Abstract
Problem
CD11cHI human decidual macrophages express several isoforms of CD1 molecules. Their expression pattern and function required investigation.
Method of Study
CD11cHI macrophages were isolated from decidua. Expression of CD1 isoforms and their ability to present lipid antigens to T cells was studied.
Results
CD1a, CD1c, and CD1d were all expressed on CD11cHI dMϕ, a pattern differing from those previously observed. Exposure of peripheral monocytes and dendritic cells to lipid isolates from decidua led to increased surface CD1a levels only. The CD1a and CD1c on dMϕ were able to present the appropriate lipid antigens to lipid antigen-specific T cells. Finally, autoreactivity of decidual T cells to CD1a was observed.
Conclusion
The unique pattern of expression of CD1 isoforms on CD11cHI dMϕ is consistent with organ-specific roles of CD1 in human T cell responses. dMϕ are able to present lipid antigens to both peripheral and decidual T cells and are major antigen presenting cells in human de-cidua.
Keywords: Cytokines, Gene Expression, Immunology, Leukocytes, Uterus
Introduction
During pregnancy the maternal immune system is presented with a unique set of challenges. The viviparous nature of mammalian reproduction, combined with the genetic diversity of the conceived offspring, means that the maternal immune system must develop an ability to tolerate fetal antigens, many of which are of paternal origin, while maintaining its the ability to defend against pathogens. In the decidua, which forms the maternal side of the maternal-fetal interface, an abundance of CD45+ leukocytes exist in close proximity to fetal tissue, particularly to extravillous trophoblasts, and represent between 30–40% of total maternal decidual cells1. Of these, decidual macrophages (dMϕ) are the second largest population and constitute approximately 15–20% of total leukocytes2,3.
The exact functions of dMϕ are still not fully understood. Macrophages occupy a unique niche within the decidual leukocyte population. They are the most abundant professional antigen-presenting cell (APC), outnumbering dendritic cells with a ratio greater than 10:14 and are thought to be involved in protection against pathological infection during pregnancy by effectuating and regulating both innate and adaptive immune responses5. The combination of these properties makes them uniquely poised to coordinate the immune systems dual roles of maintaining the integrity of the host while providing immune regulation and tolerance toward fetal antigens.
Recently two unique populations of macrophages have been identified in human decidua based on their expression of CD11c6. These two populations, termed CD11cHI and CD11cLO dMϕ, were also determined to have unique gene signatures that distinguished them from other macrophage subpopulations, such as the pro- and anti-inflammatory M1 and M2 macrophages7. Of note was the upregulation of a network of genes involved in lipid metabolism and presentation in CD11cHI dMϕ. Analysis of the lipid profiles of the two subsets revealed that CD11cHI cells also contained intracellular neutral lipid bodies that were not present in the CD11cLO population. The unusual lipid composition of CD11cHI cells was notable in light of the fact that they also showed significantly elevated expression of transcripts of at least two types of lipid antigen presenting molecules, known as CD1c and CD1d, as well as moderately increased CD1a gene expression6.
CD1 proteins are a family of non-polymorphic antigen-presenting molecules that are related to major histocompatibility complex (MHC) class I proteins. However, unlike MHC proteins that present peptide-based antigens to T cells, CD1 molecules present antigens that bind to CD1 via hydrophobic lipid tails8,9,10. In humans there are 5 isoforms of CD1, of which CD1e is only expressed intracellularly, while the other four are expressed on the cell surface and can directly present lipid antigens to T cells. These four antigen presenting CD1 isoforms are further divided into group I CD1 molecules, which include CD1a, CD1b and CD1c and group II of which CD1d is the only member11.
Unlike MHC class I proteins, which are expressed on most cells, and MHC class II proteins, which are expressed on nearly all professional APCs, CD1 expression is more restricted to individual subtypes of human APCs. For example, dendritic cells and thymocytes express all four isoforms of CD1 antigen presenting molecules, whereas macrophages and gastrointestinal epithelia express only CD1d. Langerhans cells express only CD1a, and marginal zone B cells express CD1c and CD1d12. Further, on myeloid cells, the group 1 CD1 proteins show inducible patterns of expression that are controlled by toll-like receptors (TLRs) and interleukin-1_13,14,15. The highly regulated and cell-type and tissue-specific patterns of CD1 gene and protein expression has given rise to speculation that the controlled expression of CD1 is important in regulating lipid-specific immune responses16. However, most studies to date are based on in vitro analysis of blood-derived cells, rather than tissue-derived cells that have homed to, and have been primed by, specific microenvironments.
While the number and isoforms of CD1 proteins within mammalian species varies considerably, almost all mammalian genomes encode CD1 genes17,18. Such conservation suggests that CD1 molecules both developed early in the evolution of mammalian species and play an important role in survival. CD1d and NKT cells influence the outcomes of infectious, autoimmune and neoplastic diseases in many mouse models, but group 1 CD1 molecules are not expressed in mice8. Therefore, experimental evidence for the involvement of group 1 CD1 molecules in T cell mediated immune responses has been mainly limited to human studies19. Many studies of group I CD1 isoforms have focused on foreign antigens20,21,22,23,24,25,26,27. However, direct recognition of CD1 proteins presenting self-ligands was observed28 prior to recognition of foreign lipids29. More recent studies of antigen-specific CD1-restricted T cell clones also clearly document autoreactivity, and self-lipid ligands such as sulfatides, gangliosides, and squalene have now been identified21,30,31. Furthermore, limiting dilution studies in human cohorts suggest that CD1 autoreactive T cells, especially those recognizing CD1a and CD1c, are very common in the blood, where they can comprise up to 10% of all T cells32. Using a mammalian cell line (K562) that does not express any MHC protein and has been transfected with individual CD1 isoforms, CD1a autoreactive T cells were almost universally found in the peripheral blood of the test subjects21,33. The advantage of this technique was that the transfected K562 cells likely expressed a diverse range of self-lipid antigens and as a result the caveats of using specific ligands and clonal cell lines could be avoided. Collectively these studies suggest that CD1 autoreactive cells are common in the blood of humans. However, although there is some evidence that CD1 expressing cells are capable of entering into peripheral tissues such as the skin or thyroid21,14, their potential roles in the human reproductive tract are unknown.
Given these findings, and our previous indications that CD11cHI dMϕ have both elevated CD1 mRNA levels and lipid metabolising pathways6, we set out to investigate if there was functional CD1 presentation by dMϕ and an endogenous population of responsive T cells in the decidua. We show that CD11cHI dMϕ are capable of presenting lipid antigens via CD1a and CD1c whereas CD11cLO dMϕ are not. Moreover, exposure of myeloid cells to decidual lipids leads to an increase in surface expression of CD1a and CD1c, providing a candidate mechanism of tissue-guided CD1-related differentiation in the uterus. Furthermore, utilizing the K562 system to measure autoreactivity ex vivo, we provide direct evidence of the presence of CD1a autoreactive cells at the maternal-fetal interface.
Materials and Methods
Decidual macrophage and T cell isolation
Discarded human placental and decidual material (gestational age 6–12 weeks) was obtained from women undergoing elective pregnancy termination from a local reproductive health clinic. Peripheral blood leukocytes are isolated from discarded leukopacks from healthy volunteer blood donors from the Massachusetts General Hospital in Boston, MA. All of the human tissue used for this research was de-identified, discarded clinical material. The Committee on the Use of Human Subjects (the Harvard IRB) determined that this use of placental and decidual material is Not Human Subjects Research. Decidual tissue was macroscopically identified, separated and processed as described previously6,35. Briefly, decidual tissue was washed, minced and thereafter digested with 0.1% collagenase type IV and 0.01% DNase I (Sigma-Aldrich) gently shaking in a water bath for 60 minutes at 37°C. Released lymphocytes were washed with RPMI 10% FBS (8 min, 1800 rpm) and filtered through 100μm, 70μm and 40μm sieves (BD, Labware; NJ). Lymphocytes were dissolved in 20 ml 1.023 g/ml Percoll (GE Healthcare) and layered on a Percoll gradient of (7.5ml 1.080g/ml; 12.5ml 1.053g/ml) for density gradient centrifugation (30min/800g). Lymphocytes were isolated from the 1.080 – 1.053g/ml interface and macrophages from the 1.053 -1.023 g/ml interface. Collected cells were washed twice and directly stained for Flow cytometric analysis or FACS sort. Peripheral blood T cells and monocytes from leukopacks obtained from the Massachusetts General Hospital were isolated using a RosetteSep™ T cell or monocyte enrichment cocktail (StemCell Technologies) followed by Ficoll (GE Healthcare) density gradient centrifugation (20 min, 2000 rpm).
Flow Cytometry
The following conjugated mouse anti-human antibodies were used for FACS analysis: CD3-Percp, CD11c-APC; CD11c-Percp-Cy5.5; CD14-FITC; CD14-PE; CD14-PE-Cy7, CD45-APC, CD45-Alexa700, CD56-Alexa488, CD56-Alexa700 (Biolegend). Monoclonal antibodies binding to CD1a (OKT6), CD1c (F10/21A3), and CD1d (CD1d42) were purified in the laboratory. Cells were analyzed on a FACS calibur™ (BD) or LSR-II flow cytometer (BD). Data was analyzed using FACS Diva software. Cell sorting was performed on a MoFlo Astrios high performance cell sorter (DAKO-Cytomation) or a FACS-ARIA III cell sorter (BD). dMϕ were FACS sorted based on CD45+CD14-CD56-CD3- phenotype, whereas decidual T cells were sorted based on a CD45+CD14-CD56- phenotype. CD3 staining to identify CD3+ T cells was not included to avoid non-specific activation of the T cells. A median of 7.6 × 105 (Range 1.4 – 16 × 105) T cells were isolated.
Cytokine analysis
Cell culture supernatants from cocultures of decidual T cells with CD1 transformed K562 cells were snap frozen at −80 °C and all cytokines were analyzed for IL-2, 4, 5, 5, 10, 12, 13, GM-CSF, IFNg and TNFa using a multiplex cytokine assay kit (Bio-Rad) according to manufacturer’s protocols. Data was acquired on a Luminex 200 analyzer.
Cell cultures supernatants from cocultures of CD1 transformed K562 cells with selected T cell lines were analysed for IFN-γ levels using Human IFN gamma ELISA (Thermo Fisher, Waltham, MA) in accordance with manufacturer’s protocols.
Lipid Extraction
Lipids were extracted from decidual tissue using a modified Folch extraction method35. Briefly, decidual tissue was first diluted to a volume 20-fold the volume of the tissue in 2:1 chloroform:methanol and homogenized using a Dounce homogenizer (Wheaton Science Products, Millville, NJ). The mixture was allowed to stand to create a biphasic system. The organic layer containing the lipids was extracted, dried down under nitrogen gas, and resuspended in dimethyl sulfoxide (DMSO).
Monocyte Isolation and Differentiation to DC
Peripheral monocytes (MO) were isolating using CD14+ microbeads (Miltenyi Biotec, San Diego, CA). They were differentiated to a DC phenotype in RPMI/CM containing 100U/ml of GM-CSF (Peprotech, Rocky Hill, NJ), 100U/ml of IL-4 (Peptrotech) at 2 × 106 cells/well in a 48-well plate. Decidual lipids were added at t=0. After 72h, cells were gated on a CD45+CD14+CD11c+ scheme and assessed for expression of CD1a, CD1c, or CD1d with flow cytometry.
CD1 expressing antigen presenting cell lines were made by stable transfection of K562 cells with pcDNA3.1 or pcDNA3 vector containing no insert or cDNA encoding CD1a, CD1b, CD1c or CD1d33. K562 clones were selected for high CD1 expression and low expression of HLA class I. Surface expression of HLA-DR was undetectable on all clones.
Results
CD11cHI Decidual Macrophages Have Elevated CD1 expression Compared to CD11cLO Decidual Macrophages
Previous microarray analysis of gene expression profiles in CD11cHI and CD11cLO dMϕ revealed differential expression of mRNA of several members of the CD1 family (Figure 1A)6. Specifically, CD11cHI dMϕ expressed elevated levels of CD1a, CD1c and CD1d mRNAs compared to CD11cLO cells. This preliminary result was unexpected because myeloid cells typically upregulate CD1a, CD1b, CD1c and CD1e together with constitutive expression of CD1d on myeloid DCs28,36,37. Yet, neither decidua-derived dMϕ population expressed CD1b or CD1e (Figure 1A). Therefore, we sought to determine whether this differential mRNA expression pattern also led to differences in the expression of CD1 isoforms on the surface of dMϕ using multicolour flow cytometry. Consistent with the microarray data, a significantly higher percentage of CD11cHI dMϕ, compared to CD11cLO dMϕ expressed CD1a (16.6% vs. 7%), CD1c (8% vs. 0.7%) and CD1d (7.1% vs. 0.17%) proteins (Figure 1B). Variation in expression of CD1a seen in the microarray (Figure 1A) corresponds to the variation observed in the FACS analysis (Figure 1B). Thus, the dual expression of CD1a and CD1c in the absence of CD1b was confirmed, identifying a previously unknown tissue-specific pattern of CD1 expression in decidua12.
Figure 1. CD11cHI dMϕ express high levels of CD1.
A. Heat map (left) and graph (right) of RNA expression values for CD1a, CD1b, CD1c, CD1d and CD1e within CD11cHI and CD11cLO dMϕ as found in the microarray of Houser, et al. (2011)6. Each square symbol represents a different sample/experiment. B. Representative FACS plots of CD1a, CD1c and CD1d expression within CD11cHI and CD11cLO dMϕ as compared to matched isotype controls for one individual (left). Percentage of CD1a, CD1c and CD1d positive cells within all cells meeting criteria for CD11cHI and CD11cLO dMϕ (right). IgG represents the isotype controls. Each symbol represents dMϕ from an individual decidua. *p<0.05, p<0.01,***p<0.005.
Decidual lipids increase CD1a expression on peripheral monocytes (MOs) and dendritic cells
Prior studies demonstrate a role of CD1 protein induction through the local release of TLR agonists and lipids36,15. The known factors for CD1 induction are mainly mycobacterial lipid agonists of TLR213,36, so CD1 response to lipids derived from total lipid extracts of decidua was measured as part of an effort to identify new, non-mycobacterial stimuli of CD1 expression. After treating decidua with chloroform and methanol to generate cell-free, sterile preparations that are enriched in lipids from five individual patient samples (Lipid1 – Lipid5), these were incubated with CD14+ peripheral monocytes (MOs). After 72 hours, an increased level of CD1a, but not CD1c and CD1d, was observed on the cells incubated with the decidual lipids (Figure 2A). Testing of each lipid extract with multiple patient MO populations revealed that two of the five lipid samples significantly increased CD1a expression compared to MO grown in the absence of decidual lipids. Although an overall increase in CD1a expression was observed when the other three lipid samples were added, it failed to reach significance. The fold change in CD1a expression amounted to about 3 fold. No significant changes in CD1c or CD1d were observed (Figure 2B–C).
Figure 2. CD14+ MOs incubated with decidual lipids increase surface expression of CD1a, but not CD1c or CD1d.

Peripheral monocytes were isolated and incubated with decidual lipids at t=0 (as described in Methods). After 72h, cells were stained for surface expression of CD1a, CD1c, and CD1d. Each dot represents an individual patient’s MO; The MFI depicted in the graphs has been corrected for the MFI of the isotype control. *p<0.05.
MO isolated from leukocytes derived from patient plasmapheresis were differentiated into a DC phenotype by incubation with IL-4 and GM-CSF for 72h38,39. Maturation induced by addition of IL-4 and GM-CSF increased CD1a and, to a lesser extent, CD1c but not CD1d expression (Figure 3, compare to Figure 2). The addition of decidual lipids to these culture conditions further increased the expression of CD1a and CD1c but not CD1d. Four of the five tested decidual lipid samples significantly increased CD1a expression, whereas all five decidual lipid samples resulted in a small but significant increase in CD1c expression (Figure 3, note that the Y axis scale is compressed relative to that in Figure 2). In the single case without a significant increase in CD1a, a noticeable trend of increased CD1a expression after lipid addition was evident, a pattern which matches recent studies showing that individual humans differ in the extent to which they can up-regulate CD1a proteins40. The mean increase in CD1a expression induced by decidual lipids ranged from 2 to 3 fold while the mean increase in CD1c expression was around 2 fold. These results suggest that factors within the decidual lipid extracts also up-regulate CD1a and CD1c on myeloid cells in addition to the increase in expression induced by GM-CSF and IL-4.
Figure 3. DC derived from peripheral MO were incubated with decidual lipids results in increased surface expression of CD1a and CD1c, but not CD1d.
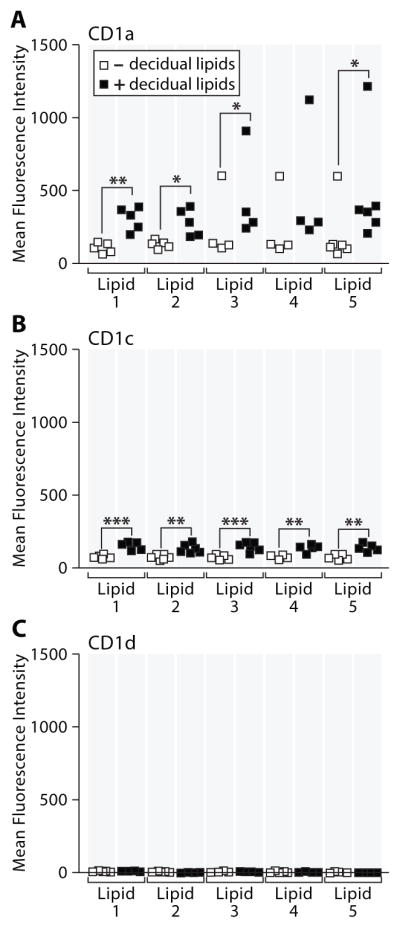
Peripheral monocytes were isolated and incubated with GM-CSF (100U/ml), IL-4 (100U/ml) and decidual lipids at t=0 (as described in Methods). After 72h, cells were stained for surface expression of CD1a, CD1c, and CD1d. Each dot represents an individual patient’s MO;. The MFI depicted in the graphs has been corrected for the MFI of the isotype control. A student’s t-test was performed to determine significance. *p<0.05, *p<0.01,***p<0.005.
Thus, the unique pattern of CD1a and CD1c expression on macrophages that home to the decidua largely matches the isoform specific pattern induced by local decidual tissue factors. These results are consistent with the existence of a local program of myeloid cell differentiation driven by decidua-derived factors.
CD11cHI Decidual Macrophages Can Present Lipid Antigens to T cells via CD1
To assess if the CD1 protein expression on CD11cHI dMϕ was sufficient to facilitate presentation of antigens to T cells, previously established T cell lines or clones that respond to lipid antigens only when presented by specific CD1 proteins were utilized. The lipid antigens and T cell lines utilised for this study were; dideoxymycobactin (DDM) and the CD8-2 line to assess CD1a presentation; mannosyl-β(1)-phosphomycoketide (MMP) and the CD8-1 line to assess CD1c presentation; and α-galactosylceramide (α-GalCer) and invariant-Natural Killer T (iNKT) cells to assess CD1d presentation25,26,40. K562 cells transfected with specific CD1 isotypes of interest were used as positive control antigen presenting cells to confirm the responsiveness of the T cell clones33.
CD11cHI and CD11cLO dMϕ isolated from first trimester donors, were preincubated with, or without, the specific lipid antigen for the CD1 molecule of interest before being incubated with specific T cell clones. Lipid antigen presentation by the two dMϕ macrophage subsets was assessed by the production of IFN-γ from the specific T cell clones in the presence of their cognate lipid antigen. CD11cHI dMϕ were able to present DDM and MPM via CD1a and CD1c respectively, as evidenced by the presence of elevated concentrations IFN-γ in supernatants when co-cultured with CD1a (CD8-2) or CD1c (CD8-1) restricted T cell clones (Figure 4A–B). However, CD11cLO dMϕ loaded with DDM and MMP failed to induce IFN-γ secretion by these T cells, demonstrating a function of CD1 in mediating the observed response. Furthermore, dMϕ that were not loaded with lipids did not induced IFN-γ secretion. Thus, the T cell clones respond to the CD1-lipid complexes, and the response is not due to possible alloreactivity to the dMϕ.
Figure 4. Presentation of lipid antigen by CD1a, CD1c, or CD1d CD11chi decidual macrophages.

to T cells with known antigen specificities. IFNγ ELISA of supernatant of CD11cLO and CD11cHI decidual macrophages incubated for 24 h with T cell lines recognizing CD1a and dideoxymycobactin (a); CD1c and mannosyl phosphomycoketide (MPM) (b); or CD1d and α-galactosylceramide(c). Five independent experiments were performed. Error bars indicate the Standard Deviation of the mean. A student’s t-test was performed to determine significance. *p<0.05.
When CD11cHI and CD11cLO were co-incubated with iNKT cells in the presence or absence of α-GalCer, the level of IFN-γ in the culture supernatants was unchanged (Figure 4C). Thus, the disparate levels of CD1a and CD1c on CD11cHI and CD11cLO dMϕ are sufficient to result in a functional difference between the two subsets, with CD11cHI dMϕ being capable of presenting lipid antigens to T cells while CD11cLO cells were not. However, unlike the other CD1 isoforms, the difference in CD1d expression levels between CD11cHI and CD11cLO cells did not result in a different ability to present α-GalCer to iNKT cells, which might reflect the usually high sensitivity and iNKT TCR affinity for CD1d-glycolipid complexes42 (Spada et al. 2000).
CD1a Autoreactive T cells Are Part of The Endogenous Decidual T Cell Population
After confirming the CD11cHI dMϕs could present lipid antigens, the question whether CD1 autoreactive T cells reside within the decidua was assessed. The newly developed in vitro system that uses K562 transfected with plasmids encoding the different human CD1 isoforms, was again utilized to allow analysis of multiple, unrelated human donors33. In this assay, the low or absent level of MHC I and MHC II on K562 cells negates any confounding MHC alloreactivity that might interfere with assessing the reactivity to CD1 molecules. Additionally these cells presumably possess and present a wide range of endogenous lipids, which allows for the analysis of T cell autoreactivity to many lipid antigens without prior knowledge of the antigens themselves, which is needed for conventional activation assays.
Decidual T cells isolated from first trimester donors were co-incubated with K562 cells expressing CD1a, CD1c, CD1d, or with an empty vector (EV3) control. After 6 days the concentrations of interleukins (IL) -2, -4, -5, -10, -12 (p70 subunit) and -13 as well as granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ and tumor necrosis factor alpha (TNFα) were analyzed in the cell culture supernatants. The cytokine concentrations in the supernatants of the decidual T cells co-cultured with K562 cells expressing the different CD1 isoforms were compared to those from the empty vector (EV3) control co-cultures. Furthermore, given the recent discovery of a population of CD1a-autoreactive T cells within the polyclonal peripheral T cell pool33, a further control group was included that comprised co-culturing T cells isolated from peripheral blood of healthy non-pregnant donors with CD1 transformed K562 cell. Of the cytokines analysed, only GM-CSF and IL-2 were at high enough concentrations to be reliably detected. GM-CSF was elevated in the supernatants of 4 of the 6 samples, obtained from co-culturing decidual T cells with K562/CD1a when compared to the same cells co-cultured with K562 transformed with the empty vector (EV3). In contrast, the co-culture of peripheral T cells from non-pregnant women with CD1a transformed K562 cells resulted in elevated supernatant GM-CSF levels in only 1 of 4 samples (Figure 5). When this experiment was performed with K562 cells transformed with CD1c or CD1d, no cytokine response was observed. IL-2 levels in supernatants of co-cultures were unchanged irrespective of exposure to CD1 transformed K562 cells. In light of the above, it appears that an endogenous population of CD1a autoreactive T cells exists at the maternal-fetal interface.
Figure 5. Secretion of GM-CSF by CD11chi decidual macrophages induced by K562/CD1a, K562/CD1c, or K562/CD1d.

T cells isolated from decidua (dT) and peripheral blood (pT) were incubated for 6 days with CD1 expressing K562 cells or control K562 cells, and supernatants were assayed for GM-CSF, using the Bioplex system. Results are indicated as fold change of the cytokine levels in the co-cultures of T cells with K562/CD1 cells relative to those with K562 control cells.
Discussion
The dynamics by which the local immune system is regulated at the maternal-fetal interface is critical to the success of pregnancy and the healthy development of the conceptus. The interaction of maternal APCs and T cells are critical not only for protection against bacterial and viral infections but also for the regulation of fetal tolerance42,43, and for the generation of an appropriate environment to facilitate and enhance placental development and invasion44. Despite this unusual importance, the mechanism by which different subsets of immune cells interact in the maternal decidual tissue during pregnancy is still poorly understood.
Of the two subsets of macrophages in the decidua, CD11cHI cells contained high levels of neutral lipid bodies and, expressed relatively high levels of mRNA for a variety of molecules involved in lipid metabolism6. In addition to these, relatively elevated expression levels of CD1c and CD1d mRNAs were present. FACS analysis confirmed that CD11cHI dMϕ did indeed have relatively high levels of surface expression of both CD1c and CD1d proteins that present glycolipids to T cells (as did CD1a, for which the mRNA level did not appear to be greatly elevated). This pattern of elevated expression of both CD1a-CD1c is distinct from the consistent pattern of CD1a-CD1b-CD1c co-regulation observed on maturing myeloid DCs35,39.
Lipidic factors present in the decidua may drive this unique pattern of decidual maturation, with the lipids potentially being of bacterial or host origin. Bacterial lipid stimuli of CD1 are known, but host lipid stimuli have not been described. Identification of the stimulus may be important in future work. A unique placental microbiome was recently described45 and could contribute to lipids presented by decidual CD1 proteins.
To investigate the functional significance of these findings, CD11cHI dMϕ were shown to be capable of presenting cognate lipid antigens to known clones of T cells via CD1a and CD1c but not CD1b or CD1d. CD11cLO dMϕ are unable to present via any of the four CD1 isotypes and failed to induce IFNγ secretion in any of the CD1 restricted T cell clones. The inability to elicit a T cell response in either the absence of an added lipid antigen, or using CD11cLO cells that express insufficient levels of CD1 molecules, confirmed that the results seen for CD11cHI dMϕ were not due to autoreactivity to the CD1 molecules themselves, or to non-specific reactivity to the presence of the lipid antigen. Thus, the CD11cHI population of dMϕ are functionally distinct in their ability to present lipid antigen to T cells via CD1a and CD1c. The ability of macrophages to present via CD1a and CD1c has, to our knowledge, not previously been demonstrated in an ex vivo context. Indeed group 1 CD1 proteins are usually used as markers of phenotypic DC maturation39.
Traditional understanding of CD1-mediated T cell responses and antigenic lipids has been largely limited to a few microbial pathogens46. Interestingly, many of these clonal T cell populations have been demonstrated also to be “self” reactive, in that they respond to CD1 expressing cells without the provision of exogenous foreign antigens32. Despite these observations, the prevalence of CD1 self-reactive T cells was reported recently. Self-reactive CD1a-restricted T cells were reported in the human skin and in peripheral blood at a prevalence of up to 1 in 50 of all self-reactive T cells32,33. Both of these papers indicate the presence of previously unappreciated populations of group I CD1 restricted self-reactive T cell clones. Four of six samples of decidual T cells but only one in four T cells isolated from the peripheral blood secreted GM-CSF when co-incubated with K562 cells that expressed CD1a. This data suggests that autoreactive T cells are part of the decidual immune response and that the frequency of CD1 restricted self-reactive T clones is increased in decidua compared to peripheral blood.
The present data may suggest that autoreactive T cell responses are also part of the decidual immune response. Decidual T cells reacted to CD1a in the absence of any exogenous lipid antigen, suggesting that at least a subpopulation within the polyclonal decidual T cell population are auto-reactive to CD1a, i.e., they can react to either CD1a molecules without addition of lipid antigen, or to CD1a molecules presenting exogenous lipid. The droplets of neutral lipids present in CD11cHI dMϕ6, would provide an abundance of potential lipid self-antigens to be presented by CD1a. The production of GM-CSF by these cells is compatible with the cytokine environment of the uterine and decidual tissue throughout pregnancy47. GM-CSF has been demonstrated to have a plurality of functions during pregnancy, including regulation of leukocyte populations such as dendritic cells48, facilitating enhanced embryo development49, as well as placental morphogenesis and function50. The autoreactivity of CD1a-restricted decidual T cells may contribute to the appropriate cytokine milieu in the pregnant uterus and aid in pregnancy success. The absence of group 1 CD1 expression in most inflamed tissues12 along with the transcriptional control of CD1 proteins is thought to regulate T cell response16. Therefore, it is notable that we observed the highest CD1 expression was that of CD1a, and the strongest autoreactive T cell response was also against CD1a. The downstream outcomes of CD1a T cell activation are not extensively studied, but recent studies of CD1a autoreactive T cells in the skin are proposed to be involved in homeostasis of this barrier tissue rather than host response51. These findings, along with the isolation of CD1-inducing lipid factors from the decidua are consistent with a model of tissue specific human myeloid cells at the maternal-fetal interface.
In conclusion, our study further confirms the functional differences between CD11cHI and CD11cHI dMϕ and provides strong support for the conclusion that the CD11 cHI dMϕ are principal antigen presenting cells of the human decidua. Preliminary data using polyclonal decidual and peripheral T cells suggests that autoreactive T cell responses are part of the decidual immune response in uncomplicated pregnancy. Understanding of how interaction of dMϕ with autoreactive T cell influences the decidual immune environment could significantly enhance insight into reproductive failure as well as reproductive success.
Acknowledgments
Work of authors L.G., B.L.H., T.T., V.W., and J.L.S. was funded by a grant from the NIAID (AI053330). J.L.S. is a consultant for King Abdulaziz University, Jeddah, KSA. Work of authors A.J. and D.B.M. was supported by grants from the NIAMS (048632), NIAID (AI054456 and AI056299), the Burroughs Wellcome Fund for Translational Research (to D.B.M.), and the National Psoriasis Foundation, USA (to A.J).
Footnotes
The authors declare there are no conflicts of interest.
References
- 1.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. The International journal of developmental biology. 2010;54:281–294. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 2.Bulmer JN, Johnson PM. Macrophage populations in the human placenta and amniochorion. Clinical and experimental immunology. 1984;57:393–403. [PMC free article] [PubMed] [Google Scholar]
- 3.Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. Journal of immunological methods. 1990;132:181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 4.Gardner L, Moffett A. Dendritic cells in the human decidua. Biology of reproduction. 2003;69:1438–1446. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 5.Singh U, Nicholson G, Urban BC, Sargent IL, Kishore U, Bernal AL. Immunological properties of human decidual macrophages--a possible role in intrauterine immunity. Reproduction. 2005;129:631–637. doi: 10.1530/rep.1.00331. [DOI] [PubMed] [Google Scholar]
- 6.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. Two unique human decidual macrophage populations. Journal of immunology. 2011;186:2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Advances in immunology. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 9.Lawton AP, Kronenberg M. The Third Way: Progress on pathways of antigen processing and presentation by CD1. Immunology and cell biology. 2004;82:295–306. doi: 10.1111/j.0818-9641.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 10.Moody DB. The surprising diversity of lipid antigens for CD1-restricted T cells. Advances in immunology. 2006;89:87–139. doi: 10.1016/S0065-2776(05)89003-0. [DOI] [PubMed] [Google Scholar]
- 11.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. European journal of immunology. 1989;19:285–292. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 12.Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. Current topics in microbiology and immunology. 2007;314:113–141. doi: 10.1007/978-3-540-69511-0_5. [DOI] [PubMed] [Google Scholar]
- 13.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nature medicine. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roura-Mir C, Catalfamo M, Cheng TY, Marqusee E, Besra GS, Jaraquemada D, Moody DB. CD1a and CD1c activate intrathyroidal T cells during Graves’ disease and Hashimoto’s thyroiditis. Journal of immunology. 2005a;174:3773–3780. doi: 10.4049/jimmunol.174.6.3773. [DOI] [PubMed] [Google Scholar]
- 15.Yakimchuk K, Roura-Mir C, Magalhaes KG, de Jong A, Kasmar AG, Granter SR, Budd R, Steere A, Pena-Cruz V, Kirschning C, et al. Borrelia burgdorferi infection regulates CD1 expression in human cells and tissues via IL1-beta. European journal of immunology. 2011;41:694–705. doi: 10.1002/eji.201040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody DB. TLR gateways to CD1 function. Nature immunology. 2006;7:811–817. doi: 10.1038/ni1368. [DOI] [PubMed] [Google Scholar]
- 17.Dascher CC. Evolutionary biology of CD1. Current topics in microbiology and immunology. 2007;314:3–26. doi: 10.1007/978-3-540-69511-0_1. [DOI] [PubMed] [Google Scholar]
- 18.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annual review of immunology. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 19.Bricard G, Porcelli SA. Antigen presentation by CD1 molecules and the generation of lipid-specific T cell immunity. Cellular and molecular life sciences : CMLS. 2007;64:1824–1840. doi: 10.1007/s00018-007-7007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 21.de Jong A, Cheng TY, Huang S, Gras S, Birkinshaw RW, Kasmar AG, Van Rhijn I, Pena-Cruz V, Ruan DT, Altman JD, et al. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nature immunology. 2014;15:177–185. doi: 10.1038/ni.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasmar AG, Van Rhijn I, Magalhaes KG, Young DC, Cheng TY, Turner MT, Schiefner A, Kalathur RC, Wilson IA, Bhati M, et al. Cutting Edge: CD1a Tetramers and Dextramers Identify Human Lipopeptide-Specific T Cells Ex Vivo. Journal of immunology. 2013 doi: 10.4049/jimmunol.1301660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki N, Rillahan CD, Cheng TY, Van Rhijn I, Macauley MS, Moody DB, Paulson JC. Targeted delivery of mycobacterial antigens to human dendritic cells via Siglec-7 induces robust T cell activation. Journal of immunology. 2014;193:1560–1566. doi: 10.4049/jimmunol.1303278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 25.Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 26.Moody DB, Young DC, Cheng TY, Rosat JP, Roura-Mir C, O’Connor PB, Zajonc DM, Walz A, Miller MJ, Levery SB, et al. T cell activation by lipopeptide antigens. Science. 2004;303:527–531. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- 27.Van Rhijn I, Ly D, Moody DB. CD1a, CD1b, and CD1c in Immunity Against Mycobacteria. Advances in experimental medicine and biology. 2013;783:181–197. doi: 10.1007/978-1-4614-6111-1_10. [DOI] [PubMed] [Google Scholar]
- 28.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 29.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 30.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. European journal of immunology. 1999;29:1667–1675. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Shamshiev A, Gober HJ, Donda A, Mazorra Z, Mori L, De Libero G. Presentation of the same glycolipid by different CD1 molecules. The Journal of experimental medicine. 2002;195:1013–1021. doi: 10.1084/jem.20011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, Mori L, De Libero G, Dellabona P, Casorati G. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. European journal of immunology. 2011;41:602–610. doi: 10.1002/eji.201041211. [DOI] [PubMed] [Google Scholar]
- 33.de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nature immunology. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilburgs T, Roelen DL, Van der Mast BJ, Van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HH, Claas FH, Scherjon SA. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Folch J, Lees M, Sloane GH. Stanley A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- 36.Roura-Mir C, Wang L, Cheng TY, Matsunaga I, Dascher CC, Peng SL, Fenton MJ, Kirschning C, Moody DB. Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. Journal of immunology. 2005;175:1758–1766. doi: 10.4049/jimmunol.175.3.1758. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. The Journal of experimental medicine. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leslie DS, Dascher CC, Cembrola K, Townes MA, Hava DL, Hugendubler LC, Mueller E, Fox L, Roura-Mir C, Moody DB, et al. Serum lipids regulate dendritic cell CD1 expression and function. Immunology. 2008;125:289–301. doi: 10.1111/j.1365-2567.2008.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seshadri C, Shenoy M, Wells RD, Hensley-McBain T, Andersen-Nissen E, McElrath MJ, Cheng TY, Moody DB, Hawn TR. Human CD1a deficiency is common and genetically regulated. Journal of immunology. 2013;191:1586–1593. doi: 10.4049/jimmunol.1300575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling PA, Frederique D, Modlin RL, Porcelli SA, Furlong ST, Brenner MB. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. Journal of immunology. 1999;162:366–371. [PubMed] [Google Scholar]
- 41.Spada FM, Borriello F, Sugita M, Watts GF, Koezuka Y, Porcelli SA. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. European journal of immunology. 2000;30:3468–3477. doi: 10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 42.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Human reproduction update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nature immunology. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 44.Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. The International journal of developmental biology. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Trasl Med. 2014;6:237. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent MS, Gumperz JE, Brenner MB. Understanding the function of CD1-restricted T cells. Nature immunology. 2003;4:517–523. doi: 10.1038/ni0603-517. [DOI] [PubMed] [Google Scholar]
- 47.Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine & growth factor reviews. 2007;18:287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Moldenhauer LM, Keenihan SN, Hayball JD, Robertson SA. GM-CSF is an essential regulator of T cell activation competence in uterine dendritic cells during early pregnancy in mice. Journal of immunology. 2010;185:7085–7096. doi: 10.4049/jimmunol.1001374. [DOI] [PubMed] [Google Scholar]
- 49.Sjoblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology. 2005;146:2142–2153. doi: 10.1210/en.2004-1260. [DOI] [PubMed] [Google Scholar]
- 50.Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 51.Colonna M. Skin function for human CD1a-reactive T cells. Nature immunology. 2010;11:1079–1080. doi: 10.1038/ni1210-1079. [DOI] [PubMed] [Google Scholar]



