Abstract
Tendon injuries are common, and the damaged tendon often turns into scar tissue and never completely regains the original biomechanical properties. Previous studies have reported that the mRNA levels of inflammatory cytokines such as IL-1β are remarkably up-regulated in injured tendons. To examine how IL-1β impacts tendon repair process, we isolated the injured tendon-derived progenitor cells (inTPCs) from mouse injured Achilles tendons and studied the effects of IL-1β on the inTPCs in vitro. IL-1β treatment strongly reduced expression of tendon cell markers such as scleraxis and tenomodulin, and also down-regulated gene expression of collagen 1, collagen 3, biglycan and fibromodulin in inTPCs. Interestingly, IL-1β stimulated lactate production with increases in hexokinase II and lactate dehydrogenase expression and a decrease in pyruvate dehydrogenase. Inhibition of lactate production restored IL-1β-induced down-regulation of collagen1 and scleraxis expression. Furthermore, IL-1β significantly inhibited adipogenic, chondrogenic and osteogenic differentiation of inTPCs. Interestingly, inhibition of tenogenic and adipogenic differentiation was not recovered after removal of IL-1β while chondrogenic and osteogenic differentiation abilities were not affected. These findings indicate that IL-1β strongly and irreversibly impairs tenogenic potential and alters glucose metabolism in tendon progenitors appearing in injured tendons. Inhibition of IL-1β may be beneficial for maintaining function of tendon progenitor cells during the tendon repair process.
Keywords: IL-1β, tendon, progenitor, injury
Introduction
Tendon is a compositionally complex tissue and has an important mechanical function that translates muscular contractions into joint movement by transmitting forces from muscle to bone [1]. Tendon injuries are common, and the damaged tendon often turns into scar tissue and never completely regains its original biomechanical properties [2]. A better understanding of biological processes of tendon repair and degeneration is required for establishing strategies that stimulate tendon repair and induce its regeneration. Previous studies have profiled expression of inflammatory cytokines in canine and rat tendon injury models [3, 4], and found that gene expression of inflammatory cytokines including IL-1β is remarkably up-regulated. In vitro studies have shown that IL-1β makes tendon fibroblasts decrease the mRNA level of collagen 1 [5], suggesting that IL-1β disturbs function of tendon fibroblasts. However, the exact impact of increases in inflammatory cytokines in injured tendons has not been fully elucidated.
Recently, the presence of tendon stem/progenitor cells (TSPCs) has been demonstrated in various species including human, horse, rabbit, rat, and mouse [6-9]. These cells express stem cell-related markers, form adherent colonies and show multipotency in vitro and/or in vivo. Because they form tendon-like tissues in nude mouse or nude rat models [6, 8], TSPCs are suggested to contribute to tendon repair. Using a mouse Achilles tendon injury model, we have recently reported that tendon progenitor-like cells (inTPCs) rapidly appear and are expanded in injured tendons [10]. These cells have characteristics as tendon progenitors and are integrated into regenerating tendon. On the other hand, they exhibit stronger spontaneous chondrogenic ability than the normal tendon progenitor cells and are integrated into heterotopic cartilaginous lesions induced in injured tendon [10]. Thus the inTPCs likely have the potential to contribute to tendon repair, but may be transdifferentiated into different cell lineages, negatively impacting tendon healing.
To understand how inflammatory cytokines affect the regenerative and degenerative potential of inTPCs, we focused on IL-1β, one of dominant cytokines that are up-regulated in injured tendons [3, 4] and examined the effects of IL-1β on function of inTPCs. IL-1β strongly inhibited tenogenic, chondrogenic and osteogenic differentiation in inTPCs and altered glucose metabolism. Interestingly, the inhibitory effect on tenogenic differentiation was not recovered after removal of IL-1β while chondrogenic and osteogenic potential were maintained. The results indicate important implications in understanding the roles of IL-1β during the tendon repair process.
Materials and Methods
Animals
All aspects of the research were conducted in accordance with the guidelines set by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia. CD-1 female mice (6-8 weeks-old of age) were purchased from Charles River Laboratories (Charles River Laboratories International, Inc., Wilmington, MA).
Isolation of inTPCs
Injured tendon-derived progenitor cells (inTPCs) were isolated from the Achilles tendons of CD-1 mouse as we previously reported [10]. Briefly, the fibrous tissues that formed in the incised Achilles tendons were dissected 1 week after surgery. The dissociated cells were plated at a density of 140 cells/cm2 in 100-mm dishes and cultured in DMEM containing 20% FBS (Gemini BioProducts, West Sacramento, CA). The inTPCs at passage 3 or 4 were used in the following experiments. We usually isolated inTPCs from 4 injured Achilles tendons and plated on 2 100-mm dishes.
Cultures of inTPCs
To perform cell proliferation assay, inTPCs were plated at 104 cells/well in a 96-well plate and allowed to adhere overnight. DMEM containing 10% FBS medium was supplemented with 1, 5 and 10 ng/ml mouse IL-1β (R&D Systems, Inc., Minneapolis, MN) for 3 days. Proliferation activity was then determined by measurement of cellular DNA contents using the CyQUANT NF Cell Proliferation Assay Kit (Life Technologies, Grand Island, NY) following the manufacturer's protocol. To evaluate tenogenic differentiation, inTPCs were plated on collagen 1 substrate (Cellmatrix, Nitta Gelatin Inc., Osaka, Japan) at a density of 1.5×105/well in a 12 well plate and were cultured in DMEM containing 10% FBS for 7 days in the presence or absence of IL-1β at concentrations of 1, 5 or 10 ng/ml with or without dichloroacetate (DCA, 1mM, Santa Cruz Biotechnology, Inc. Santa Cruz, CA). For adipogenic or osteogenic induction, the inTPCs were plated on collagen 1 substrate at a density of 1.5×105/well in a 12 well plate and were cultured in DMEM containing 10% FBS and mouse StemXVivo Adipogenic or Osteogenic Supplement, respectively (R&D Systems, Inc., Minneapolis, MN) for 2 weeks in the presence or absence of IL-1 β at a concentration of 5 ng /ml. For chondrogenic induction, the cells were spotted on collagen 1 substrate at a density of 1.5×105/15 μl/well in a 12 well plate and cultured in DMEM containing 10% FBS and 50 μg/ml ascorbic acid for 7 days in the presence or absence of IL-1β at a concentration of 5 ng/ml. The inTPCs were also pre-treated with IL-1β in monolayer for 7 days in 100 mm dishes and then tested for tenogenic, chondrogenic, osteogenic and adipogenic differentiation under each condition described above. We used 2-3 different batches of inTPCs and obtained similar results. The concentrations of IL-1β used in this study were 1-10 ng/ml that have been usually used in other studies. Because 1-10 ng/ml of IL-1β significantly affected gene expression of tendon-associated markers in tenogenesis experiment, a single dose (5 ng/ml, the median dose) was used in some experiments.
RNA isolation and gene expression assay
Total RNA was isolated using an RNeasy Mini kit (Qiagen, Valencia, CA) following the manufacturer's protocol and reverse-transcribed into cDNA. The resulting cDNA was subjected to a quantitative polymerase chain reaction (qPCR) assay. The qPCR was performed with an Applied Biosystems 7900HT Sequence Detection Systems running SDS 2.1 software using SYBR green reagents (Applied Biosystems, Foster City, CA). The average threshold cycle value (Ct value) was calculated from quadruplicate reactions. Standard curves were generated using 10-fold serial dilutions of cDNA of each gene with a correlation coefficient of >0.98. Relative expression levels were calculated based on a standard curve and normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh). The primer sequences used in this research are listed in Table 1.
Table 1. Primers for qPCR.
| Gene | Forward primers | Reverse primers | Accession No. |
|---|---|---|---|
| Scx | 5′-TCAGCAACCAGAGAAAGTTGAGCAA- 3′ | 5′-GGGTCAGTGTTCGGCTGCTTAGAGT- 3′ | NM_198885 |
| Mkx | 5′-AGTAAAGACAGTCAAGCTGCCACTG -3′ | 5′-TCCTGGCCACTCTAGAAGCG-3′ | NM_177595 |
| Egr1 | 5′-CAGCGCCTTCAATCCTCAAG-3′ | 5′-GCGATGTCAGAAAAGGACTCTGT-3′ | NM_007913 |
| Col3 | 5′-CAGAGCAACGGTCATACTCATTCACC-3′ | 5′-CAGCAACAGCAGAAGAGAAGCACC-3′ | NM_009930 |
| Col1 | 5′-GACATGTTCAGCTTTGTGGACCTC-3′ | 5′-GGGACCCTTAGGCCATTGTGTA-3′ | NM_007729 |
| Tnmd | 5′-AACACTTCTGGCCCGAGGTAT-3′ | 5′-AAGTGTGCTCCATGTCATAGGTTTT-3′ | NM_022322 |
| Bgn | 5′-TTTCTGAGCTTCGCAAGGATG-3′ | 5′-GGGCGTAGAGGTGCTGGAG-3′ | NM_007542 |
| Dcn | 5′-CTATGTGCCCCTACCGATGC-3′ | 5′-CAGAACACTGCACCACTCGAAG-3′ | NM_007833 |
| Fmod | 5′-CTCCAACCCAAGGAGACCAG-3′ | 5′-GGATCCACCAGTGAGAGTCTTC-3′ | NM_021355 |
| Lum | 5′-TCGAGCTTGATCTCTCCTAT-3′ | 5′-TGGTCCCAGGTCTTACAGAA-3′ | NM_008524 |
| MMP13 | 5′-TCAGTCTCTTCACCTCTTTTGGGAATCC-3′ | 5′-TCAGTTTCTTTATGGTCCAGGCGATG-3′ | NM_008607 |
| Agg | 5′-TCTGGAAATGACAACCCCAAGCAC-3′ | 5′-TGGCGGTAACAGTGACCCTGGAACT-3′ | NM_007424 |
| SOX9 | 5′-AGTTTGACCAATACTTGCCACCCAAC-3′ | 5′-TCCGTCTTGATGTGCGTTCGCT-3′ | NM_011448 |
| Osx | 5′-CCAGCCTCTGGCTATGCAAA-3′ | 5′-AGGAAATGAGTGAGGGAAGGGT-3′ | NM_130458 |
| Runx2 | 5′-GGTCCCCGGGAACCAA-3′ | 5′-GGCGATCAGAGAACAAACTAGGTTT-3′ | NM_001145920 |
| Cebpa | 5′-CAAGAACAGCAACGAGTACCG-3′ | 5′-GTCACTGGTCAACTCCAGCAC-3′ | NM_007678 |
| Cebpg | 5′-CAGCACGGAAACTACAGCGA-3′ | 5′-ACTGCCCTGGGTTATCAGAAT-3′ | NM_009884 |
| Gapdh | 5′-ATGAGCCCTTCCACAATGCCAAAG-3′ | 5′-AAGCCCATCACCATCTTCCAGGAG-3′ | NM_008084 |
Immunoblot analysis
The inTPCs plated on a density of 1.5×105/well in a 12 well plate and were cultured in DMEM containing 10% FBS in the presence or absence IL-1β at the concentration of 5 ng/ml for 6 or 24 hours and then lysed in SDS sample buffer. Hexokinase II (HKII), lactate dehydrogenase (LDHA), pyruvate dehydrogenase (PDHA) and α-tubulin contents were examined by Immunoblot using the corresponding antibodies as described previously [11]. The anti-hexokinase II rabbit monoclonal antibody (#2867), anti-lactate dehydrogenase rabbit monoclonal antibody (#3582), anti-pyruvate dehydrogenase rabbit monoclonal antibody (#3205) were purchased from Cell Signaling Technology (Danvers, MA). The anti-α-tubulin mouse monoclonal antibody were purchased from Sigma-Aldrich (St. Louis, MO).
Lactate measurement
The inTPCs plated on a density of 1.5×105/well in a 12 well plate and were cultured in DMEM containing 10% FBS in the presence or absence IL-1β at the concentration of 5 ng/ml for 6, 24, 48 and 72 hours with or without 1 mM DCA. The culture media were collected at each time point and lactate concentration in the conditioned medium was determined using the L-Lactate Assay Kit (Colorimetric) (Abcam Inc., Cambridge, MA) according to the manufacturer's protocol.
Statistical Analysis
Results were analyzed using InStat 3 version 3.1a (GraphPad Software, Inc., La Jolla, CA). Student's T Test or one-way ANOVA followed by Dunnett tests were used to identify the differences. The threshold for significance for all tests was set as p<0.05.
Results
Effects on tenogenic differentiation and glucose metabolism
We first examined the effect of IL-1β on tenogenic differentiation of inTPCs. The cells were treated with 1, 5 or 10 ng/ml IL-1β for 7 days and analyzed gene expression of tendon cell markers, Scleraxis (Scx) and Tenomodulin (Tnmd) [12, 13]. Treatment with IL-1β significantly reduced the expression of Scx and Tnmd genes (Figs. 1A and 1B). IL-1β also down-regulated gene expression of early growth response gene 1 (Egr1), collagen 1 (Col1) and collagen 3 (Col3) while it up-regulated Mohawk (MKx) and matrix metalloproteinase 13 (Mmp13) expression (Fig.1C). It has been demonstrated that small leucine-rich proteoglycans play important roles in tendon repair and maintenance [14, 15]. We therefore examined gene expression of these molecules and found decreases in Biglycan (Bgn) and Fibromoduline (Fmod) expression and a great increase in Decorin (Dcn) expression in the IL-1β-treated cultures (Fig. 1C). There are no significant differences for the gene expression of Lumican (Lum) between control and IL-1β-treated cultures (Fig. 1C).
Figure 1.
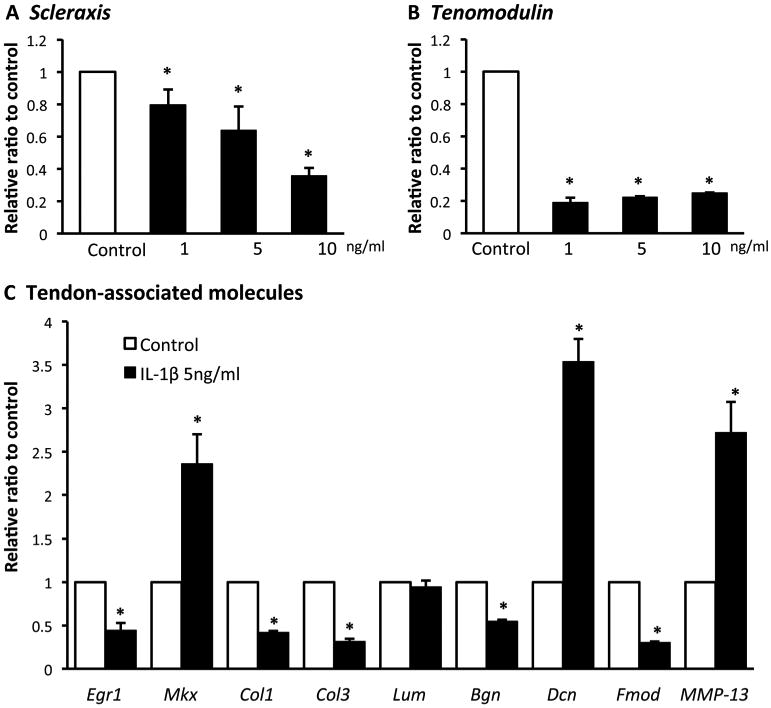
Effects of IL-1β on tenogenic differentiation of inTPCs. The inTPCs were treated with the indicated concentrations of IL-1β for 7 days and subjected to qPCR for gene expression of Scleraxis (A), Tenomodulin (B) and tendon-associated molecules(C). Values are average and SD for 4 samples. *P < 0.05 to Control.
IL-1β did not affect cell proliferation of inTPCs significantly (Fig. 2A), but we noticed that the medium of IL-1β-treated culture became acidic rapidly as determined by the medium color (Fig. 2B) and pH measurement (data not shown). To examine whether this change is due to changes in glycolysis activity and lactate synthesis, we measured the concentration of lactate in the culture medium 24, 48 and 72 hours after treatment with IL-1β. IL-1β significantly increased lactate production over time (Fig. 2C). The increase in lactate production by IL-1β was completely abolished by co-treatment with dichroloacetate (DCA) that stimulates TCA cycle and indirectly inhibits lactate synthesis [16] (Fig. 2C), suggesting that IL-1β shifted glucose metabolism toward an anaerobic respiration mode. Consistently, the contents of hexokinaseII (HK II) that catalyzes the first rate-limiting step of glucose catabolism and lactate dehydrogenase (LDHA) that catalyzes the conversion of pyruvate to lactate were increased by IL-1β. In contrast, pyruvate dehydrogenase (PDHA) that catalyzes the conversion of pyruvate and coenzyme A into acetyl coenzyme A was inhibited by IL-1β after 24 hours (Fig. 2D). Furthermore, DCA treatment recovered IL-β inhibitory effects on collagen 1 and scleraxis gene expression (Fig. 2E).
Figure 2.
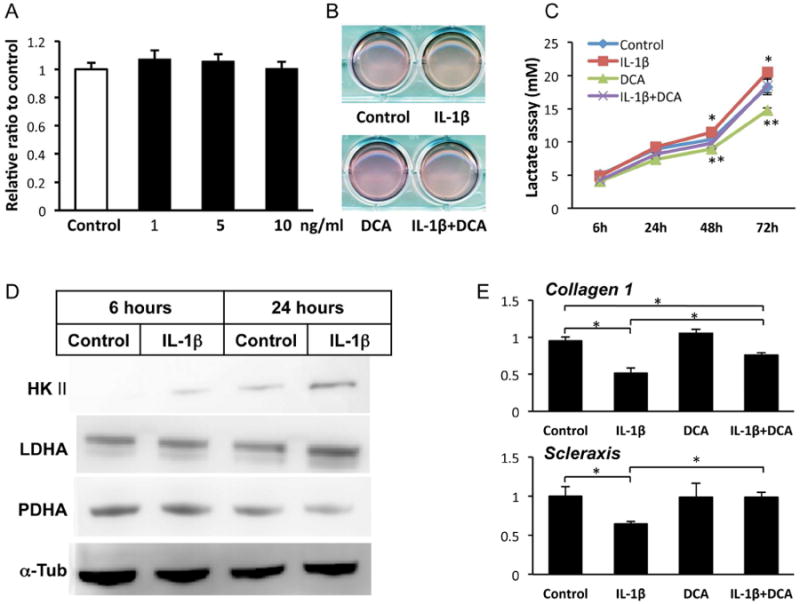
IL-1β did not affect cell proliferation and stimulated lactate production with alterations of glucose metabolic enzymes in inTPCs. A, The inTPCs were treated with the indicated concentrations of IL-1β for 7 days and subjected to CYQUANT cell proliferation assay. B, Pictures of inTPC cultures treated in absence of presence of 5 ng/ml IL-1β and/or 1 mM DCA for 3 days. C, The media of inTPCs cultures were collected after treatment with vehicle (Control) or 5 ng/ml IL-1β for 6, 24, 48 or 72 hours and subjected to lactate assay. *, p<0.05 Control to IL-1β at 48 and 72 h; **, p<0.05 DCA group to either Control or IL-β+DCA group at 48 and 72 h. D, The inTPC cultures were treated with vehicle (Control) or 5 ng/ml IL-1β and subjected to Immunoblot analysis for hekisokinase II (HKII), lactate dehydrogenase (LDHA), pyruvate dehydrogenase (PDHA) or α-tubulin (α-Tub). E, The inTPCs were treated with or without IL-1β (5 ng/ml) and DCA (1mM) for 7 days and subjected to qPCR for gene expression of Collagen 1 and Scleraxis. Values are average and SD for 4 samples. *, p< 0.05 to Control.
Effects on multipotency
Next, we studied whether IL-1β modulates multipotency of inTPCs. The cells were cultured under chondrogenic, osteogenic or adipogenic conditions in the presence or absence of IL-1β and subjected to gene expression analysis for corresponding differentiation markers: Aggrecan (Agg) and Sex-determining region Y-box 9 (SOX9) for chondrogenesis; Osterix (Osx) and Runt-related transcription factor 2 (Runx2) for osteogenesis; and CCAAT/enhancer-binding protein alpha (Cebpa) and CCAAT/enhancer binding protein gamma (Cebpg) for adipogenesis. The results showed that IL-1β decreased expression of chondrogenic, osteogenic and adipogenic differentiation markers (Figs. 3A, 3B and 3C, respectively).
Figure 3.
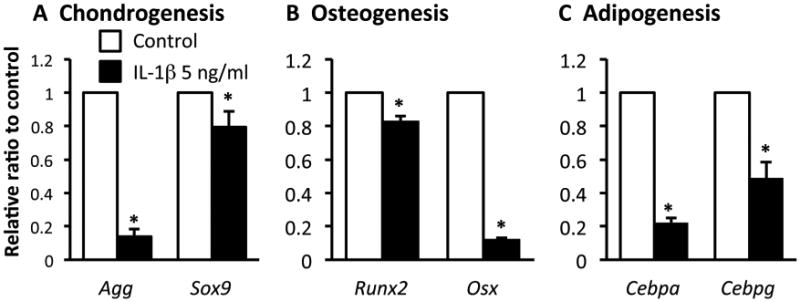
Effects of IL-1β on gene expression of chondrogenic, ostegenic and adipogenic differentiation in inTPCs. The inTPCs were cultured in micromass (A) or monolayer (B and C) under chondrogenic (A), osteogenic (B) or adipogenic (C) inductive condition in presence or absence of IL-1β (5 ng/ml). Total RNAs were prepared from the cultures after 7 days (A) or 14 days (B and C) and subjected to gene expression analysis for indicated genes. Values are average and SD for 4 samples. *, p< 0.05 to Control.
Gene expression of inflammatory cytokines is initially up-regulated in the injured site of tendon, but would eventually decrease. We asked whether the phenotype of inTPCs is consequently altered once they are exposed to IL-1β or not. To ask this question, we pretreated inTPCs with IL-1β for 7 days and then re-plated them and cultured under various differentiation inductive conditions in the absence of IL-1β. The IL-1β pre-treated inTPCs showed decreases in gene expression levels of Scx, Tnmd, Col1 and Col3 compared to those in the control culture that had not been pre-treated with IL-1β (Fig. 4A). The expression levels of chondrogenic and osteogenic differentiation markers in the pre-treated inTPCs were comparable with those in the control culture without IL-1β pre-treatment (Figs. 4B and 4C). In contrast, expression levels of adipogenic differentiation markers were lower in the IL-1β pretreated cells than those in the control (Fig. 4D).
Figure 4.
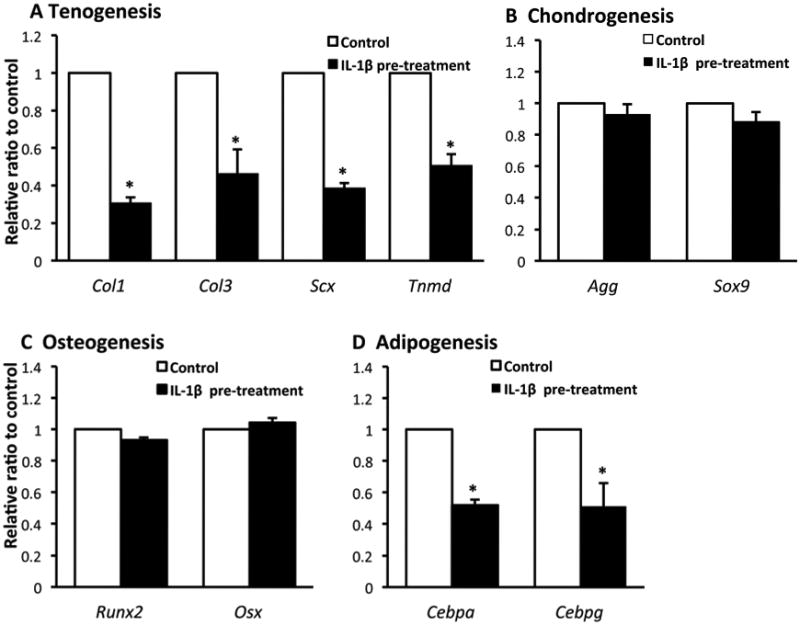
Effects of IL-1β pre-treatment on function of inTPCs. The inTPCS were cultured with (IL-1β pre-treatment) or without (Control) 5 ng/ml IL-1β for 7 days in monolayer and re-plated in monolayer culture (A, C and D) or micromass culture (B) without IL-1β under tenogenic (A), chondrogenic (B), osteogenic (C) or adipogenic (D) induction. Total RNAs were prepared from the cultures after 7 days (A and B) or 14 days (C and D) and subjected to gene expression analysis for indicated genes. Values are average and SD for 4 samples. *P < 0.05 to Control.
Discussion
It has been acknowledged that the use of anti-inflammatory drugs should be carefully considered for therapies of tendon disorders [17]. Treatment with anti-inflammatory drugs may result in negative effects on the biomechanical properties of affected tendons [18, 19]. In turn, the in vitro results indicate that inhibition of inflammatory cytokine actions would be beneficial to tendon healing. The inflammatory cytokines such as IL-1β cause matrix destruction and loss of tendon biomechanical properties by inducing inflammatory mediators such as cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2), and increase expression or activities of matrix metalloproteinases (MMPs) such as MMP1, MMP3 and MMP13 in tenocytes [5, 20-22]. However, current information on the effects of inflammatory cytokines on tendon cells is largely limited to the cells isolated from uninjured tendons.
Our results showed that IL-1β up-regulated gene expression of MMP13 in tendon progenitors derived from injured tendons. Furthermore, IL-1β reduced expression of tenogenic differentiation markers (Scx and Tnmd), the main tendon associated collagens (Col1 and Col3) and Egr-1, a transcriptional factor that plays an important role in tendon [23]. More interestingly, the inhibitory effect of IL-1β on tenogenic differentiation is likely irreversible, and IL-1β-pretreated cells continued showing inferior tenogenic ability even after IL-1β was removed. This irreversible response was detected for adipogenic differentiation, but not for chondrogenic and osteogenic differentiation. These findings indicate that exposure of tendon progenitors to IL-1β at an early stage of tendon injury permanently impairs tenogenic differentiation of tendon progenitor cells. In contrast, the exposed progenitor cells likely maintain the ability to differentiate into chondrogenic and osteogenic cells. Interestingly, we found that IL-1β stimulated lactate-producing glycolysis, presumably by up-regulation of key enzymes such as HKII and LDHA and down-regulation of PDHA. These findings indicate that IL-1β affects glucose metabolic mode of inTPCs, possibly resulting in inhibition of collagen 1 synthesis.
Our results indicate that expression of Bgn and Fmod was inhibited in inTPCs by IL-1β. These actions of IL-1β would alter the microenvironment of the niche of tendon progenitors and impair the recovery of biomechanical properties of regenerating tendons. In contrast, we detected up-regulation of expression of Dcn and Mkx which is a homeobox gene involved in tendon development. Importance of Dcn in formation of tendon collagen fibers has been demonstrated by different lines of evidence [24-26]. Ito et al. have reported that Mkx mutant mice have hypoplastic tendons throughout the body and smaller collagen fibril diameters; they have also indicated that Mkx plays a critical role in tendon development by regulating type I collagen production [27]. Significance of Dcn and Mkx by IL-1β needs to be further investigated. Tendon mineralization can be found as a result of tendon injuries or a feature of tendinopathy [28, 29]. Previous studies reported that mineralization was found in 14–62% of cases following percutaneous or open repair of the Achilles tendon [30] and is thought to be a cause of pain and tendon weakness [31, 32]. Our data showed that IL-1β suppressed the chondrogenic and osteogenic differentiation ability in inTPCs, indicating that IL-1β may not directly participate in tendon mineralization in injured tendons, but rather may play an inhibitory action on tendon mineralization during the inflammatory stage. Chondrogenic and osteogenic differentiation of inTPCs was not affected after the removal of IL-1β while their tenogenic differentiation was suppressed, suggesting that tendon progenitor cells irreversibly decrease the tenogenic ability, but preserve chondrogenic and osteogenic potential after exposure to IL-1β. Thus once IL-1β concentration would decrease, the progenitors would contribute to these inductive events.
In summary, our study showed that IL-1β irreversibly inhibits tenogenic differentiation of inTPCs, indicating that inflammatory cytokines, at least IL-1β proteins, strongly affect function of tendon progenitor cells appearing in injured tendons. It is therefore suggested that proper timing of the control of inflammatory cytokine actions is critical for stimulation of tendon regeneration.
IL-1β inhibited gene expression of tendon-associated genes in inTPCs.
IL-1β altered the glucose metabolism in inTPCs, possibly linked to inhibition of tenogenesis.
IL-1β inhibited adipogenic, chondrogenic and osteogenic differentiation of inTPCs.
The IL-1β irreversibly inhibited expression of tenocyte-associated genes in inTPCs.
Chondrogenic and osteogenic potential of inTPCs was not affected by IL-1β pretreatment.
Acknowledgments
We thank Miss L. Cantley, A. T. Gunawardena and R. Berger for technical assistance. This study was supported by the Penn Center for Musculoskeletal Disorders Pilot and Feasibility Grant (NIH/NIAMS P30AR050950), the NIH R21AR062193 Grant and the interdepartmental fund of the Children's Hospital of Philadelphia.
Footnotes
Authors contribution: Research design (K. Z and M. E.-I.); the acquisition and analysis of data (K. Z.); the interpretation of data (K.Z., M. E.-I., S. A. and B.Y.); drafting the paper or revising it critically (K.Z., M. E.-I., S. A. and B. Y.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 2.Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11:223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 3.Sugg KB, Lubardic J, Gumucio JP, et al. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32:944–51. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning CN, Havlioglu N, Knutsen E, et al. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J Orthop Res. 2014;32:645–52. doi: 10.1002/jor.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thampatty BP, Li H, Im HJ, et al. EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1 beta treatment. Gene. 2007;386:154–61. doi: 10.1016/j.gene.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rui YF, Lui PP, Li G, et al. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549–58. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 9.Lovati AB, Corradetti B, Lange Consiglio A, et al. Characterization and differentiation of equine tendon-derived progenitor cells. J Biol Regul Homeost Agents. 2011;25:S75–84. [PubMed] [Google Scholar]
- 10.Asai S, Otsuru S, Candela ME, et al. Tendon Progenitor Cells in Injured Tendons Have Strong Chondrogenic Potential: The CD105-Negative Subpopulation Induces Chondrogenic Degeneration. Stem Cells. 2014 doi: 10.1002/stem.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuhara R, Ohta Y, Yuasa T, et al. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91:1739–52. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 13.Shukunami C, Takimoto A, Oro M, et al. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–47. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Ezura Y, Chakravarti S, Oldberg A, et al. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–88. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 16.Stacpoole PW, Nagaraja NV, Hutson AD. Efficacy of dichloroacetate as a lactate-lowering drug. J Clin Pharmacol. 2003;43:683–91. [PubMed] [Google Scholar]
- 17.Ferry ST, Dahners LE, Afshari HM, et al. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35:1326–33. doi: 10.1177/0363546507301584. [DOI] [PubMed] [Google Scholar]
- 18.Elder CL, Dahners LE, Weinhold PS. A cyclooxygenase-2 inhibitor impairs ligament healing in the rat. Am J Sports Med. 2001;29:801–5. doi: 10.1177/03635465010290062101. [DOI] [PubMed] [Google Scholar]
- 19.Connizzo BK, Yannascoli SM, Tucker JJ, et al. The detrimental effects of systemic Ibuprofen delivery on tendon healing are time-dependent. Clin Orthop Relat Res. 2014;472:2433–9. doi: 10.1007/s11999-013-3258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuzaki M, Guyton G, Garrett W, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–64. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 21.Archambault J, Tsuzaki M, Herzog W, et al. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36–9. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 22.Sun HB, Li Y, Fung DT, et al. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466:1555–61. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerquin MJ, Charvet B, Nourissat G, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123:3564–76. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–49. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura N, Hart DA, Boorman RS, et al. Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J Orthop Res. 2000;18:517–23. doi: 10.1002/jor.1100180402. [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Toriuchi N, Yoshitaka T, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Natl Acad Sci U S A. 2010;107:10538–42. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien EJ, Frank CB, Shrive NG, et al. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol. 2012;93:319–31. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliva F, Via AG, Maffulli N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012;10:95. doi: 10.1186/1741-7015-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ateschrang A, Gratzer C, Weise K. Incidence and effect of calcifications after open-augmented Achilles tendon repair. Arch Orthop Trauma Surg. 2008;128:1087–92. doi: 10.1007/s00402-007-0441-5. [DOI] [PubMed] [Google Scholar]
- 31.Tsujii A, Tanaka Y, Yonetani Y, et al. Symptomatic calcification of the anterior cruciate ligament: A case report. Knee. 2012;19:223–5. doi: 10.1016/j.knee.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Richards PJ, Braid JC, Carmont MR, et al. Achilles tendon ossification: pathology, imaging and aetiology. Disabil Rehabil. 2008;30:1651–65. doi: 10.1080/09638280701785866. [DOI] [PubMed] [Google Scholar]


