Abstract
Atrial natriuretic peptide (ANP) is a cardiac hormone that regulates salt-water balance and blood pressure by promoting renal sodium and water excretion and stimulating vasodilation. ANP also has an anti-hypertrophic function in the heart, which is independent of its systemic blood pressure-lowering effect. In mice, ANP deficiency causes salt-sensitive hypertension and cardiac hypertrophy. Recent studies have shown that ANP plays an important role in regulating vascular remodeling and energy metabolism. Variants in the human NPPA gene, encoding the ANP precursor, are associated with hypertension, stroke, coronary artery disease, heart failure (HF) and obesity. ANP and related peptides are used as biomarkers for heart disease. Recombinant proteins and small molecules that enhance the ANP pathway have been developed to treat patients with HF. In this review, we discuss the role of ANP in cardiovascular biology and disease.
Keywords: ANP, Corin, Heart failure, Hypertension, Natriuretic peptides, Sodium homeostasis
1. Introduction
Maintaining salt-water balance is of fundamental importance for all animals. The discovery of the natriuretic activity in rat atrial extracts by de Bold et al. established a previously suspected cardiac endocrine function in regulating body fluid homeostasis (de Bold et al., 1981). The responsible molecule later was found to be atrial natriuretic factor (ANF), also called atrial natriuretic peptide (ANP) (de Bold, 1985). Under high blood volume and pressure, heart muscle cells release ANP into the circulation. In the kidney, ANP enhances salt and water excretion. In the blood vessel, ANP promotes vasodilation. As such, ANP acts as a cardiac hormone to regulate blood volume and pressure. Defects in the ANP pathway now are known to contribute to major diseases such as hypertension, cardiac hypertrophy and heart failure (HF). More recently, ANP and related peptides have been implicated in lipid metabolism and metabolic disease. In this review, we discuss the role of ANP in cardiovascular biology and disease.
2. The NPPA gene
In mammals, the natriuretic peptide family has three members: ANP, brain or B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) (Potter et al., 2006; Wu et al., 2009). These peptides are encoded by three separate genes evolved from an ancestral natriuretic peptide gene in primitive vertebrates (Inoue et al., 2003; Takei et al., 2006). The human NPPA gene, encoding the ANP precursor, is on the short arm of chromosome 1 (1p36.21). The gene consists of 3 exons and spans more than 2 kb in length. The NPPB gene, encoding the BNP precursor, has a similar genomic structure and is located ~10 kb upstream of the NPPA gene. Most likely, these genes were derived from gene duplication. In contrast, the human NPPC gene, encoding the CNP precursor, is on the long arm of chromosome 2 (2q37.1), indicating that it was segregated from the NPPA and NPPB genes during evolution (Takei et al., 2006).
The NPPA gene is expressed primarily in the heart, where the expression level is higher in atria than ventricles (Potter et al., 2006). Low levels of ANP expression have been detected in other tissues, including the lung, aorta, brain, adrenal gland, kidney and uterus. Transcription factors including GATA-4, NF-AT-3 and TBX5 are critical for NPPA expression in the heart (Durocher and Nemer, 1998). These transcription factors also play a role in the expression of other cardiac genes (Molkentin et al., 1998; Pan et al., 2002). To date, the transcriptional regulation of NPPA expression in non-cardiac tissues is not well understood.
3. ANP biosynthesis and processing
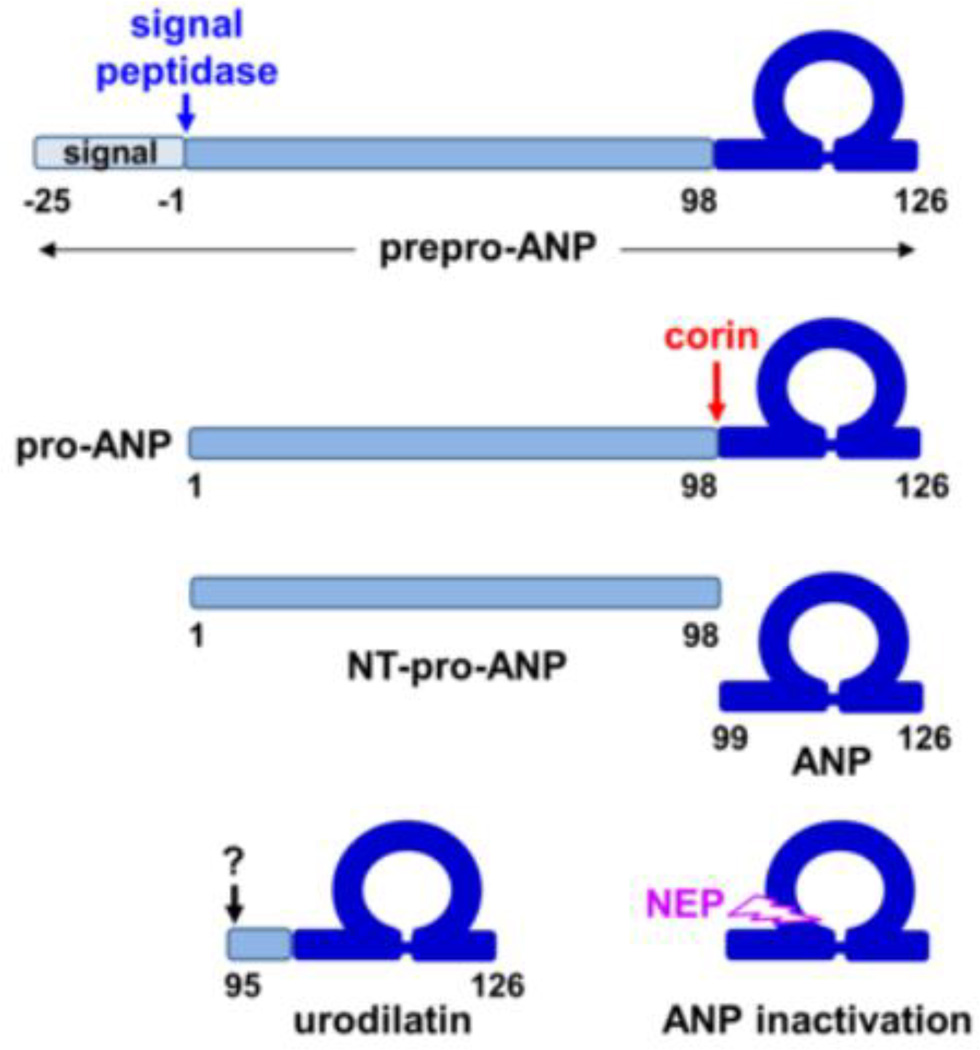
ANP is synthesized as a precursor, i.e. prepro-ANP, a polypeptide of 151 amino acids (Fig. 1). In the endoplasmic reticulum, signal peptidase removes the 25-amino-acid signal peptide, which may be further processed by signal peptide peptidase and released from the cell. Small fragments derived from the ANP signal peptide have been detected in human plasma (Pemberton et al., 2012). After the removal of the signal peptide, the 126-amino-acid pro-ANP is stored in the granules in cardiomyocytes. When the cells are stimulated, for example, by mechanical stretch, pro-ANP is released and converted to mature ANP by corin, a serine protease anchored on the cell surface (Wu et al., 2002; Yan et al., 1999). The corin-mediated activation cleavage occurs at Arg-98↓Ser-99 in pro-ANP (Yan et al., 2000), generating a 98-amino-acid N-terminal peptide, i.e. NT-pro-ANP, and a 28-amino-acid C-terminal peptide, i.e. ANP. In mice, disrupting the Corin gene abolished pro-ANP processing and caused spontaneous hypertension (Chan et al., 2005; Wang et al., 2012b), indicating that corin is the primary pro-ANP convertase in vivo. In humans, CORIN variants and mutations that impair corin activity have been identified in patients with hypertension and heart disease (Dong et al., 2013; Dries et al., 2005; Wang et al., 2012a; Wang et al., 2008; Zhang et al., 2014).
Fig. 1.
ANP synthesis and processing. ANP is synthesized as prepro-ANP. The signal peptide is removed by signal peptidase. Pro-ANP is converted to active ANP by corin. A disulfide bond connects two Cys residues, forming a ring structure in ANP. Neutral endopeptidase (NEP) inactivates ANP. In the kidney, alternative processing by an unknown enzyme generates urodilatin.
ANP is degraded by at least two different mechanisms. After binding to the natriuretic peptide receptor C (NPR-C), also called the clearance receptor, which is expressed in many tissues, ANP is internalized and degraded in lysosomes (Koller and Goeddel, 1992; Potter et al., 2006). Alternatively, ANP is degraded by neutral endopeptidase (NEP), also called neprilysin, a zinc-dependent protease that inactivates ANP at Cys-105↓Phe-106 and other sites (Kenny and Stephenson, 1988) (Fig. 1). Inhibition of neprilysin increases ANP levels. In HF patients, combined inhibition of neprilysin and angiotensin receptor represents a novel therapeutic strategy to reduce the mortality and morbidity (McMurray et al., 2014).
In non-cardiac tissues, pro-ANP may be processed at alternative sites. Urodilatin, for example, is a 32-amino-acid ANP isoform from human urine, which contains four additional amino acids (Thr-Ala-Pro-Arg) at the N-terminus (Feller et al., 1989) (Fig. 1). In animal models and healthy volunteers, urodilatin exhibited potent natriuretic and diuretic activities (Forssmann et al., 2001). Urodilatin appeared more resistant than ANP to NEP-mediated degradation (Gagelmann et al., 1988). To date, the proteolytic enzyme responsible for producing urodilatin in the kidney remains unclear. Because urodilatin is cleaved at Leu-94↓Thr-95, it is unlikely that the enzyme is a trypsin-like protease such as corin, which cleaves after basic residues (Knappe et al., 2003).
4. Biological functions of ANP
The primary function of ANP is to promote natriuresis and diuresis in the kidney and to relax vascular smooth muscles, thereby regulating blood volume and pressure. This function is mediated by the natriuretic peptide receptor A (NPR-A), also called guanylyl cyclase A, a transmembrane receptor containing an intracellular guanylyl cyclase domain (Koller and Goeddel, 1992; Potter et al., 2006). Upon ANP binding, the receptor is activated, promoting cyclic guanosine monophosphate (cGMP) production. Increased intracellular cGMP levels activate cGMP-dependent protein kinases (PKGs), which in turn increase glomerular filtration rate, inhibit sodium and water reabsorption in the proximal tubules and collecting ducts, and suppress renin secretion from the juxtaglomerular cells (Theilig and Wu, 2015; Zeidel, 1990). In smooth muscles, PKG activation lowers intracellular Ca2+ concentration and decreases the sensitivity of the contractile apparatus to Ca2+, thereby relaxing the muscle cells (Carvajal et al., 2000). ANP also inhibits aldosterone production in the adrenal gland (Kudo and Baird, 1984; Maack et al., 1984). Consistently, knockout mice lacking Nppa or Npr1 gene developed hypertension (John et al., 1995; Lopez et al., 1995) and had high levels of plasma and renal renin activities (Melo et al., 1998; Shi et al., 2001).
ANP and BNP are believed to share the same receptor. The binding affinity for BNP to NPR-A, however, is ~10-fold weaker than that for ANP (Koller and Goeddel, 1992), suggesting that ANP is the primary ligand for NPR-A. In mice, ANP- and BNP-deficiencies resulted in different phenotypes; ANP knockout mice were hypertensive, whereas BNP knockout mice were normotensive but developed cardiac fibrosis (John et al., 1995; Tamura et al., 2000). These results suggest that ANP and BNP may not substitute each other for the same receptor in vivo. It has been reported that another BNP receptor may exist (Goy et al., 2001). To date, however, such a receptor remains unidentified.
In the heart, ANP has a local anti-hypertrophic function that is independent of its systemic blood pressure lowering effect. In Nppa and Npr1 knockout mice, dietary or pharmacological treatments lowered blood pressure, but did not prevent cardiac hypertrophy (Feng et al., 2003; Knowles et al., 2001). Conversely, overexpression of NPR-A in the heart did not alter blood pressure, but reduced cardiac myocyte size in both wild-type and Npr1 knockout mice (Kishimoto et al., 2001). Moreover, heart-specific Npr1 knockout mice had normal blood pressure, but exhibited marked cardiac hypertrophy (Holtwick et al., 2003). It has been shown that ANP-stimulated cGMP production leads to cGMP-dependent protein kinase-I activation and phosphorylation of transient receptor potential canonical-6 Ca2+ channels and the regulator of signaling-2, an angiotensin II receptor type 1 downstream molecule, thereby antagonizing hypertrophic responses in cardiomyocytes (Kinoshita et al., 2010; Klaiber et al., 2011; Klaiber et al., 2010; Tokudome et al., 2005). A recent study indicated that ANP-mediated cGMP increase caused subcellular redistribution of phosphodiesterases 2 and 3, which modulated cardiac contractility by enhancing β1-adrenergic receptor/cAMP signaling and decreasing β2-adrenergic receptor/cAMP signaling (Perera et al., 2015). Such an alteration between β1- and β2- adrenergic receptor/cAMP signaling may serve as a compensatory mechanism in response to hypertrophic stress in the heart (Kuhn, 2015).
Vascular remodeling is an important physiological process (Korshunov et al., 2007; Mulvany et al., 1996). ANP has been shown to inhibit vascular smooth muscle cell proliferation (Hutchinson et al., 1997; Itoh et al., 1990) and regulate endothelial cell growth, migration and permeability (Itoh et al., 1992; Lara-Castillo et al., 2009; Sabrane et al., 2005), which are important in angiogenic processes (Kuhn et al., 2009; Tokudome et al., 2009). During pregnancy, spiral artery remodeling in the uterus is critical for increasing uteroplacental blood flow. Impaired spiral artery remodeling has been identified as an underlying mechanism in pregnancy-induced hypertension (Pijnenborg et al., 2006). Studies have indicated that corin-mediated ANP production in the uterus is important for spiral artery remodeling (Zhou and Wu, 2013). In Corin and Nppa knockout mice, uterine spiral artery remodeling was impaired, causing gestational hypertension and proteinuria (Armstrong et al., 2013; Cui et al., 2012). In humans, CORIN variants associated with preeclampsia were reported in a Caucasian population (Stepanian et al., 2014) and CORIN mutations reducing corin activity were identified in preeclamptic patients (Cui et al., 2012; Dong et al., 2014). Consistent with these findings, low levels of plasma corin were associated with the risk of preeclampsia (Khalil et al., 2015).
It has been shown that ANP is also involved in energy metabolism. In isolated adipocytes and healthy volunteers, ANP promoted lipolysis in a cGMP-dependent manner (Lafontan et al., 2008; Moro et al., 2004; Sengenes et al., 2000; Sengenes et al., 2003). In adipocytes, ANP enhanced mitochondrial respiration and the brown fat thermogenic program via a PKG-p38 mitogen-activated protein kinase (MAPK)-mediated pathway (Bordicchia et al., 2012). Moreover, ANP stimulated fat oxidation in skeletal muscles (Engeli et al., 2012; Miyashita et al., 2009). These ANP actions may be important in preventing metabolic diseases. Consistently, low plasma ANP levels were associated with obesity and type 2 diabetes (Magnusson et al., 2012; Wang et al., 2007; Wang et al., 2004b). In patients with hypertension and HF, low plasma ANP levels were associated with the metabolic syndrome (Hsieh et al., 2013; Wang et al., 2013). More recently, glucagon-like peptide-1, a gut-derived hormone, was found to increase ANP secretion from the heart, thereby lowering blood pressure in mice (Buglioni and Burnett, 2013; Kim et al., 2013). Thus, ANP may serve as a part of the extended endocrine network that regulates cardiovascular and metabolic homeostasis.
5. NPPA variants in cardiovascular disease
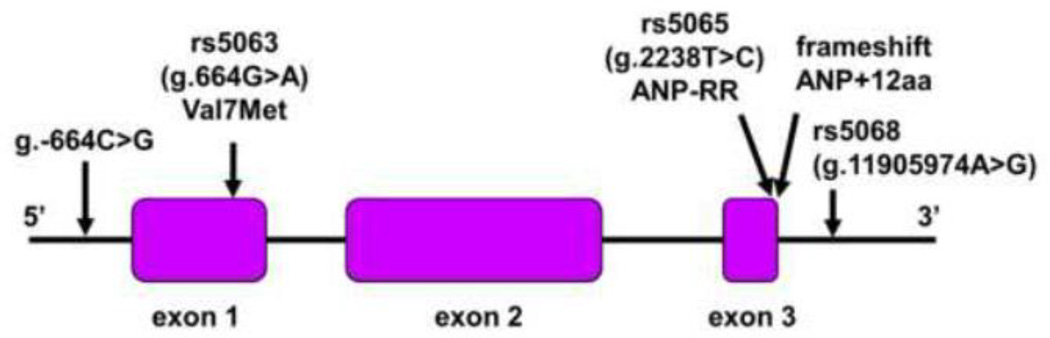
NPPA variants are associated with plasma ANP concentrations, blood pressure levels and cardiovascular diseases (Lynch et al., 2009; Rubattu et al., 2014b). One of the variants, g.–664C>G, is in the 5’ promoter region (Fig. 2). In a European population, the G allele was associated with low plasma ANP levels and high risks of hypertension and cardiac hypertrophy (Rubattu et al., 2006; Rubattu et al., 2007). A similar finding was reported in a Japanese population (Kato et al., 2000).
Fig. 2.
Variants and a frameshift mutation in NPPA. The human NPPA gene consists of three exons and two introns. NPPA variants in the 5’ promoter region, exons 1 and 3, and the 3’ untranslated region are indicated. A frameshift mutation abolishes the stop codon, creating a mutant ANP with 12 extra amino acids at the C-terminus (ANP+12aa).
The variant rs5063, i.e. g.664G>A, is in NPPA exon 1, causing Val7Met substitution (Fig. 2). The variant did not appear to alter NPPA expression, as similar plasma ANP levels were found in individuals with the G and A alleles (Kato et al., 2000). The minor A allele was associated with low diastolic blood pressure (Zhang et al., 2005) and a low risk of hypertension (Conen et al., 2009; Conen et al., 2007). Conversely, the common G allele was linked to a high risk of cardiovascular disease (Lynch et al., 2008). In another study, however, the minor A allele predicted a high risk of stroke (Rubattu et al., 1999). In patients with familial hypercholesterolemia, the minor A allele was associated with lower levels of ApoA1 and HDL (Dedoussis et al., 2006).
The variant rs5065, i.e. g.2238T>C, is in NPPA exon 3 (Fig. 2), which alters the stop codon, creating a variant (ANP-RR) with two extra C-terminal Arg residues. This variant resembles ANP molecules in other mammalian sepiecs such as porcine, rat and mouse, which all contain two Arg residues at the C-terminus (Koller and Goeddel, 1992). The minor C allele was associated with high risks of hypertension (Niu, 2011), stroke (Rubattu et al., 2004), coronary artery disease (Barbato et al., 2012a), and myocardial infarction (Cannone et al., 2013b). In other studies, however, such an association was not confirmed (Kato et al., 2002; Lynch et al., 2008). More recent studies showed that the ANP-RR variant enhanced NPR-C signaling, causing endothelial dysfunction (Cannone et al., 2013b; Sciarretta et al., 2013) and oxidative stress in vascular smooth muscles (Rubattu et al., 2014a).
The variant rs5068, i.e. g.11905974A>G, is in the NPPA 3’ untranslated region (Fig. 2). Individuals with the minor G allele had higher plasma ANP levels, lower systolic and diastolic blood pressures, and a reduced risk of hypertension, compared with those with two A alleles (Arora et al., 2013; Newton-Cheh et al., 2009). This minor G allele also predicted low risks of left ventricular hypertrophy (Arora et al., 2013; Newton-Cheh et al., 2009) and metabolic syndrome (Cannone et al., 2011; Cannone et al., 2013a). Apparently, the A to G change abolished a binding site for microRNA-425 that inhibited NPPA mRNA expression in cardiac myocytes, which resulted in higher plasma ANP levels (Arora et al., 2013).
Atrial fibrillation (AF) is a common disease characterized by irregular heart rhythm. ANP deposits were reported in atrial amyloidosis, a pathological lesion linked to AF (Rocken et al., 2002). An NPPA frameshift mutation was reported in an AF patient family (Hodgson-Zingman et al., 2008). The mutation abolished the stop codon, adding 12 extra amino acids at the C-terminus of ANP (Fig. 2). The mutant ANP exhibited an enhanced biological activity and was more resistant to proteolytic degradation than wild-type ANP (Dickey et al., 2009; McKie et al., 2009). Patients with the mutation had elevated plasma ANP levels compared with that in normal controls (Hodgson-Zingman et al., 2008). It remains unclear how the mutant ANP contributed to AF in the patients.
6. ANP as a biomarker
The NPPA gene is up-regulated under physiological and pathological conditions. Mechanical stretch of the atrial wall is one of the most potent stimuli for ANP expression and release (Edwards et al., 1988). The signaling molecules in this process include integrins, p38 MAPK and focal adhesion kinase (Kerkela et al., 2011; Peng et al., 2008). To date, many growth factors and vasoactive molecules have been reported to enhance ANP expression and secretion (Ogawa and de Bold, 2014; Potter et al., 2006).
Peptide fragments derived from prepro-ANP have been detected in human plasma (Goetze et al., 2015). In population-based studies, elevated plasma NT-pro-ANP levels predicted the risk of cardiovascular events and death (Wang et al., 2004a). In an elderly European male population, high plasma ANP levels predicted the risk of AF (Mandalenakis et al., 2014). In patients with stroke, coronary artery disease, myocardial infarction and HF, elevated plasma NT-pro-ANP and ANP levels were associated with poor clinical outcomes (Barbato et al., 2012b; Makikallio et al., 2005; Sabatine et al., 2012; Volpe et al., 2010). These data indicate that ANP and related peptides are useful biomarkers in the diagnosis and risk stratification of cardiovascular diseases.
Most common methods to measure natriuretic peptide levels are immunoassays. Antibodies in these assays often have cross-reactivity between pro-ANP and its cleaved fragments (Xu-Cai and Wu, 2010). For example, antibodies against NT-pro-ANP are likely to recognize pro-ANP. Similarly, antibodies against ANP may bind to pro-ANP. As a result, an ANP assay may in fact measure both ANP and pro-ANP. Similar cross-reactivity has been reported for pro-BNP, NT-pro-BNP and BNP assays (Luckenbill et al., 2008). It is important, therefore, to consider the assay cross-reactivity when interpreting the data from these studies. A recent study showed that NPPA variants may alter the epitope recognized by the antibodies in the immunoassays, which may affect data analysis (Newton-Cheh et al., 2009).
7. ANP as a therapeutic agent
Given its unique pleiotropic functions in promoting natriuresis, diuresis and vasodilation and inhibiting aldosterone and renin secretion, ANP represents a promising drug candidate for cardiovascular disease such as heart failure. In fact, a recombinant form of human ANP, carperitide, has been approved in Japan to treat HF patients (Mitaka et al., 2011; Saito, 2010). The peptide also showed therapeutic benefits in patients with myocardial infarction and kidney disease (Kasama et al., 2007; Kitakaze et al., 2007; Morikawa et al., 2009; Sezai et al., 2010). Because of its short plasma half-life (<5 minutes), carperitide is given by intravenous infusion. Currently, additional efforts are ongoing to develop ANP derivatives with improved pharmacological properties and therapeutic efficacies (Anker et al., 2015; de Bold et al., 2012; McKie et al., 2012; Zakeri and Burnett, 2011).
8. Conclusions
Since the discovery of ANP in the 1980s, we have gained considerable insights into the gene expression, biosynthesis, post-translational processing and biological function of this peptide hormone. In addition to its role in promoting natriuresis, diuresis and vasodilation, ANP also regulates cardiac function, vascular remodeling and energy metabolism. These functions are of significant importance in maintaining cardiovascular and metabolic homeostasis. To date, common NPPA variants have been identified that are associated with circulating ANP levels and hypertensive disease in the general population. Community-based studies indicate that plasma natriuretic peptide levels may be used to predict the risk of cardiovascular disease. The knowledge gained in understanding ANP biology has led to new therapies for heart disease. Currently, recombinant ANP is used as a parenteral drug to treat acute HF in patients. In recent clinical trials, a small molecule compound inhibiting the angiotensin II receptor type 1 and NEP, which degrades the natriuretic peptides, exhibited therapeutic benefits in HF patients (McMurray et al., 2014). These data indicate that enhancing natriuretic peptide activity can be used as a new therapeutic strategy for cardiovascular diseases. Further studies will be important to test if such a strategy may be extended to treat metabolic diseases.
Highlights.
Atrial natriuretic peptide (ANP) is a hormone that regulates salt-water balance.
ANP acts in the heart to prevent cardiac hypertrophy.
ANP also regulates vascular remodeling and energy metabolism.
ANP variants are associated with cardiovascular and metabolic diseases.
Acknowledgements
This review and the corresponding Gene Wiki article are written as part of the Cardiac Gene Wiki Review series--a series resulting from a collaboration between the journal GENE, the Gene Wiki Initiative, and the BD2K initiative. The Cardiac Gene Wiki Initiative is supported by National Institutes of Health (GM083924 and GM114833). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The authors would like to thank Nancy Fiordalisi for critical reading of this manuscript. This work was also supported in part by NIH grants R01HD064634 and R01HL126697 and by the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Abbreviations
- AF
atrial fibrillation
- ANF
atrial natriuretic factor
- ANP
atrial natriuretic peptide
- BNP
B-type or brain natriuretic peptide
- cGMP
cyclic guanosine monophosphate
- CNP
C-type natriuretic peptide
- NEP
neutral endopeptidase
- HF
heart failure
- MAPK
mitogen-activated protein kinase
- NPR-A
natriuretic peptide receptor A
- NPR-C
natriuretic peptide receptor C
- NT
N-terminal
- PKG
cGMP-dependent protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker SD, Ponikowski P, Mitrovic V, Peacock WF, Filippatos G. Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. Eur Heart J. 2015;36:715–723. doi: 10.1093/eurheartj/ehu484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DW, Tse MY, O'Tierney-Ginn PF, Wong PG, Ventura NM, Janzen-Pang JJ, Matangi MF, Johri AM, Croy BA, Adams MA, et al. Gestational hypertension in atrial natriuretic peptide knockout mice and the developmental origins of salt-sensitivity and cardiac hypertrophy. Regul Pept. 2013;186:108–115. doi: 10.1016/j.regpep.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Arora P, Wu C, Khan AM, Bloch DB, Davis-Dusenbery BN, Ghorbani A, Spagnolli E, Martinez A, Ryan A, Tainsh LT, et al. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest. 2013;123:3378–3382. doi: 10.1172/JCI67383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbato E, Bartunek J, Mangiacapra F, Sciarretta S, Stanzione R, Delrue L, Cotugno M, Marchitti S, Iaccarino G, Sirico G, et al. Influence of rs5065 atrial natriuretic peptide gene variant on coronary artery disease. J Am Coll Cardiol. 2012a;59:1763–1770. doi: 10.1016/j.jacc.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Barbato E, Bartunek J, Marchitti S, Mangiacapra F, Stanzione R, Delrue L, Cotugno M, Di Castro S, De Bruyne B, Wijns W, et al. NT-proANP circulating level is a prognostic marker in stable ischemic heart disease. Int J Cardiol. 2012b;155:311–312. doi: 10.1016/j.ijcard.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglioni A, Burnett JC., Jr A gut-heart connection in cardiometabolic regulation. Nat Med. 2013;19:534–536. doi: 10.1038/nm.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, Heublein DM, Lahr BD, Bailey KR, Averna M, et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 2011;58:629–636. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannone V, Cefalu AB, Noto D, Scott CG, Bailey KR, Cavera G, Pagano M, Sapienza M, Averna MR, Burnett JC., Jr The atrial natriuretic peptide genetic variant rs5068 is associated with a favorable cardiometabolic phenotype in a Mediterranean population. Diabetes Care. 2013a;36:2850–2856. doi: 10.2337/dc12-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannone V, Huntley BK, Olson TM, Heublein DM, Scott CG, Bailey KR, Redfield MM, Rodeheffer RJ, Burnett JC., Jr Atrial natriuretic peptide genetic variant rs5065 and risk for cardiovascular disease in the general community: a 9-year follow-up study. Hypertension. 2013b;62:860–865. doi: 10.1161/HYPERTENSIONAHA.113.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen D, Cheng S, Steiner LL, Buring JE, Ridker PM, Zee RY. Association of 77 polymorphisms in 52 candidate genes with blood pressure progression and incident hypertension: the Women's Genome Health Study. J Hypertens. 2009;27:476–483. doi: 10.1097/hjh.0b013e32832104c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen D, Glynn RJ, Buring JE, Ridker PM, Zee RY. Natriuretic peptide precursor a gene polymorphisms and risk of blood pressure progression and incident hypertension. Hypertension. 2007;50:1114–1119. doi: 10.1161/HYPERTENSIONAHA.107.097634. [DOI] [PubMed] [Google Scholar]
- Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- de Bold MK, Sheffield WP, Martinuk A, Bhakta V, Eltringham-Smith L, de Bold AJ. Characterization of a long-acting recombinant human serum albumin-atrial natriuretic factor (ANF) expressed in Pichia pastoris. Regul Pept. 2012;175:7–10. doi: 10.1016/j.regpep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Dedoussis GV, Maumus S, Skoumas J, Choumerianou DM, Pitsavos C, Stefanadis C, Visvikis-Siest S. Natriuretic peptide Val7Met substitution and risk of coronary artery disease in Greek patients with familial hypercholesterolemia. J Clin Lab Anal. 2006;20:98–104. doi: 10.1002/jcla.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey DM, Yoder AR, Potter LR. A familial mutation renders atrial natriuretic Peptide resistant to proteolytic degradation. J Biol Chem. 2009;284:19196–19202. doi: 10.1074/jbc.M109.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Fang C, Jiang Y, Zhou T, Liu M, Zhou J, Shen J, Fukuda K, Qin J, Wu Q. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J Biol Chem. 2013;288:7867–7874. doi: 10.1074/jbc.M112.411512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Zhou T, Zhang Y, Liu M, Li H, Huang X, Liu Z, Wu Y, Fukuda K, Qin J, et al. Corin mutations K317E and S472G from preeclamptic patients alter zymogen activation and cell surface targeting. J Biol Chem. 2014;289:17909–17916. doi: 10.1074/jbc.M114.551424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- Durocher D, Nemer M. Combinatorial interactions regulating cardiac transcription. Dev Genet. 1998;22:250–262. doi: 10.1002/(SICI)1520-6408(1998)22:3<250::AID-DVG7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Edwards BS, Zimmerman RS, Schwab TR, Heublein DM, Burnett JC., Jr Atrial stretch, not pressure, is the principal determinant controlling the acute release of atrial natriuretic factor. Circ Res. 1988;62:191–195. doi: 10.1161/01.res.62.2.191. [DOI] [PubMed] [Google Scholar]
- Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller SM, Gagelmann M, Forssmann WG. Urodilatin: a newly described member of the ANP family. Trends Pharmacol Sci. 1989;10:93–94. doi: 10.1016/0165-6147(89)90199-5. [DOI] [PubMed] [Google Scholar]
- Feng JA, Perry G, Mori T, Hayashi T, Oparil S, Chen YF. Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin Exp Pharmacol Physiol. 2003;30:343–349. doi: 10.1046/j.1440-1681.2003.03836.x. [DOI] [PubMed] [Google Scholar]
- Forssmann W, Meyer M, Forssmann K. The renal urodilatin system: clinical implications. Cardiovasc Res. 2001;51:450–462. doi: 10.1016/s0008-6363(01)00331-5. [DOI] [PubMed] [Google Scholar]
- Gagelmann M, Hock D, Forssmann WG. Urodilatin (CDD/ANP-95-126) is not biologically inactivated by a peptidase from dog kidney cortex membranes in contrast to atrial natriuretic peptide/cardiodilatin (alpha-hANP/CDD-99-126) FEBS Lett. 1988;233:249–254. doi: 10.1016/0014-5793(88)80436-8. [DOI] [PubMed] [Google Scholar]
- Goetze JP, Hansen LH, Terzic D, Zois NE, Albrethsen J, Timm A, Smith J, Soltysinska E, Lippert SK, Hunter I. Atrial natriuretic peptides in plasma. Clin Chim Acta. 2015;443:25–28. doi: 10.1016/j.cca.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Goy MF, Oliver PM, Purdy KE, Knowles JW, Fox JE, Mohler PJ, Qian X, Smithies O, Maeda N. Evidence for a novel natriuretic peptide receptor that prefers brain natriuretic peptide over atrial natriuretic peptide. Biochem J. 2001;358:379–387. doi: 10.1042/0264-6021:3580379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett JC, Jr, Olson TM. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Wang JH, Lee CJ, Chen YC, Liou HH, Hsu BG. Low serum long-acting natriuretic peptide level correlates with metabolic syndrome in hypertensive patients: a cross-sectional study. Arch Med Res. 2013;44:215–220. doi: 10.1016/j.arcmed.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Hutchinson HG, Trindade PT, Cunanan DB, Wu CF, Pratt RE. Mechanisms of natriuretic-peptide-induced growth inhibition of vascular smooth muscle cells. Cardiovasc Res. 1997;35:158–167. doi: 10.1016/s0008-6363(97)00086-2. [DOI] [PubMed] [Google Scholar]
- Inoue K, Naruse K, Yamagami S, Mitani H, Suzuki N, Takei Y. Four functionally distinct C-type natriuretic peptides found in fish reveal evolutionary history of the natriuretic peptide system. Proc Natl Acad Sci U S A. 2003;100:10079–10084. doi: 10.1073/pnas.1632368100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Pratt RE, Dzau VJ. Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest. 1990;86:1690–1697. doi: 10.1172/JCI114893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Pratt RE, Ohno M, Dzau VJ. Atrial natriuretic polypeptide as a novel antigrowth factor of endothelial cells. Hypertension. 1992;19:758–761. doi: 10.1161/01.hyp.19.6.758. [DOI] [PubMed] [Google Scholar]
- John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity and left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol. 2007;49:667–674. doi: 10.1016/j.jacc.2006.09.048. [DOI] [PubMed] [Google Scholar]
- Kato N, Ikeda K, Nabika T, Morita H, Sugiyama T, Gotoda T, Kurihara H, Kobayashi S, Yazaki Y, Yamori Y. Evaluation of the atrial natriuretic peptide gene in stroke. Atherosclerosis. 2002;163:279–286. doi: 10.1016/s0021-9150(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Kato N, Sugiyama T, Morita H, Nabika T, Kurihara H, Yamori Y, Yazaki Y. Genetic analysis of the atrial natriuretic peptide gene in essential hypertension. Clin Sci (Lond) 2000;98:251–258. [PubMed] [Google Scholar]
- Kenny AJ, Stephenson SL. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett. 1988;232:1–8. doi: 10.1016/0014-5793(88)80375-2. [DOI] [PubMed] [Google Scholar]
- Kerkela R, Ilves M, Pikkarainen S, Tokola H, Ronkainen VP, Majalahti T, Leppaluoto J, Vuolteenaho O, Ruskoaho H. Key roles of endothelin-1 and p38 MAPK in the regulation of atrial stretch response. Am J Physiol Regul Integr Comp Physiol. 2011;300:R140–R149. doi: 10.1152/ajpregu.00853.2009. [DOI] [PubMed] [Google Scholar]
- Khalil A, Maiz N, Garcia-Mandujano R, Elkhouli M, Nicolaides KH. Longitudinal changes in maternal corin and mid-regional proatrial natriuretic peptide in women at risk of pre-eclampsia. Ultrasound Obstet Gynecol. 2015;45:190–198. doi: 10.1002/uog.14685. [DOI] [PubMed] [Google Scholar]
- Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Kuwahara K, Nishida M, Jian Z, Rong X, Kiyonaka S, Kuwabara Y, Kurose H, Inoue R, Mori Y, et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ Res. 2010;106:1849–1860. doi: 10.1161/CIRCRESAHA.109.208314. [DOI] [PubMed] [Google Scholar]
- Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci U S A. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- Klaiber M, Dankworth B, Kruse M, Hartmann M, Nikolaev VO, Yang RB, Volker K, Gassner B, Oberwinkler H, Feil R, et al. A cardiac pathway of cyclic GMP-independent signaling of guanylyl cyclase A, the receptor for atrial natriuretic peptide. Proc Natl Acad Sci U S A. 2011;108:18500–18505. doi: 10.1073/pnas.1103300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiber M, Kruse M, Volker K, Schroter J, Feil R, Freichel M, Gerling A, Feil S, Dietrich A, Londono JE, et al. Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol. 2010;105:583–595. doi: 10.1007/s00395-010-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J Biol Chem. 2003;278:52363–52370. doi: 10.1074/jbc.M309991200. [DOI] [PubMed] [Google Scholar]
- Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, Rockman HA, Maeda N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86:1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- Kudo T, Baird A. Inhibition of aldosterone production in the adrenal glomerulosa by atrial natriuretic factor. Nature. 1984;312:756–757. doi: 10.1038/312756a0. [DOI] [PubMed] [Google Scholar]
- Kuhn M. Cardiac actions of atrial natriuretic Peptide: new visions of an old friend. Circ Res. 2015;116:1278–1280. doi: 10.1161/CIRCRESAHA.115.306325. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Volker K, Schwarz K, Carbajo-Lozoya J, Flogel U, Jacoby C, Stypmann J, van Eickels M, Gambaryan S, Hartmann M, et al. The natriuretic peptide/guanylyl cyclase--a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119:2019–2030. doi: 10.1172/JCI37430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–137. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Lara-Castillo N, Zandi S, Nakao S, Ito Y, Noda K, She H, Ahmed M, Frimmel S, Ablonczy Z, Hafezi-Moghadam A. Atrial natriuretic peptide reduces vascular leakage and choroidal neovascularization. Am J Pathol. 2009;175:2343–2350. doi: 10.2353/ajpath.2009.090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- Luckenbill KN, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, Tate J, Wu AH, Ler R, Apple FS. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem. 2008;54:619–621. doi: 10.1373/clinchem.2007.097998. [DOI] [PubMed] [Google Scholar]
- Lynch AI, Boerwinkle E, Davis BR, Ford CE, Eckfeldt JH, Leiendecker-Foster C, Arnett DK. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. Jama. 2008;299:296–307. doi: 10.1001/jama.299.3.296. [DOI] [PubMed] [Google Scholar]
- Lynch AI, Claas SA, Arnett DK. A review of the role of atrial natriuretic peptide gene polymorphisms in hypertension and its sequelae. Curr Hypertens Rep. 2009;11:35–42. doi: 10.1007/s11906-009-0008-7. [DOI] [PubMed] [Google Scholar]
- Maack T, Marion DN, Camargo MJ, Kleinert HD, Laragh JH, Vaughan ED, Jr, Atlas SA. Effects of auriculin (atrial natriuretic factor) on blood pressure, renal function, and the renin-aldosterone system in dogs. Am J Med. 1984;77:1069–1075. doi: 10.1016/0002-9343(84)90190-6. [DOI] [PubMed] [Google Scholar]
- Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, Morgenthaler NG, Nilsson P, Newton-Cheh C, Wang TJ, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab. 2012;97:638–645. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makikallio AM, Makikallio TH, Korpelainen JT, Vuolteenaho O, Tapanainen JM, Ylitalo K, Sotaniemi KA, Huikuri HV, Myllyla VV. Natriuretic peptides and mortality after stroke. Stroke. 2005;36:1016–1020. doi: 10.1161/01.STR.0000162751.54349.ae. [DOI] [PubMed] [Google Scholar]
- Mandalenakis Z, Eriksson H, Welin L, Caidahl K, Dellborg M, Rosengren A, Lappas G, Hedner J, Johansson S, Svardsudd K, et al. Atrial natriuretic peptide as a predictor of atrial fibrillation in a male population study. The Study of Men Born in 1913 and 1923. Int J Cardiol. 2014;171:44–48. doi: 10.1016/j.ijcard.2013.11.042. [DOI] [PubMed] [Google Scholar]
- McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC., Jr A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Cardiol. 2009;54:1024–1032. doi: 10.1016/j.jacc.2009.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie PM, Ichiki T, Burnett JC., Jr M-atrial natriuretic peptide: a novel antihypertensive protein therapy. Curr Hypertens Rep. 2012;14:62–69. doi: 10.1007/s11906-011-0244-5. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- Melo LG, Veress AT, Chong CK, Pang SC, Flynn TG, Sonnenberg H. Salt-sensitive hypertension in ANP knockout mice: potential role of abnormal plasma renin activity. Am J Physiol. 1998;274:R255–R261. doi: 10.1152/ajpregu.1998.274.1.R255. [DOI] [PubMed] [Google Scholar]
- Mitaka C, Kudo T, Haraguchi G, Tomita M. Cardiovascular and renal effects of carperitide and nesiritide in cardiovascular surgery patients: a systematic review and meta-analysis. Crit Care. 2011;15:R258. doi: 10.1186/cc10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S, Sone T, Tsuboi H, Mukawa H, Morishima I, Uesugi M, Morita Y, Numaguchi Y, Okumura K, Murohara T. Renal protective effects and the prevention of contrast-induced nephropathy by atrial natriuretic peptide. J Am Coll Cardiol. 2009;53:1040–1046. doi: 10.1016/j.jacc.2008.10.061. [DOI] [PubMed] [Google Scholar]
- Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C, Lafontan M, Berlan M. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. Faseb J. 2004;18:908–910. doi: 10.1096/fj.03-1086fje. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension. 1996;28:505–506. [PubMed] [Google Scholar]
- Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W. The Relationship between Natriuretic Peptide Precursor a Gene T2238C Polymorphism and Hypertension: A Meta-Analysis. Int J Hypertens. 2011;2011:653698. doi: 10.4061/2011/653698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, de Bold AJ. The heart as an endocrine organ. Endocr Connect. 2014;3:R31–44. doi: 10.1530/EC-14-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–38398. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- Pemberton CJ, Siriwardena M, Kleffmann T, Ruygrok P, Palmer SC, Yandle TG, Richards AM. First identification of circulating prepro-A-type natriuretic peptide (preproANP) signal peptide fragments in humans: initial assessment as cardiovascular biomarkers. Clin Chem. 2012;58:757–767. doi: 10.1373/clinchem.2011.176990. [DOI] [PubMed] [Google Scholar]
- Peng X, Wu X, Druso JE, Wei H, Park AY, Kraus MS, Alcaraz A, Chen J, Chien S, Cerione RA, et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci U S A. 2008;105:6638–6643. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RK, Sprenger JU, Steinbrecher JH, Hubscher D, Lehnart SE, Abesser M, Schuh K, El-Armouche A, Nikolaev VO. Microdomain Switch of cGMP-Regulated Phosphodiesterases Leads to ANP-Induced Augmentation of beta-Adrenoceptor-Stimulated Contractility in Early Cardiac Hypertrophy. Circ Res. 2015;116:1304–1311. doi: 10.1161/CIRCRESAHA.116.306082. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Rocken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, Roessner A, Goette A. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091–2097. doi: 10.1161/01.cir.0000034511.06350.df. [DOI] [PubMed] [Google Scholar]
- Rubattu S, Bigatti G, Evangelista A, Lanzani C, Stanzione R, Zagato L, Manunta P, Marchitti S, Venturelli V, Bianchi G, et al. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48:499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Rubattu S, Evangelista A, Barbato D, Barba G, Stanzione R, Iacone R, Volpe M, Strazzullo P. Atrial natriuretic peptide (ANP) gene promoter variant and increased susceptibility to early development of hypertension in humans. J Hum Hypertens. 2007;21:822–824. doi: 10.1038/sj.jhh.1002228. [DOI] [PubMed] [Google Scholar]
- Rubattu S, Marchitti S, Bianchi F, Di Castro S, Stanzione R, Cotugno M, Bozzao C, Sciarretta S, Volpe M. The C2238/alphaANP variant is a negative modulator of both viability and function of coronary artery smooth muscle cells. PLoS One. 2014a;9:e113108. doi: 10.1371/journal.pone.0113108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rubattu S, Ridker P, Stampfer MJ, Volpe M, Hennekens CH, Lindpaintner K. The gene encoding atrial natriuretic peptide and the risk of human stroke. Circulation. 1999;100:1722–1726. doi: 10.1161/01.cir.100.16.1722. [DOI] [PubMed] [Google Scholar]
- Rubattu S, Sciarretta S, Volpe M. Atrial natriuretic peptide gene variants and circulating levels: implications in cardiovascular diseases. Clin Sci (Lond) 2014b;127:1–13. doi: 10.1042/CS20130427. [DOI] [PubMed] [Google Scholar]
- Rubattu S, Stanzione R, Di Angelantonio E, Zanda B, Evangelista A, Tarasi D, Gigante B, Pirisi A, Brunetti E, Volpe M. Atrial natriuretic peptide gene polymorphisms and risk of ischemic stroke in humans. Stroke. 2004;35:814–818. doi: 10.1161/01.STR.0000119381.52589.AB. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, Solomon SD, Pfeffer MA, Braunwald E. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation. 2012;125:233–240. doi: 10.1161/CIRCULATIONAHA.111.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y. Roles of atrial natriuretic peptide and its therapeutic use. J Cardiol. 2010;56:262–270. doi: 10.1016/j.jjcc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Sciarretta S, Marchitti S, Bianchi F, Moyes A, Barbato E, Di Castro S, Stanzione R, Cotugno M, Castello L, Calvieri C, et al. C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ Res. 2013;112:1355–1364. doi: 10.1161/CIRCRESAHA.113.301325. [DOI] [PubMed] [Google Scholar]
- Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. Faseb J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- Sengenes C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M, Galitzky J. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278:48617–48626. doi: 10.1074/jbc.M303713200. [DOI] [PubMed] [Google Scholar]
- Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, Fujita K, Takayama T, Kasamaki Y, Hirayama A, et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose Human ANP Infusion Therapy during cardiac surgery) for left ventricular dysfunction. J Am Coll Cardiol. 2010;55:1844–1851. doi: 10.1016/j.jacc.2009.11.085. [DOI] [PubMed] [Google Scholar]
- Shi SJ, Nguyen HT, Sharma GD, Navar LG, Pandey KN. Genetic disruption of atrial natriuretic peptide receptor-A alters renin and angiotensin II levels. Am J Physiol Renal Physiol. 2001;281:F665–673. doi: 10.1152/ajprenal.2001.281.4.F665. [DOI] [PubMed] [Google Scholar]
- Stepanian A, Alcais A, de Prost D, Tsatsaris V, Dreyfus M, Treluyer JM, Mandelbrot L. Highly significant association between two common single nucleotide polymorphisms in CORIN gene and preeclampsia in Caucasian women. PLoS One. 2014;9:e113176. doi: 10.1371/journal.pone.0113176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Kawakoshi A, Tsukada T, Yuge S, Ogoshi M, Inoue K, Hyodo S, Bannai H, Miyano S. Contribution of comparative fish studies to general endocrinology: structure and function of some osmoregulatory hormones. J Exp Zool A Comp Exp Biol. 2006;305:787–798. doi: 10.1002/jez.a.309. [DOI] [PubMed] [Google Scholar]
- Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilig F, Wu Q. ANP-induced signalling cascade and its implications in renal pathophysiology. Am J Physiol Renal Physiol. 2015;28 doi: 10.1152/ajprenal.00164.2014. ajprenal 00164 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokudome T, Horio T, Kishimoto I, Soeki T, Mori K, Kawano Y, Kohno M, Garbers DL, Nakao K, Kangawa K. Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides. Circulation. 2005;111:3095–3104. doi: 10.1161/CIRCULATIONAHA.104.510594. [DOI] [PubMed] [Google Scholar]
- Tokudome T, Kishimoto I, Yamahara K, Osaki T, Minamino N, Horio T, Sawai K, Kawano Y, Miyazato M, Sata M, et al. Impaired recovery of blood flow after hind-limb ischemia in mice lacking guanylyl cyclase-A, a receptor for atrial and brain natriuretic peptides. Arterioscler Thromb Vasc Biol. 2009;29:1516–1521. doi: 10.1161/ATVBAHA.109.187526. [DOI] [PubMed] [Google Scholar]
- Volpe M, Francia P, Tocci G, Rubattu S, Cangianiello S, Elena Rao MA, Trimarco B, Condorelli M. Prediction of long-term survival in chronic heart failure by multiple biomarker assessment: a 15-year prospective follow-up study. Clin Cardiol. 2010;33:700–707. doi: 10.1002/clc.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Lee CJ, Hsieh JC, Chen YC, Hsu BG. Inverse association of long-acting natriuretic peptide with metabolic syndrome in congestive heart failure patients. Diabetol Metab Syndr. 2013;5:19. doi: 10.1186/1758-5996-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–1353. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004a;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004b;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- Wang W, Cui Y, Shen J, Jiang J, Chen S, Peng J, Wu Q. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension. 2012a;60:1352–1358. doi: 10.1161/HYPERTENSIONAHA.112.201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, Wu Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012b;82:26–33. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–16905. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Cai YO, Wu Q. Molecular forms of natriuretic peptides in heart failure and their implications. Heart. 2010;96:419–424. doi: 10.1136/hrt.2008.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri R, Burnett JC. Designer natriuretic peptides: a vision for the future of heart failure therapeutics. Can J Physiol Pharmacol. 2011;89:593–601. doi: 10.1139/y11-048. [DOI] [PubMed] [Google Scholar]
- Zeidel ML. Renal actions of atrial natriuretic peptide: regulation of collecting duct sodium and water transport. Annu Rev Physiol. 1990;52:747–759. doi: 10.1146/annurev.ph.52.030190.003531. [DOI] [PubMed] [Google Scholar]
- Zhang S, Mao G, Zhang Y, Tang G, Wen Y, Hong X, Jiang S, Yu Y, Xu X. Association between human atrial natriuretic peptide Val7Met polymorphism and baseline blood pressure, plasma trough irbesartan concentrations, and the antihypertensive efficacy of irbesartan in rural Chinese patients with essential hypertension. Clin Ther. 2005;27:1774–1784. doi: 10.1016/j.clinthera.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li H, Zhou J, Wang A, Yang J, Wang C, Liu M, Zhou T, Zhu L, Zhang Y, et al. A corin variant identified in hypertensive patients that alters cytoplasmic tail and reduces cell surface expression and activity. Sci Rep. 2014;4:7378. doi: 10.1038/srep07378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wu Q. Role of corin and atrial natriuretic peptide in preeclampsia. Placenta. 2013;34:89–94. doi: 10.1016/j.placenta.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]