Abstract
Background
The risk of thrombotic complications such as deep vein thrombosis (DVT) during tumor development is well known. Tumors release into circulation procoagulant microparticles (MPs) that can participate in thrombus formation following vessel injury. The importance of this MP tissue factor (TF) in the initiation of cancer-associated DVT remains uncertain.
Objective
To address how pancreatic cancer MPs promote DVT in vivo.
Methods
We combined a DVT mouse model where thrombosis is induced by flow restriction of the inferior vena cava with one of subcutaneous pancreatic cancer in C57BL/6J mice. We infused high and low TF tumor MPs to determine the importance of TF in experimental cancer-associated DVT.
Results
Both tumor-bearing mice and mice infused with tumor MPs submitted to 3 hours of partial flow restriction developed an occlusive thrombus; fewer than a third of the control mice did. We observed that MPs adhered to neutrophil extracellular traps (NETs), functionally important players during DVT, whereas neither P-selectin nor GPIb were required for the MP recruitment in DVT. The thrombotic phenotype induced by MP infusion was suppressed by hirudin suggesting the importance of thrombin generation. TF carried by tumor MPs was essential to promote DVT as mice infused with low TF tumor MPs had less thrombosis than mice infused with high TF tumor MPs.
Conclusions
TF expressed on tumor MPs contributes to the increased incidence of cancer-associated venous thrombosis in mice in vivo. These MPs may adhere to NETs formed at the site of thrombosis.
Keywords: Cancer, Cell-derived Microparticles, Neutrophils, Tissue Factor, Venous Thrombosis
INTRODUCTION
The prevalence of deep vein thrombosis (DVT) in cancer patients is up to six-fold higher than in the general population [1–3]. Cancer patients are also more susceptible to recurrent venous thromboembolism (VTE) [4–8] and this risk is particularly high in patients with pancreatic cancer [9–11]. VTE events contribute greatly to morbidity and mortality and also challenge physicians by delaying, interrupting or interfering with cancer therapy [12].
Tumor cells induce and take advantage of the hypercoagulable state, which promotes tumor growth [13], angiogenesis [14], and metastasis [15–17]. The ability of cancer cells to induce platelet aggregation was first observed by Gasic and colleagues more than thirty years ago [18], and has been the focus of several studies [19–21]. However, the factors that promote cancer-associated DVT require further studies.
In a model of ferric chloride-induced injury, it was previously demonstrated that tumor-bearing mice have shortened occlusion times in mesenteric venules and arterioles compared to control mice [22]. This work indicated that the thrombotic phenotype was due to the shedding of procoagulant microparticles (MPs) from cancer cells into blood. These MPs express a higher density of active tissue factor (TF) than the parental cell (> 100-fold), conferring an ability to promote vessel injury-induced thrombosis [22,23]. The presence of tumor MPs has been observed in the circulation of both mice and humans and several studies suggest a positive correlation between circulating TF-bearing MPs and the occurrence of thrombotic events in cancer patients. However, available data are ambiguous [11,24–26]. Thaler et al. have shown that MP-TF activity was not associated with future VTE but rather to ongoing or past VTE and mortality. However, no serial measurements were done, with only one blood sample taken on the day of study initiation. It is still possible that MP-TF activity rose at a later stage of the disease or before patients developed VTE. In the present study, we investigated whether tumors and/or tumor microparticles could promote DVT initiation in mice. We used an established murine model of DVT by producing stenosis, in the inferior vena cava (IVC). The model recapitulates blood flow conditions existing in human deep vein valves: turbulence, whirling, lack of laminarity and local hypoxia. With these conditions as a common denominator, we superposed cancer cells or MPs as a variable. The mouse model approximates but cannot mimic DVT pathogenesis in cancer patients but it addresses the effect of cancer on the venous thrombosis process. In contrast to most DVT models, our model maintains blood flow in the IVC and does not induce endothelial injury or denudation [27]. Also the IVC side branches are ligated as not to influence thrombus development [28]. The pathological venous thrombi form by a complex process involving endothelial release of von Willebrand factor (VWF) [27], and recruitment and cooperation of platelets, neutrophils, monocytes and red blood cells [27,29–31]. In the absence of cancer, studies have shown that neutrophils play an essential role in this thrombotic process by forming neutrophil extracellular traps (NETs) [29,30,32].
However, the mechanisms behind the increased risk of DVT during cancer development and whether circulating tumor microparticles can trigger DVT in stenotic vessels is not known. Wang and colleagues previously reported in nude mice that TF of tumor origin can affect venous thrombosis in a ferric chloride model of saphenous vein injury [33]. Using a C57Bl/6J syngeneic cancer model, DVT was tested in wild-type C57Bl/6J mice with or without murine pancreatic cancer [22], giving us the advantage of using immunocompetent mice. In addition, we were able to show that infusion of tumor MPs promotes DVT even in healthy mice.
MATERIALS AND METHODS
Mice
Experiments were performed using wild-type (WT) C57BL/6J mice purchased from the Jackson Laboratory or P-selectin −/− mice [34] of the same genetic background. All mice were 3- to 8-week-old males housed in the animal facility at Boston Children’s Hospital. Tumor cells (or control PBS) were injected subcutaneously in 3-week-old mice and DVT was then induced at 8 weeks of age in the same mice. Experimental procedures were approved by the Animal Care and Use Committee of Boston Children’s Hospital.
Cell lines
The Panc02 cell line was derived from a pancreatic ductal adenocarcinoma induced in a C57BL/6 mouse [35]. Panc02 cells were grown in RPMI 1640 medium supplemented with 10% FCS, 2mM L-glutamine, 100U/ml penicillin, 100μg/ml streptomycin and 0.1% fungizone. Blasticidin was used as the selection antibiotic for the Panc02 low-TF cell line described below. Cells were grown at 37°C in a humidified atmosphere with 5% CO2.
The Panc02-LowTF clone was described previously [23]. The Panc02 cells were stably transfected with the TF-silencing vector using Lipofectamine 2000 and PLUS reagent (Life Technologies). Cells were sorted for GFP expression (which was a function of the TF knock-down level) using a FACS Aria III (BD Biosciences). TF expression was further assessed by RT-PCR, immunofluorescence and by TF activity measurement described in a previously published study [23].
Microparticle preparation
The isolation of MPs was done as described [22] and their concentration determined by protein quantification using the bicinchoninic acid assay method (Thermo Fisher Scientific). The concentration of MPs used in vivo (0.2 μg/g mouse) has been determined by flow cytometry analysis and shown to correspond to 18,000 MPs/g mouse [22].
Induction of ectopic tumors
Induction of ectopic tumors using Panc02 cells was as described [22] by subcutaneous injection in the right flank of 2×105 cells in 3-week-old mice and the tumors were grown for 5 weeks.
IVC stenosis model
The flow restriction model was as described [27,29]. Briefly, mice were anesthetized by isoflurane-oxygen mixture and, after laparotomy, the IVC was separated from the aorta and ligated below the left renal vein over a spacer (0.26 mm in diameter) that was removed immediately after ligation. All visible side branches were ligated. This procedure decreases vascular lumen by about 90% (in 8 week-old mice) and produces no detectable endothelial denudation. Mice were euthanized 1, 3 or 48 hours after stenosis, and thrombi developed below the suture (in the caudal direction). In this DVT model, IVC ligation followed by quick removal of the suture does not induce thrombus development in 48 hours nor endothelial activation in 6 hours [27], indicating that thrombosis is induced by flow restriction and not by the ligation procedure. Some mice were given intravenous Panc02-derived MPs (0.2 μg of MP-associated proteins per gram of body weight) or PBS right after stenosis induction.
GPG290 treatment was done as described [27]. Values for weight and length of each thrombus were deemed as 0 when no thrombus was detected in the IVC.
Intravital microscopy and quantification of accumulated microparticles
Mice were anesthetized by isoflurane-oxygen mixture and kept under anesthesia for the whole experiment. To visualize microparticle accumulation during thrombus formation in the IVC, calcein-AM-labeled cancer cell-derived MPs (0.2 μg/g of body weight) were injected intravenously, just after induction of IVC stenosis, via a catheter implanted into the left jugular vein. Accumulation of fluorescent MPs in the IVC was assessed by intravital fluorescence microscopy 10, 30, and 60 minutes after induction of stenosis and MP injection. The fluorescent background was recorded before MP injection and was digitally removed from each acquisition following injection. Fluorescent images were acquired using a Zeiss Axioplan upright fluorescence microscope with a LED 4-Color Light Engine (Lumencor), in conjunction with a VGA CCD Camera (Hamamatsu) and an image intensifier (Videoscope). Slidebook 5.0 software (Intelligent Imaging Innovations, Inc.) was used for control of all hardware components, digital acquisition and data analysis.
Adhesion assay of microparticles to NETs
Neutrophils were isolated on a Percoll gradient from WT mouse bone marrow. Neutrophils were then stimulated with 50 μM Platelet Activating-Factor-16 (PAF, Merck Chemicals), a potent inducer of NETosis released by many cells involved in host defense [36]. The cells were washed with PBS, incubated with DiD-labeled Panc02-derived MPs (2μg/ml final) for 10 minutes at 37°C and washed again. The preparation was then fixed and stained with Hoescht-33342 before visualization on a motorized Leica DMI6000 B fluorescence microscope in conjunction with a CCD Hamamatsu Orca Er2 camera. For each independent experiment, 3 wells were analyzed per condition and at least 3 random photographs were taken per well. Representative pictures were then chosen for each condition. Flow experiments were performed in flow chambers (μ-Slide IV, IBIDI) at a flow rate of 100s−1. If indicated, DNase-1 (50U/ml, Pulmozyme, Roche) was added before MP perfusion. MP binding was visualized using a motorized Leica DMi8 fluorescence microscope in conjunction with a Leica DFC3000G camera. MetaMorph software (Molecular Devices) was used for digital acquisition.
Statistical analysis
We assessed statistical significance for non-parametric distributions with the two-tailed Mann-Whitney test. The proportions of mice that developed a thrombus were compared between the different experimental groups using the Fisher test. Difference was considered significant at P < 0.05.
RESULTS
Tumor or tumor-derived MPs promote DVT
To analyze whether cancer cell-derived microparticles promote DVT initiation in vivo, we used an established murine model of Trousseau’s syndrome. In this model, a subcutaneous injection of pancreatic cancer cells leads to the development of an ectopic tumor and the presence of circulating tumor-derived MPs in the blood [22]. To determine if the model was suitable to study cancer-associated DVT, we combined it with a model of DVT induced by inferior vena cava (IVC) stenosis. Using the two models together, we observed that all the tumor-bearing mice (8 of 8) developed a thrombus within 3 hours after flow restriction of the IVC whereas less than a third of mice without tumor (2 of 7) developed a thrombus (Fig. 1). We previously showed that these tumors shed TF-bearing MPs which alone can accelerate thrombus formation following vessel injury [22]. Interestingly, when Panc02 cell-derived MPs (0.2 μg/g mouse) where infused into the bloodstream of healthy mice 5 minutes after IVC stenosis, the occurrence of thrombus formation (10 of 10) was identical to tumor-bearing mice (8 of 8) (Fig. 1A), and the medians of thrombi weights and sizes were similar to the tumor-bearing mouse group (Fig. 1B–C). To determine whether cancer cell–derived MPs accumulate at the IVC stenosis site, MPs were isolated, labeled with the calcein-AM fluorochrome, and infused into a recipient mouse after stenosis induction. Using intravital microscopy, within minutes we detected the presence of fluorescently labeled MPs accumulating at the site of the growing thrombus (Fig. 1D). These results suggest that blood flow restriction induces tumor MP accumulation at the site of thrombosis and that these could promote the formation of cancer-associated DVT in vivo.
Fig. 1.
(A–C) Three-hour IVC stenosis in control WT mice (WT control, n=7), tumor-bearing mice (Cancer, n=8) and mice infused with cancer cell-derived MPs (MPs, n=10). (A) Percentage of mice with a thrombus. (B and C) Values for weight and length of each thrombus. Horizontal bars in dot plots represent median. (D) Representative images of fluorescence depicting accumulation of calcein-AM-labeled Panc02-derived MPs within the thrombosis area (n=3 mice). The pictures show the IVC part immediately below stenosis.
P-selectin and GPIb are not required for MP promotion of DVT while MPs bind to NETs
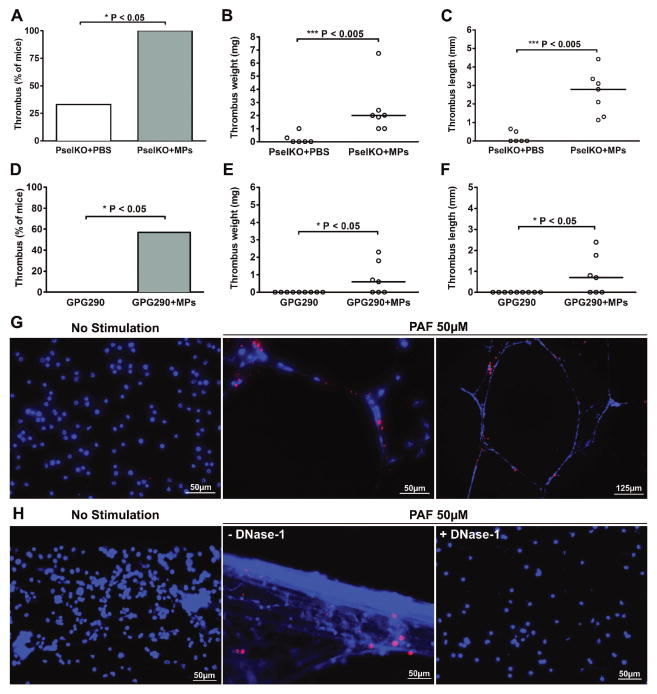
Tissue-factor-bearing tumor MPs used in this study have been shown to target sites of vessel injury through interaction of P-selectin glycoprotein ligand-1 (PSGL-1) expressed at their surface to P-selectin (Psel) expressed at a site of vessel injury [22]. Blood flow restriction induces endothelial activation below the stenosis site leading to local degranulation of Weibel-Palade bodies (WPB) from endothelial cells [27]. Because P-selectin and VWF are stored in WPB, in a blinded experiment, we first tested whether cancer cell-derived MPs were still able to promote DVT when the Psel/PSGL-1 axis was disrupted. We infused cancer cell-derived MPs in the circulation of Psel−/− mice and induced stenosis of the IVC. After 3 hours of blood flow restriction, all the Psel−/− mice infused with MPs (n = 7) developed DVT while only a third of the Psel−/− mouse group infused with PBS did (2 out of 6) (Fig. 2A). Thrombi from MPs-infused Psel−/− mice had medians in thrombus weight (Fig. 2B) and length (Fig. 2C) comparable to thrombi observed in MP-infused WT mice. These data suggest that cancer cell-derived MPs do not require P-selectin for their recruitment to promote DVT in this model.
Fig. 2.
(A–C) Three-hour IVC stenosis in control P-selectin deficient mice (PselKO + PBS, n=6) and P-selectin deficient mice infused with cancer cell-derived MPs (PselKO + MPs, n=7). (A) Percentage of mice with a thrombus. (B and C) Values for weight and length of each thrombus. Horizontal bars in dot plots represent median. (D–F) One-hour IVC stenosis in GPG290 pretreated mice (GPG290, n=9) and in GPG290-pretreated mice infused with cancer cell-derived MPs (GPG290 + MPs, n=7). (D) Percentage of mice with a thrombus. (E and F) Values for weight and length of each thrombus. (G and H) Representative images depicting Panc02-derived MPs interacting with NETs but not with unstimulated neutrophils. MPs were labeled with DiD (red) and NET DNA was stained with Hoechst 33342 (blue) after incubation of MPs with washed mouse neutrophils pre-incubated in absence (no stimulation) or in presence of 50 μM PAF to induce NET formation. (G) Experiment performed under static conditions. The photo on the right is at a higher magnification as indicated by bar. (H) Experiment performed in a flow chamber, at a shear rate of 100s−1. The flow direction was from left to right. The photo on the right shows the absence of MP binding after treatment with DNase-1.
Next we examined whether the MPs could be recruited by platelet GPIb which binds to Mac-1, P-selectin, and other ligands such as VWF [37–39]. Concomitantly with the MPs, we infused GPG290, a mutant glycoprotein Ib-immunoglobulin chimera that inhibits interaction of the VWF A1 domain and likely competes with other GPIb-mediated interactions. This chimera has been shown to prevent thrombosis in WT healthy mice after 48 hours of stenosis using this DVT model [27]. However, we observed that when mice were infused with tumor MPs and GPG290, thrombosis was still observed in mice as early as 1 hour after stenosis (Fig. 2D–F), suggesting that MPs do not require GPIb or the VWF A1 domain to promote DVT and can induce DVT even if VWF-mediated platelet adhesion is prevented. As an internal control, we verified that in this study GPG290 also inhibited thrombus formation in healthy WT mice after 48 hours of IVC stenosis (Supplemental Fig. 1).
It was recently shown that NETs are an important part of deep vein thrombi [29,30] and that NETs form early in the DVT process [32]. Interestingly, we now observed that cancer cell-derived MPs can adhere to NETs in vitro both under static (Fig. 2G) or flow conditions (Fig. 2H). Treatment with DNase-1 prevented MP adhesion, further indicating that the MPs bound to NETs (Fig. 2H). These results suggest that NETs could recruit MPs to the site of the pathological thrombosis.
TF carried by cancer cell-derived MPs is essential for their DVT-promoting activity
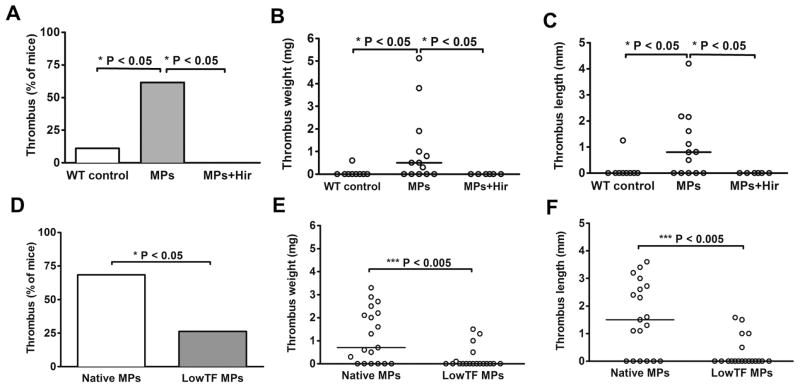
In the mouse model of DVT used in this study, thrombus formation is associated with endothelium activation without any exposure of the subendothelial matrix. TF-dependent thrombin generation has been shown to be of importance in DVT [32,40,41]. Because cancer cell-derived MPs are very rich in active TF, we examined whether they were able to trigger DVT independently of TF expressed by long term activated endothelial cells. We therefore performed a short (1 hour) stenosis, a time likely too brief for induction of synthesis and expression of TF [42]. We observed that nearly 2/3 of the mice infused with cancer cell-derived MPs (8 out of 13) still developed a thrombus after 1 hour of stenosis whereas only 1 out of 9 of the control mice did (Fig. 3). Not surprisingly, we observed that cancer cell-derived MP-induced DVT was completely abolished when thrombin generation was inhibited by hirudin infusion (Fig. 3A). In order to further establish the importance of TF expressed on cancer cell-derived MPs, we isolated MPs from low TF Panc02 cells [23]. Interestingly, the perfusion of low TF MPs significantly reduced the incidence of thrombus formation in this model and also significantly reduced thrombus weight and size when compared to mice perfused with unmodified Panc02-derived MPs. Altogether these observations show that, in our model, cancer cell-derived MPs express enough TF at their surface to significantly increase the incidence of DVT in vivo.
Fig. 3.
(A–C) One-hour IVC stenosis in control WT mice (WT control, n=9), mice infused intravenously with cancer cell-derived MPs (MPs, n=13) and in mice infused with cancer cell-derived MPs and pretreated with hirudin (MPs + Hir, hirudin 8U/g, n=6). (A) Percentage of mice with a thrombus. (B and C) Values for weight and length of each thrombus. Horizontal bars in dot plots represent median. (D–F) One-hour IVC stenosis in WT mice infused with unmodified Panc02-derived MPs (Native MPs, n=19) and in mice infused with LowTF MPs (LowTF MPs, n=19), (D) Percentage of mice with a thrombus. (E and F) Values for weight and length of each thrombus. Horizontal bars in dot plots represent median.
DISCUSSION
In order to better understand how cancer promotes DVT, we used a mouse model of DVT induced by IVC stenosis in combination with a mouse model of pancreatic cancer. In contrast to most DVT models [43–45], our model maintains blood flow and does not induce injury [27]. Here, we show that all tumor-bearing mice already formed an occlusive thrombus after 3 hours of stenosis, whereas the incidence of thrombus formation was low in mice without cancer. Previous studies have shown that these pancreatic tumors release into the circulation MPs that accelerate thrombus formation following injury [29,30]. The MPs express PSGL-1, allowing them to bind P-selectin presented by activated endothelium or platelets, and active tissue factor that can trigger blood coagulation [22]. We now observed that, when infused into healthy mice, MPs also trigger DVT. However, in the model of blood flow restriction, the MPs did not require P-selectin for their recruitment and activity, because P-selectin deficient mice were as susceptible to MP-induced DVT as WT. MP recruitment could possibly be supported by integrins as Panc02-derived MPs have recently been shown to express CD29 (alphaV), CD51 (beta1) and CD61 (beta3) whose inhibition by RGDV peptide prevented their binding to fibrinogen under flow [23]. We previously observed that free circulating Panc02 MPs have a half-life of approximately 10 minutes in mice [22]. However, these MPs expressing PSGL-1 [22] and integrins [23] at their surface, could stick to activated endothelium, platelets, leukocytes or fibrin. This might explain the increased accumulation of fluorescent tumor MPs in the thrombus over time. In addition, we observed that the cancer MPs bound avidly to NETs known to be formed early in this DVT model and to play a crucial role in the thrombosis process [29,30,32]. The observation of tumor MPs interacting with NETs is consistent with a study that showed that histones, that are part of NETs, bind phospholipids such as phosphatidylserine and phosphatidylethanolamine present on MPs [46]. Our results may explain a recent finding in a cancer patient by Thaler and colleagues of TF-positive cerebral and myocardial thrombi containg both epithelial markers (likely from tumor MPs) and citrullinated histone H3, indicative of the presence of NETs [47].
The present study complements well that of Wang and colleagues who observed that human tumors expressing TF implanted in nude mice activated coagulation in these mice and increased thrombosis in a ferric chloride model of saphenous vein injury [33]. However in their limited study of DVT without ligation of side branches [28], they did not detect difference in thrombus weight. Our larger study using syngeneic murine tumors or tumor MPs shows the direct involvement of TF expressed on cancer cell-derived MPs on DVT incidence. We chose not to evaluate thrombosis in mice bearing low-TF Panc02 tumors because tumor growth is much reduced in these mice [23], which would complicate data interpretation. These mouse studies may help to explain the observed correlation between circulating MP-TF activity and the heightened occurrence of VTE events in certain types of cancer patients [11]. It is possible that patients develop VTE following a rise in plasmatic MP-TF activity which could trigger thrombosis. Interestingly, the surface expression of TF on cancer cells may increase with the malignancy grade of the tumor [48], making it a marker of poor prognosis.
The importance of MP-linked TF in the triggering of venous thrombosis is further supported by the fact that phospholipids present on MPs, such as phosphatidylethanolamine and phosphatidylserine, strongly increase their procoagulant activity [49] and we show that their procoagulant activity may replace that of platelets when platelet recruitment is decreased by GPG290 (Fig. 2D–F and Supplemental Fig. 1). However, the observation that thrombus formation still occurs in MP-injected mice despite GPIbα blockade, does not necessarily indicate that MPs are driving thrombus formation without contribution of platelets. TF+ circulating MPs may rapidly activate the coagulation system after injection, leading to thrombin generation and platelet activation. A recent work from Davila and collaborators has confirmed this hypothesis. The authors observed that injecting pancreatic cancer-derived MPs into mice, rapidly induced thrombin generation as shown by elevated TAT levels, and platelet activation leading to thrombocytopenia and signs of shock [50].
Other cancers, such as glioblastoma for example, can induce a procoagulant state even if they express lower levels of TF. Here, other pathways must be employed to heighten the thrombotic risk. The secretion by the tumor of cytokines or growth factors can affect thrombosis through thrombocytosis [51], leukocytosis [52,53], and NETosis [36], a process crucial for DVT [54].
Clinical studies suggest an advantage of long-term low molecular weight heparin (LMWH) over vitamin K antagonists such as warfarin for patients with VTE in the setting of cancer [55]. This could be explained by the fact that, among its other effects, heparin can dismantle NETs’ scaffold [31] and prevent histone-mediated platelet aggregation [56].
Cancer and its treatment affect the three components of the Virchow’s triad in which misbalance can lead to venous thrombosis: release of procoagulant factors into circulation, alteration in blood flow and damage/activation to endothelial cells. In our DVT model, cancer cell-derived MPs do not seem to require selectin interaction to trigger thrombus formation. However, we believe that like NETs, P-selectin might be important later in the pathologic recruitment of cancer cell-derived MPs in the thrombus and in thrombus stabilization. For instance, P-selectin has been shown to be implicated in a mouse IVC stasis model at later time points and in the absence of cancer [57]. It would not be surprising that tumor MPs could have a worse impact on thrombosis in patients with cancer who are treated by chemotherapy as such drugs can damage the vascular endothelium [58,59] and induce NETosis [60]. NETs induced by chemotherapy [60] may contribute to MP recruitment and thus to thrombosis. Chemotherapy could also affect the risk of venous thrombosis [61,62] by inducing the production of TF-positive MPs following cancer cells’ apoptosis in response to treatment [63,64] just as leukocyte-derived MPs have been found to be involved in venous thrombogenesis in vivo [57]. In addition, several studies have shown that platelet activation and thrombin generation as well as extracellular DNA can further promote tumor progression and spread [65,66].
Altogether our results support the hypothesis that tumor microparticles and their TF help trigger cancer-associated venous thrombosis. Thus, targeting their recruitment or activity could provide strategies to prevent morbidity associated with cancer-related thrombotic complications.
Supplementary Material
Acknowledgments
We are grateful to Lesley Cowan for help with preparation of the manuscript. We thank Ken Ketman for technical assistance with cell sorting and Dr. Gray D. Shaw from Wyeth Research (Cambridge, MA, USA) for the gift of GPG290. We thank Pr. Françoise Dignat-George for her help and scientific support and the common research service of the URMITE UMR 6236, CNRS-IRD (Marseilles, France) and Pascal Weber for access to and help with the Leica fluorescence microscope. This work was supported by National Heart, Lung and Blood Institute of the National Institutes of Health grant RO1 HL102101 (D.D.W).
Footnotes
ADDENDUM
G. M. Thomas designed and performed research, collected, analyzed and interpreted the data, and wrote the paper; A. Brill designed and performed research and interpreted the data; S. M. Mezouar contributed to the preparation and characterization of the low-TF Panc02 cells, L. Crescence performed research, M. Gallant provided valuable technical assistance, C. Dubois provided crucial reagents and supervised the study. D. D. Wagner designed and supervised the study, and wrote the paper.
DISCLOSURE OF CONFLICT OF INTERESTS
G. M. Thomas and D. D. Wagner report grants from National Institutes of Health, NHLBI, during the conduct of the study.
Other authors state that they have no conflict of interests.
References
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 3.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 4.Prandoni P, Lensing AWA, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–8. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 5.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60–8. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 6.Schiavetti A, Foco M, Ingrosso A, Bonci E, Conti L, Matrunola M. Venous thrombosis in children with solid tumors. J Pediatr Hematol Oncol. 2008;30:148–52. doi: 10.1097/MPH.0b013e31815f88b7. [DOI] [PubMed] [Google Scholar]
- 7.Connolly GC, Khorana AA. Emerging risk stratification approaches to cancer-associated thrombosis: risk factors, biomarkers and a risk score. Thromb Res. 2010;125(Suppl 2):S1–7. doi: 10.1016/S0049-3848(10)00227-6. [DOI] [PubMed] [Google Scholar]
- 8.Kakkar AK. Cancer-associated thrombosis. Br J Cancer. 2010;102(Suppl 1):S1. doi: 10.1038/sj.bjc.6605598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blom JW, Vanderschoot JPM, Oostindiër MJ, Osanto S, van der Meer FJM, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–47. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharthuar A, Khorana AA, Hutson A, Wang J-G, Key NS, Mackman N, Iyer RV. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132:180–4. doi: 10.1016/j.thromres.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Camidge R, Reigner B, Cassidy J, Grange S, Abt M, Weidekamm E, Jodrell D. Significant effect of capecitabine on the pharmacokinetics and pharmacodynamics of warfarin in patients with cancer. J Clin Oncol. 2005;23:4719–25. doi: 10.1200/JCO.2005.09.129. [DOI] [PubMed] [Google Scholar]
- 13.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Caunt M, Huang Y-Q, Brooks PC, Karpatkin S. Thrombin induces neoangiogenesis in the chick chorioallantoic membrane. J Thromb Haemost. 2003;1:2097–102. doi: 10.1046/j.1538-7836.2003.00426.x. [DOI] [PubMed] [Google Scholar]
- 15.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300. [PubMed] [Google Scholar]
- 16.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 17.Chang C-Y, Lin S-C, Su W-H, Ho C-M, Jou Y-S. Somatic LMCD1 mutations promoted cell migration and tumor metastasis in hepatocellular carcinoma. Oncogene. 2012;31:2640–52. doi: 10.1038/onc.2011.440. [DOI] [PubMed] [Google Scholar]
- 18.Gasic GJ, Koch PA, Hsu B, Gasic TB, Niewiarowski S. Thrombogenic activity of mouse and human tumors: effects on platelets, coagulation, and fibrinolysis, and possible significance for metastases. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1976;86:263–77. doi: 10.1007/BF00286945. [DOI] [PubMed] [Google Scholar]
- 19.Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51:6073–8. [PubMed] [Google Scholar]
- 20.Alonso-Escolano D, Strongin AY, Chung AW, Deryugina EI, Radomski MW. Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. Br J Pharmacol. 2004;141:241–52. doi: 10.1038/sj.bjp.0705606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina C, Jurasz P, Santos-Martinez MJ, Jeong SS, Mitsky T, Chen R, Radomski MW. Platelet aggregation-induced by caco-2 cells: regulation by matrix metalloproteinase-2 and adenosine diphosphate. J Pharmacol Exp Ther. 2006;317:739–45. doi: 10.1124/jpet.105.098384. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–27. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer. 2014;136:462–75. doi: 10.1002/ijc.28997. [DOI] [PubMed] [Google Scholar]
- 24.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 25.Tesselaar MET, Romijn FPHTM, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 26.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010;125:511–2. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–7. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandt M, Schönfelder T, Schwenk M, Becker C, Jäckel S, Reinhardt C, Stark K, Massberg S, Münzel T, von Brühl M-L, Wenzel P. Deep vein thrombus formation induced by flow reduction in mice is determined by venous side branches. Clin Hemorheol Microcirc. 2014;56:145–52. doi: 10.3233/CH-131680. [DOI] [PubMed] [Google Scholar]
- 29.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–44. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110:8674–9. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von Brühl M-L, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J-G, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–52. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 35.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Jr, Schabel FM., Jr Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–26. [PubMed] [Google Scholar]
- 36.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109:13076–81. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon DI, Chen Z, Xu H, Li CQ, Dong JF, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, López JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, Li CQ, McIntire LV, Berndt MC, López JA. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med. 1999;190:803–14. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grainick HR, Williams SB, Coller BS. Asialo von Willebrand factor interactions with platelets. Interdependence of glycoproteins Ib and IIb/IIIa for binding and aggregation. J Clin Invest. 1985;75:19–25. doi: 10.1172/JCI111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y-D, Ye B-Q, Zheng S-X, Wang J-T, Wang J-G, Chen M, Liu J-G, Pei X-H, Wang L-J, Lin Z-X, Gupta K, Mackman N, Slungaard A, Key NS, Geng J-G. NF-kappaB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J Biol Chem. 2009;284:4473–83. doi: 10.1074/jbc.M806010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–8. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 42.Ishii H, Horie S, Kizaki K, Kazama M. Retinoic acid counteracts both the downregulation of thrombomodulin and the induction of tissue factor in cultured human endothelial cells exposed to tumor necrosis factor. Blood. 1992;80:2556–62. [PubMed] [Google Scholar]
- 43.Wakefield TW, Greenfield LJ, Rolfe MW, DeLucia A, 3rd, Strieter RM, Abrams GD, Kunkel SL, Esmon CT, Wrobleski SK, Kadell AM. Inflammatory and procoagulant mediator interactions in an experimental baboon model of venous thrombosis. Thromb Haemost. 1993;69:164–72. [PubMed] [Google Scholar]
- 44.Diaz JA, Wrobleski SK, Hawley AE, Lucchesi BR, Wakefield TW, Myers DD., Jr Electrolytic inferior vena cava model (EIM) of venous thrombosis. J Vis Exp. 2011:e2737. doi: 10.3791/2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wrobleski SK, Farris DM, Diaz JA, Myers DD, Jr, Wakefield TW. Mouse complete stasis model of inferior vena cava thrombosis. J Vis Exp. 2011 doi: 10.3791/2738. pii: 2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fürnrohr BG, Groer GJ, Sehnert B, Herrmann M, Voll RE. Interaction of histones with phospholipids--implications for the exposure of histones on apoptotic cells. Autoimmunity. 2007;40:322–6. doi: 10.1080/08916930701356457. [DOI] [PubMed] [Google Scholar]
- 47.Thalin C, Blomgren B, Mobarrez F, Lundstrom A, Laska AC, von Arbin M, von Heijne A, Rooth E, Wallen H, Aspberg S. Trousseau’s syndrome, a previously unrecognized condition in acute ischemic stroke associated with myocardial injury. J Investig Med High Impact Case Rep. 2014;2 doi: 10.1177/2324709614539283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg. 1995;82:1101–4. doi: 10.1002/bjs.1800820831. [DOI] [PubMed] [Google Scholar]
- 49.Yates KR, Welsh J, Echrish HH, Greenman J, Maraveyas A, Madden LA. Pancreatic cancer cell and microparticle procoagulant surface characterization: involvement of membrane-expressed tissue factor, phosphatidylserine and phosphatidylethanolamine. Blood Coagul Fibrinolysis. 2011;22:680–7. doi: 10.1097/MBC.0b013e32834ad7bc. [DOI] [PubMed] [Google Scholar]
- 50.Davila M, Robles-Carrillo L, Unruh D, Huo Q, Gardiner C, Sargent IL, Adam M, Woodhams BJ, Francis JL, Bogdanov VY, Amirkhosravi A. Microparticle association and heterogeneity of tumor-derived tissue factor in plasma: is it important for coagulation activation? J Thromb Haemost. 2014;12:186–96. doi: 10.1111/jth.12475. [DOI] [PubMed] [Google Scholar]
- 51.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–9. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 52.Ahlbrecht J, Dickmann B, Ay C, Dunkler D, Thaler J, Schmidinger M, Quehenberger P, Haitel A, Zielinski C, Pabinger I. Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2012;30:3870–5. doi: 10.1200/JCO.2011.40.1810. [DOI] [PubMed] [Google Scholar]
- 53.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–76. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee AYY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123–9. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 56.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–14. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myers DD, Hawley AE, Farris DM, Wrobleski SK, Thanaporn P, Schaub RG, Wagner DD, Kumar A, Wakefield TW. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38:1075–89. doi: 10.1016/s0741-5214(03)01033-4. [DOI] [PubMed] [Google Scholar]
- 58.Yamaç D, Elmas C, Ozoğul C, Keskil Z, Dursun A. Ultrastructural damage in vascular endothelium in rats treated with paclitaxel and doxorubicin. Ultrastruct Pathol. 2006;30:103–10. doi: 10.1080/01913120500406335. [DOI] [PubMed] [Google Scholar]
- 59.Bar-Joseph H, Ben-Aharon I, Tzabari M, Tsarfaty G, Stemmer SM, Shalgi R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS ONE. 2011;6:e23492. doi: 10.1371/journal.pone.0023492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9:2313–21. doi: 10.1111/j.1538-7836.2011.04465.x. [DOI] [PubMed] [Google Scholar]
- 61.Sud R, Khorana AA. Cancer-associated thrombosis: risk factors, candidate biomarkers and a risk model. Thromb Res. 2009;123(Suppl 4):S18–21. doi: 10.1016/S0049-3848(09)70137-9. [DOI] [PubMed] [Google Scholar]
- 62.Falanga A, Marchetti M. Anticancer treatment and thrombosis. Thromb Res. 2012;129:353–9. doi: 10.1016/j.thromres.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 63.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boles JC, Williams JC, Hollingsworth RM, Wang J-G, Glover SL, Owens AP, Barcel DA, Kasthuri RS, Key NS, Mackman N. Anthracycline treatment of the human monocytic leukemia cell line THP-1 increases phosphatidylserine exposure and tissue factor activity. Thromb Res. 2012;129:197–203. doi: 10.1016/j.thromres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 65.Snyder KM, Kessler CM. The pivotal role of thrombin in cancer biology and tumorigenesis. Semin Thromb Hemost. 2008;34:734–41. doi: 10.1055/s-0029-1145255. [DOI] [PubMed] [Google Scholar]
- 66.Wen F, Shen A, Choi A, Gerner EW, Shi J. Extracellular DNA in pancreatic cancer promotes cell invasion and metastasis. Cancer Res. 2013;73:4256–66. doi: 10.1158/0008-5472.CAN-12-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.