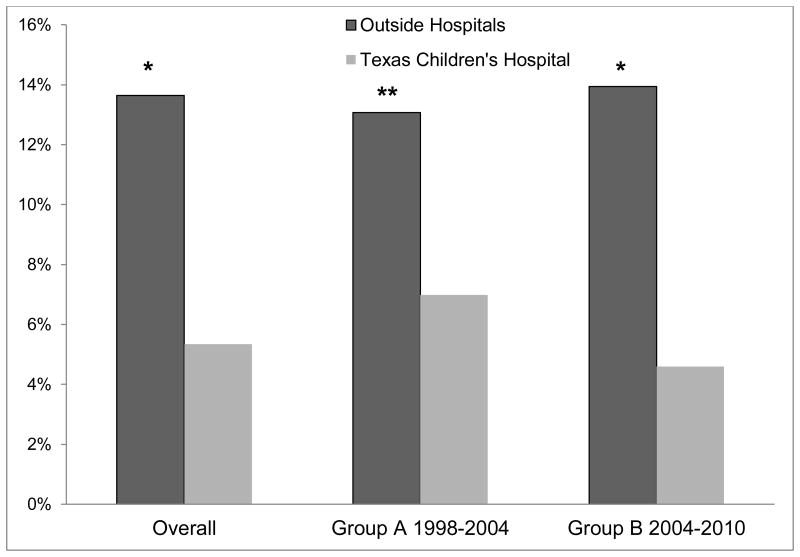
There was nearly a three-fold higher rate of suspected clinical cerebral edema in children who received their initial fluid resuscitation at outside hospitals compared to those initially seen at TCH. Overall, 13.6% of cases transferred from an outside hospital were designated to have suspected clinical cerebral edema versus only 5.3% initially managed at TCH (p< 0.001). Similar results were observed in Group A (13.1 vs 7.0%, p< 0.05) and Group B (13.9 vs 4.6%, p< 0.001) (Figure 1).
Figure 1. Suspected Clinical Cerebral Edema Based on Initial Site of Care.
The percent of children and adolescents who were initially treated at either outside hospitals or at Texas Children's Hospital who developed suspected clinical cerebral edema. Overall depicts inclusion of all children 1998 to 2010. *p-value< 0.0001 and **p-value< 0.05 for comparison of outside hospitals versus Texas Children's Hospital
Discussion
This study included 1868 hospitalizations for DKA over a 12 year period at a single institution and is one of the largest reported case series. In addition, it is unique in that it reports the results of an abrupt change in fluid treatment which occurred on August 1, 2004 in response to the LWPES/ESPE/ISPAD consensus statement. The intended rate of rehydration was lowered to 2500 mL-m-2-day-1 and the tonicity of the IV fluids was increased by changing from ½ NS to Lactated Ringers for the rehydration fluid. A previous small retrospective study reported changes in rates and tonicity of rehydration fluid. This involved 520 episodes of DKA of which 120 records were randomly selected and analyzed. No difference in outcomes were observed.(13) However, to our knowledge no center has reported such a deliberate and institution-wide change in its management of DKA and compared outcomes before and after such a change in their totality of patients. Although there are flaws in the complete recovery of medical records, the incidence of all cause severe adverse outcomes in this series is lower than those previously reported using a traditional definition of DKA(1, 2) and similar to the low rates recently reported by White and Dickson.(5) Despite our large case series, we were unable to prove statistical superiority for our change in fluid management, early anticipatory intervention involving further fluid restriction, and early use of osmotic agents. Even using a less stringent indicator of adverse outcomes, suspected clinical cerebral edema, it is difficult to determine whether the substantial and systematic change in our fluid management made any dramatic difference in outcome in those children with suspected clinical cerebral edema. Our study supports a previous report of a the low incidence of morbidity and mortality associated with DKA.(5)
Although the White and Dickson report used 0.675% NaCl at 3750 mL-m-2-day-1, both therapeutic approaches in our study utilized relative restriction in fluid administration rates with Lactated Ringer's keeping with other early reports of severe adverse outcomes using higher rates of fluid administration.(8, 9) It is of historic interest to note that Sidney Ringer invented what is now known as Ringer's Solution in 1880 for in vitro incubation of tissue. This was subsequently modified by Alex Hartman who added lactate and published his experience in the treatment of children with DKA in 1936.(14) Thus it would appear that we have come full circle to the findings of these early pioneers.
Of equal importance is the increased incidence of suspected clinical cerebral edema in patients who initially presented to outside hospitals. It is unknown whether these hospitals had any consistent rehydration regimen for children with DKA. In 2008, a retrospective review of 135 episodes of DKA in 127 children examined the variations in DKA management at outside facilities prior to transfer to a tertiary care center. They documented variations in the initial laboratory evaluation, insulin dosing, fluid therapy, and the administration of sodium bicarbonate.(15) In addition, Glaser, et al. reported that the management of pediatric DKA varied depending on physician training and that these differences inhibit our ability to study this process in a systematic manner.(16) It is of interest to note that children initially treated in the TCH emergency room had an overall lower frequency of suspected clinical cerebral edema after the initiation of our new protocol (Group B) despite more than double the number of admissions of children and adolescents with either DKA or suspected clinical cerebral edema. We attribute the greater number of hospitalizations in Group B to the growth of the Houston area, expansion of the hospital, and an increase in the number of pediatric endocrinology providers from 3 to 22 over this period of time. In addition, TCH serves as the primary referral center for the management and education of children with diabetes serving a population base in excess of 5 million people. Although not statistically significant, there was a decrease in the incidence of adverse outcomes in Group B compared to Group A after the institution of our new fluid guidelines.
We speculate that the differences in the incidence of suspected clinical cerebral edema in both Groups A and B based on the site of initial observed management is the result of some combination of factors. Outside hospitals could easily be more likely to refer sicker patients particularly those having altered mental status. These hospitals may also be more likely to order more extensive testing such as imaging studies or conversely TCH physicians may be more likely to order imaging studies due to the uncertainty of prior management. These data together with the report of Glaser et al. (16) strongly suggest large referral centers should play a greater role in the development of treatment guidelines or protocols for the initial fluid resuscitation in children and adolescents presenting with DKA increasing their awareness of the risks, signs, and symptoms of cerebral edema.
The cause of cerebral edema associated with DKA is not fully understood. There are a number of proposed etiologic mechanisms for cerebral edema in children with DKA. A thorough review of these is beyond the scope of this discussion and we refer the interested reader to a number of excellent reviews.(7, 17-19) Nevertheless, the only way truly to prevent this potentially devastating complication is to prevent children with diabetes from developing DKA. Many studies suffer from small sample sizes, heterogeneity in patient management, and inconsistency in diagnosing cerebral edema. Typically, cerebral edema occurs between 4 and 12 hours following presentation, but cases have been reported prior to and as late as 24-28 hours after the initiation of therapy.(20) This typical timing correlates well with changes in lumbar spinal fluid pressure observed over the first 10 hours of therapy in adults presenting with DKA.(21) Unfortunately, timing of actual initiation of therapy for suspected cerebral edema could not be consistently found during our records retrieval. Cerebral imaging studies may show focal or diffuse cerebral edema, but up to 40% of initial CT scans of children presenting with DKA are reported as “cerebral edema” despite the absence of obvious clinical symptoms different from those without such findings and unremarkable outcomes.(20) In a small series of children presenting with DKA, brain CT at the time of diagnosis and days later demonstrated an increase in ventricular size suggesting that an element of cerebral edema may exist to some degree in children presenting with severe DKA and dehydration.(22) However, multiple retrospective studies have led to the association of risk factors and treatment recommendations for cerebral edema related to DKA.(2, 6, 23)
Either one change or a combination of both changes to the tonicity of the rehydration fluid and a decrease the rate of fluid administration may have contributed to the low morbidity and mortality rate in this report. In a small pilot study, DKA treatment protocols using different rates of fluid infusion were not associated with large or obvious differences in MRI measures of cerebral swelling.(24) Moreover, early recognition of signs and symptoms of cerebral edema and early intervention with mannitol (or 3% saline) may be an even more important factor in decreasing morbidity and mortality as described by Harris and Fiordalisi.(25, 26) The question remains whether or not treatment itself contributes to the development of cerebral edema or whether it is the ultimate clinical manifestation of something that to some degree occurs in all children with severe DKA. Several smaller studies have attempted to elucidate differences in clinical outcomes comparing differences in tonicity and rates of administration.(13, 27) Although differences were observed in the incidence of cerebral edema, these studies were dramatically underpowered to address the question. Currently, the PECARN Fluid Therapies Under Investigation in DKA (“FLUID”) study is the first prospective randomized controlled clinical trial to investigate the impact of fluid rehydration regimens on neurological and neurocognitive outcomes in children with DKA. The study has a total of 4 arms (2 rates of rehydration with either 0.9% saline or 0.45% saline).(28) However, projecting from our data and those of White and Felner (13) in the initial DKA management among providers, centers, and referral hospitals, it will be difficult, if not futile, to design an appropriate study to define a definitive treatment protocol to avoid the devastating outcome of cerebral edema during recovery from DKA. The true incidence and etiology of brain swelling, the extreme of which is clinical cerebral edema, will remain unknown unless there are clearer definitions of the clinical diagnosis of cerebral edema and a reporting mechanism to accumulate accurate and meaningful data over time in the United States.
Conclusion
Despite our large numbers of very ill children and adolescents with DKA, the number of adverse outcomes was fortunately small. We cannot exclude that increased awareness and early intervention in treating children with suspected clinical cerebral edema may have in and of itself improved our outcomes. However, it is clear that after the institution of our DKA fluid protocol using a 30% lower rehydration rate and a 60% increase in the tonicity of the replacement fluids, the increased awareness of cerebral edema, and the aggressive management of those with suspected clinical cerebral edema, was not superior to our previous protocol but gave comparable outcomes in the morbidity or mortality rate due to DKA-related cerebral edema. Finally, children transferred from outside hospitals had a higher incidence of suspected clinical cerebral edema than those initially managed in our emergency room and points to the need to more readily share management protocols and clinical experience developed at tertiary referral centers with local referring hospitals to potentially decrease the incidence of suspected clinical cerebral edema and adverse outcomes in children transferred with DKA.
Acknowledgments
We would like to thank the staff and physicians of the Texas Children's Hospital Emergency Department, the Sections of Critical Care Medicine and Pediatric Diabetes and Endocrinology for their dedication both day and night, their competency and care for these critically ill children and adolescents with diabetic ketoacidosis.
Dr. Hsia is now at the Pennington Biomedical Research Center, Baton Rouge, Louisiana and Dr. Tarai is now at the University of Indiana, Department of Pediatrics
A portion of the data was presented as a poster at the Pediatric Endocrine Society Annual Meeting in Boston, MA in May 2012.
This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, TX).
Funding source: The project was supported by NIH grant RO1DK 55478 (MWH), USDA/ARS 6250-5100 (MWH), and NIH grant 5T32DK 063873-10 (DSH).
Abbreviations
- DKA
diabetic ketoacidosis
- NS
normal saline
- OSH
outside hospitals
- TCH
Texas Children's Hospital
References
- 1.Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004 Feb;113(2):e133–40. doi: 10.1542/peds.113.2.e133. [DOI] [PubMed] [Google Scholar]
- 2.Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001 Jan 25;344(4):264–9. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 3.Brown TB. Cerebral oedema in childhood diabetic ketoacidosis: is treatment a factor? Emergency medicine journal : EMJ. 2004 Mar;21(2):141–4. doi: 10.1136/emj.2002.001578. Epub 2004/02/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbloom AL. Intracerebral crises during treatment of diabetic ketoacidosis. Diabetes Care. 1990 Jan;13(1):22–33. doi: 10.2337/diacare.13.1.22. Epub 1990/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 5.White PC, Dickson BA. Low Morbidity and Mortality in Children with Diabetic Ketoacidosis Treated with Isotonic Fluids. J Pediatr. 2013 Mar 15; doi: 10.1016/j.jpeds.2013.02.005. Epub 2013/03/19. Eng. [DOI] [PubMed] [Google Scholar]
- 6.Edge JA, Hawkins MM, Winter DL, Dunger DB. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001 Jul;85(1):16–22. doi: 10.1136/adc.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris GD, Fiordalisi I, Harris WL, Mosovich LL, Finberg L. Minimizing the risk of brain herniation during treatment of diabetic ketoacidemia: a retrospective and prospective study. J Pediatr. 1990 Jul;117(1 Pt 1):22–31. doi: 10.1016/s0022-3476(05)82439-4. [DOI] [PubMed] [Google Scholar]
- 8.Duck SC, Weldon VV, Pagliara AS, Haymond MW. Cerebral edema complicating therapy for diabetic ketoacidosis. Diabetes. 1976 Feb;25(2):111–5. doi: 10.2337/diab.25.2.111. Epub 1976/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 9.Duck SC, Wyatt DT. Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr. 1988 Jul;113(1 Pt 1):10–4. doi: 10.1016/s0022-3476(88)80521-3. Epub 1988/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney CP, Vlcek BW, DelAguila M. Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatr Neurol. 1999 Oct;21(4):721–7. doi: 10.1016/s0887-8994(99)00079-x. [DOI] [PubMed] [Google Scholar]
- 11.Wolfsdorf JI. The International Society of Pediatric and Adolescent Diabetes guidelines for management of diabetic ketoacidosis: do the guidelines need to be modified? Pediatr Diabetes. 2014 Jun;15(4):277–86. doi: 10.1111/pedi.12154. [DOI] [PubMed] [Google Scholar]
- 12.Muir AB, Quisling RG, Yang MC, Rosenbloom AL. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care. 2004 Jul;27(7):1541–6. doi: 10.2337/diacare.27.7.1541. [DOI] [PubMed] [Google Scholar]
- 13.Felner EI, White PC. Improving management of diabetic ketoacidosis in children. Pediatrics. 2001 Sep;108(3):735–40. doi: 10.1542/peds.108.3.735. Epub 2001/09/05. eng. [DOI] [PubMed] [Google Scholar]
- 14.White SA, Goldhill DR. Is Hartmann's the solution? Anaesthesia. 1997 May;52(5):422–7. doi: 10.1111/j.1365-2044.1997.090-az0082.x. [DOI] [PubMed] [Google Scholar]
- 15.Bradley P, Tobias JD. An evaluation of the outside therapy of diabetic ketoacidosis in pediatric patients. Am J Ther. 2008 Nov-Dec;15(6):516–9. doi: 10.1097/MJT.0b013e318172771b. [DOI] [PubMed] [Google Scholar]
- 16.Glaser NS, Kuppermann N, Yee CK, Schwartz DL, Styne DM. Variation in the management of pediatric diabetic ketoacidosis by specialty training. Archives of pediatrics & adolescent medicine. 1997 Nov;151(11):1125–32. doi: 10.1001/archpedi.1997.02170480055008. Epub 1997/11/25. eng. [DOI] [PubMed] [Google Scholar]
- 17.Glaser NS, Wootton-Gorges SL, Marcin JP, Buonocore MH, Dicarlo J, Neely EK, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004 Aug;145(2):164–71. doi: 10.1016/j.jpeds.2004.03.045. Epub 2004/08/04. eng. [DOI] [PubMed] [Google Scholar]
- 18.Vavilala MS, Richards TL, Roberts JS, Chiu H, Pihoker C, Bradford H, et al. Change in blood-brain barrier permeability during pediatric diabetic ketoacidosis treatment. Pediatr Crit Care Med. 2010 May;11(3):332–8. doi: 10.1097/PCC.0b013e3181c013f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wootton-Gorges SL, Buonocore MH, Kuppermann N, Marcin JP, Barnes PD, Neely EK, et al. Cerebral proton magnetic resonance spectroscopy in children with diabetic ketoacidosis. AJNR Am J Neuroradiol. 2007 May;28(5):895–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfsdorf J, Glaser N, Sperling MA. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006 May;29(5):1150–9. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 21.Clements RS, Jr, Blumenthal SA, Morrison AD, Winegrad AI. Increased cerebrospinal-fluid pressure during treatment of diabetic ketosis. Lancet. 1971 Sep 25;2(7726):671–5. doi: 10.1016/s0140-6736(71)92245-8. [DOI] [PubMed] [Google Scholar]
- 22.Krane EJ, Rockoff MA, Wallman JK, Wolfsdorf JI. Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N Engl J Med. 1985 May 2;312(18):1147–51. doi: 10.1056/NEJM198505023121803. [DOI] [PubMed] [Google Scholar]
- 23.Glaser N. Cerebral edema in children with diabetic ketoacidosis. Current diabetes reports. 2001 Aug;1(1):41–6. doi: 10.1007/s11892-001-0009-7. Epub 2003/05/24. eng. [DOI] [PubMed] [Google Scholar]
- 24.Glaser NS, Wootton-Gorges SL, Buonocore MH, Tancredi DJ, Marcin JP, Caltagirone R, et al. Subclinical cerebral edema in children with diabetic ketoacidosis randomized to 2 different rehydration protocols. Pediatrics. 2013 Jan;131(1):e73–80. doi: 10.1542/peds.2012-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris GD, Fiordalisi I. Physiologic management of diabetic ketoacidemia. A 5-year prospective pediatric experience in 231 episodes. Archives of pediatrics & adolescent medicine. 1994 Oct;148(10):1046–52. doi: 10.1001/archpedi.1994.02170100044009. [DOI] [PubMed] [Google Scholar]
- 26.Fiordalisi I, Novotny WE, Holbert D, Finberg L, Harris GD. An 18-yr prospective study of pediatric diabetic ketoacidosis: an approach to minimizing the risk of brain herniation during treatment. Pediatr Diabetes. 2007 Jun;8(3):142–9. doi: 10.1111/j.1399-5448.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 27.Mel JM, Werther GA. Incidence and outcome of diabetic cerebral oedema in childhood: are there predictors? Journal of paediatrics and child health. 1995 Feb;31(1):17–20. doi: 10.1111/j.1440-1754.1995.tb02905.x. Epub 1995/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 28.Glaser NS, Ghetti S, Casper TC, Dean JM, Kuppermann N, Pediatric Emergency Care Applied Research Network DKAFSG Pediatric diabetic ketoacidosis, fluid therapy, and cerebral injury: the design of a factorial randomized controlled trial. Pediatr Diabetes. 2013 Sep;14(6):435–46. doi: 10.1111/pedi.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]