Abstract
Beclin 1 is an essential regulator of autophagy that is induced in response to cellular stress and serves to maintain cell survival in established tumors. We recently demonstrated that Beclin 1 suppression can sensitize colorectal cancer cells to radiation-induced DNA damage and apoptosis. Therefore, we hypothesized that the level of Beclin 1 expression may be associated with radiation sensitivity in vivo. We determined the association of Beclin 1 expression in pre-treatment rectal cancer tissues with response to neoadjuvant chemoradiation in surgical resection specimens. Consecutive stage II and III (n=96) rectal adenocarcinoma patients were treated with neoadjuvant chemoradiation followed by surgical resection with curative intent. Beclin 1 was analyzed by immunohistochemistry and the expression level was dichotomized at the median value with categorization into low and high groups. We identified 56 (58.3%) and 40 (41.7%) patients with high vs low level Beclin 1 expression, respectively. Patients with high vs low Beclin 1 expression were significantly less likely to be downstaged after chemoradiation treatment [45% (25/55) vs 58% (22/38); p=0.02]. In a multivariable analysis adjusted for age, sex, histological grade and baseline TNM stage, the impact of Beclin 1 expression on tumor downstaging remained statistically significant (p=0.03). The association of the level of Beclin 1 expression with the rate of tumor downstaging after chemoradiation is consistent with in vitro data, and suggests that Beclin 1 may be a predictive biomarker for the efficacy of chemoradiation in rectal cancer patients.
Keywords: Beclin 1, rectal cancer, chemoradiation, autophagy, biomarker
Beclin 1 is an essential regulator of autophagy where it is required for autophagosome formation and maturation.1 During autophagy, cytosolic proteins and organelles are engulfed into autophagosomes that fuse with lysosomes where they are degraded and recycled.2 Autophagy is upregulated in tumor cells in response to metabolic, hypoxic or cytotoxic stress to maintain cell survival.3 Allelic loss of Beclin 1 and defective autophagy were shown to sensitize cells to metabolic stress and to activate the DNA damage response in association with aneuploidy in immortalized murine epithelial cells and in mammary tumors.4, 5 Beclin 1 interacts with multiple proteins including the product of UV-irradiation-resistance-associated gene (UVRAG) to form core complexes that regulate autophagy. In a recent study in colorectal cancer (CRC) cells, we found that Beclin 1 and UVRAG expression can protect against radiation-induced DNA double strand breaks with a resultant decrease in tumor cell apoptosis.6 These data suggest that Beclin 1 overexpression in CRCs can contribute to radiation resistance. Conversely, suppression of Beclin 1 or inhibition of autophagy has been shown to sensitize CRC cells to radiation7 or chemotherapy8 which establishes autophagy as a therapeutic target in this malignancy.3
Nearly one-third of new diagnoses of CRC are due to cancers of the rectum.9 Patients whose tumors show transmural invasion of the rectal wall (T3,4) or have any depth of invasion with regional lymph node metastases (TxN1,2) routinely receive concurrent chemotherapy and radiation prior to surgical resection. Preoperative or neoadjuvant chemoradiation is preferred to postoperative treatment since it allows for tumor downstaging that can improve resectability and local control, as well as reducing toxicity.10, 11 Tumor downstaging is defined as a decrease in the pathologically-determined tumor (T) and lymph node (N) categories and is commonly utilized as a measurement of treatment response. Downstaging is determined by comparing preoperative staging data with endoscopic ultrasonography and/or imaging with the surgical resection specimen. In rectal cancer patients, tumor downstaging by chemoradiation has been shown to predict long-term outcomes.12 In this study, we tested the hypothesis that the level of Beclin 1 expression in pretreatment rectal cancer tissues is associated with the response to neoadjuvant chemoradiation therapy. To date, a predictive biomarker for the efficacy of chemoradiation therapy in rectal cancer is lacking13–15, and such a biomarker has the potential enable personalized therapy including novel therapeutic strategies.
MATERIALS AND METHODS
Patient cohort
Consecutive patients with locally advanced rectal adenocarcinoma (T3-4, N0 or Tx, N1-2) whose pre-treatment tumor tissues were available from our biorepository in the Center for Cell Signaling in Gastroenterology were included. All patients were treated with neoadjuvant chemoradiation and underwent surgical resection with curative intent between 1997 and 2009 at the Mayo Clinic in Rochester, MN. Pre-treatment tumor staging was performed in all patients using endoscopic ultrasonography, abdominal/pelvic computed tomography, and chest imaging. Neoadjuvant chemoradiation therapy consisting of infusional 5-fluorouracil (5-FU)[225 mg/m2] and external beam radiation therapy (50.4 Gy in 28 fractions). Four to six weeks after completion of chemoradiation, all patients underwent surgical resection with negative surgical margins (R0 resections) being achieved in all. The study was approved by the Mayo Clinic Institutional Review Board.
Assessment of Treatment Response and Tumor Downstaging
Pathological examination of surgical resection specimens was performed in accordance with the TNM classification where T stage, number of involved regional lymph nodes, and histologic grade were determined. We classified the pathological treatment response following chemoradiation in each patient into the following categories: pathological complete response (pCR), residual microscopic disease, or residual macroscopic tumor. Tumor downstaging was determined by a comparison between pretreatment TNM staging and re-staging by pathological examination of the surgical specimen.16
Beclin 1 expression and scoring in tumor specimens
Tissue sections (4–6 µm) from formalin-fixed, paraffin-embedded tumors were deparaffinized and antigen retrieval was performed in a preheated 0.1mM EDTA, pH 8.0 buffer for 30 min. After blocking endogenous peroxidase activity, slides were incubated (30 min) with a primary anti-Beclin 1 rabbit polyclonal antibody (ABCAM, Cambridge, MA; diluted 1: 250). After rinsing in TBST wash buffer (DAKO, Carpenteria, CA), a secondary incubation was performed (15 min) using a DUAL+/HRP labeled polymer (K4061, DAKO). Slides were subsequently placed in 3,3’-diaminobenzidine (5 min) and counterstained with a modified Schmit's hematoxylin. Negative controls omitted the primary antibody, but included all other procedural steps. Each slide contained a unique number that enabled blinding with respect to clinical data.
Beclin 1 protein expression was scored in tumor biopsy specimens obtained prior to chemoradiation therapy using criteria determined a priori. Tumors were considered positive if > 5% of tumor cells stained for Beclin 1 with a staining intensity ≥ 1+. Intensity was graded as follows: 0=not detectable, 1=weak; 2=moderate; 3=strong. Staining extent was defined as the percentage of positive tumor cells and scored: 0: <5%; 1: 5–24%; 2: 25–49%; 3: 50–74%; 4: ≥75%. Staining intensity and extent were multiplied to produce a weighted score17 for each tumor whose values were dichotomized at the median for subsequent analyses. All specimens were analyzed by a gastrointestinal pathologist (TTW) without knowledge of clinical information.
Statistical analysis
For Beclin 1 data, categorical variables were analyzed using the Chi-square test and continuous variables using the t-test. The paired differences for the staging variable were compared between Beclin 1 expression levels (low vs high) using a 2-sample t-test. A multivariable linear regression model was eveloped for predicting the staging paired differences between Beclin 1 expression levels after adjusting for age, gender, histologic grade, and baseline TNM stage. Descriptive statistics and graphical methods were used to summarize the data. All statistical tests were two-sided with a significance level defined at p< 0.05. Data were analyzed using JMP 10.0 (SAS Institute).
RESULTS
Patient Characteristics
Among 96 patients with locally advanced (T3-4, N0 or Tany, N1-2) rectal cancer, tumor location included the distal rectum (0 to 5 cm from anal verge) (n=38; 39.6%), midrectum (5–10 cm) (n=32; 33.3%) and proximal rectum (>10 cm) (n=26; 27.1%). Forty-seven patients (49.0%) underwent a low anterior resection and 49 (51.0%) underwent an abdominoperineal resection. Mean patient age was 58.7 years (range, 25–92 years) and 69.8% were men. Characteristics of the study population stratified by the dichotomized pre-treatment Beclin 1 expression level in tumor tissue are shown in Table 1.
Table 1.
Patient Characteristics
| All patients (%) |
Low Beclin 1 expression |
High Beclin 1 expression |
P value* |
|
|---|---|---|---|---|
| No. of patients | 96 (100) | 40 (100) | 56 (100) | |
| Mean age, range (years) | 58.7 (25–92) | 59.9 (38–92) | 57.8 (25–88) | 0.41** |
| Gender | ||||
| Male | 67 (69.8) | 25 (62.5) | 42 (75.0) | 0.19 |
| Female | 29 (30.2) | 15 (37.5) | 14 (25.0) | |
| Histologic grade | ||||
| Well/moderate | 26 (27.1) | 13 (32.5) | 13 (23.2) | 0.31 |
| Poor | 70 (72.9) | 27 (67.5) | 43 (76.8) | |
| Pre-treatment TNM staging | ||||
| T3-4, NO | 19 (19.8) | 7 (17.5) | 12 (21.4) | 0.69 |
| T1-4, N1-2 | 74 (77.1) | 31 (77.5) | 43 (76.8) | |
| Unknown*** | 3 (3.1) | 2 (5.0) | 1 (1.8) |
2-sided Chi-square p value
2-sample t-test p value
Treated as missing for Chi-square test
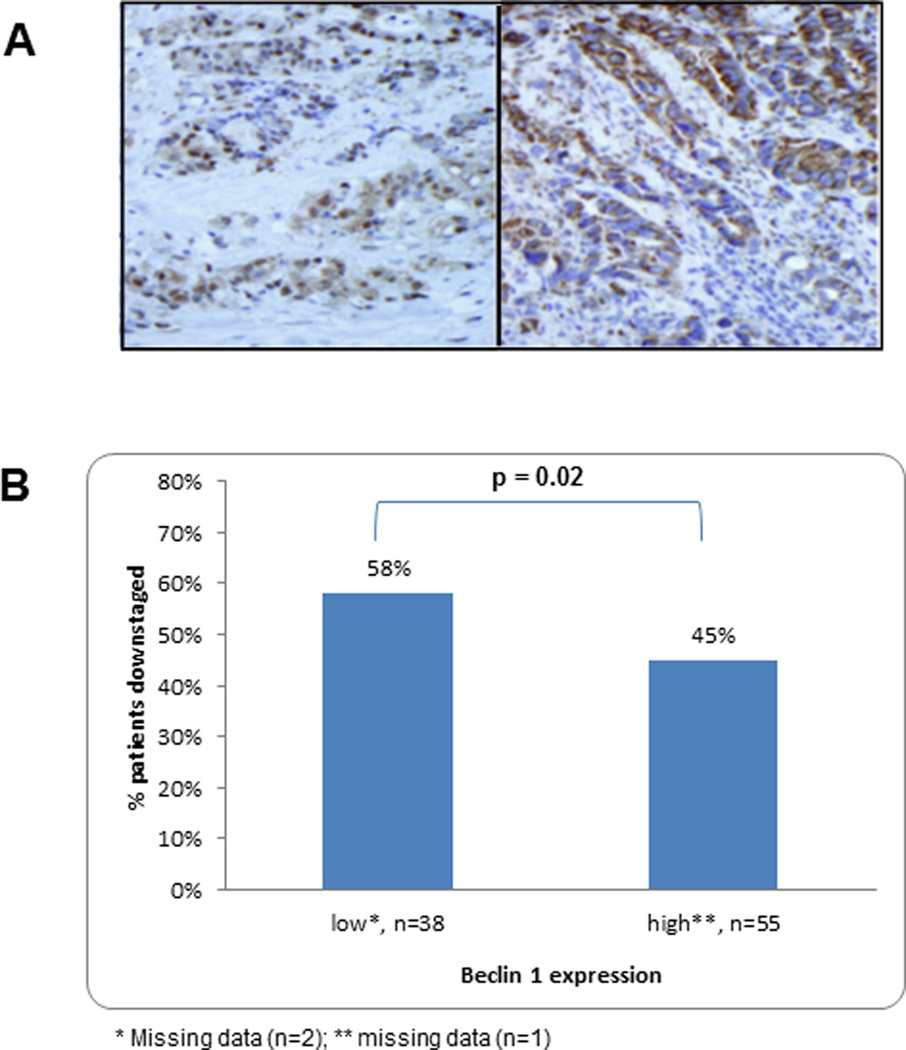
Beclin 1 protein expression was analyzed in carcinoma tissues prior to chemoradiation. Cytoplasmic expression (Fig. 1A) was dichotomized at the median value of the weighted score and Beclin 1 scores at the median were included in the high expression group. High vs low level Beclin 1 expression was detected in 56 (58.3%) and 40 (41.7%) tumors, respectively (Table 1). Beclin 1 expression was not significantly associated with the clinicopathological variables such as age, gender, tumor grade, or pre-treatment TNM stage (all p >0.05) (Table 1).
Figure 1.
A) Immunostaining for Beclin 1 protein expression was performed in rectal carcinomas. Representative tumors with low (left) vs high (right) level expression of Beclin 1 in the tumor cell cytoplasm (see Methods) are shown from pre-treatment biopsies (×400). B) Percentage of patients with rectal cancers who are downstaged after neoadjuvant chemoradiation therapy according to the level of Beclin 1 expression in pre-treatment tumor biopsies.
Beclin 1 Expression and Response to Chemoradiation
Patients with high vs low Beclin 1 expression were significantly less likely to achieve a pathologic response to neoadjuvant chemoradiation, defined as pCR or microscopic residual disease [8 (14.3%) vs 16 (66.7%); p=0.02]. An analysis of tumor downstaging based upon the TNM staging system revealed that patients whose tumors had high vs low Beclin 1 expression were significantly less likely to be downstaged after chemoradiation treatment [45% (25/55) vs 58% (22/38); mean (median) downstaging: −0.64 (0) vs −1.16 (−1); (p=0.02)] (Fig. 1B). In a multivariable analyses that adjusted for age, gender, histologic grade, and baseline TNM stage, the association of the Beclin 1 expression level with tumor downstaging was maintained (p=0.03) (Table 2). Specifically, tumor downstaging by chemoradiation was significantly predicted by both the Beclin 1 expression level and by the TNM stage at baseline in contrast to other clinicopathological variables (Table 2).
Table 2.
Multivariate Regression Model Predicting for Changes in TNM Stage From Baseline
| Variable | Estimate* (standard error) | p value |
|---|---|---|
| Beclin 1 level expression | −0.49 (0.23) | 0.03 |
| Gender | −0.06 (0.25) | 0.82 |
| Histologic grade | −0.14 (0.25) | 0.58 |
| TNM stage (at baseline) | 0.57 (0.28) | 0.04 |
| Age | 0.005 (0.009) | 0.60 |
Estimate is the differences in the means between the groups after adjusting for the other variables. For example, for the Beclin 1 expression variable, the −0.49 estimate is the low Beclin 1 expression mean minus the high Beclin 1 expression mean after adjusting for the other variables (−1.01 – (−0.52)).
DISCUSSION
The treatment of choice in patients with locally advanced rectal carcinoma is preoperative chemoradiation therapy followed by surgical resection.10, 11 In patients receiving such therapy, studies have suggested that the degree of tumor downstaging is clinically important in that it can be used for treatment monitoring and as a prognostic parameter.18 In this regard, tumor downstaging has been proposed as a surrogate marker for favorable long-term outcome in rectal cancer patients.19, 20 A range of pathological response to chemoradiotherpy occurs that includes pCR (ypT0N0), with no viable tumor cells left (currently 10% to 25% of patients), to microscopic residual disease, to virtually no tumor regression or even tumor progression during therapy.21 Based upon preclinical data, we evaluated the level of Beclin 1 expression as a potential predictive marker of pathological response and downstaging by neoadjuvant chemoradiation. Consistent with in vitro data 6, we found that a high level of Beclin 1 expression in pre-treatment rectal carcinoma tissues was associated with a significantly reduced rate of tumor downstaging by chemoradiation therapy compared to tumors with a low level of Beclin 1 expression. This finding for Beclin 1 remained statistically significant in a multivariable analysis after adjustment for relevant covariates. We also found that the pathological tumor response to chemoradiation was significantly reduced in tumors with high vs low level Beclin 1 expression. Together, these data suggest that Beclin 1 overexpression can contribute to treatment failure and may, therefore, enable tumor progression. Further support for these data derives from a study whereby the expression of Beclin 1 was associated with poor survival after chemoradiation in patients with esophageal squamous cell carcinoma compared to tumors lacking Beclin 1.22 Since all patients received 5-FU and concurrent radiotherapy, we were unable to determine the effect of Beclin 1 expression on 5-FU alone. Preclinical studies have shown that siRNA knockdown of Beclin 1 or Atg5 can sensitize human colorectal cancer cells to 5-FU.23 In patients with stages II and III colon carcinoma treated with 5-FU-based adjuvant chemotherapy, Beclin 1 overexpression was associated with reduced survival.24 While conflicting data have been reported regarding the prognostic impact of Beclin 1 in CRC, a recent meta-analysis found that patients whose CRCs had high Beclin 1 expression had a poor prognosis.25
Radiation has been shown to promote autophagy as a mechanism of stress tolerance in cultured tumor cell lines. However, the role of autophagy or its key regulator Beclin 1 in radiation resistance in human cancers remains unclear. Beclin 1-deficient cells have been reported to display increase susceptibility to radiation-induced cell death.26 Furthermore, knockdown of Beclin 1 and autophagy related 5 (ATG5) genes were shown to increase radiosensitization induced by a mTOR/PI3K inhibitor (NVP-BEZ235).7 In CRC cell lines, we recently reported that Beclin 1 expression can confer resistance to radiation-induced DNA double strand breaks that can be reversed by siRNA knockdown of Beclin 1 to promote cell death.6 These observations are clinically relevant in that the autophagy inhibitor chloroquine has been shown to strongly promote radiation-induced cell death in highly radioresistant cancer stem cells, and is currently being studied in clinical trials in cancer patients.27
Strengths of our study are uniform preoperative staging procedures that included endoscopic ultrasonography in all patients performed at a major tertiary referral center. Study limitations including the relatively small sample size and retrospective design. We recognize the inherent limitations related to comparison of pre-therapeutic imaging and surgical pathology findings post chemoradiation that include the potential for overstaging rectal cancers by endoscopic ultrasonography28. Our series included a higher than expected number of rectal cancers with poor differentiation. However, Beclin 1 expression did not differ by histologic grade in this study nor in most prior reports in this malignancy.25 Other limitations include interpretation of IHC by one expert pathologist, although scoring criteria were established a priori. It will be important to confirm the association of the dichotomization of Beclin 1 expression used in our study with clinical outcome. A formal method to assess neoadjuvant treatment response by grading histologic changes in the resected specimen has been proposed29, although correlation with disease prognosis awaits further study and external validation is needed.
In conclusion, we found that the level of Beclin 1 expression in rectal carcinomas was significantly associated with pathological response and rates of tumor downstaging following neoadjuvant chemoradiation therapy. If validated prospectively, our findings support a role for utilizing Beclin 1 as a predictive biomarker for the efficacy of neoadjuvant chemoradiation therapy in patients with rectal cancer.
Novelty and Impact of Work?
The essential autophagy protein Beclin 1 was recently shown to protect against radiation-induced DNA damage with a resultant decrease in colorectal cancer cell apoptosis. In locally advanced rectal cancer patients, we found that high vs low level Beclin 1 expression in pretreatment tumor tissues can predict the response to chemoradiation therapy with a significantly reduced rate of tumor downstaging. These data suggest the potential utility of Beclin 1 expression as a predictive biomarker.
ACKNOWLEDGEMENTS
This work was supported in part by a National Cancer Institute Senior Scientist Award (Grant No. K05CA-142885 to F.A.S). Dr. Aziz Zaanan is a recipient of a fellowship (Bourse Robert Tournut) from the French National Society of Gastroenterology (SNFGE).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27(Suppl 1):S137–S148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- 5.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JM, Tougeron D, Huang S, Okamoto K, Sinicrope FA. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS One. 2014;9:e100819. doi: 10.1371/journal.pone.0100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerniglia GJ, Karar J, Tyagi S, Christofidou-Solomidou M, Rengan R, Koumenis C, Maity A. Inhibition of autophagy as a strategy to augment radiosensitization by the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Mol Pharmacol. 2012;82:1230–1240. doi: 10.1124/mol.112.080408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer. 2010;46:1900–1909. doi: 10.1016/j.ejca.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 10.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 12.Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, Hu CY, Chang GJ. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Aguilar J, Chen Z, Smith DD, Li W, Madoff RD, Cataldo P, Marcet J, Pastor C. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg. 2011;254:486–492. doi: 10.1097/SLA.0b013e31822b8cfa. discussion 92-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negri FV, Campanini N, Camisa R, Pucci F, Bui S, Ceccon G, Martinelli R, Fumagalli M, Losardo PL, Crafa P, Bordi C, Cascinu S, et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. Br J Cancer. 2008;98:143–147. doi: 10.1038/sj.bjc.6604131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuchi M, Mikami T, Sato T, Tokuyama W, Araki K, Watanabe M, Saigenji K, Okayasu I. High Ki67, Bax, and thymidylate synthase expression well correlates with response to chemoradiation therapy in locally advanced rectal cancers: proposal of a logistic model for prediction. Br J Cancer. 2009;101:116–123. doi: 10.1038/sj.bjc.6605105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, Khanduja KS. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895–903. doi: 10.1007/s10350-004-6325-7. [DOI] [PubMed] [Google Scholar]
- 17.Sinicrope FA, Rego RL, Okumura K, Foster NR, O'Connell MJ, Sargent DJ, Windschitl HE. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14:5810–5818. doi: 10.1158/1078-0432.CCR-07-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stipa F, Chessin DB, Shia J, Paty PB, Weiser M, Temple LK, Minsky BD, Wong WD, Guillem JG. A pathologic complete response of rectal cancer to preoperative combined-modality therapy results in improved oncological outcome compared with those who achieve no downstaging on the basis of preoperative endorectal ultrasonography. Ann Surg Oncol. 2006;13:1047–1053. doi: 10.1245/ASO.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S, Mattia FO, Friso ML, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Janjan NA, Crane C, Feig BW, Cleary K, Dubrow R, Curley S, Vauthey JN, Lynch P, Ellis LM, Wolff R, Lenzi R, Abbruzzese J, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107–112. doi: 10.1097/00000421-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Chetty R, Gill P, Bateman AC, Driman DK, Govender D, Bateman AR, Chua YJ, Greywoode G, Hemmings C, Imat I, Jaynes E, Lee CS, et al. Pathological grading of regression: an International Study Group perspective. J Clin Pathol. 2012;65:865–866. doi: 10.1136/jclinpath-2012-201054. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Li X, Wu X, He C, Guo L, Zhang S, Xiao Y, Guo W, Tan B. Autophagy-related proteins LC3 and Beclin-1 impact the efficacy of chemoradiation on esophageal squamous cell carcinoma. Pathol Res Pract. 2013;209:562–567. doi: 10.1016/j.prp.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Sui X, Kong N, Wang X, Fang Y, Hu X, Xu Y, Chen W, Wang K, Li D, Jin W, Lou F, Zheng Y, et al. JNK confers 5-fluorouracil resistance in p53-deficient and mutant p53-expressing colon cancer cells by inducing survival autophagy. Sci Rep. 2014;4:4694. doi: 10.1038/srep04694. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Park JM, Huang S, Wu TT, Foster NR, Sinicrope FA. Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther. 2013;14:100–107. doi: 10.4161/cbt.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Y, Xue XF, Shen HG, Guo XB, Wang X, Yuan B, Guo XP, Kuang YT, Zhi QM, Zhao H. Prognostic significance of Beclin-1 expression in colorectal cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:4583–4587. doi: 10.7314/apjcp.2014.15.11.4583. [DOI] [PubMed] [Google Scholar]
- 26.Sato N, Mizumoto K, Nakamura M, Ueno H, Minamishima YA, Farber JL, Tanaka M. A possible role for centrosome overduplication in radiation-induced cell death. Oncogene. 2000;19:5281–5290. doi: 10.1038/sj.onc.1203902. [DOI] [PubMed] [Google Scholar]
- 27.Firat E, Weyerbrock A, Gaedicke S, Grosu AL, Niedermann G. Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote gamma-irradiation-induced cell death in primary stem-like glioma cells. PLoS One. 2012;7:e47357. doi: 10.1371/journal.pone.0047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marusch F, Ptok H, Sahm M, Schmidt U, Ridwelski K, Gastinger I, Lippert H. Endorectal ultrasound in rectal carcinoma--do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43:425–431. doi: 10.1055/s-0030-1256111. [DOI] [PubMed] [Google Scholar]
- 29.MacGregor TP, Maughan TS, Sharma RA. Pathological grading of regression following neoadjuvant chemoradiation therapy: the clinical need is now. J Clin Pathol. 2012;65:867–871. doi: 10.1136/jclinpath-2012-200958. [DOI] [PubMed] [Google Scholar]