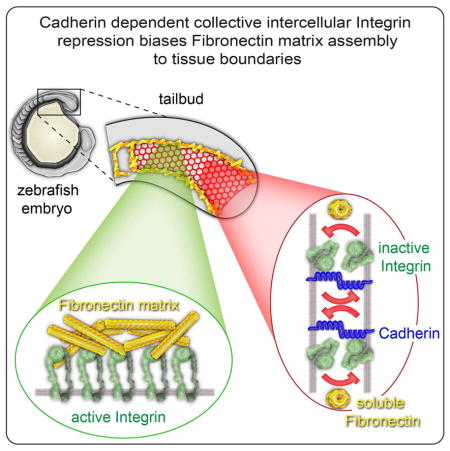
Summary
The diverse morphologies of animal tissues are underlain by different configurations of adherent cells and extracellular matrix (ECM). Here, we elucidate a cross-scale mechanism for tissue assembly and ECM remodeling involving Cadherin 2, the ECM protein Fibronectin and its receptor Integrin α5. Fluorescence crosscorrelation spectroscopy within the zebrafish paraxial mesoderm mesenchyme reveals a physical association between Integrin α5 on adjacent cell membranes. This Integrin-Integrin complex correlates with conformationally inactive Integrin. Cadherin 2 stabilizes both the Integrin association and inactive Integrin conformation. Thus, Integrin repression within the adherent mesenchymal interior of the tissue biases Fibronectin fibrillogenesis to the tissue surface lacking cell-cell adhesions. Along nascent somite boundaries, Cadherin 2 levels decrease becoming anti-correlated with levels of Integrin α5. Simultaneously, Integrin α5 clusters and adopts the active conformation and then commences ECM assembly. This cross-scale regulation of Integrin activation organizes a stereotypic pattern of ECM necessary for vertebrate body elongation and segmentation.
Graphical abstract
Introduction
The ECM is continually synthesized and remodeled throughout embryonic development, adult tissue homeostasis and wound healing (Hynes, 2002). Inappropriate ECM assembly leads to fibrosis, and solid tumors remodel the ECM during tumor progression and metastasis. Conversely, synthetically regulated ECM assembly has the potential to be harnessed for tissue engineering(Lu et al., 2011). In order to better understand how ECM and tissue topology are governed in vivo, we performed an integrated molecular-, cellular- and tissue-level examination of patterned Fibronectin matrix assembly during early vertebrate development.
Fibronectin (FN) is secreted as a soluble dimer and subsequently converted into insoluble, interlinked fibers by its Integrin receptors. Integrins are heterodimeric transmembrane proteins composed of an α and a β subunit that link the actin cytoskeleton to the ECM. Integrin α5 (Itgα5) and Integrin αV are the α subunits most responsible for FN matrix assembly. Integrins signal bidirectionally across the plasma membrane and play roles in cell migration, adhesion, and cell signaling. Signals transduced from the extracellular space to the cytoplasm, called “outside-in signaling,” rearrange the cytoskeleton and modulate gene expression. Cytoplasmic signals initiate “inside-out” signaling by separating the cytoplasmic tails of the α and β subunits, thereby inducing an allosteric change to the extended, high-affinity ligand-binding conformation (Hynes, 2002). Matrix assembly proceeds via Integrin binding to FN and cross-linking of FN. The cross-linking depends upon the application of tension from the actin cytoskeleton through the Integrin, which alters the conformation of FN dimers and exposes additional self-association domains (Schwarzbauer and DeSimone, 2011).
Within the trunk and tail of the zebrafish embryo, FN matrix is most prominent on the surface of the paraxial mesoderm and somite boundaries (Crawford et al., 2003). Somites are vertebrate mesodermal segments that give rise to the vertebrae, skeletal muscle and dermis. Somites form in bilateral pairs and in an anterior to posterior sequence concomitant with elongation of the vertebrate embryo. Paraxial mesoderm and somite morphogenesis are dependent upon itgα5, itgαV and fn in mouse, zebrafish, Xenopus and chick (Dray et al., 2013; George et al., 1993; Georges-Labouesse et al., 1996; Jülich et al., 2005; Jülich et al., 2009; Koshida et al., 2005; Kragtorp and Miller, 2007; Latimer and Jessen, 2010; Rifes et al., 2007; Takahashi et al., 2007; Yang et al., 1999). In zebrafish, cell-Fibronectin interactions propel trunk elongation through paraxial mesoderm tissue mechanics and not via cell migration. In addition, the FN matrix, not cell-cell adhesion, mechanically couples the paraxial mesoderm to the adjacent tissues (Dray et al., 2013). Indeed, the formation of this cortex of FN matrix may be an intrinsic property of the paraxial mesoderm (Jülich et al., 2009).
itgα5 and fibronectin are broadly expressed in the paraxial mesoderm but FN matrix assembly is restricted to this tissue’s surface and somite borders. The two zebrafish fibronectin genes, fn1 and fn1b, are both maternally deposited, and fn1 is zygotically expressed in the mesoderm during gastrulation and the posterior tailbud during body elongation (Jülich et al., 2005; Koshida et al., 2005; Latimer and Jessen, 2010; Trinh and Stainier, 2004). fn1b is transcribed throughout the paraxial mesoderm during body elongation (Jülich et al., 2005). itgα5 mRNA is maternally deposited, ubiquitously expressed during gastrulation and transcribed in the posterior tailbud and adaxial cells during body elongation (Crump et al., 2004; Jülich et al., 2005; Koshida et al., 2005). The maternally deposited itgα5 activity partially rescues the somite defect in homozygous itgα5 mutant embryos (Jülich et al., 2009).
Somite morphogenesis commences with a mesenchymal to epithelial transition as border cells polarize and assemble a FN matrix along their basal surface. In zebrafish, the receptor tyrosine kinase ephA4 is segmentally expressed along the posterior of the nascent somite border while its membrane bound ligands ephrinB2a and ephrinA1 are transcribed along the anterior of the border (Barrios et al., 2003; Durbin et al., 1998). This juxtaposed expression of the receptor and ligand concentrates activated, phosphorylated EphA4 along nascent somite borders (Jülich et al., 2009). In turn, Eph/Ephrin signaling can induce the mesenchymal to epithelial transition, inside-out Itgα5 activation and FN matrix assembly (Barrios et al., 2003; Durbin et al., 1998; Durbin et al., 2000; Jülich et al., 2009; Watanabe et al., 2009). The small GTPase rap1b, which regulates epithelial morphology and activates Integrins in other contexts, also functions with itgα5 to promote FN assembly at the somite border (Lackner et al., 2013).
N-Cadherin, now named cadherin 2 (cdh2), is a classical Cadherin and component of adherens junctions that mediate cell-cell adhesion. Adherens junctions are present throughout the paraxial mesoderm (Crawford et al., 2003). Cdh2 is necessary both for normal body elongation and coordinated cell motion in the zebrafish tailbud and for normal somite morphogenesis in mouse embryos (Harrington et al., 2007; Horikawa et al., 1999; Lawton et al., 2013; Lele et al., 2002; Warga and Kane, 2007).
Here, we delineate a comprehensive in vivo multi-scale mechanism that establishes the topology of adherent cells and ECM during tissue morphogenesis. We define this mechanism across molecular, cellular and tissue scales using in vivo fluorescence crosscorrelation spectroscopy, a conformation-specific Itgα5 antibody, activated and ligand-binding deficient Itgα5 variants and live imaging of a zebrafish FN-GFP transgenic. Itgα5 suppresses Matrix fibrillogenesis within the tissue mesenchyme, and we find that Itgα5 expressed on adjacent cell membranes physically interact. This Integrin-Integrin association correlates with conformationally inactive Itgα5. Cadherin 2 stabilizes the physical interactions between Itgα5 on adjacent cells and the inactive Itgα5 conformation. The Integrin repression is missing on the tissue surface due to the lack of an interface with adherent Itgα5 expressing cells. Thus, in this self-organizing mechanism, Itgα5 on the paraxial mesoderm surface coats the tissue in FN matrix. Segmental activation of Itgα5 within the tissue overrides the repression and leads to both de novo FN fibrillogenesis and FN matrix remodeling to create a contiguous FN matrix along the somite boundaries and tissue surface. This stereotypical tissue and ECM topology is essential for vertebrate body elongation and segmentation.
Results
Reciprocal intercellular repression of Itgα5 by Itgα5
Both the surface of the paraxial mesoderm and the somite boundaries are coated in FN matrix (Figure 1A–1E and Movie S1). We probed the regulation leading to this cell-ECM topology using genetic mosaics created by blastoderm stage cell transplantation (Figure 1F). In host embryos lacking segmental pattern within the somitic mesoderm, juxtaposition of cells lacking Itgα5 next to cells expressing Itgα5 causes activation of the Integrins and FN matrix fibrillogenesis along clone boundaries (Figure 1G). These experiments suggest that inactive Itgα5 expressed on adjacent cells reciprocally maintain Integrin repression to bias FN fibrillogenesis to cell surfaces free of this repression, i.e. the tissue surfaces or boundary of a clone. However, this repression does not require a 1:1 stoichiometry since mosaic 3X overexpression of Itgα5 does not lead to ECM assembly (Jülich et al., 2009). In normal embryos, segmental patterning overcomes the repression to activate Itgα5 along somite boundaries. Two hypotheses stemming from this model are that Itgα5 on adjacent cells physically associate to maintain Itgα5 repression and that Itgα5 is predominantly in the bent inactive conformation within the mesenchyme of the paraxial mesoderm.
Figure 1. Regulation of FN matrix fibrillogenesis within the paraxial mesoderm.
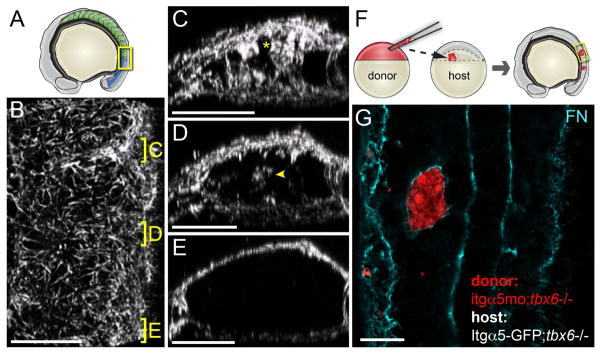
(A) A schematic showing the somites (green) and mesenchymal presomitic mesoderm (blue). The yellow box indicates the regions shown in B–E. (B–E) show a 3D reconstruction of the FN matrix in the paraxial mesoderm (see Movie S1). (A) A dorsal view of the tissue with anterior at the top. Positions of sections of the mature (C) and nascent (D) borders and presomitic mesoderm are indicated. Asterisk in C indicates somite border matrix contiguous with the dorsal surface of the tissue. Arrowhead in D indicates de novo matrix assembly within the nascent boundary. For the Fibronectin IHC analysis z-stacks were acquired and analyzed for 13 embryos from 3 independent stainings. In panels C–E, dorsal is up. (F) A schematic of cell transplantation from a labeled donor blastula into host blastula. The tbx6−/− background is used since they make somite boundaries. Embryos develop until the 8–16 somite stage and clones are examined for FN matrix. (G) Isolated paraxial mesoderm clones lacking itgα5, due to itgα5 morpholino injection, are coated with FN by Itgα5 expressed on adjacent host paraxial mesoderm cells. Scale bars are 40 microns.
Intercellular association of Itgα5 is independent of direct FN binding
To test for physical intercellular association of Itgα5, we employed fluorescence correlation spectroscopy (FCS) and fluorescence crosscorrelation spectroscopy (FCCS). FCS is a highly sensitive quantitative microscopy technique that can be used to calculate diffusion coefficients of proteins in complex in vivo contexts by measuring fluctuations in fluorescence intensity within a defined focal volume. In FCCS, the crosscorrelation in fluctuations of two different fluorophores in the focal volume can be used to detect physical association (Ries et al., 2009). In other words, if an Itgα5-GFP were to physically interact with an Itgα5-RFP, the fluctuations in GFP and RFP fluorescence will show a crosscorrelation in FCCS experiments.
We performed FCS and FCCS on Cadherin 2 (Cdh2) to provide a positive control for intercellular homotypic interaction in the presomitic mesoderm. A systems level analysis of tailbud cell movement in the cdh2 mutant indicated that it is required for ordered cell motion within the presomitic mesoderm (Lawton et al., 2013). The affinity of Cadherin homotypic binding has been measured in vitro using the E-Cadherin ectodomain (Haussinger et al., 2004; Koch et al., 1997). However, our Cdh2 experiments provide the first in vivo measurement of intercellular homotypic adhesion between full length, membrane anchored Cadherin.
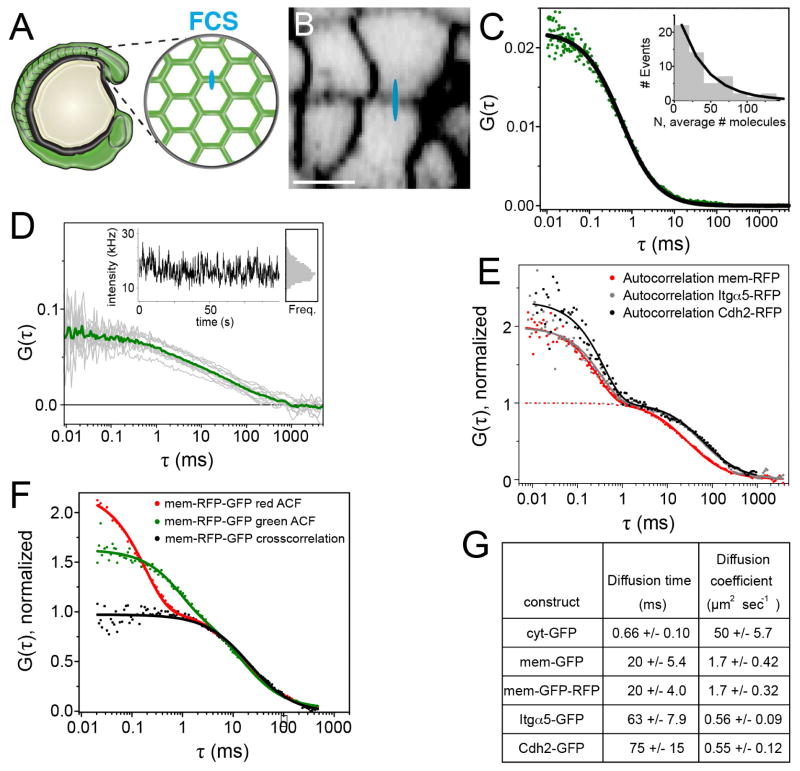
We developed an FCS protocol to measure the diffusion of Itgα5-GFP, Itgα5-RFP, Cdh2-GFP and Cdh2-RFP within the cell cortex of mesenchymal cells of the PSM (Figure 2A and 2B). We used maternal zygotic itgα5 mutant embryos (MZ itgα5−/−)(Jülich et al., 2009) to remove all endogenous non-GFP-tagged Itgα5 thereby eliminating interference with the Itgα5 FCCS measurements. To remove endogenous Cdh2, we used a cdh2 morpholino shown to recapitulate the null mutant phenotype (Lele et al., 2002; Warga and Kane, 2007). The concentration of fluorescent proteins must be relatively low in order to attain accurate FCS measurements. Thus, we expressed sub-physiological levels of Itgα5-GFP in MZ itgα5−/− embryos via mRNA injection at the one cell stage and performed FCS in PSM cells of live 8–10 somite stage embryos to quantify the movement of Itgα5-GFP (Figure 2C, inset). Control measurements of the diffusion coefficients of cytosolic GFP (Figure 2C and 2G) and intracellular membrane-anchored GFP (Figure 2E and 2G) were consistent with published FCS measurements (Schaaf et al., 2009; Shi et al., 2009).
Figure 2. Quantification of Itgα 5 dynamics in the presomitic mesoderm.
(A) A schematic of FCS data collection for fluorescently tagged proteins in the presomitic mesoderm. For FCS, the confocal volume (drawn approximately to scale in blue in A and B) was statically positioned in a cell membrane oriented orthogonal to the optical axis. (B) Confocal image of a zebrafish embryo expressing membrane RFP (mem-RFP). Scale bar is 10 μm. (C) Autocorrelation curve of cytosolic GFP (cyt-GFP), fit to 3D diffusion (Equation 1 supplemental methods). Inset, histogram of measured number of Itgα5-GFP molecules. (D) Representative set of 10 autocorrelation curves (gray) of Itgα5-GFP with the averaged curve used in fitting (green). Inset, the intensity fluctuations and binned intensities from this measurement. (E) Auto-correlation measurements of mem-RFP, Itgα5-RFP, and Cdh2-RFP. Fits to 2D diffusion and fluorophore fluctuation (solid line, Equation 3 supplemental methods) and to 2D diffusion only (dotted line, Equation 2). To facilitate comparison, diffusion components are normalized to 1. (F) Autocorrelation curves of mem-GFP-RFP illustrating the fast decay component is fluorophore specific, but the slow decay, i.e. diffusion, is identical in both autocorrelation curves as well the crosscorrelation curve. (G) Table of measured diffusion times and calculated diffusion coefficients ±SD.
Itgα5-GFP exhibited a diffusion coefficient of 0.56 ±0.09 μm2/s in the membranes of live mesenchymal PSM cells (Figure 2D, 2E and 2G). This value contrasts with a diffusion coefficient of 0.02 to 0.04 μm2/s for the FN receptor in cultured chick somite cells measured by FRAP (Duband et al., 1988). Cdh2-GFP exhibited a comparable diffusion coefficient of 0.55 ±0.12 μm2/s (Figure 2E and 2G). Our Itgα5-GFP and Cdh2-GFP measurements are similar to recent FCS quantification of diffusion of other transmembrane proteins in live zebrafish embryos (Ries et al., 2009; Shi et al., 2009; Yu et al., 2009).
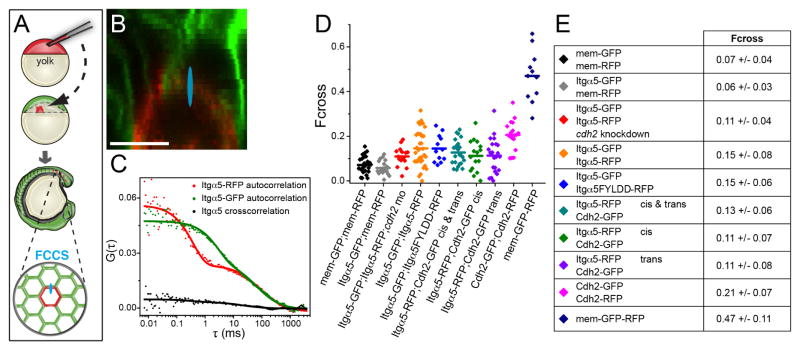
To specifically measure intercellular interactions between proteins expressed on opposing cell membranes, we created mosaic embryos via cell transplantation (Figure 3A). We performed FCCS on juxtaposed cell membranes of GFP and RFP expressing cells in the presomitic mesoderm of live embryos to measure the crosscorrelation (Figure 3A and 3B). We report normalized crosscorrelation values as Fcross, the fraction of molecules crosscorrelating. A negative control was provided by genetic mosaics in which cells expressing intracellular myristoylated membrane-anchored RFP (mem-RFP) were transplanted into host embryos expressing intracellular myristoylated membrane-anchored GFP (mem-GFP) and exhibited an Fcross of 0.07 ±0.04 (Figure 3B, 3D and 3E). A positive control for crosscorrelation was a membrane bound GFP-RFP fusion protein (mem-GFP-RFP) which exhibited an Fcross of 0.47 ±0.11 (Figure 2F, 3D and 3E). These Fcross values correspond to the minimum and maximum possible values observed in the absence of crosscorrelation and in the presence of 100% crosscorrelation, respectively. For the Cdh2 analysis, we transplanted cells from embryos coinjected with the cdh2 morpholino and cdh2-GFP mRNA into cdh2 morpholino injected embryos expressing cdh2-RFP. Cdh2 exhibits a significant crosscorrelation with an Fcross of 0.21 ±0.07 (Figure 3D and 3E). The apparent Kd for the Cdh2 interaction, extrapolated from the crosscorrelation measurements, is 200 ±100 nM (Figure S1).
Figure 3. Quantification of protein association in the presomitic mesoderm.
(A) a schematic of cell transplantation and FCCS data collection. For FCCS, the confocal volume (drawn approximately to scale in blue in A and B) was positioned at a membrane interface between RFP and GFP expressing cells. (B) Confocal image of a mosaic embryo comprised of cells expressing mem-RFP and mem-GFP. Scale bar is 10μm. (C) Auto- and crosscorrelation measurements of Itgα5-RFP and Itgα5-GFP in a mosaic interface show low crosscorrelation. (D) Plots of crosscorrelation from individual measurements and mean values. (E) Table of crosscorrelation (Fcross) magnitude ±SD. Significance was examined using both t-test (two tailed, unequal variance) and Mann- Whitney U. There were no statistically significant differences in mem-GFP/mem-RFP compared to Itgα5-GFP/mem-RFP and Itgα5-GFP/Itgα5-RFP compared to Itgα5-GFP/Itgα5FYLDD-RFP. Notably, either mem-GFP/mem-RFP or Itgα5-GFP/mem-RFP compared to either Itgα5-GFP/Itgα5-RFP or Itgα5-GFP/Itgα5FYLDD-RFP differed with p<0.002. The Itgα5-RFP/Cdh2-GFP “trans” Fcross differs from the negative control (p<0.05) but not from the Itgα5-RFP/Cdh2-GFP “cis” Fcross. The Itgα5-GFP/Itgα5-RFP Fcross in the absence of Cdh2 is significantly lower (p<0.05) than the Itgα5-GFP/Itgα5-RFP Fcross in the presence of Cdh2. See Figure S1 for apparent Kd calculations.
For the Itgα5 analysis, we transplanted cells from MZ itgα5−/− embryos expressing Itgα5-GFP into MZ itgα5−/− embryos expressing Itgα5-RFP. The Itgα5 experiments revealed a significant crosscorrelation with an Fcross of 0.15 ±0.08 (Figure 3C–3E). This Fcross was significantly larger than both the negative control Fcross between mem-GFP and mem-RFP and an additional negative control Fcross between Itgα5-GFP and mem-RFP (p<0.002)(Figure 3D and 3E). These data do not indicate whether the physical association is direct or if the Integrins are part of a larger protein complex. To test whether the crosscorrelation between Integrins was mediated by direct FN binding, we measured the Fcross between Itgα5-GFP and ligand-binding deficient Itgα5FYLDD-RFP (Jülich et al., 2009). This Fcross was indistinguishable from that between wild-type Integrins indicating that the interaction is not mediated by direct coupling to FN (Figure 3D and 3E). This association also parallels the inhibitory function as Itgα5FYLDD can repress FN fibrillogenesis by Itgα5 expressed on adjacent cells (Jülich et al., 2009). In total, these data suggest that Integrin α5β1 expressed on adjacent cells have intercellular physical association, independent of direct FN binding, with an apparent Kd of 750 ±100 nM (Figure S1).
Cdh2 is necessary for physical association of Itgα5
Cdh2 and Itgα5 have been shown to colocalize within the same cell membrane (Canonici et al., 2008; Chattopadhyay et al., 2003; Lefort et al., 2011). We thus examined whether Itgα5 and Cdh2 on adjacent cells interact and whether the interaction between Itgα5 on adjacent cells requires cdh2.
We first examined whether any crosscorrelation could be detected between Itgα5-RFP and Cdh2-GFP by ubiquitous co-expression via mRNA injection. These experiments will detect Itgα5 and Cdh2 associations within the same cell membrane, i.e. in “cis”, as well as associations between Cdh2 expressed on one cell membrane and Itgα5 expressed on the adjacent cell membrane, i.e. in “trans”. The fraction of crosscorrelating molecules, Fcross, is 0.13 ± 0.06. We next sought to determine whether this Itgα5-Cdh2 association is in cis, in trans or both. To measure cis interactions within the same cell membrane, we injected a single blastomere at the 32 cell stage with mRNAs coding for Itgα5-RFP and Cdh2-GFP. After these embryos reached mid-somitogenesis, we performed FCCS on injected cells within the presomitic mesoderm which were surrounded by uninjected cells. The cis Fcross is 0.11 ± 0.07 (Figure 3D and 3E). We examined Itgα5-Cdh2 association in trans by transplanting Cdh2-GFP expressing cells into Itgα5-RFP expressing hosts. We performed FCCS along the interface of juxtaposed Itgα5-RFP and Cdh2-GFP expressing cell membranes in the presomitic mesoderm (Figure 3D and 3E). The trans Fcross is 0.11 ± 0.08, which is significantly greater than the negative control. There is no statistically significant difference between the “cis” and “trans” Fcross values, thus the “trans” association is not the result of a much stronger cis association. The Itgα5-Cdh2 Fcross values are also not statistically different than the Itgα5-Itgα5 Fcross, but are different than the Cdh2-Cdh2 Fcross. The apparent Kds of Itgα5-Cdh2 interactions are between 400 and 650 nM (Figure S1).
We next asked whether the physical association of Itgα5 expressed on adjacent cells requires cdh2 function. Genetic mosaics of hosts expressing Itgα5-RFP and donors expressing Itgα5-GFP were created. Both donor and host embryos lacked cdh2. The Fcross between the Itgα5 was 0.11 ± 0.04, a significant reduction relative to the Fcross between Itgα5 in the presence of Cdh2 (p<0.05)(Figure 3D and 3E). Thus, Cdh2 is stabilizes the physical association of Itgα5 expressed on adjacent cell membranes.
Itgα5 within the PSM is primarily in the inactive conformation
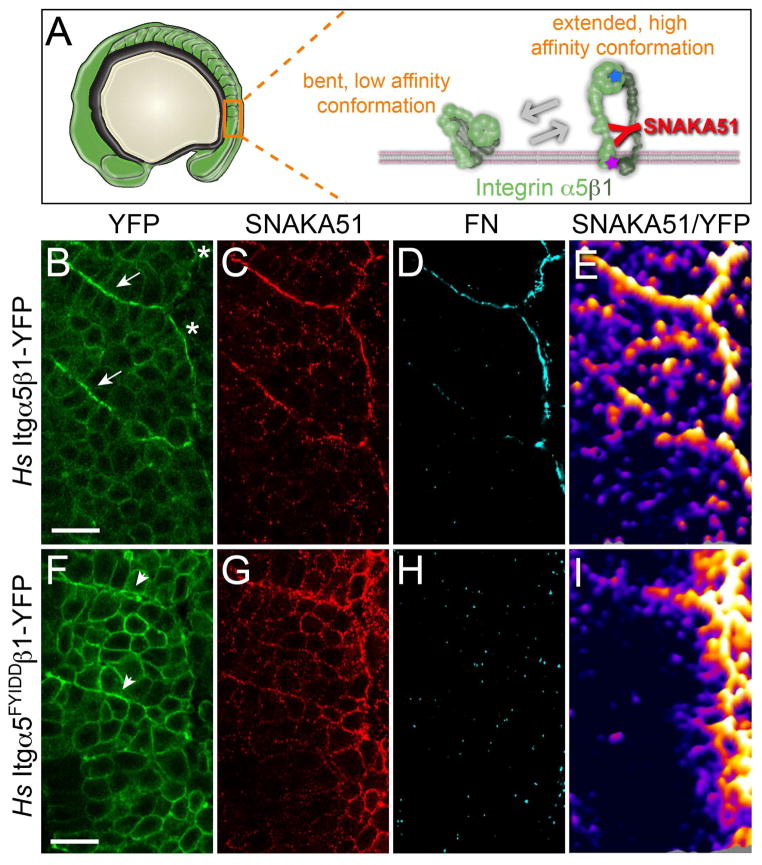
The second hypothesis stemming from our model is that Itgα5 primarily exists in the inactive conformation within the mesenchyme of the PSM. We experimentally examined Itgα5 conformation via immunohistochemistry (IHC) using a monoclonal antibody (SNAKA51) that recognizes an epitope in the calf-1/calf-2 domain of human Itgα5. This epitope is exposed in the fully extended and ligand bound Itgα5β1 heterodimer (Figure 4A) (Askari et al., 2010; Clark et al., 2005). SNAKA51 does not recognize zebrafish Itgα5. However, using Venus YFP Bimolecular Fluorescence Complementation (BiFC), we found that human Itgα5 (Hs Itgα5) heterodimerizes with zebrafish Itgβ1 (Figure 4B) and rescues somite boundary morphogenesis in zebrafish MZ itgα5−/− embryos (Jülich et al., 2009). To validate the specificity of SNAKA51 for the activated Itgα5 conformation within the zebrafish presomitic mesoderm, we confirmed that increasing actomyosin contractility elevated SNAKA51 binding (Figure S2).
Figure 4. Itgα 5 adopts the active, extended conformation independently of ligand binding.
(A) A schematic of the bent and extended Itgα5β1. The SNAKA51 antibody recognizes an epitope on the extracellular domain of Itgα5 that is exposed when the Itgα5β1 adopts the extended, active conformation (see also Figure S2). The blue star represents the five amino acid substitutions in Itgα5FYIDD that eliminate ligand binding. The magenta star represents the two amino acid substitutions in Itgα5GAAKR that promote adoption of the extending active conformation. (B–E) Confocal images of MZ itgα5−/− mutant embryos expressing Hs Itgα5β1-YFP (B) immunostained with the conformation-specific anti-Hs Itgα5 antibody SNAKA51 (C) and anti-FN antisera (D). Arrows in B indicate somite borders and the asterisks denote the lateral surface of the tissue. 6 experiments were performed with n=55 embryos analyzed. (E) To normalize the SNAKA51 signal to the level of YFP, a ratiometric topological heat map was generated. Warmer colors indicate higher relative levels of extended, active Itgα5β1. The same pattern of Itgα5 activation is observed in the absence of BiFC when using Hs Itgα5-GFP for ratio imaging (not shown). (F–I) show confocal images of a parallel analysis of MZ itgα5−/− mutant embryos expressing Hs Itgα5FYIDD ligand binding deficient Integrin. Arrowheads in (F) indicate ephemeral Itgα5FYIDDβ1 clustering. 6 experiments were performed with n=52 embryos analyzed. In all panels, anterior is up and lateral is right. Scale bars are 20 μm.
We next examined Itgα5 conformation before and after initiation of somite border morphogenesis. We fixed MZ itgα5−/− embryos expressing Hs Itgα5β1-YFP and performed IHC using SNAKA51. The IHC revealed high levels of conformationally extended Itgα5 on the surface of the tissue and along somite boundaries (Figure 4B and 4C). These sites colocalize with deposits of FN matrix (Figure 4D). Only low levels of extended Itgα5 are observed within the mesenchymal presomitic mesoderm. We normalized the SNAKA51 signal to levels of Hs Itgα5β1-YFP to identify locations where the proportion of activated Itgα5 is high (Figure 4E). The ratiometric heat maps reveal that even after normalizing for Integrin clustering, the sites of FN matrix assembly exhibit higher ratios of extended Itgα5. By contrast, most Itgα5 internally within the presomitic mesoderm is in the inactive conformation.
Itgα5 clusters and adopts the extended conformation prior to FN binding
The data suggest that within the mesenchymal PSM, Itgα5 in the inactive conformation physically associate to repress FN fibrillogenesis within the tissue. We next examined the transition from the inactive to the active conformation along nascent somite boundaries. To determine whether ligand binding is required for Itgα5 to adopt the extended conformation, we created a mutant Integrin, Hs Itgα5FYIDDβ1-YFP that is unable to bind either the RGD or synergy site of FN (Figure 4A)(Irie et al., 1995; Jülich et al., 2009; Mould et al., 2003). Unlike the wild-type Hs Itgα5β1-YFP, Hs Itgα5FYIDDβ1-YFP does not rescue somite boundary formation in MZ itgα5−/− embryos due to the inability to bind ligand. However, the mutant Integrin adopts the extended conformation as detected by SNAKA51 and clusters to nascent boundaries lacking detectable FN matrix (Figure 4F–4H). Since FN matrix is required for stabilization of the morphological boundary, both these boundaries and the Itgα5FYIDDβ1-YFP clustering are transient and disintegrate over time (Jülich et al., 2005; Jülich et al., 2009; Koshida et al., 2005). The ratiometric heat map shows that after normalizing for Integrin clustering, there is more extended Hs Itgα5FYIDDβ1-YFP along the tissue surface and transient segment boundary (Figure 4I). Thus, inside-out signaling is sufficient to elevate levels of extended Itgα5β1 in the absence of FN binding. These observations suggest that inside-out regulation of both Itgα5 conformation and Itgα5 clustering govern the pattern of FN fibrillogenesis along the nascent somite boundary.
The bent Itgα5 conformation suppresses FN matrix assembly in the PSM
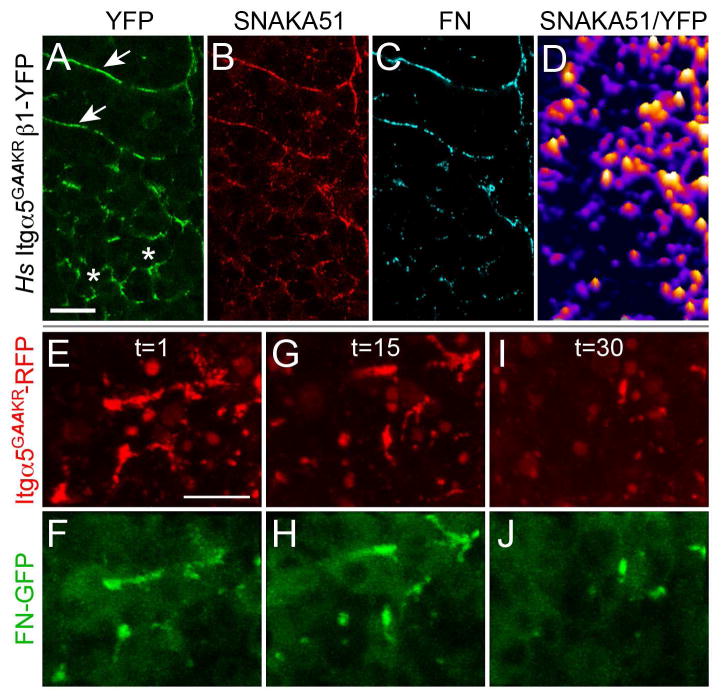
We next asked whether Itgα5 conformation is regulated to prevent ectopic FN fibrillogenesis in the PSM by using an activated Itgα5 (Hs Itgα5GAAKR). This Integrin harbors two amino acid substitutions at residues 984 and 985 (GFFKR>GAAKR) in the cytoplasmic juxtamembrane domain that destabilizes the bent, inactive conformation (Figure 4A) (O’Toole et al., 1994; Zhu et al., 2009). Expression of Hs Itgα5GAAKRβ1-YFP in MZ itgα5−/− embryos results in inappropriate clustering in the presomitic mesoderm (Figure 5A) and elevated SNAKA51 signal compared to Hs Itgα5β1-YFP and Hs Itgα5FYIDDβ1-YFP (Figure 5B and 5D). Moreover, Hs Itgα5GAAKRβ1-YFP leads to ectopic FN aggregation in the PSM (Figure 5C) and along clone boundaries in genetic mosaics (Figure S3). The activated Integrins colocalize with thickening of the actin cortex but do not appear to alter adherens junctions (Figure S3). Together, these data indicate that regulation of Itgα5 conformation suppresses FN fibrillogenesis in the mesenchyme of the PSM and promotes matrix assembly along nascent somite boundaries. On the other hand, FN fibrillogenesis is still most prominent along the somite boundaries in embryos expressing Hs Itgα5GAAKRβ1-YFP. Thus, even the activated Hs Itgα5GAAKRβ1-YFP responds to segmental signals to cluster along nascent segment boundaries and thereby bias FN fibrillogenesis to the boundary.
Figure 5. Itgα5 conformation regulates Itgα5 clustering and FN matrix assembly in the presomitic mesoderm.
Itgα5GAAKR contains two amino acid substitutions that bias towards the extended, active conformation. (A) Hs Itgα5GAAKRβ1-YFP clusters along somite boundaries (arrows) in MZ itgα5−/− embryos but also inappropriately clusters in the presomitic mesoderm (asterisks). This inappropriate clustering colocalizes with Hs Itgα5GAAKRβ1-YFP adoption of the active conformation (B and D) and FN aggregation (C). SNAKA51 and FN IHC were examined in 51 Hs Itgα5GAAKRβ1-YFP expressing MZ itgα5−/− embryos (5 experiments). (E–J) Hs Itgα5GAAKRβ1-YFP clustering and FN-GFP aggregation in the presomitic mesoderm is unstable. Time-lapses of three Tg(tbx6:fn1a-GFP) embryos co-injected with the itgα5 morpholino and itgα5GAAKR-RFP mRNA were acquired and analyzed. Three time points t=1 (E and F), t=15 (G and H) and t=30 (I and J) show the correlated changes in Itgα5GAAKR-RFP clustering FN-GFP aggregation in the anterior presomitic mesoderm. Scale bars are 20 μm. The images are from a 94 minute time-lapse, with a z-stack spanning 14 μm acquired every 4 minutes (see Movie S2).
We sought to determine how the unpatterned FN matrix induced by Hs Itgα5GAAKRβ1-YFP in the presomitic mesoderm resolves into a relatively normal segmental FN matrix (Figure 5C). For this experiment, we needed to more clearly define the temporal dynamics of FN matrix in live embryos. Therefore, we generated FN-GFP transgenic zebrafish to image matrix assembly and remodeling. A number of approaches have been used to visualize FN matrix dynamics in real time (Benazeraf et al., 2010; Davidson et al., 2004; Ohashi et al., 2002; Zamir et al., 2006; Zamir et al., 2008). We adapted a previous strategy to fuse emerald GFP in frame within the coding sequence of zebrafish FN1a (Ohashi et al., 2002) and created a stable transgenic line Tg(tbx6:fn1a-GFP). The tbx6 enhancer drives transgene expression in the paraxial mesoderm progenitors in the posterior tailbud (Dray et al., 2013; Szeto and Kimelman, 2004). While only cells in the posterior tailbud actively secrete the FN-GFP, we observe the soluble FN-GFP being incorporated into the FN matrix throughout the trunk and tail.
We co-injected the itgα5 morpholino with itgα5GAAKR-RFP mRNA into Tg(tbx6:fn1a-GFP) embryos and performed 3D time-lapse imaging (Figure 5E–5J and Movie S2). These experiments revealed the flux of this unpatterned FN matrix in the anterior PSM, in contrast to the relatively stable matrix along the somite border. Examination of the onset of outside-in signaling indicates that this transient matrix correlates with a failure to activate Focal Adhesion Kinase (FAK) (Figure S3). Therefore, while adoption of the active Itgα5 conformation causes premature aggregation of Itgα5 and FN matrix, stable FN matrix fibrillogenesis and Integrin signaling is primarily confined to the somite boundaries and tissue surface.
Cdh2 is necessary for repression of Itgα5 within the PSM mesenchyme
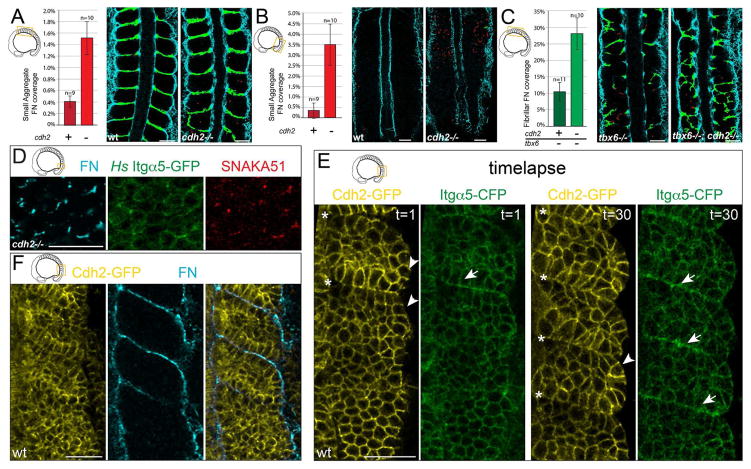
Given the association between Itgα5 and Cdh2 as well as the requirement for Cdh2 for the association of Itgα5 expressed on adjacent cells, we asked whether Cdh2 suppresses Itgα5 activation and FN matrix assembly. Indeed, cdh2−/− embryos produce small aggregates of FN in the mesenchyme of the paraxial mesoderm. We quantified FN in both cdh2−/− embryos and wild-type siblings. We divided FN matrix into two categories based on their size: large FN fibrils, similar to that found at somite boundaries and small aggregates of FN. We found a 3.7-fold increase of small aggregates of FN within the core of formed somites in cdh2−/− embryos (Figure 6A). We further found a 10.2-fold increase in small aggregates of FN within the interior mesenchyme of the presomitic mesoderm in cdh2−/− embryos (Figure 6B). These data indicate that Cdh2 inhibits ECM formation within the mesenchyme of the paraxial mesoderm.
Figure 6. Cdh2 represses Itgα5 activation.
(A) cdh2−/− embryos produce significantly more ectopic small aggregates of FN matrix (red) in the mesenchyme of both the anterior (p<0.005, t-test) and (B) the posterior paraxial mesoderm (p<0.02, t-test). (C) tbx6−/−;cdh2−/− embryos produce significantly more FN fibrils (green) in the anterior paraxial mesoderm mesenchyme than tbx6−/− controls (p<0.01, t-test). Mean values ± SEM are shown. (D) Ectopic FN assembly, Itgα5 clustering and adoption of the active Itgα5 conformation is observed in the PSM mesenchyme of cdh2 mutants, n=38 embryos. (E) The first and last timepoints of a 90 minute timelapse (1 frame every 3 min) of Cdh2-GFP and Itgα5-CFP localization during somite morphogenesis (see also Movie S3). Cdh2-GFP levels decrease at somite boundaries (asterisks) while Itgα5-CFP levels increase (arrows) due to clustering. Cdh2-GFP is also diminished along the surface of the paraxial mesoderm (arrowheads), n=4 timelapses. (F) Cdh2-GFP levels anti-correlate with site of FN assembly along somite boundaries and the tissue surface, n=8 embryos. Scale bars =40 μm.
In order to decouple ECM assembly from segmentation, we investigated the role of Cdh2 in regulating ECM formation using tbx6 mutants, which do not form somites (van Eeden et al., 1996). Compared to tbx6−/− embryos, tbx6−/−;cdh2−/− double mutants exhibit a 2.7 fold increase in large FN fibrils in the anterior paraxial mesoderm accompanied by formation of multiple irregular morphological boundaries (Figure 6C and Figure S4). These data indicate that Cdh2 suppresses ECM synthesis within the mesenchyme of the paraxial mesoderm.
We directly examined Itgα5 activation in the PSM of cdh2 mutants. Indeed, we observed irregular clusters of Itgα5-GFP that colocalize with the active conformation and FN matrix assembly (Figure 6D). Lastly, we examined localization of Cdh2 using a transgene driving fluorescently tagged Cdh2 under the control of its endogenous promoter (Revenu et al., 2014). Cdh2-GFP localization is anti-correlated with Itgα5 clustering and FN assembly along somite boundaries and the surface of the paraxial mesoderm (Figure 6E and 6F, and Movie S3). Cumulatively, the data indicate that cdh2 stabilizes the physical association of Itgα5 expressed on adjacent cells, biases Itgα5 to the inactive conformation and prevents ectopic Itgα5 clustering and FN matrix assembly within the mesenchyme of the paraxial mesoderm.
FN matrix fibrillogenesis and remodeling along the somite boundary
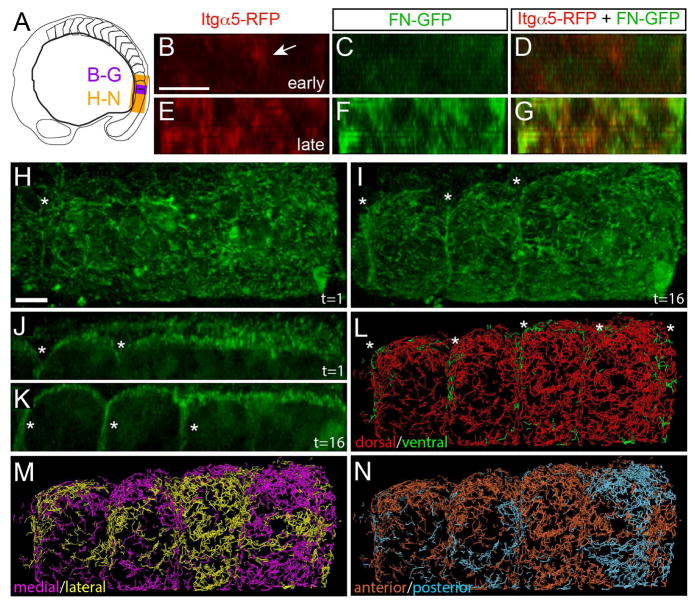
In the chick embryo, IHC studies suggest that the FN matrix along the surface of the PSM translocates to the forming somite border in a manner similar to that reported for salivary gland morphogenesis (Larsen et al., 2006; Martins et al., 2009; Rifes and Thorsteinsdottir, 2012). Our IHC studies suggested that FN matrix is assembled de novo subsequent to Itgα5 clustering during zebrafish somite morphogenesis (Figure 1D)(Jülich et al., 2009). To examine the relationship between Itgα5 clustering and FN matrix fibrillogenesis in live embryos, we expressed Itgα5-RFP in the Tg(tbx6:fn1a-GFP) transgenic line and performed 4D time-lapse confocal imaging of border formation (Figure 7A–7G and Movie S4). The time-lapses consistently show Itgα5-RFP clustering prior to the appearance of FN-GFP matrix. These PSM cells are no longer actively producing FN-GFP. Thus, this FN matrix assembly involves soluble dimers secreted by the posterior tailbud. Altogether, both FN immunolocalization and live imaging of the FN-GFP transgenic support the model that FN matrix is assembled de novo within the nascent somite boundary following Integrin clustering. We performed a second series of 3D time-lapses of the Tg(tbx6:fn1a-GFP) transgenic line to examine FN matrix dynamics on the surface of the tissue during border morphogenesis (Figure 7A, 7H–7K and Movie S5). Here, it appears that some of the FN-GFP from the dorsal surface involutes into the nascent somite boundary. To quantify FN motion, we performed particle tracking on the matrix fibrils. When the tracking data are color coded to reveal the direction of FN movement, ventral translocation of the dorsal tissue surface FN into the forming somitic cleft is evident (Figure 7L). We did not consistently observe FN flowing into the somite boundary from the medial or lateral boundary (Figure 7M). We also did not observe a regular segmental pattern of FN-GFP movement along the anterior posterior axis (Figure 7N).
Figure 7. FN matrix fibrillogenesis and remodeling at the somite boundary.
(A) A schematic indicating the regions shown in subsequent figure panels. (B–G) show digital transverse sections of a 93 minute time-lapse of a nascent somite border in an Itgα5-RFP; Tg(tbx6:fn1a-GFP) embryo with 11 μm z-stacks of 1 μm intervals acquired every 3 minutes. Early: time-point 5 (12 minutes into the movie), late: time-point 31 (90 minutes into the movie). Arrow in B indicates Itgα5-RFP clustering prior to the appearance of detectable levels of FN matrix. See also Movie S4. Eleven time-lapses of itgα5-RFP; Tg(tbx6:fn1a-GFP) embryos were acquired and analyzed. (H–N) We acquired seven time-lapses of Tg(tbx6:fn1a-GFP) embryos. Shown are images from a 3D reconstruction of a 195 minute time-lapse with 40 μm z-stacks taken every 6.5 minutes. (H and J) timepoint 1. (I and K) timepoint 16, 97 minutes into the movie. Asterisks indicate boundaries. J and K are parasagittal sections while all other panels are dorsal views. Anterior is left in all panels. See also Movie S5. (L–N) Motion of the FN matrix was examined using particle tracking. Individual tracks were color coded to indicate whether they exhibited a net displacement along the dorsal-ventral axis (L), medial-lateral axis (M) or anterior-posterior axis (N). The ventral motion displays a segmental pattern corresponding to the somite boundaries (asterisks). Scale bars are 40 μm.
Discussion
This study delineates a cross-scale mechanism linking molecular and cellular level regulation to self-organizing tissue level topology of cells and ECM. We found evidence of physical association of Itgα5 on adjacent cells 3–4 fold weaker than the association between Cadherin 2 molecules. Using a conformation specific antibody, we further found that most Itgα5 within the tissue is in the bent, inactive conformation. Cadherin 2 stabilizes the physical interaction between Itgα5 expressed on adjacent cells and is necessary to maintain repression of Itgα5 within the paraxial mesoderm mesenchyme. A series of assays using wild-type, ligand binding deficient and activated Itgα5 delineated the transition from inactive Itgα5 to activation along the nascent somite border. Upon activation, FN matrix assembled along the somite boundary integrates with involuting FN from the tissue surface to form a contiguous ECM throughout the tissue.
Integrin activation is perhaps best understood for the process of platelet adhesion which has been extensively studied in vitro (Boettiger, 2012). The zebrafish embryo does not allow the same resolution of an in vitro system. However, it provides an opportunity to study Integrin regulation and ECM assembly in a natural 3D environment, which is important given the differences in cellular mechanobiology and Integrin regulation in 3D versus 2D contexts (Baker and Chen, 2012). While there is no generally accepted mechanism to explain Integrin clustering, the most common hypothesis is that ligand binding precedes and is required for clustering (Boettiger, 2012). As there is clear data for ligand-dependent clustering of ItgαVβ3 (Cluzel et al., 2005), our observations of ligand-independent clustering and conformational activation of Itgα5β1 suggest that the clustering mechanism may vary for different Integrin heterodimers or among dissimilar cellular contexts, e.g. cells migrating over an ECM versus de novo synthesis of an ECM.
We examined the dynamics of cell adhesion and cell-ECM adhesion proteins in the mesenchymal cells of the zebrafish presomitic mesoderm. Ordered cell motion in the presomitic mesoderm requires Cadherin 2 function but not Itgα5 and ItgαV (Dray et al., 2013; Lawton et al., 2013). We measured both the diffusion coefficients and intercellular homotypic association for Cadherin 2 and Integrin α5 in the mesenchyme of the PSM. Using FCCS, we found that the Kd of intercellular homotypic binding of Cadherin 2 on adjacent cells was 200 ±100 nM, which is tighter than Kds of 80 ±20 μM and 720 μM deriving from in vitro measurements with soluble E-Cadherin ectodomains (Haussinger et al., 2004; Koch et al., 1997). The tighter in vivo Kd likely stems from the constrained anti-parallel arrangement of Cadherins in the membranes of adjacent cells. We also observed a significant, though weaker, crosscorrelation between Integrin α5β1 heterodimers expressed on the surface of adjacent cells. From these data, we calculated an apparent Kd of 750 ±100 nM. At present, we do not know whether the Integrin heterodimers directly interact or whether other proteins present in the Integrin α5β1 adhesion complex mediate the physical association. However, prior data suggest that the Integrin trans-inhibition does not require a 1:1 stoichiometry (Jülich et al., 2009). We also find that Cadherin 2 is a component of the cell surface complex that regulates ECM assembly in the paraxial mesoderm. Thus, the Integrin α5 trans-inhibition is mediated by a multi-protein adhesion complex and is not simply the result of competitive Integrin-Integrin binding versus Integrin-Fibronectin binding.
The SNAKA51 IHC data suggest that the majority of the Integrin α5β1 heterodimers within the presomitic mesoderm are in the bent conformation, and our FCCS experiments show that the physical association is not due to direct coupling of the Integrins via Fibronectin binding. Thus, the physical association may predominantly involve inactive Integrin α5, though at present, our data do not rule out the possibility of intercellular association of active Integrin α5. However, the simplest explanation of the mechanism of trans-inhibition is that Cadherin 2 promotes the physical association of Integrin α5 on adjacent cells which in turn stabilizes the inactive conformation.
Is it possible for Integrins on adjacent cells to directly interact if the cells are bound by adherens junctions? In vitro, Cadherins link lipid spheres with a gap of 23 nm membrane to membrane (Lambert et al., 2005). Structural analysis of ligand bound EphA4 suggest that plasma membranes must be ≤ 18 nm apart for Eph/Ephrin signaling complexes to form (Xu et al., 2013), and we know that Eph/Ephrin signaling takes place along nascent somite boundaries. Indeed, an extracellular gap of ~18 nm could be narrow enough to allow direct physical association of Integrins on adjacent cells. The bent Integrin conformation is 10–13 nm high while activated Integrins are ~11 to 23 nm in height according the experiments supporting the deadbolt and switchblade models, respectively (Eng et al., 2011; Ye et al., 2008). These observations suggest that the extracellular space should be narrow enough in adherent paraxial mesoderm cells to allow direct interaction of Integrins on opposing membranes.
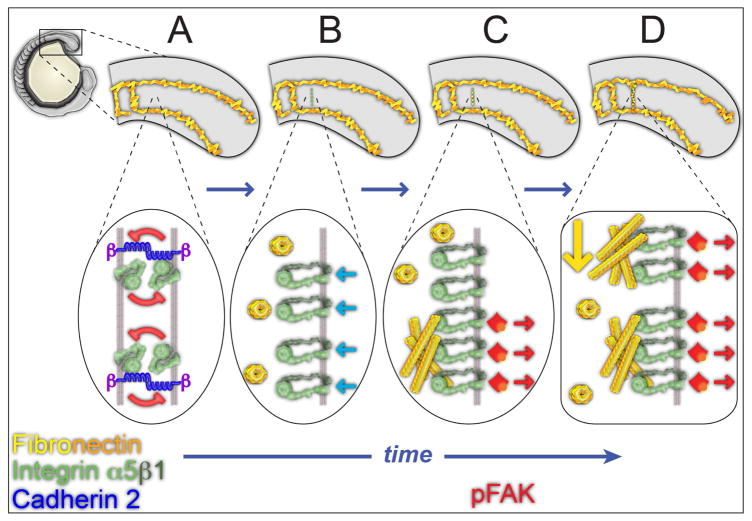
Our data suggest a model in which collective intercellular association of Cadherin 2 and Integrin α5 in the mesenchymal cells of the PSM help maintain the bent, inactive conformation thereby biasing FN matrix assembly to the tissue surface (Figure 8A). There is a low level of sporadic activated Integrin α5 within the mesenchyme. Along the tissue surface there is little Cadherin 2. Thus, activated Integrin α5 in the presomitic mesoderm is predominantly found on the tissue surface and colocalizes with the FN matrix that coats the tissue. This FN matrix pattern gives rise to the tissue mechanics of the paraxial mesoderm required for body elongation (Dray et al., 2013). Inside-out signaling at the nascent somite boundary induces Integrin α5 to adopt the extended, active conformation and to cluster along the future boundary (Figure 8B). At the same time, Cadherin 2 levels decrease along the nascent boundary. Following clustering, we observe accumulation of both FN and phosphorylated FAK (Y397), the latter indicating the onset of outside-in Integrin signaling (Figure 8C). Using both immunohistochemistry and FN-GFP transgenic zebrafish, we find that de novo assembly of FN matrix occurs within the somite boundary. These events initiate a positive feedback loop that stabilizes the FN matrix and the segment boundary. The newly synthesized matrix along the nascent boundary fuses with FN matrix that involutes from the dorsal tissue surface during somite epithelialization to form a contiguous matrix between the somite boundary and tissue cortex (Figure 8D). This bimodal mechanism of ECM remodeling may function in other contexts where a patchy or damaged matrix gives rise to a dense contiguous ECM. Altogether, these data delineate a cross-scale mechanism of Integrin activation and ECM fibrillogenesis during in vivo tissue morphogenesis.
Figure 8. From collective Integrin repression to Integrin activation and Fibronectin matrix fibrillogenesis.
(A–D) represent successive time points during somite border formation. Top images show tissue level changes in Fibronectin matrix while lower images show the underlying molecular processes at the cell membrane. (A) Within the unsegmented PSM, Integrin α5β1 is in the bent conformation and repression is maintained by physical interaction between Integrins on adjacent cells. Cdh2 promotes the association of Itgα5 on adjacent cell membranes and stabilizes the inactive Itgα5 conformation. Fibronectin dimers remain in soluble form in the extracellular space. This collective repression prevents FN fibrillogenesis internally within the tissue biasing fibrillogenesis to the tissue surface where collective repression is absent. (B) Cytoplasmic signals downstream of EphA4/Ephrinb2a, (light blue arrows) activate the Integrin heterodimer at the nascent somite border by inducing the extended conformation as well as Integrin clustering. Cdh2 levels diminish at the nascent boundary. (C) Activated Integrins bind soluble Fibronectin and assemble the dimers into an insoluble matrix. The Fibronectin matrix stabilizes Integrin clustering and induces Integrin signaling via Focal Adhesion Kinase (red). (D) Fibronectin matrix from the dorsal surface of the paraxial mesoderm involutes (yellow arrow) along the nascent border where it is integrated with the newly synthesized matrix to form a contiguous ECM between the tissue surface and the somite border.
Materials and Methods
Zebrafish care and strains
Zebrafish were maintained in accordance with standard protocols approved by Yale University IACUC. Wild-type strains used are Tübingen and AB. The Itgα5 mutant allele used is bfethl30 (Jülich et al., 2005). The Tg(tbx6:fn1a-emeraldGFP) strain was generated using a modified published strategy for GFP-tagging Fibronectin (Ohashi et al., 2002). The Cadherin 2 transgenic was kindly provided by Darren Gilmour (Revenu et al., 2014). MZitgα5−/−;cdh2mo embryos were created by injecting 50μM cdh2mo (Lele et al., 2002; Warga and Kane, 2007) into MZitgα5−/−embryos. cdh2−/−, tbx6−/−, tbx6−/−;cdh2−/− and wild-type controls were generated by incrossing tbx6+/−;cdh2+/− parents and sorting progeny by phenotype.
in vitro mRNA synthesis and cell transplantations
For mRNA synthesis, plasmids were linearized with NotI, the mRNA in vitro transcribed with the Sp6 mMessage mMachine kit (Ambion), and cleaned with the RNaid kit (MPbio). With the exception of caMRLC2, mRNA was injected into one-cell stage embryos. caMRLC2 mRNA was injected into 1 to 2 cells at the 16 to 32 cell stage to create mosaic expression. Mosaic embryos were generated using standard methods.
Confocal Microscopy and Immunohistochemistry
Acquisition of time-lapses and images of immunohistochemically stained embryos were performed on a Zeiss LSM510. The primary mouse monoclonal antibody SNAKA51 (a gift from Martin Humphries)(Clark et al., 2005) was diluted 1:50 and rabbit anti-Fibronectin (Sigma F3648) used at 1:100. The secondary antibodies (Alexa fluor 555 donkey anti-rabbit IgG, Alexa Fluor 647 goat anti-mouse IgG, Molecular Probes/Invitrogen) were diluted 1:200. The primary antibody (rabbit anti-FAK pY397 phosphospecific antibody, Invitrogen) was diluted 1:100.
For the SNAKA51/YFP ratiometric analysis, a Matlab routine, written by Oleksii Sliusarenko, was used to apply a Gaussian filter and then the SNAKA51 levels were divided by total YFP levels. A mask was used to exclude points were YFP levels were 0 and the topological heat map was generated by ImageJ.
FN immunolocalization was quantified using an ImageJ macro which removed noise, converted the micrograph into a binary image, and divided the signal into small FN aggregates (5–30 pixels) or FN fibrils (>31 pixels) objects. Data are presented as means ± SEM and analyzed via t-test.
FN matrix tracking was performed using Imaris software (Bitplane). Briefly, the points of interest with a radius of 1.5μm and were tracked using the connected components algorithm. Tracks with durations of at least 900 seconds were analyzed. The tracks were segregated based on displacement in the x-, y- and z-planes.
FCS and FCCS
All measurements were made on a custom-built instrument based on an inverted Olympus IX-71 microscope, as described previously (LaRochelle et al., 2015; Trexler and Rhoades, 2009). For FCS measurements, the signal from the APD was hardware correlated (Flex03LQ-12, Correlator.com). Autocorrelation curves were fit to an equation modeling both 2D diffusion and fluorophore blinking (Schwille and Haustein, 2009). FCCS curves were fitted with a diffusion-only model. Diffusion times and diffusion coefficients are reported ±SD. We report normalized cross-correlation values as Fcross, the fraction of molecules crosscorrelating ±SD (Triffo et al., 2012). Differences in Fcross significance were examined using both t-test (two tailed, unequal variance) and Mann-Whitney U. Calculation of diffusion times, diffusion coefficients, Fcross and fitting are detailed in the Supplemental Methods.
Detailed methods are provided in supplemental material.
Supplementary Material
Highlights.
In vivo FCCS reveals a physical association between Integrin α5 on adjacent cells.
This physical association represses integrin activity in the tissue mesenchyme.
Cadherin 2 stabilizes the integrin association and inactive integrin conformation.
This regulation biases extracellular matrix formation to tissue boundaries.
Acknowledgments
We thank Tomoo Ohashi for advice, Joe Wolenski for microscopy support, Nicolas Dray for help with the image data analysis and Abeer Obaid for comments on the manuscript. Support provided by an ACS Research Scholar Award, NSF IOS-1051839 and NIH R01GM107385A-01A1 to SAH, funding from the Beverly and Raymond Sackler Institute to ER and NIH Predoctoral Training Grants T32GM067543 to GC, T32GM007223 to PM and T32HD07180-29 to AKL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askari JA, Tynan CJ, Webb SE, Martin-Fernandez ML, Ballestrem C, Humphries MJ. Focal adhesions are sites of integrin extension. J Cell Biol. 2010;188:891–903. doi: 10.1083/jcb.200907174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Chen CS. Deconstructing the third dimension - how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D. Mechanical control of integrin-mediated adhesion and signaling. Curr Opin Cell Biol. 2012 doi: 10.1016/j.ceb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Canonici A, Steelant W, Rigot V, Khomitch-Baud A, Boutaghou-Cherid H, Bruyneel E, Van Roy F, Garrouste F, Pommier G, Andre F. Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. International journal of cancer Journal international du cancer. 2008;122:572–582. doi: 10.1002/ijc.23164. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Wang Z, Ashman LK, Brady-Kalnay SM, Kreidberg JA. alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J Cell Biol. 2003;163:1351–1362. doi: 10.1083/jcb.200306067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Pankov R, Travis MA, Askari JA, Mould AP, Craig SE, Newham P, Yamada KM, Humphries MJ. A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J Cell Sci. 2005;118:291–300. doi: 10.1242/jcs.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J Cell Biol. 2005;171:383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–3081. doi: 10.1091/mbc.E02-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Kimmel CB. An Integrin-Dependent Role of Pouch Endoderm in Hyoid Cartilage Development. PLoS Biol. 2004;2:E244. doi: 10.1371/journal.pbio.0020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Keller R, DeSimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev Dyn. 2004;231:888–895. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- Dray N, Lawton AK, Nandi A, Jülich D, Emonet T, Holley SA. Cell-Fibronectin interactions propel vertebrate trunk elongation via tissue mechanics. Curr Biol. 2013;23:1335–1341. doi: 10.1016/j.cub.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband JL, Nuckolls GH, Ishihara A, Hasegawa T, Yamada KM, Thiery JP, Jacobson K. Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988;107:1385–1396. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin L, Sordino P, Barrios A, Gering M, Thisse C, Thisse B, Brennan C, Green A, Wilson S, Holder N. Anteriorposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development. 2000;127:1703–1713. doi: 10.1242/dev.127.8.1703. [DOI] [PubMed] [Google Scholar]
- Eng ET, Smagghe BJ, Walz T, Springer TA. Intact alphaIIbbeta3 integrin is extended after activation as measured by solution X-ray scattering and electron microscopy. J Biol Chem. 2011;286:35218–35226. doi: 10.1074/jbc.M111.275107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207:145–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Harrington MJ, Hong E, Fasanmi O, Brewster R. Cadherin-mediated adhesion regulates posterior body formation. BMC Dev Biol. 2007;7:130. doi: 10.1186/1471-213X-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussinger D, Ahrens T, Aberle T, Engel J, Stetefeld J, Grzesiek S. Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography. Embo J. 2004;23:1699–1708. doi: 10.1038/sj.emboj.7600192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Radice G, Takeichi M, Chisaka O. Adhesive subdivisions intrinsic to the epithelial somites. Dev Biol. 1999;215:182–189. doi: 10.1006/dbio.1999.9463. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Irie A, Kamata T, Puzon-McLaughlin W, Takada Y. Critical amino acid residues for ligand binding are clustered in a predicted beta-turn of the third N-terminal repeat in the integrin alpha 4 and alpha 5 subunits. Embo J. 1995;14:5550–5556. doi: 10.1002/j.1460-2075.1995.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülich D, Geisler R, Holley SA Consortium, T.S. Integrina5 and Delta/Notch Signalling have Complementary Spatiotemporal Requirements during Zebrafish Somitogenesis. Dev Cell. 2005:575–586. doi: 10.1016/j.devcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Jülich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- Koch AW, Pokutta S, Lustig A, Engel J. Calcium binding and homoassociation of E-cadherin domains. Biochemistry. 1997;36:7697–7705. doi: 10.1021/bi9705624. [DOI] [PubMed] [Google Scholar]
- Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S. Integrinalpha5-Dependent Fibronectin Accumulation for Maintenance of Somite Boundaries in Zebrafish Embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kragtorp KA, Miller JR. Integrin alpha5 is required for somite rotation and boundary formation in Xenopus. Dev Dyn. 2007;236:2713–2720. doi: 10.1002/dvdy.21280. [DOI] [PubMed] [Google Scholar]
- Lackner S, Schwendinger-Schreck J, Jülich D, Holley SA. Segmental assembly of Fibronectin matrix requires rap1b and integrin α5. Dev Dyn. 2013;242:122–131. doi: 10.1002/dvdy.23909. [DOI] [PubMed] [Google Scholar]
- Lambert O, Taveau JC, Him JL, Al Kurdi R, Gulino-Debrac D, Brisson A. The basic framework of VE-cadherin junctions revealed by cryo-EM. Journal of molecular biology. 2005;346:1193–1196. doi: 10.1016/j.jmb.2004.12.053. [DOI] [PubMed] [Google Scholar]
- LaRochelle JR, Cobb GB, Steinauer A, Rhoades E, Schepartz A. Fluorescence correlation spectroscopy reveals highly efficient cytosolic delivery of certain penta-arg proteins and stapled peptides. J Am Chem Soc. 2015;137:2536–2541. doi: 10.1021/ja510391n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- Latimer A, Jessen JR. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 2010;29:89–96. doi: 10.1016/j.matbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Lawton AK, Nandi A, Stulberg MJ, Dray N, Sneddon MW, Pontius W, Emonet T, Holley SA. Regulated tissue fluidity steers zebrafish body elongation. Development. 2013;140:573–582. doi: 10.1242/dev.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort CT, Wojciechowski K, Hocking DC. N-cadherin cell-cell adhesion complexes are regulated by fibronectin matrix assembly. J Biol Chem. 2011;286:3149–3160. doi: 10.1074/jbc.M110.115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele Z, Folchert A, Concha M, Rauch GJ, Geisler R, Rosa F, Wilson SW, Hammerschmidt M, Bally-Cuif L. parachute/n-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development. 2002;129:3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins GG, Rifes P, Amandio R, Rodrigues G, Palmeirim I, Thorsteinsdottir S. Dynamic 3D cell rearrangements guided by a fibronectin matrix underlie somitogenesis. PLoS One. 2009;4:e7429. doi: 10.1371/journal.pone.0007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould AP, Symonds EJ, Buckley PA, Grossmann JG, McEwan PA, Barton SJ, Askari JA, Craig SE, Bella J, Humphries MJ. Structure of an integrin-ligand complex deduced from solution x-ray scattering and site-directed mutagenesis. J Biol Chem. 2003;278:39993–39999. doi: 10.1074/jbc.M304627200. [DOI] [PubMed] [Google Scholar]
- O’Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, Erickson HP. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J Cell Sci. 2002;115:1221–1229. doi: 10.1242/jcs.115.6.1221. [DOI] [PubMed] [Google Scholar]
- Revenu C, Streichan S, Dona E, Lecaudey V, Hufnagel L, Gilmour D. Quantitative cell polarity imaging defines leader-to-follower transitions during collective migration and the key role of microtubule-dependent adherens junction formation. Development. 2014;141:1282–1291. doi: 10.1242/dev.101675. [DOI] [PubMed] [Google Scholar]
- Ries J, Yu SR, Burkhardt M, Brand M, Schwille P. Modular scanning FCS quantifies receptor-ligand interactions in living multicellular organisms. Nat Methods. 2009;6:643–645. doi: 10.1038/nmeth.1355. [DOI] [PubMed] [Google Scholar]
- Rifes P, Carvalho L, Lopes C, Andrade RP, Rodrigues G, Palmeirim I, Thorsteinsdottir S. Redefining the role of ectoderm in somitogenesis: a player in the formation of the fibronectin matrix of presomitic mesoderm. Development. 2007;134:3155–3165. doi: 10.1242/dev.003665. [DOI] [PubMed] [Google Scholar]
- Rifes P, Thorsteinsdottir S. Extracellular matrix assembly and 3D organization during paraxial mesoderm development in the chick embryo. Dev Biol. 2012;368:370–381. doi: 10.1016/j.ydbio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Koopmans WJ, Meckel T, van Noort J, Snaar-Jagalska BE, Schmidt TS, Spaink HP. Single-molecule microscopy reveals membrane microdomain organization of cells in a living vertebrate. Biophys J. 2009;97:1206–1214. doi: 10.1016/j.bpj.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwille P, Haustein E. Fluorescence Correlation Spectroscopy: An Introduction to Its Concepts and Applications. Spectroscopy. 2009:1–33. [Google Scholar]
- Shi X, Teo LS, Pan X, Chong SW, Kraut R, Korzh V, Wohland T. Probing events with single molecule sensitivity in zebrafish and Drosophila embryos by fluorescence correlation spectroscopy. Dev Dyn. 2009;238:3156–3167. doi: 10.1002/dvdy.22140. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Kimelman D. Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development. 2004;131:3751–3760. doi: 10.1242/dev.01236. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, et al. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol. 2007;178:167–178. doi: 10.1083/jcb.200703021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler AJ, Rhoades E. Alpha-synuclein binds large unilamellar vesicles as an extended helix. Biochemistry. 2009;48:2304–2306. doi: 10.1021/bi900114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triffo SB, Huang HH, Smith AW, Chou ET, Groves JT. Monitoring lipid anchor organization in cell membranes by PIE-FCCS. J Am Chem Soc. 2012;134:10833–10842. doi: 10.1021/ja300374c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- van Eeden FJM, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, et al. Mutations affecting somite formation and patterning in the zebrafish Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- Warga RM, Kane DA. A role for N-cadherin in mesodermal morphogenesis during gastrulation. Dev Biol. 2007;310:211–225. doi: 10.1016/j.ydbio.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Tzvetkova-Robev D, Xu Y, Goldgur Y, Chan YP, Himanen JP, Nikolov DB. Insights into Eph receptor tyrosine kinase activation from crystal structures of the EphA4 ectodomain and its complex with ephrin-A5. Proc Natl Acad Sci U S A. 2013;110:14634–14639. doi: 10.1073/pnas.1311000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol. 1999;215:264–277. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- Ye F, Liu J, Winkler H, Taylor KA. Integrin alpha IIb beta 3 in a membrane environment remains the same height after Mn2+ activation when observed by cryoelectron tomography. Journal of molecular biology. 2008;378:976–986. doi: 10.1016/j.jmb.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SR, Burkhardt M, Nowak M, Ries J, Petrasek Z, Scholpp S, Schwille P, Brand M. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature. 2009;461:533–536. doi: 10.1038/nature08391. [DOI] [PubMed] [Google Scholar]
- Zamir EA, Czirok A, Cui C, Little CD, Rongish BJ. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc Natl Acad Sci U S A. 2006;103:19806–19811. doi: 10.1073/pnas.0606100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir EA, Rongish BJ, Little CD. The ECM moves during primitive streak formation--computation of ECM versus cellular motion. PLoS Biol. 2008;6:e247. doi: 10.1371/journal.pbio.0060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo BH, Barth P, Schonbrun J, Baker D, Springer TA. The structure of a receptor with two associating transmembrane domains on the cell surface: integrin alphaIIbbeta3. Mol Cell. 2009;34:234–249. doi: 10.1016/j.molcel.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.