Abstract
US28, a constitutively active G-protein-coupled receptor encoded by the human cytomegalovirus, leads to mechanistically unknown programmed cell death. Here we show that expression of wild type US28 in human melanoma cells leads to apoptotic cell death via caspase 3 activation along with reduced cell proliferation. Reduced tumor growth upon US28 expression was observed in a xenograft mouse model. The signaling mute US28R129A showed a reduced anti-proliferative effect. On evaluating different G-proteins coupled to US28 for signal transduction, Gα13 was identified as the main G-protein executing the apoptotic effect. Silencing of Gα13 but not Gαq resulted in a substantial increase in cell survival. Over-expression of Gα13 but not Gαq and their GTPase deficient forms Gα13Q226L and GαqQ209L, respectively, confirmed the requirement of Gα13 for US28 mediated cell death. Increasing expression of Gα13 alone induced cell death underscoring its relay function for US28 mediated decreased cell viability. Further reduced expression of Gα13 in melanoma cell lines isolated from advanced lesions and melanoma tissue was observed. These findings identified Gα13 as crucial for US28 induced cell death, substantiating that the effect of US28 on cell fate depends on preferred G-protein binding.
Keywords: US28, viral receptor, cell death, GNA13, melanoma
INTRODUCTION
Viral homologues of chemokine receptors have been identified in a number of human and animal herpes viruses (1). Recently US28, encoded by human cytomegalovirus (HCMV) has been shown to induce apoptosis in different cell types including HeLa, Cos7 or 293T cells (2). US28 signals in a highly constitutive, ligand-independent manner through coupling to heterotrimeric G-proteins, for which US28 shows promiscuity (3–5). Signaling through different G-proteins allows US28 to exert functional diversity through many pathways. Activation of different G-proteins may also account for the observation that in mouse models US28 shows oncogenic properties (6, 7).
Here we provide evidence that the apoptosis inducing property of US28 is dependent on availability of Gα13 for signaling. Expression of US28 resulted in apoptosis through activation of caspase 3 and PARP. Cell death was only observed in human cell lines but not murine cells. In elucidating the G-protein responsible for the observed apoptotic inducing properties of US28 we identified Gα13 being crucial for these effects. Further, single transfection with Gα13, but not Gαq, led to apoptosis in cancer cells. We propose a model of US28 induced apoptosis based on coupling to Gα13, which suggests that availability of particular G-proteins for US28 signaling determines cell fate.
MATERIALS AND METHODS
An expanded Materials and Methods section is available as Online Supplemental Material.
Vectors and DNA constructs
US28 constructs with the N-terminal tag of the HA peptide YPYDVPDYA for wild type US28 (wtUS28) and US28R129A in the vector backbone of pDEF (5) were subcloned into pLXRN (Clontech, Mountain View, CA) for retroviral transduction. US28 ORFs as well as GFP were sub-cloned into the pcDNA3.1 vector. GNA constructs, GNAQ, GNAQQ209L, GNA13, and GNA13Q226L were kindly provided by Dr. Boris Bastian (UCSF, CA, USA) and Dr. Joaquin Teixido (CSIC, Madrid, Spain).
Cell culture, transfection, and transduction
Human melanoma cell lines SBcl2, WM793, WM35, WM1366, WM9, WM164, and 451Lu were cultured in RPMI 1640 (Sigma Aldrich, St. Louis, MO) supplemented with 2% (v/v) FCS and 2% (w/v) L-Glutamine (PAA, Pasching, Austria). The HEK-293 derived cell line GP-293 (Clontech) was maintained in DMEM (Sigma) containing 10% (v/v) FCS and 2% (w/v) L-glutamine. African green monkey COS-7 cells and mouse fibroblasts NIH3T3 were cultured in DMEM Nutrient Mixture F-12 HAM (Sigma), supplemented with 4% FCS and 2% L-Glutamine. B16/BL6 mouse melanoma cells were maintained in minimum essential medium supplemented with 5% (v/v) FBS, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, and 1% (w/v) nonessential amino acids (all from Invitrogen). Cells were transfected with pcDNA3.1-GFP, pcDNA3.1-GNA-constructs or pcDNA3.1-HAUS28 constructs and maintained in RPMI medium till further analysis at 48 and 72 hrs. Retroviruses were produced by two-plasmid co-transfection of GP293 cells with pLXRN-CMV1- or pQCXIN- constructs and the envelope protein-coding plasmid pVSV-G (Clontech) using the ProFection Mammalian Transfection System (Promega). siRNAs targeting mRNAs of Gαq and Gα13 were obtained from Santa Cruz Biotechnology Inc. Control siRNA was purchased from Qiagen, Vienna, Austria. 150pmol siRNA were transfected into cells seeded in 6-well-plates using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s protocol.
Statistics
Statistical analysis was performed using Student’s t-test. Results were calculated as the mean ± SD of three different experiments. P values less than 0.05 were considered significant, less than 0.01 highly significant. Data from in vivo experiments were subjected to a one-way-ANOVA analysis with a Bonferroni’s Multiple Comparison Post-test. * indicates a significant difference with a p-value of < 0.05, ** with a p-value of < 0.01.
RESULTS AND DISCUSSION
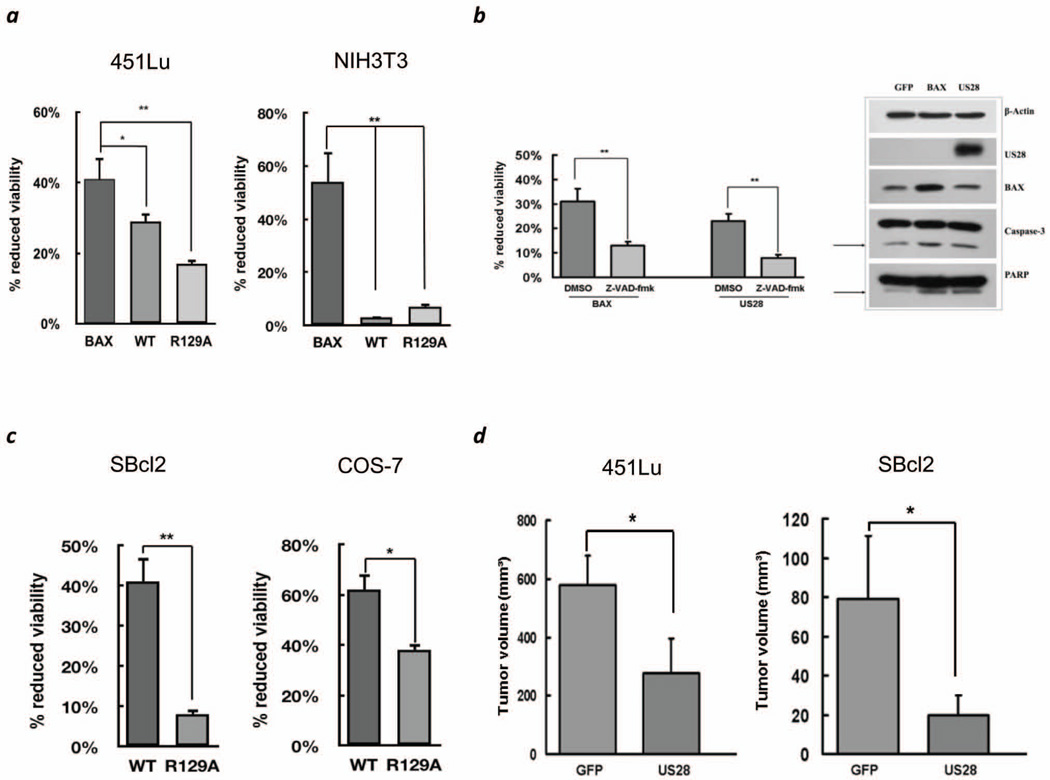
Recent studies suggest that US28 induces oncogenic transformation in murine NIH3T3 cells and plays a prooncogenic role in intestinal tumorigenesis in mice (6, 7), which is opposed to its apoptosis inducing effect in human cell lines (2). To test for alternate effects of US28, human 451Lu melanoma cells isolated from an advanced stage and mouse NIH3T3 cells were transfected with wild type (wt) US28, the signaling mute US28R129A, GFP or pro-apoptotic BAX. Transfection efficiency of US28 was confirmed 48hrs later (Figure S1A). 72hrs post transfection the MTT assay indicated that US28 expression reduces cell viability only in 451Lu but not NIH3T3 cells compared to BAX (Figure 1A). Additionally, for probing the effect of US28 in the mouse melanoma cell line B16/BL6, wtUS28 cDNA was subcloned into pENTR3C and transferred to RCASBP-M by genetic recombination (8, 9), which is species compatible. No effect on B16/BL6 survival was observed (Figure S1B). These data suggest that the cell death inducing properties of US28 are confined to human rather than mouse cell lines. We then investigated the cell death inducing properties of US28 in human cell lines. There was absence of any detectable apoptosis 48hrs post transfection in 451Lu cells (data not shown). 72hrs post transfection caspase mediated apoptosis by US28 was confirmed by detection of activated caspase 3 and the cleaved caspase substrate PARP (Figure 1B, right). Induction of apoptosis was reversible in the presence of the pan-caspase inhibitor z-VAD-fmk (Figure 1B, left). Cell death was also observed in another melanoma cell line from an early stage, SBcl2 as well as COS-7 (Figure 1C). Like in 451Lu transfection with the signaling mute US28R129A resulted in increased viability compared to US28wt (Figure 1A,C). Overall these experiments indicate an apoptotic effect of US28 upon expression in human cell lines and particularly two melanoma cell lines representing an early and advanced stage. In a human melanoma xenograft model employing SCID mice inoculated with melanoma cell lines 451Lu wtUS28, SBcl2 wtUS28 or GFP transduced cells tumor growth was observed after 14 days or 7 weeks, respectively, which increased over the period of 30 days and 15 weeks. Retarded tumor growth was observed for wtUS28 compared to GFP transduced cells for both cell lines with significantly smaller tumors at day 30 and week 15 for 451Lu and SBcl2, respectively, in mice injected with wtUS28 transduced cells (Figure 1D, Figure S2).
Figure 1.
(a) Proliferation assays at 72 hrs post-transfection with BAX, wtUS28 or the signaling mute R129A for the human melanoma cell 451Lu and NIH3T3 cells. Percent reduced cell viability to control GFP. (b) (left) Pan-caspase inhibition assay indicating a caspase dependent induction of apoptosis for wtUS28 similar to BAX. (right) Lysates of 451Lu cells transfected with GFP, BAX or US28 were immunoblotted for control β-actin, HA antibody for US28 expression, caspase 3 activation, BAX expression and PARP cleavage. (c) Proliferation assays for COS-7 and the human melanoma cell line SBcl2 after transfection with wtUS28 or US28R129A. (d) Reduced tumor growth of 451Lu and SBcl2 melanoma cells transduced with wtUS28 (US28) compared to GFP. 2×106 SBcl2 or 451Lu cells were injected s.c. in SCID CB-17 mice. Tumor volumes for 451Lu at 30 days post injection (left) and 15 weeks post injection for SBcl2 (right). Error bars indicate S.D. of triplicates. *p<0.05, **p<0.01.
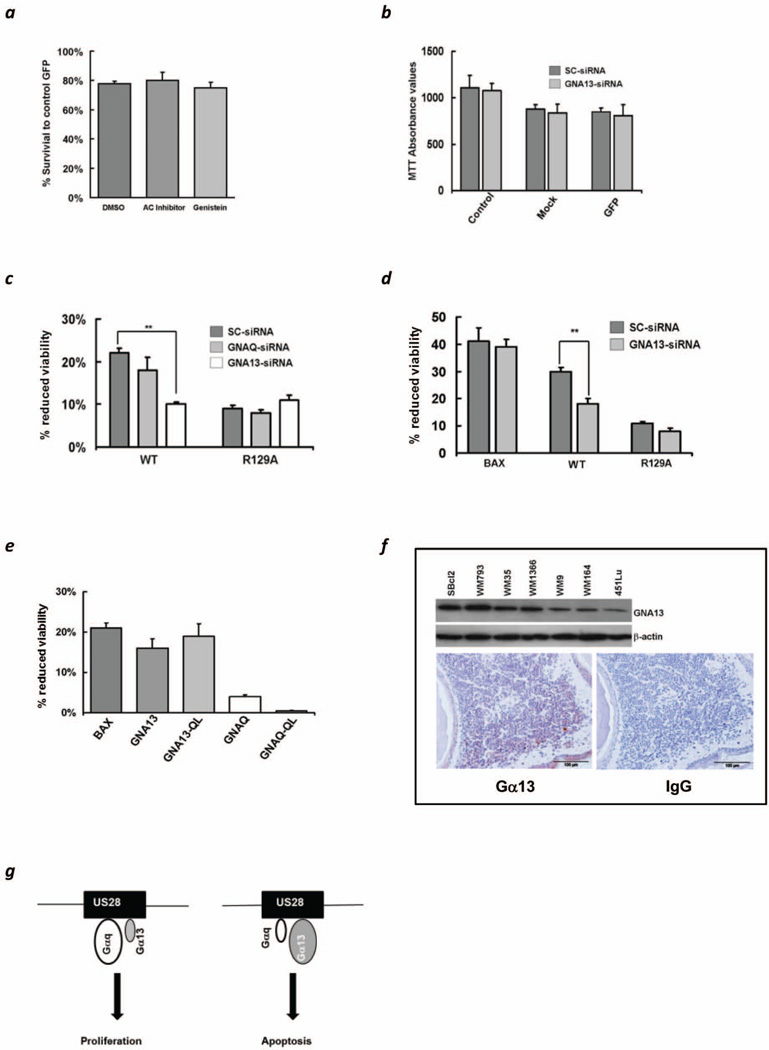
Cell death induced by US28 might depend on proper signaling as demonstrated for US28R129A (3–5). US28 has evolved to interact with different ligands and is also suspected to be associated with multiple Gα-proteins, like Gαq/11, Gαi, Gαs, or Gα12/13 (3–5, 10) (Figure S3). It has been proposed that the apoptotic effect of US28 in 293T, Cos-7 and HeLa cells is mediated via Gαq/11 in a PKC dependent manner (2). In order to elucidate which of the G-proteins is involved, we embarked on selectively inhibiting pathways known to be activated by US28 (2, 11). Neither an inhibitor of adenylate cyclase nor genistein was able to reverse the effect of US28 on 451Lu cells thus indirectly excluding the role of several pathways, in particular the Gαs activation pathway (Figure 2A). The relevance of Gαq for tumor growth is underscored by a high number of Gαq mutations in uveal melanomas (12). To further determine whether Gαq is in fact involved in US28 mediated apoptosis (2), GNAQ and GNA13 were individually silenced (Figure S4A). MTT-absorbance values of cells treated with scrambled and GNA13 siRNA, followed by mock and GFP transfection showed no difference after 72 hrs (Figure 2B), indicating that the silencing of GNA13 itself is not responsible for the change in proliferation. Gene silencing of GNAQ did not substantially alter the apoptotic effect of US28, silencing of GNA13, however, led to a highly significant increase in viability for the wild type construct, but not for the signaling mute R129A in case of both G-proteins with a minor increase in cell death if GNA13 was silenced (Figure 2C). The effect of BAX on 451Lu survival was unchanged despite using scrambled or Gα13 siRNA silencing, suggesting that the apoptosis inducing effect of US28 via Gα13 is specific (Figure 2D, left). Furthermore, 451Lu cells were transfected with wild type GNA13, a constitutively active form of GNA13 (GNA13Q226L), wild type GNAQ and its constitutively active mute GNAQQ209L (13, 14). Gα13Q226L but not GαqQ209L decreased survival confirming our previous finding, suggesting that active Gα13 induces apoptosis while active Gαq lacks any such effect (Figure 2E). Overall the silencing and overexpression studies of selected Gα-proteins clarified the important role of Gα13 for the apoptosis inducing and anti-proliferative effects of US28.
Figure 2.
(a) MTT assay of 451Lu cells at 72hrs post transfection with US28 and exposed either to vehicle control (DMSO), an adenylate cyclase inhibitor (30µM) or genistein (30µM). (b) Comparative MTT absorbance values of control, mock transfected and GFP transfected cells at 72 hrs post transfection. The cells were subjected to siRNA silencing prior to transfection. (c) MTT assay showing comparative effects on cell viability of scrambled (sc)-siRNA, GNAQ-siRNA and GNA13-siRNA in both US28-WT and US28-R129A 451Lu transfected cells. Error bars indicate S.D. of triplicates. **p<0.01. (d) MTT assay of 451Lu cells transfected with BAX, wtUS28 (WT) and US28R129A. GNA13 silencing only affects US28 induced apoptosis but not of BAX. Error bars indicate S.D. of triplicates. **p<0.01. (e) MTT assay of 451Lu at 72 hrs post-transfection with the following constructs: BAX, Gα13 (GNA13), Gα13Q226L (GNA13-QL), Gαq (GNAQ) and GαqQ209L (GNAQ-QL). (f) Immunoblot of 1×106 melanoma cells derived from different stages for endogenous levels of Gα13 (upper panel). Immunohistochemical analysis of a primary melanoma for Gα13 positivity compared to control IgG (lower panel). (g) A model of US28 determining cell fate relying on G-protein signaling as exemplified for Gαq and Gα13. Predominance of Gαq might result in survival and proliferation, whereas high levels of Gα13 lead to cell death.
Increased susceptibility towards the apoptosis inducing effect of US28 was observed in SBcl2 compared to 451Lu melanoma cells (Figure S4B). We questioned, whether endogenous levels of Gα13 could be predictive for differences in susceptibility, and in fact, a decrease of Gα13 in a panel of melanoma cell lines was found with highest expression in cells from early lesions (e.g. SBcl2, WM793, WM35) and lowest from advanced (e.g. WM164, 451Lu) (Figure 2F, upper panel). Whether this finding is, in principal, transmissible to tissue from melanocytic lesions was determined by immunohistochemistry. Similar to in vitro, some positive staining for Gα13 was only found in melanoma nests at the papillary dermis close to the basal membrane from early lesions, whereas melanoma cells in the dermis from advanced lesions and metastases remained almost unstained (Figure 2F, lower panel; Figure S4C). Overall this suggests that expression of Gα13 in a given cellular context might determine whether US28 exerts its apoptosis inducing effect. The importance of Gα13 as being crucial for tumor growth and dissemination has recently been shown by Muppidi et al. (15). By generating transgenic mice lacking Gα13 the development of germinal center B-cell-derived lymphomas was observed. Deficient Gα13 germinal center B-cells were devoid of suppressing phosphorylation of Akt and migration. This suggests that the expression of Gα13 might be tumor suppressive and additionally prevents the spread of tumor cells supporting the findings from this study.
Dissecting US28 activation pathways led to the understanding that expression of constitutively active forms of different G-proteins, like Gαq, Gα16, Gαs or Gα11 are capable of exerting pro-survival and proliferative signals (16–17). With regard to the apoptotic properties of US28 diverse observations have been made in human or murine cells. In addition to the apoptotic effect of US28 in 293T, COS-7 and HeLa cells, Pleskoff et al. reported that attempts to develop stable US28 expressing cell lines for human HEK293T, THP1, U937, HL60, K562, and U373-MG cells failed. In contrary they found that murine NIH3T3 and SVEC cells expressing US28 could be maintained in culture showing a proliferative advantage with increased cyclin-D1 levels (2). Besides an increased growth rate US28 expression led to a transformed phenotype, induction of angiogenesis and tumorigenicity in a NIH3T3 mouse model (6). In both studies the authors claim that US28 mediated Gαq activation might be responsible for the observed effects based on the upregulation of VEGF (6) and the use of genistein and a PI3K inhibitor (2). Further, constitutively active forms of Gαq and Gα16 in US28 expressing cells are both capable of inducing IP3 accumulation (16) and Gα11 is sufficient to induce NFkB activation in COS-7 cells (17). Based on these studies we only can speculate on the mechanisms leading to the different effects of US28 in human versus murine cells but availability or predominance of Gαq or Gα16 might be one reason. Members of the Gα12/13 subfamily are known for their involvement in motility and migration. Their role in SMC migration due to activation by US28 has been demonstrated (18). Apart from the effects on motility and migration two studies also described their role in inducing apoptosis. Transfection of COS-7 cells with constitutively active Gα13 led to profound apoptosis involving the JNK pathway via ASK1 (19). In HEK293 cells Gα12 stimulated JNK1 increased IκB expression, which possibly contributes to Gα12-mediated apoptosis (20). However, more in depth investigations have to be performed to delineate the exact mechanisms of apoptosis induction of US28 via Gα13. We propose a model whereupon US28 mediated effects may depend on availability and preferred activation of G-proteins. Specifically, predominance of Gαq may lead to proliferation, whereas higher levels of Gα13 to apoptosis, respectively (Figure 2G). This model might also explain why a low expression of Gα13 in tumor tissue might favor a pro-tumorigenic effect of US28.
Supplementary Material
Acknowledgements
We would like to thank the staff of the Center for Medical Research (ZMF), Graz, for technical assistance. This work was supported by grants from the National Cancer Institute (R01CA121118 to SLH), and the Austrian Science Fund (P18723 to MW, P15591 and P18630 to HS). S. Joshi was funded by the PhD program ‘Molecular Medicine’ of the Medical University of Graz, Austria, by the Jubiläumsfonds der Österreichischen Nationalbank (12552) and by the Austrian Science Fund (P15591-B18 and P21156-B18).
Footnotes
Author Contributions
H.S. supervised the project; H.S. and S.J. designed the research; S.J. and C.W. performed all of the experiments except IHC and the in vivo experiments; M.F-K. performed in vivo experiments and contributed to the study design; C.B-S. examined immunohistochemistry; S.L.H., M.H., M.O. and M.W. analyzed data; H.S. wrote the manuscript. All authors discussed the results.
REFERENCES
- 1.Rosenkilde MM, Waldhoer M, Luttichau HR, Schwartz TW. Virally encoded 7TM receptors. Oncogene. 2001;20:1582–1593. doi: 10.1038/sj.onc.1204191. [DOI] [PubMed] [Google Scholar]
- 2.Pleskoff O, Casarosa P, Verneuli L, Ainoun F, Beisser P, Smit M, Leurs R, Schneider P, Michelson S, Ameisen JC. The human cytomegalovirus-encoded chemokine rexceptor US28 induces caspase-dependet apoptosis. FEBS J. 2005;272:4163–4177. doi: 10.1111/j.1742-4658.2005.04829.x. [DOI] [PubMed] [Google Scholar]
- 3.Waldhoer M, Kledal TN, Farrell H, Schwartz TW. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol. 2002;76:8161–8168. doi: 10.1128/JVI.76.16.8161-8168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, Whistler JL, Schwartz TW. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278:19473–19482. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- 5.Tschische P, Moser E, Thompson D, Vischer HF, Parzmaier GP, Pommer V, Platzer W, Schwarzbraun T, Schaider H, Smit MJ, Martini L, Whistler JL, Waldhoer M. The G-protein coupled receptor associated sorting protein GASP-1 regulates the signaling and trafficking of the viral chemokine receptor US28. Traffic. 2010;11:660–674. doi: 10.1111/j.1600-0854.2010.1045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maussang D, Verzijl D, van WM, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci USA. 2006;103:13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongers G, Maussang D, Muniz LR, Noriega VM, Fraile-Ramos A, Barker N, Marchesi F, Thirunarayanan N, Vischer HF, Qin L, Mayer L, Harpaz N, Leurs R, Furtado GC, Clevers H, Tortorella D, Smit MJ, Lira SA. The cytomegalovirus-encoded chemokine receptor US28 promotes intestinal neoplasia in transgenic mice. J Clin Invest. 2010;120:3969–3978. doi: 10.1172/JCI42563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JP, Vanbrocklin MW, Lastwika KJ, McKinney AJ, Brandner S, Holmen SL. Activated MEK cooperates with Ink4a/Arf loss or AKT activation to induce gliomas in vivo. Oncogene. 2011;30:1341–1350. doi: 10.1038/onc.2010.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barsov EV, Payne WS, Hughes SH. Adaptation of chimeric retroviruses in vitro and in vivo: isolation of avian retroviral vectors with extended host range. J Virol. 2001;75(11):4973–4983. doi: 10.1128/JVI.75.11.4973-4983.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraile-Ramos A, Kohout TA, Waldhoer M, Marsh M. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic. 2003;4:243–253. doi: 10.1034/j.1600-0854.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 11.Vomaske J, Melnychuk RM, Smith PP, Powell J, Hall L, DeFilippis V, Früh K, Smit M, Schlaepfer DD, Nelson JA, Streblow DN. Differential ligand binding to a human cytomegalovirus chemokine receptor determines cell type-specific motility. PLoS Pathogens. 2009;5:1–12. doi: 10.1371/journal.ppat.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field MG, Harbour JW. GNAQ/11 mutations in uveal melanoma: is YAP the key to targeted therapy? Cancer Cell. 2014;25(6):714–715. doi: 10.1016/j.ccr.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartolome RA, Wright N, Molina-Ortiz I, Sanchez-Luque FJ, Teixido J. Activated G(alpha)13 impairs cell invasiveness through p190RhoGAP-mediated inhibition of RhoA activity. Cancer Res. 2008;68:8221–8230. doi: 10.1158/0008-5472.CAN-08-0561. [DOI] [PubMed] [Google Scholar]
- 14.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muppidi JR, Schmitz R, Green JA. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature. 2014 Sep 28; doi: 10.1038/nature13765. 2014 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moepps B, Tulone C, Kern C, Minisini R, Michels G, Vatter P, Wieland T, Gierschik P. Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Galpha(q/11) and Galpha(16) Cell Signal. 2008;20:1528–1537. doi: 10.1016/j.cellsig.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276:1133–1137. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- 18.Melnychuk RM, Streblow DN, Smith PP, Hirsch AJ, Pancheva D, Nelson JA. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Galpha12. J Virol. 2004;78:8382–8391. doi: 10.1128/JVI.78.15.8382-8391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berestetskaya YV, Faure MP, Ichijo H, Voyno-Yasenetskaya TA. Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J Biol Chem. 1998;273:27816–27823. doi: 10.1074/jbc.273.43.27816. [DOI] [PubMed] [Google Scholar]
- 20.Yanamadala V, Negoro H, Gunaratnam L, Kong T, Denker BM. Galpha12 stimulates apoptosis in epithelial cells through JNK1-mediated Bcl-2 degradation and up-regulation of IkappaBalpha. J Biol Chem. 2007;282:24352–24363. doi: 10.1074/jbc.M702804200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.