Abstract
A novel, reusable, cofactor-free, and mediator-free human liver microsomal bioreactor constructed on carbon nanostructure electrodes for stereoselective green syntheses of drug metabolites and specialty chemicals is reported here for the first time. Drug metabolites are useful for examining pharmaceutical and pharmacological properties of new drugs in development.
Human liver microsomes (HLM) are subcellular fractions containing the major drug metabolizing cytochrome P450 (CYP) enzymes and their redox partner protein CYP-NADPH reductase (CPR).1 Due to the broad range of biocatalytic reactions catalyzed by CYP enzymes (57 isoforms known in the liver) with inherent stereoselectivity, chemists have focused on the structure-function, biosensing, and catalytic applications of this unique class of enzymes.2-7 For new drug development, HLM are being used as in vitro systems to study drug metabolism, inhibition, and drug-drug interactions and to identify the specific isoforms of CYPs involved in these processes.8 These in vitro assays use NADPH as the electron source. The electrons derived from NADPH are mediated by CPR via its flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) cofactors to reduce CYP enzymes in their heme iron-FeIII state to heme iron-FeII, which facilitates dioxygen binding. Following the second electron reduction from CPR, the strong oxidant formed (i.e., the ferryloxy-CYP cation radical) can oxygenate bound drugs.1 Herein we describe the first liver microsomal electrocatalysis achieved on carbon nanostructure electrodes to convert a drug into its metabolite at enhanced yields.
The efficacy and pharmacokinetic properties of a drug depend on the biological activity of the metabolites formed in the liver and other organs mainly via CYP-catalyzed drug metabolism.9 Formation of reactive metabolites from a drug can cause hepato-toxicity by damaging DNA and other cellular protein-protein interactions. Hence, it is imperative to study the physicochemical and toxicity properties of new drugs for which sufficient drug metabolites are required.10,11 This report is significant and novel, as it demonstrates that the biocatalytic reactions of liver microsomes immobilized on high surface area nanostructure electrodes will allow design of viable bioreactors for drug metabolite synthesis requiring only a small amount of microsomes.
Distinguished prior contributions by Arnold and co-workers include bioengineering of CYP enzymes to favorably tune the catalytic specificity and activity towards converting a desired substrate into products.12 Rusling et al. pioneered the CYP direct electrochemistry and electrocatalysis in films of polyions and surfactants. They additionally reported layer-by-layer films of genetically engineered specific CYP with CPR or rat liver microsomes or HLM assembled with polyions for direct electrochemistry and chemical toxicity evaluations.13 Gilardi et al. engineered CYP-fused CPR proteins to enhance catalytic activity by controlling the lifetime of the active ferryloxy oxidant form of CYP.14 Mie et al. designed thiolated gold electrodes with hydrophobic units to immobilize supersomes and demonstrated electrocatalytic properties.15
Recently, our laboratory examined the influences of various carbon electrode materials in the direct electron transfer and electrocatalytic properties of immobilized HLM.16 However, achieving highly enhanced electrocatalytic metabolite production from simple adsorption of complex HLM directly onto ‘3D’ carbon nanostructures with sufficient electrocatalytic stability and reusability features has not been reported before. This novel, in vivo mimic biocatalytic system has the potential to realize the development of practically useful green bioreactors for stereoselective metabolite production to assess physicochemical, toxicological, and biochemical properties of new drugs in development.
Specifically, we have discovered that HLM can be adsorbed onto multiwalled carbon nanotubes (MWNT) coated on edge plane pyrolytic graphite electrodes (PGEs) in bioactive form to offer enhanced production of drug metabolites by direct electrocatalysis. This finding simplifies the design of drug metabolizing CYP enzyme bioreactors because it eliminates the need for tedious, expensive, and time-consuming purification of CYP enzymes, and additionally allows identification of a specific liver CYP isoform involved in the metabolism of new drugs. Uniquely, we show that the designed HLM bioreactor on PGE/MWNT is reusable for metabolite generation with good stability and does not require expensive cofactors and electron transfer mediators. Scheme 1 illustrates the electrocatalysis by HLM bound to PGE/MWNT designed in this study for the first time.
Scheme 1.
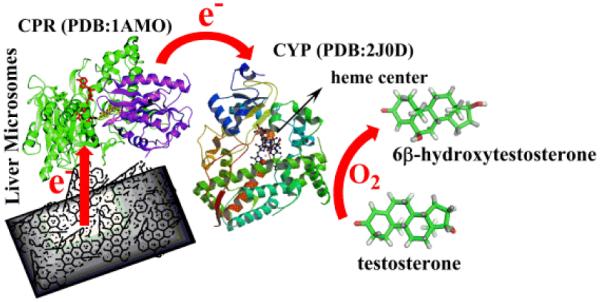
Electrocatalysis by liver microsomes bound to carbon nanostructures.
Briefly, 10 μL of 1 mg mL−1 MWNT dispersion in dimethyl formamide (obtained by 4 h ultrasonication in a water bath) were dry coated on PGEs (geometric area 0.2 cm2).17 Next, 20 μL of HLM (Xenotech LLC, Lenexa, KS) were placed on the PGE/MWNT surface and adsorbed for 30 minutes at 4 °C. The electrodes were rinsed in water and stored overnight at 4 °C before being used in the electrochemical and electrocatalytic experiments. Overnight storage provided better film stability than electrodes that were used immediately after adsorbing HLM.
The surface morphologies of PGEs, PGE/MWNT electrodes, and HLM-coated PGE/MWNT electrodes were characterized by scanning electron microscopy (SEM, Figure S1). The characteristic surface defects of PGEs were covered by the MWNT, and subsequent physical adsorption of HLM led to the formation of a newly coated layer around the MWNT. This finding confirmed the immobilization of HLM on the PGE/MWNT surface (Figures S1 A-C). The cyclic voltammograms of the HLM adsorbed onto PGE/MWNT electrodes displayed a redox pair at a formal potential of −0.46 V vs. Ag/AgCl (Figure 1), which is in agreement with the formal potential of only microsomal CPR film adsorbed on similar electrodes (Figure S2) and with results of other studies.13,18 No redox peaks at this potential region were observed for control electrodes of PGE adsorbed with a phospholipid (PL) layer or PGE/MWNT in the absence of HLM (Figure S3a,b). Thus, the cyclic voltammetry results confirmed the existence of direct electronic communication between HLM and MWNT-modified electrodes.
Figure 1.
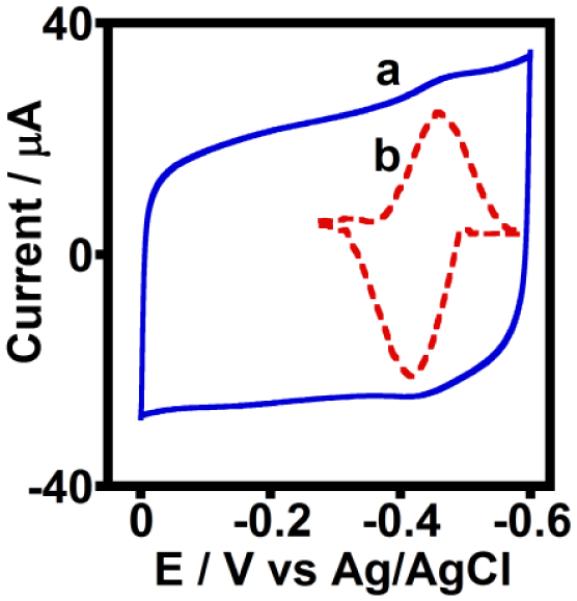
Cyclic voltammograms of (a) unsubtracted and (b) background subtracted PGE/MWNT/HLM electrodes in argon atmosphere, phosphate buffer (pH 7.0) at 25 °C, scan rate 0.3 V s−1.
Figure S4 shows the cyclic voltammogram of a HLM film directly adsorbed onto a PGE in the absence of MWNT. The electroactive charge was calculated to be 340 ± 30 nC for microsomal proteins on PGE/MWNT/HLM electrodes, which was comparable to that observed for PGE/HLM films in the absence of coated MWNT (400 ± 45 nC). In contrast, the electrocatalytic oxygen reduction currents catalyzed by the microsomal heme proteins on PGE/MWNT/HLM electrodes was about two times greater than that of the PGE/HLM film (Figure 2a,b). Control PGE/PL and PGE/MWNT electrodes in the absence of adsorbed HLM showed small reduction currents arising from the electrocatalytic property of the edge planes of pyrolytic graphite and those present in MWNT (Figure 2c,d).19
Figure 2.
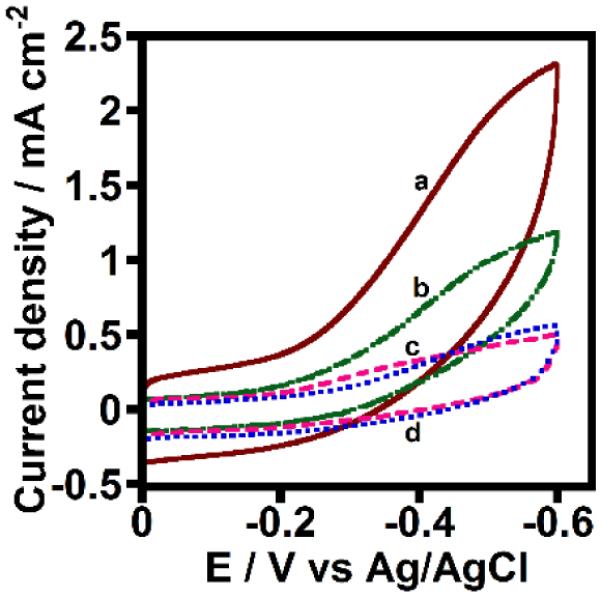
Rotating disc catalytic oxygen reduction voltammograms of (a) PGE/MWNT/HLM, (b) PGE/HLM, (c) PGE/MWNT, and (d) PGE/PL films in saturated oxygen, phosphate buffer (pH 7.0) at 25 °C, 300 rpm rotation rate, scan rate 0.3 V s−1.
The electroactive coverage infers that compared to the enhancement factor in catalytic currents for the PGE/MWNT/HLM over the PGE/HLM electrodes, the direct electron transfer (ET) currents are not proportionately increased in PGE/MWNT/HLM. This could be due to the larger capacitive currents of MWNT-modified electrodes compared to bare PGEs (Figure S3), which could act as a barrier to observing unhindered non-turnover currents from the PGE/MWNT/HLM electrodes in argon atmosphere. This finding also emphasizes the high enzyme turnover rates in the presence of a substrate, and it shows that the associated large catalytic currents can overcome the charging current effect of MWNT-coated electrodes (Figure 2a). In addition to the enhanced oxygen reduction currents, the PGE/MWNT/HLM electrodes exhibited greater electrode-driven bioactivity in converting testosterone to 6#x03B2;-hydroxytestosterone, which is a characteristic of CYP enzymes present in HLM as explained below.
The biocatalytic properties of microsomal enzymes on PGE/MWNT/HLM and PGE/HLM electrodes were studied by bulk electrolysis at an applied potential of −0.6 V vs. Ag/AgCl in the presence of oxygen in phosphate buffer (pH 7.0) containing dissolved testosterone (250 μM). The reaction mixture was analyzed by high performance liquid chromatography. The identification of the reactant and product in the chromatograms was accomplished by running standard solutions of testosterone and 6β-hydroxytestosterone under similar conditions (Figure S5). CYP 2C19, 2C9, and 3A4 present in HLM have been shown to hydroxylate testosterone.20
Figure 3A shows the chromatograms of 6β-hydroxytestosterone product formation from testosterone conversion electrocatalyzed by PGE/MWNT/HLM (curve a) and PGE/HLM electrodes (curve e). The absence of product formation by the control PGE/PL and PGE/MWNT electrodes (Figure S6) clearly confirms the role of CYP enzymes present in HLM in catalyzing testosterone conversion in the PGE/MWNT/HLM and PGE/HLM electrodes. The reusability of PGE/MWNT/HLM electrodes was examined by replenishing fresh testosterone solutions following each electrolysis and by repeating the reaction under an applied potential of −0.6 V vs. Ag/AgCl (curves b-d).
Figure 3.
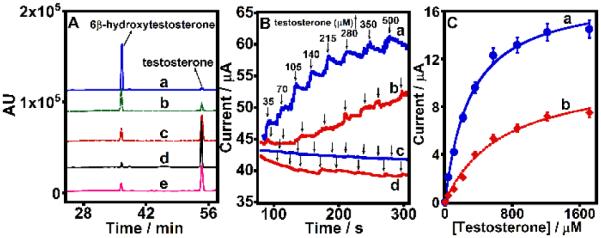
(A) HPLC chromatograms of 250 μM testosterone in phosphate buffer (pH 7.0) after every 1 h bulk electrolysis of (a-d) PGE/MWNT/HLM electrodes or (e) PGE/HLM electrodes at −0.6 V vs. Ag/AgCl under saturated oxygen conditon at 25 °C. For b-d, fresh testosterone solution was supplied following the removal of each prior electrolysis reaction mixture to examine the reusability of PGW/MWNT/HLM electrodes. (B) Amperometric evidence of testosterone electrocatalysis by (a) PGE/MWNT/HLM and (b) PGE/HLM films at −0.6 V vs. Ag/AgCl, 25 °C, pH 7.0 buffer, 150 rpm electrode rotation rate, atmospheric air condition. Amperometric curves for control electrodes (c) PGE/MWNT and (d) PGE/PL in the absence of HLM under similar conditions. (C) Electrochemical Michaelis-Menten kinetics of testosterone hydroxylation by (a) PGE/MWNT/HLM and (b) PGE/HLM films.
Figure 3B shows the increase in currents with increasing testosterone concentration monitored by chronoamperometry at an applied constant potential of −0.6 V vs. Ag/AgCl under atmospheric air condition at 25 °C for PGE/MWNT/HLM and PGE/HLM electrodes (a and b, respectively). Control electrodes of only PGE/MWNT and PGE/PL in the absence of HLM did not show any increase in currents as shown in Figure 3B(c and d) suggesting the role of HLM in testosterone catalytic currents. We found that the large background currents from saturated oxygen condition interfered with the detection of current signals from added testosterone electrolysis in chronoamperometry. Hence, the use of atmospheric air (~ 21 % oxygen) was identified to offer small, but constant baseline currents and allowed monitoring subsequent increases in currents with testosterone concentration (Figure 3B). Electrochemical Michaelis-Menten fitting of the testosterone catalytic currents offered apparent Michaelis-Menten affinity constants (KMapp) of 290 ± 33 μM for PGE/MWNT/HLM and 480 ± 51 μM PGE/HLM electrodes towards testerone substrate (Figure 3C a and b).13a The lower KMapp of PGE/MWNT/HLM indicates the stronger affinity of HLM adsorbed to MWNT for testosterone.
Results suggest that the testosterone hydroxylation by microsomal CYP enzymes in HLM may involve electron mediation by reductases from the electrode to CYPs heme centers.13,15 By obtaining the calibration plot of standard 6β-hydroxytestosterone (Figure S7), we were able to determine that 2.2 ± 0.3 and 0.45 ± 0.06 nmol of metabolite were formed by the PGE/MWNT/HLM and PGE/HLM electrodes, respectively, per unit PGE geometric area (in cm2). This suggests a 5-fold enhancement in the amount of metabolite produced by HLM immobilized on MWNT-modified electrodes. Figure 3A(b) shows that 45–50 % of the initial product yield was obtained upon reuse of the PGE/MWNT/HLM electrodes. Subsequent reuses of the electrodes decreased the amount of metabolite formation to 20–25% [2nd reuse, Figure 3A(c)] and 10% [3rd reuse, Figure 3A(d)]. Each reaction was carried out for duplicate measurements, and the reproducibility of product levels was good (standard deviation was within 15%).
Figure S8 presents the film stability of HLM coated on the catalytically superior PGE/MWNT electrode in the presence of oxygen examined by chronoamperometry at an applied potential of −0.6 V vs. Ag/AgCl in phosphate buffer (pH 7.0). The half-life of the PGE/MWNT/HLM electrode was found to be 10 h. Thus, the stability of the HLM film on PGE/MWNT electrodes did not appear to affect the catalytic yields significantly with the number of reuses. This is because within 4 h, i.e., the time sufficient to complete the biocatalysis with the reuse of electrodes for three times (Figure 3A), only about 20% loss in catalytic current was noted (Figure S8).
Using peroxide assay strips (Millipore Inc., MA, USA), we found that ~ 3 μmol of H2O2 were formed in the electrolytic reaction after 1 h electrolysis of PGE/MWNT/HLM electrodes in the presence of oxygen and testosterone. On the other hand, no detectable H2O2 amount was formed in the absence of HLM on PGE/MWNT electrodes under similar electrolysis conditions, suggesting the catalytic role of microsomal heme proteins in forming colorimetrically measurable peroxide levels from oxygen reduction (Figure 2).13 We suggest the possible role of H2O2 towards microsomal lipid peroxidation that could cause a detrimental effect on the organization of membrane-bound microsomal enzyme films and associated electrocatalytic bioactivity.21,22 Thus, the observed decrease in product yield with number of reuses of the PGE/MWNT/HLM electrodes was likely due to the extensive lipid peroxidation by repeated H2O2 stress caused by the electrolysis process. Detailed investigation is required to understand this presumed possibility.
Designing stable electrocatalytic enzyme films is a huge challenge, and we expected that doing so using complex liver microsomes on nanostructure electrodes would be even more challenging. Despite this presumption, we observed reasonably good stability for HLM films on PGE/MWNT electrodes. This suggests the favorable secondary interactions of microsomal membranes with predominantly hydrophobic MWNT to retain the intact membrane-bound protein structures with drug metabolizing bioactivity as uniquely demonstrated in this report. The favorable interaction of phospholipid membranes of HLM with MWNT appears to be also reflected in the lower KMapp of PGE/MWNT/HLM than PGE/HLM for testosterone substrate. Moreover, the larger oxygen reduction currents observed in MWNT/HLM over the unmodified PGE/HLM electrode suggests the availability of higher proportion of ferryloxy states of cyt P450 enzymes in MWNT/HLM to readily bind smaller concentration of testosterone and thus offer greater affinity and lower KM (Figure 3C).
In summary, we demonstrated a novel electrochemical liver microsomal bioreactor on carbon nanostructure electrodes for the first time. This system offers direct bioelectronic communication between the microsomal proteins and ‘3D’ MWNT modified electrodes, direct electrocatalysis, sufficient catalytic stability, enhanced product yields, and reusability. Our approach provides a new direction in the design of practically viable enzyme bioreactors that do not require purified enzymes and that can be used for green chemical synthesis and biosensing applications.
Supplementary Material
Acknowledgements
Research reported in this publication was partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R15DK103386. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are also grateful for the start-up funds by Oklahoma State University (OSU) and partial financial support by the OSU-Technology Development Center.
Footnotes
Electronic Supplementary Information (ESI) available: Detailed Experimental section and Figures S1-S8. See DOI: 10.1039/c000000x/
References
- 1.Ortiz de Montellano PR. Cytochrome P450. 3rd Kluwer/Plenum; New York: 2005. [Google Scholar]
- 2.(a) Guengerich FP, Munro AW. J. Biol. Chem. 2013;288:17065–17073. doi: 10.1074/jbc.R113.462275. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guengerich FP. J. Biochem. Mol. Toxicol. 2007;21:163–168. doi: 10.1002/jbt.20174. [DOI] [PubMed] [Google Scholar]
- 3.Zanger UM, Schwab M. Pharmacol. Ther. 2013;138:103–41. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Li AP. Drug Discov. Today Technol. 2005;2:179–185. doi: 10.1016/j.ddtec.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 5.(a) Krishnan S, Bajrami B, Hvastkovs EG, Choudhary D, Schenkman JB, Rusling JF. Anal. Chem. 2008;80:5279–5285. doi: 10.1021/ac800763r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wasalathanthri DP, Li D, Song D, Zheng Z, Choudhary D, Jansson I, Lu X, Schenkman JB, Rusling JF. Chem. Sci. 2015;6:2457–2468. doi: 10.1039/c4sc03401e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider E, Clark DS. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelec. 2013;39:1–13. doi: 10.1016/j.bios.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 7.O'Reilly E, Koehler V, Flitsch S, Turner N. Chem. Commun. 2011;47:2490–2501. doi: 10.1039/c0cc03165h. [DOI] [PubMed] [Google Scholar]
- 8.Wienkers LC, Heath TG. Nat. Rev. Drug Discov. 2005;4:825–833. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- 9.Ma Q, Lu AYH. Pharmacol. Rev. 2011;63:437–459. doi: 10.1124/pr.110.003533. [DOI] [PubMed] [Google Scholar]
- 10.Park BK, Boobis A, Clarke S, et al. Nat. Rev. Drug Discov. 2011;10:292–306. doi: 10.1038/nrd3408. [DOI] [PubMed] [Google Scholar]
- 11.Deavall DG, Martin EA, Horner JM, Roberts R. J. Toxicol. 2012;2012 doi: 10.1155/2012/645460. Article ID 645460, 13 pages. doi:10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung ST, Lauchli R, Arnold FH. Curr. Opin. Biotechnol. 2011;22:809–817. doi: 10.1016/j.copbio.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Krishnan S, Schenkman JB, Rusling JF. J. Phys. Chem. B. 2011;115:8371–8380. doi: 10.1021/jp201235m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Krishnan S, Wasalathanthri D, Zhao L, Schenkman JB, Rusling JF. J. Am. Chem. Soc. 2011;133:1459–1465. doi: 10.1021/ja108637s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sultana N, Schenkman JB, Rusling JF. Electroanalysis. 2007;19:2499–2506. [Google Scholar]
- 14.Dodhia VR, Sassone C, Fantuzzi A, Nardo GD, Sadeghi SJ, Gilardi G. Electrochem. Commun. 2008;10:1744–1747. [Google Scholar]
- 15.Mie Y, Suzuki M, Komatsu Y. J. Am. Chem. Soc. 2009;131:6646–6647. doi: 10.1021/ja809364r. [DOI] [PubMed] [Google Scholar]
- 16.Walgama C, Nerimetla R, Materer NF, Schildkraut D, Elman JF, Krishnan S. Anal. Chem. doi: 10.1021/ac5044362. http://dx.doi.org/10.1021/ac5044362. [DOI] [PubMed]
- 17.(a) Krishnan S, Armstrong FA. Chem. Sci. 2012;3:1015–1023. [Google Scholar]; (b) Walgama C, Krishnan S. J. Electrochem. Soc. 2014;161:H47–H52. [Google Scholar]
- 18.Shukla A, Gillam EMJ, Bernhardt PV. Electrochem. Commun. 2006;8:1845–1849. [Google Scholar]
- 19.Banks CE, Compton RG. Analyst. 2006;131:15–21. doi: 10.1039/b512688f. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki H, Shimada T. Arch. Biochem. Biophys. 1997;346:161–169. doi: 10.1006/abbi.1997.0302. [DOI] [PubMed] [Google Scholar]
- 21.Ursini F, Maiorino M, Ferri L, Valente M, Gregolin C. J. Inorg. Biochem. 1981;15:163–169. doi: 10.1016/s0162-0134(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal M, Okazaki Y, Okada S. Teratog. Carcinog. Mutagen. 2003;23:151–160. doi: 10.1002/tcm.10070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


