Abstract
Background/Objective
Rapid postnatal weight gain is a potentially modifiable risk factor for obesity and metabolic syndrome. To identify markers of rapid infancy weight gain and childhood obesity, we analyzed the metabolome in cord blood from infants differing in their postnatal weight trajectories.
Methods
We performed a nested case-control study within Project Viva, a longitudinal cohort of mothers and children. We selected cases (n=26) based on top quartile of change in weight-for-age 0-6 mo and BMI >85th percentile in mid-childhood (median 7.7 years). Controls (n=26) were age- and sex-matched, had normal postnatal weight gain (2nd or 3rd quartile of change in weight-for-age 0-6 mo) and normal mid-childhood weight (BMI 25th-75th percentile). Cord blood metabolites were measured using untargeted LC/MS; individual metabolites and pathways differing between cases vs. controls were compared in categorical analyses. We adjusted metabolites for maternal age, maternal BMI, and breastfeeding duration (linear regression), and assessed whether metabolites improved the ability to predict case-control status (logistic regression).
Results
Of 415 detected metabolites, 16 were altered in cases vs. controls (T-test, nominal P<0.05). 3 metabolites were related to tryptophan: serotonin, tryptophan betaine, and tryptophyl leucine (46%, 48% and 26% lower in cases, respectively, P<0.05). Mean levels of 2 methyl donors, dimethylglycine and N-acetylmethionine, were also lower in cases (18% and 16% respectively, P=0.01). Moreover, the glutamine:glutamate ratio was reduced by 33% (P<0.05) in cases. Levels of serotonin, tryptophyl leucine, and N-acetylmethionine remained significantly different after adjustment for maternal BMI, age, and breastfeeding. Adding metabolite levels to logistic regression models including only clinical covariates improved the ability to predict case vs. control status.
Conclusions
Several cord blood metabolites are associated with rapid postnatal weight gain. Whether these patterns are causally linked to childhood obesity is not clear from this cross-sectional analysis, but will require further study.
Introduction
Early infancy represents a window of developmental plasticity during which environmental exposures can modulate chronic disease risk. Accelerated postnatal weight gain trajectories are associated with increased risk of diabetes, obesity, and cardiovascular disease both in humans (1-3) and mammalian models (4;5). These data have implications for public health, as the postnatal period offers a window during which optimizing nutrition and/or growth rates could reap lifelong benefits. Thus, identifying early markers of rapid infancy weight gain, a potentially modifiable risk factor for obesity and type 2 diabetes (T2D), may eventually permit interventions targeting children at high risk for metabolic disease.
Metabolomic analysis allows comprehensive quantification of hundreds of nutrients, metabolic intermediates, and small molecules from biological samples and has proven a powerful tool for biomarker discovery. Recent metabolomic analyses have linked higher plasma levels of branched-chain and aromatic amino acids to subsequent development of T2D in adolescents and adults (6;7). We are not aware of any studies linking cord blood metabolomics to obesity or its early life risk factors. However, the cord blood metabolome does appear to be related to important clinical outcomes. For example, distinct cord blood signatures characterize infants with intrauterine growth restriction, notably higher phenylalanine and citrulline, and lower glutamine, choline, and glucose (8). Reductions in cord blood phospholipids have also been reported to predict early childhood type 1 diabetes risk (9).
We hypothesized that the cord blood metabolome would differ in newborns with subsequently development of early childhood obesity. To identify early biomarkers of rapid weight gain during infancy and childhood, we analyzed plasma metabolomic patterns in venous umbilical cord blood collected at delivery from infants who subsequently experienced or did not experience accelerated postnatal weight gain and childhood obesity.
Methods
Study population and measurements
We analyzed a subset of participants in Project Viva, a longitudinal cohort study of mothers and children. Detailed experimental protocols for Project Viva have been previously published (10). We recruited pregnant women during the first trimester from 8 obstetric offices of Harvard Vanguard Medical Associates in Boston, MA, between 1999 and 2002. Eligibility criteria included singleton pregnancy, ability to answer questions in English, and initial prenatal visit before 22 weeks gestation. In-person study visits occurred at recruitment (median 9.9 weeks gestation), mid-pregnancy (median 28.1 weeks gestation), just after delivery, during infancy (median 6.3 months), early childhood (median 3.3 years), and mid-childhood (median 7.7 years); detailed survey data were collected annually at intervening time points. At each visit, research assistants measured weight with a digital scale, and length (newborn, infancy) or height (early childhood, mid-childhood) using a calibrated stadiometer. We used 2000 CDC reference data (http://www.cdc.gov/growthcharts/) to calculate weight-for-age Z-scores and BMI percentiles. We assessed body composition in mid-childhood by dual-energy X-ray absorptiometry (DXA, Discovery A, Hologic, Bedford, MA). The DXA scanner was monitored for quality control daily by scanning a standard synthetic spine. We used Hologic software QDR version 12.6 for analysis. A single trained investigator checked all scans for positioning, movement, and artifacts, and defined body regions for analysis. Intrarater reliability on duplicate measurements was high (r=0.99).
We obtained written informed consent from the mother at each encounter and verbal assent from children at the mid-childhood visit. The Harvard Pilgrim Health Care Institutional Review Board approved all procedures.
Identification of cases and controls
We identified 26 cases based on accelerated early postnatal weight gain, defined as the top-quartile of change in weight for age from 0-6 months in Project Viva and overweight status (BMI>85th percentile according to CDC 2000) in mid-childhood. Controls were sex-matched and selected based on normal early postnatal weight gain, defined as the 2nd or 3rd quartile of change in weight for age from 0-6 months in Project Viva and normal weight (BMI between 25th and 75th percentile, CDC 2000) in mid-childhood.
Cord blood collection
Using a standard protocol, obstetricians and midwives collected blood from the umbilical cord vein at delivery. Whole blood was collected in EDTA tubes, refrigerated for <24 hours, and centrifuged at 2000 rpm at 4°C for 10 min. Plasma aliquots were stored in liquid nitrogen until shipment on dry ice to Metabolon (Durham, NC) for metabolomic analysis. Aliquots of cord blood were also used for analysis of adiponectin and leptin concentrations using commercial assay kits (RIA, Linco Research Inc., St. Charles, MO).
Metabolomic analysis
We performed untargeted metabolomic profiling using multi-platform mass spectroscopy (Metabolon, Durham, NC). Detailed descriptions of sample preparation, mass spectroscopy, and automated metabolite identification procedures have been previously published (11;12). We obtained semi-quantitative concentrations (expressed in arbitrary units) for 415 known biochemicals. We replaced undetectable values with 0.5*minimum value for each metabolite. We then log2-transformed the metabolite concentrations to minimize the influence of outliers. We excluded from the analysis 27 metabolites with undetectable values in >50% of samples.
Statistical analysis
To determine which cord blood metabolites differed in cases vs. controls, we calculated fold-changes for each biochemical (calculated by exponentiating the mean log2-metabolite level in cases, divided by the exponentiated mean log2-metabolite level in controls) and performed 2-sided unpaired Student’s T-tests using JMP Pro v10 software (SAS, Cary, NC). We calculated false discovery rate Q-values to account for multiple comparisons (http://genomics.princeton.edu/storeylab/qvalue/) (13); Q-value < 0.25 was considered significant. We next performed metabolite set enrichment analysis to identify pathways enriched among the most differentially regulated metabolites between cases and controls (MetaboAnalyst 2.0, www.metaboanalyst.ca) (14).
To assess the overall variability within our dataset, we performed principal components analysis (PCA) using R (www.r-project.org), including only imputed, log-transformed metabolite concentrations and case/control status. Then, to determine to what extent the metabolome distinguished cases from controls, we performed unsupervised hierarchical clustering (MetaboAnalyst 2.0) (14). To assess which metabolites contributed most strongly to distinguishing cases from controls, we next used partial-least-squares discriminant analysis (PLS-DA) using R.
We adjusted for potential confounders through multivariate linear and logistic regression analysis (JMP Pro v 10, SAS, Cary, NC). After determining which clinical covariates were associated with case vs. control status using bivariate logistic regression, we next built multivariable models including those covariates with the strongest association with case-control status, namely, maternal pre-pregnancy BMI, maternal age, breastfeeding duration, and paternal BMI. Because paternal BMI did not contribute to the predictive power (whole-model R2) of the multivariable models, we omitted it from subsequent models. We used linear regression to calculate metabolite concentrations adjusted for maternal BMI, maternal age, and breastfeeding duration. To test whether cord blood metabolite levels were associated with odds of case vs. control status after adjusting for clinical covariates, we then built models incorporating individual metabolites in addition to maternal BMI, maternal age and breastfeeding duration.
Results
Demographic, pregnancy, and hormonal characteristics in infants with accelerated postnatal weight gain
Mothers of cases were younger than controls (29.9±5.4 vs. 34.9±4.4 years), and had higher pre-pregnancy BMI (27.9±6.7 vs. 23.6±3.8 kg/m2). Paternal BMI was also higher among cases (27.6±3.7 vs. 25.3±3.5 kg/m2). 13 (50%) of the cases were non-white, vs. 7 (27%) of the controls.
Case infants tended to have lower birth weight (3275±458 vs. 3487±349 g), and a trend for lower birth weight Z-scores adjusted for gestational age and sex (−0.19 0.79 vs. 0.11±0.67). As expected based on our selection strategy, cases had significantly higher weight for age Z-score in infancy (1.25±0.86 vs. 0.26±0.59, P<0.0001) and higher BMI percentile in mid-childhood (95.5±4.7 vs. 51.5±15.5, P<0.0001). In mid-childhood, both total fat mass and lean mass were higher in cases vs. controls. Cases had a shorter duration of breastfeeding (4.0±3.1 vs. 8.2±4.0 months, P<0.001). Demographic, pregnancy, and infancy characteristics are summarized in Table 1.
Table 1. Maternal, pregnancy, and early childhood characteristics.
Maternal, pregnancy, and early childhood characteristics of 26 cases of accelerated postnatal weight gain and 26 controls with normal postnatal growth from Project Viva. If not otherwise noted, P-value refers to unpaired Student’s T-test. For categorical data, P-value calculated using Fisher’s exact test.
|
Maternal & pregnancy
characteristics |
Cases (n=26) | Controls (n=26) |
P-value |
|---|---|---|---|
| Mean (SD) or N (%) | |||
| Maternal age, years | 29.9 (5.4) | 34.9 (4.4) | 0.001 |
| Race/ethnicity, N (%) | |||
| White | 13 (50) | 19 (73) | 0.13 |
| Black | 8 (31) | 6 (23) | |
| Hispanic | 4 (15) | 0 (0) | |
| Other or more than 1 race | 1 (4) | 1 (4) | |
| Smoking status, N (%) | |||
| Never | 17 (65) | 19 (73) | 0.58 |
| Former smoker | 4 (15) | 5 (19) | |
| Smoked during pregnancy | 5 (19) | 2 (8) | |
| Pre-pregnancy BMI, kg/m2 | 27.9 (6.7) | 23.6 (3.8) | 0.01 |
| Pre-pregnancy BMI category, N (%) |
|||
| Lean | 11 (42) | 20 (77) | 0.04 |
| Overweight | 7 (27) | 3 (11.5) | |
| Obese | 8 (31) | 3 (11.5) | |
| Gestational weight gain, kg | 15.5 (5.5) | 15.8 (5.2) | 0.82 |
| Glucose challenge test, mg/dl | 110 (22) | 113 (25) | 0.67 |
| Glucose tolerance, N (%) | |||
| Normal | 22 (85) | 23 (88) | 0.7 |
| GDM | 3 (12) | 1 (4) | |
| Transient hyperglycemia | 1 (4) | 2 (8) | |
| Blood pressure status, N (%) | |||
| Normal blood pressure | 19 (79) | 24 (96) | 0.11 |
| Gestational hypertension | 4 (15) | 1 (4) | |
| Pre-eclampsia | 1 (4) | 0 (0) | |
| Caesarian delivery | |||
| No, N (%) | 22 (85) | 22 (85) | 1 |
| Yes, N (%) | 4 (15) | 4 (15) | |
| Father’s BMI, kg/m2 (SD) | 27.6 (3.7) | 25.3 (3.5) | 0.03 |
| Early childhood characteristics | |||
| Gestational age, weeks | 39.2 (2.1) | 39.8 (1.5) | 0.2 |
| Neonatal anthropometry | |||
| Birth weight, grams | 3275 (458) | 3487 (349) | 0.07 |
| Birth weight for gestational age and sex, Z-score |
−0.19 (0.79) | 0.11 (0.67) | 0.15 |
| Length for age and sex, Z-score | −0.38 (0.81) | 0.00 (0.58) | 0.16 |
| Weight for length, Z-score | 0.37 (0.94) | 0.43 (0.78) | 0.85 |
| Breastfeeding duration, months | 4.0 (3.1) | 8.2 (4.0) | 0.0002 |
| Thyroid hormone (T4), microg/dl, newborn |
18.7 (6.1) | 17.8 (3.9) | 0.68 |
| Leptin, ng/ml, cord blood | 6.4 (3.3) | 8.0 (4.9) | 0.19 |
| Adiponectin, microg/ml, cord blood |
27.4 (6.3) | 26.9 (7.9) | 0.83 |
| Anthropometry, age 6 months | |||
| Weight for age, Z-score | 1.25 (0.86) | 0.26 (0.59) | <0.0001 |
| Length for age, Z-score | 0.19 (1.22) | −0.19 (0.75) | 0.21 |
| Weight for length, Z-score | 1.52 (1.00) | 0.74 (0.60) | 0.002 |
| Change in weight for age Z-score 0-6 mo |
1.64 (0.66) | 0.24 (0.41) | <0.0001 |
| Anthropometry at mid-childhood | |||
| Weight, kg | 40.6 (12.8) | 26.4 (3.4) | <0.0001 |
| Height, cm | 132.2 (10.1) | 128.5 (6.6) | 0.12 |
| BMI, percentile | 95.5 (4.7) | 51.5 (15.5) | <0.0001 |
| Total fat mass (DEXA), kg | 13.2 (4.7) | 6.0 (1.2) | <0.0001 |
| Total lean mass (DEXA), kg | 16.6 (5.4) | 20.4 (3.1) | <0.0001 |
Unsupervised analysis: cord metabolites do not clearly discriminate cases and controls
We detected a total of 415 biochemicals, including 99 amino acids, 33 carbohydrates, 18 cofactors and vitamins, 42 xenobiotics, and 153 lipids in cord blood (full list, Supplemental Table 1). To better understand the structure of the cord blood metabolome in cases vs. controls, we used unsupervised principal components analysis (PCA) to identify metabolites contributing the most to observed differences in the dataset. PCA did not clearly separate the two groups (Supplemental Figure 1A). Similarly, unsupervised hierarchical clustering did not fully separate cases from controls, although visual inspection suggests that subsets of cases and controls did cluster together (Supplemental Figure 1B). We next used partial least squares discriminant analysis (PLS-DA) to identify metabolites predictive of case vs. control status. Two distinct clusters were observed (Supplemental Figure 1C), but the model was not statistically significant, with permuted p-value = 0.11.
Individual cord blood metabolites associated with case vs. control status
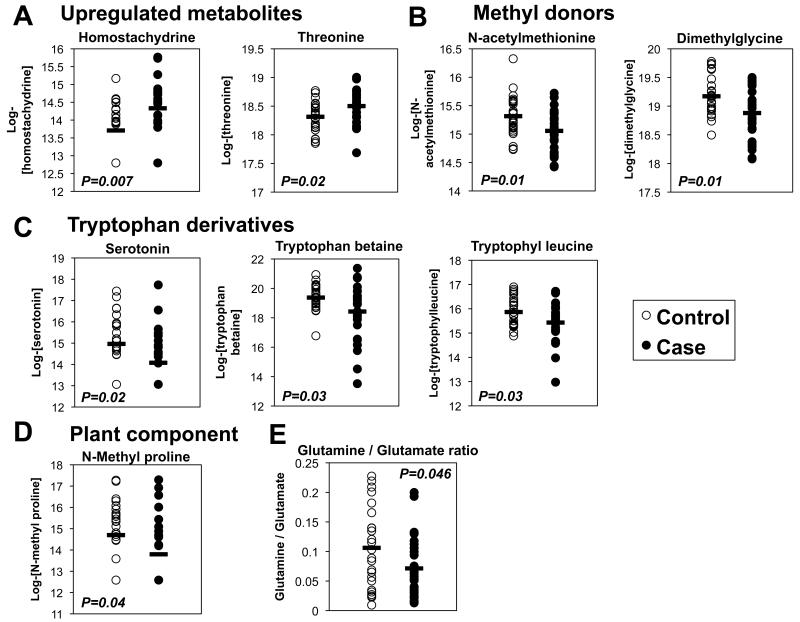
Mean levels of 16 biochemicals were significantly different between cases and controls (nominal P<0.05); none reached a false-discovery-rate (FDR) threshold of <0.25. 2 metabolites, threonine and homostachydrine, were significantly higher in cases, while 14 metabolites were lower (Figure 1A-D and Table 2). The metabolite with the greatest fold-reduction (50% lower, P=0.01) in cases was 4-vinylphenol sulfate. Two of the most significantly reduced metabolites in cases, dimethylglycine (18% lower, P=0.01) and N-acetyl methionine (16% lower, P=0.01), are methyl donors involved in one-carbon metabolism (Figure 1B). Three of the metabolites with lower levels in cases vs. controls are related to tryptophan or its metabolism: tryptophan betaine (48% lower, P=0.03), tryptophyl leucine (26% lower, P=0.03), and serotonin (46% lower, P=0.02, Figure 1C). Three of the top-ranking metabolites were xenobiotics found in plants, including homostachydrine (abundant in alfalfa and citrus; 54% higher in cases, P=0.007), N-methyl proline (citrus; 33% higher in cases, P=0.04), and tryptophan betaine (legumes).
Figure 1. Selected top-ranking metabolites in categorical analysis of cases vs. controls.
- Upregulated metabolites. N=26/group, P<0.05.
- Methyl donors. N=26/group, P<0.05.
- Tryptophan derivatives. N=26/group, P<0.05.
- Plant components. N=26/group, P<0.05.
- Glutamine to glutamate ratio. N=25 controls and 26 cases, P<0.05.
Open circles denote controls; filled circles denote cases.
Table 2. Top-ranking cord blood metabolites altered in cases vs. controls.
| A. Log2-transformed metabolite levels (missing values replaced with 0.5*minimum value detected) in cord blood from 26 cases of accelerated early postnatal weight gain and 26 controls with normal growth trajectories from Project Viva. Shown are the 16 metabolites with unadjusted P<0.05, out of the 415 metabolites detected. Fold-change was calculated as [2^(log mean Cases)]/[2^(log mean Controls)]. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Fold- change |
P-valuve | FDR | ||||
| BIOCHEMICAL | N | Mean (SD) |
N | Mean (SD) |
Case/ Control |
Unadjusted ** | Adjusted *** | Q- value |
| Homostachydrine | 22 | 14.33 (0.82) |
16 | 13.71 (0.78) |
1.54 | 0.007 | 0.02 | 0.97 |
| 4-vinylphenol sulfate | 26 | 15.8 (1.39) |
26 | 16.81 (1.27) |
0.5 | 0.009 | 0.04 | 0.97 |
| Dimethylglycine | 26 | 18.88 (0.45) |
26 | 19.17 (0.34) |
0.82 | 0.01 | 0.14 | 0.97 |
| N-acetylmethionine | 26 | 15.06 (0.36) |
26 | 15.32 (0.36) |
0.84 | 0.01 | 0.01 | 0.97 |
| Threonine | 26 | 18.50 (0.30) |
26 | 18.31 (0.25) |
1.14 | 0.02 | 0.23 | 0.97 |
| Serotonin (5HT) | 12 * | 14.08 (1.31) |
20 | 14.96 (1.32) |
0.54 | 0.02 | 0.03 | 0.97 |
| Pyridoxate | 26 | 17.26 (1.51) |
26 | 18.08 (0.91) |
0.57 | 0.02 | 0.11 | 0.97 |
| 3-carboxy-4-methyl-5-propyl- 2-furanpropanoate (CMPF) |
26 | 17.40(1.51) | 26 | 18.27 (1.15) |
0.55 | 0.02 | 0.45 | 0.97 |
| Tryptophylleucine | 25 | 15.43 (0.79) |
26 | 15.87 (0.58) |
0.74 | 0.03 | 0.02 | 0.97 |
| Tryptophan betaine | 25 | 18.42 (1.96) |
26 | 19.37 (0.86) |
0.52 | 0.03 | 0.22 | 0.97 |
| Sphingosine | 26 | 17.56 (0.94) |
26 | 18.13 (0.94) |
0.68 | 0.03 | 0.08 | 0.97 |
| Maltose | 22 | 18.83 (1.56) |
26 | 19.67 (1.22) |
0.56 | 0.03 | 0.12 | 0.97 |
| N-methyl proline | 11 * | 13.80 (1.60) |
19 | 14.70 (1.53) |
0.54 | 0.04 | 0.74 | 0.97 |
| Alanylalanine | 10 | 11.40 (1.63) |
17 | 12.35 (1.71) |
0.52 | 0.04 | 0.28 | 0.97 |
| 2-docosahexaenoyl- glycerophosphocholine |
26 | 18.31 (0.36) |
26 | 18.53 (0.41) |
0.86 | 0.04 | 0.06 | 0.97 |
| 1-stearoyl-glycerophosphoethanolamine | 25 | 18.35 (0.84) |
26 | 18.79 (0.72) |
0.73 | 0.05 | 0.04 | 0.97 |
| B. Log-2 transformed mean metabolite levels in cord blood from 26 cases of accelerated early postnatal weight gain and 26 controls with normal growth trajectories from Project Viva, adjusted for maternal BMI, maternal age, and infant breastfeeding duration. Shown are the 17 metabolites with adjusted P<0.05, out of the 415 metabolites detected. Fold-change was calculated as [2^(log mean Cases)]/[2^(log mean Controls)]. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Conntrol | Fold Change |
P-value | FDR | ||||
| BIOCHEMICAL | N | Mean (SD) |
N | Mean (SD) |
Case/ control |
Unadjusted ** | Adjusted *** | Q- value |
| Alpha-hydroxyisovalerate | 26 | 20.30 (0.52) |
26 | 20.54 (0.34) |
0.85 | 0.06 | 0.005 | 0.97 |
| N-acetylmethionine | 26 | 15.06 (0.36) |
26 | 15.32 (0.36) |
0.84 | 0.01 | 0.01 | 0.97 |
| Homostachydrine | 22 | 14.33 (0.82) |
16 | 13.71 (0.78) |
1.54 | 0.01 | 0.02 | 0.97 |
| Erythro-sphingosine-1- phosphate |
25 | 16.43 (0.84) |
26 | 16.75 (0.74) |
0.8 | 0.15 | 0.02 | 0.97 |
| Tryptophylleucine | 25 | 15.43 (0.79) |
26 | 15.87 (0.58) |
0.74 | 0.03 | 0.02 | 0.97 |
| Serotonin (5HT) | 12 * | 14.08 (1.31) |
20 | 14.96 (1.32) |
0.54 | 0.02 | 0.03 | 0.97 |
| Beta-alanine | 19 * | 14.76 (1.35) |
25 | 15.39 (0.84) |
0.65 | 0.05 | 0.03 | 0.97 |
| Tryptophan | 26 | 24.22 (0.26) |
26 | 24.32 (0.24) |
0.94 | 0.17 | 0.03 | 0.97 |
| 2-aminobutyrate | 26 | 18.59 (0.42) |
26 | 18.64 (0.27) |
0.97 | 0.65 | 0.03 | 0.97 |
| Alpha-hydroxyisocaproate | 26 | 13.72 (0.81) |
26 | 13.90 (0.84) |
0.88 | 0.44 | 0.04 | 0.97 |
| Nicotinamide | 26 | 16.75 (0.82) |
26 | 17.08 (0.53) |
0.8 | 0.09 | 0.04 | 0.97 |
| Leucine | 26 | 24.18 (0.28) |
26 | 24.23 (0.21) |
0.97 | 0.47 | 0.04 | 0.97 |
| 4-vinylphenol sulfate | 20 | 15.80 (1.39) |
26 | 16.81 (1.27) |
0.5 | 0.01 | 0.01 | 0.97 |
| N-acetyltryptophan | 19 | 12.31 (1.16) |
24 | 12.47 (0.74) |
0.89 | 0.54 | 0.04 | 0.97 |
| 1-stearoyl-glycerophosphoethanolamine | 25 | 18.35 (0.84) |
26 | 18.79 (0.72) |
0.73 | 0.05 | 0.04 | 0.97 |
| Caprylate (8:0) | 26 | 17.39 (0.41) |
26 | 17.57 (0.40) |
0.88 | 0.11 | 0.05 | 0.97 |
| Indolelactate | 26 | 19.89 (0.40) |
26 | 19.96 (0.33) |
0.95 | 0.46 | 0.05 | 0.97 |
refers to metabolites for which the number of detectable samples (N) differed between cases and controls (Fisher’s exact test).
Unadjusted P-value refers to T-test in cases vs. controls.
Adjusted P-value derived from linear regression, adjusted for maternal age, BMI and child breastfeeding duration.
Given that several clinical characteristics linked to childhood obesity risk differed between cases and controls (i.e. maternal BMI, maternal age, and breastfeeding duration), we next used linear regression to calculate metabolite concentrations adjusted for these potential confounders. A total of 17 metabolites were significantly altered in cases vs. controls at adjusted P<0.05 (Table 2B). Several of the metabolites identified in our raw comparison of cases vs. controls remained significant after adjustment, including homostachydrine, 4-vinylphenol sulfate, N-acetylmethionine, serotonin, tryptophyl leucine, and 1-stearoyl-GPE (adjusted P<0.05). Of note, 5 of the 17 top-ranking metabolites in the adjusted analysis were related to tryptophan, including serotonin, tryptophan, tryptophyl leucine, N-acetyltryptophan, and indolelactate. We also observed lower levels of metabolites related to branched chain amino acids, including leucine, alpha-hydroxyisovalerate, and alpha-hydroxyisocaproate, in cases.
Pathway analysis of cord metabolites associated with case vs. control status
To test whether cord blood metabolites that differed in cases with accelerated postnatal weight gain vs. controls belonged to known biological pathways, we performed metabolite set enrichment analysis. Several pathways were enriched (false discovery rate <0.25). The most significantly enriched pathways were “Tryptophan Metabolism” (P=0.02) and “Excitatory neural signaling through 5HTR4/6/7 and serotonin” (P=0.02) (Supplemental Table 2). Also highly enriched were pathways associated with metabolism of other amino acids, including aspartate and beta-alanine (P=0.02 and P=0.03, respectively). Metabolites involved in metabolism of betaine, an organic osmolyte and methyl donor, were also significantly enriched (P=0.02).
Given that several pathways related to amino acid metabolism were altered, we examined the ratio of glutamine to glutamate, which reflects glutamine synthase activity, and has been linked to risk of obesity, insulin resistance and diabetes in humans (15). Plasma from cases tended to have a lower glutamine : glutamate ratio (P=0.08), a difference that became significant (33% lower, P=0.046) after excluding an outlier in the control group with glutamine levels >6 standard deviations below the mean (Figure 1E).
Cord blood metabolites predicted case vs. control status above and beyond clinical risk factors
Because infants with accelerated postnatal weight gain and overweight status in mid-childhood (‘cases’) differed from controls with regard to maternal pre-pregnancy BMI, maternal age, breastfeeding duration, and other covariates that could influence postnatal obesity risk, we next investigated whether cord blood metabolites were associated with the odds of being a case independent of these potential confounders. We therefore used logistic regression to identify clinical covariates predictive of case vs. control status and then tested whether cord blood metabolite levels independently contributed to the risk of being a case above and beyond the clinical risk factors. Using bivariate logistic regression, we found that breastfeeding duration (OR: 0.74, 95%CI: 0.62-0.89, P=0.001) and maternal age (OR: 0.81, 95%CI: 0.71-0.93, P=0.003) were associated with reduced odds of being a case, whereas pre-pregnancy maternal BMI (OR: 1.19, 95%CI: 1.04-1.36, P=0.01) and paternal BMI (OR: 1.21, 95%CI: 1.01-1.45, P=0.04) were the most significant positive predictors of case vs. control status (Table 3A). We next built stepwise multivariate logistic regression models, and found that breastfeeding duration, maternal age, and pre-pregnancy BMI were independently associated with case vs. control status, whereas the addition of covariates such as newborn weight, race, sex, and paternal BMI contributed little to the strength of the models. We sequentially added each of the top-ranking metabolites identified in our adjusted and unadjusted analyses (Table 2A/2B) to these logistic regression models to determine whether cord blood metabolites improved the ability to predict case vs. control status.
Table 3. Associations of maternal and infant characteristics with odds of case vs. control status.
A. Bivariate logistic regression models of parental and perinatal covariates associated with odds of case vs. control status. B. Multivariable logistic regression models incorporating clinical and metabolite data.
| A: Bivariate logistic regression | ||
|---|---|---|
| Covariate | OR (95% CI) | P |
| Breastfeeding duration, months | 0.74 (0.62-0.89) | 0.001 |
| Mat age at enrollment, years | 0.81 (0.71-0.93) | 0.003 |
| Maternal pre-pregnancy BMI, kg/m2 | 1.19 (1.04-1.36) | 0.01 |
| Father’s BMI, kg/m2 | 1.21 (1.01-1.45) | 0.04 |
| Birth weight, grams | 1 (1.00-1.00) | 0.08 |
| Non-white race/ethnicity | 2.71 (0.85-8.64) | 0.09 |
| Birth weight for gestational age and sex, Z-score | 0.56 (0.26-1.23) | 0.15 |
| Leptin, ng/ml, cord blood | 0.91 (0.79-1.05) | 0.19 |
| Gestational age at delivery, wks | 0.80 (0.56-1.14) | 0.21 |
| T4, microg/dl, newborn | 1.04 (0.88-1.23) | 0.64 |
| Glucose, maternal | 0.99 (0.97-1.02) | 0.66 |
| Gestational weight gain, kg | 0.99 (0.89-1.10) | 0.81 |
| Adiponectin, microg/ml, cord blood | 1.01 (0.93-1.09) | 0.82 |
| Newborn weight for length, Z-score | 0.91 (0.39-2.15) | 0.84 |
| B: Multivariate logistic regression | ||
|---|---|---|
|
| ||
| Model | OR (95% CI) | P (chi-square) |
|
| ||
| Model A: R2 = 0.39, P<0.0001 | ||
| Maternal age at enrollment, years | 0.83 (0.68-0.98) | 0.02 |
| Maternal pre-pregnancy BMI, kg/m2 | 1.21 (1.04-1.49) | 0.01 |
| Breastfeeding duration, months | 0.73 (0.57-0.89) | 0.001 |
|
| ||
| Model A + homostachydrine: R2 = 0.46, P<0.0001 Homostachydrine |
3.72 (1.20-15.7) | 0.04 |
|
| ||
| Model A + N-acetylmethionine: R2 = 0.54, P<0.0001 N-acetylmethionine |
0.006 (5.3×10−5-0.17) | 0.01 |
|
| ||
| Model A + tryptophyl leucine: R2 = 0.47, P<0.0001 Tryptophyl leucine |
0.18 (0.03-0.76) | 0.04 |
|
| ||
| Model A + 1-stearoyl-GPE: R2 = 0.49, P<0.0001 1-stearoyl-GPE |
0.19 (0.03-0.66) | 0.02 |
|
| ||
| Model A + alpha-hydroxyisovalerate: R2 = 0.53, P<0.0001 Alpha-hydroxyisovalerate |
0.02 (0.0002-0.26) | 0.02 |
|
| ||
| Model A + tryptophan: R2 = 0.45, P<0.0001 Tryptophan |
0.03 (0.0004-0.71) | 0.047 |
|
| ||
| Model A + 2-aminobutyrate: R2 = 0.47, P<0.0001 2-aminobutyrate |
0.04 (0.002 – 0.59) | 0.03 |
|
| ||
| Model A + alpha-hydroxyisocaproate: R2 = 0.46, P<0.0001 Alpha-hydroxyisocaproate |
0.28 (0.07-0.87) | 0.04 |
|
| ||
| Model A + nicotinamide: R2 = 0.46, P<0.0001 Nicotinamide |
0.26 (0.07-0.81) | 0.03 |
|
| ||
| Model A + leucine: R2 = 0.44, P<0.0001 Leucine |
0.04 (0.001-0.91) | 0.046 |
|
| ||
| Model A + indolelactate: R2 = 0.45, P<0.0001 Indolelactate |
0.09 (0.007-0.81) | 0.046 |
Many of the top-ranking metabolites substantially improved the strength of the logistic regression models. For example, adding cord blood levels of N-acetyl methionine to a model including breastfeeding duration, maternal age, and pre-pregnancy BMI improved the ability to discriminate cases from controls (R-squared for model without metabolite: 0.39, P<0.0001; R-squared for model with N-acetyl methionine: 0.54, P<0.0001, Table 3B). Similarly, adding metabolites related to tryptophan (e.g. tryptophyl leucine, tryptophan, indolelactate) also improved prediction ability, as did metabolites related to branched chain amino acid metabolism (e.g. alpha-hydroxyisocaproate, alpha-hydroxyisovalerate, leucine). Moreover, the associations between such metabolites and the odds of case vs. control status were independent of the clinical covariates (Table 3B). Only the metabolites that improved the strength of the logistic regression models and contributed independently to the odds of case/control status are included in Table 3B.
Discussion
Among cases with rapid infancy weight gain and overweight in mid-childhood vs. controls without rapid weight gain and no childhood overweight, we found that mean levels of 16 cord blood metabolites were significantly different. Pathway analysis revealed that biochemicals related to tryptophan and one-carbon metabolism were enriched among the differentially-regulated metabolites. Moreover, the cord blood ratio of glutamine:glutamate was lower in cases vs. controls. Our analysis is limited by the fact that several clinical characteristics associated with childhood BMI, including maternal age, maternal BMI, and breastfeeding duration, differed between cases and controls. While many of the differentially regulated metabolites survived adjustment for identified covariates, we cannot exclude the possibility that additional factors related to maternal metabolism or breastfeeding that we did not measure or adjust for might have contributed to the observed differences. Moreover, no individual metabolites in our categorical analysis had significant false discovery rates. Logistic regression demonstrated that maternal age, pre-pregnancy BMI, and breastfeeding duration were associated with increased odds of being a case vs. a control; adding cord blood metabolites related to one-carbon and tryptophan metabolism to these prediction models improved the ability to differentiate cases with early childhood obesity risk from controls. Our results therefore suggest that cord blood metabolic signatures may be associated with early childhood obesity trajectories. However, the cross-sectional nature of our analysis and the potential influence of maternal or dietary factors on the cord metabolome preclude assessment of whether these metabolic pathways are causally related to infancy weight gain.
While many groups have used metabolomic profiling to identify disease biomarkers in adults at risk for insulin resistance (15), diabetes (7), and cardiovascular disease (16), few studies have examined plasma metabolomic patterns in infancy or childhood (6;17;18). A recent study found that cord blood phospholipids were predictive of early-onset type 1 diabetes (9). Cord blood metabolomic markers are also linked to neonatal hypoxia, prematurity, and intrauterine growth retardation (8;19;20). To our knowledge, our study is the first to characterize the cord blood metabolome of infants at risk for childhood obesity, prior to obesity onset.
Our data suggest that differences in maternal diet are associated with postnatal weight gain trajectories in infancy. Indeed, several of the top-ranking metabolites altered between cases and controls were food constituents – mostly plant products. For example, homostachydrine, higher in cases, is linked to dietary intake of citrus (21) and dairy products (22); N-methyl proline, reduced in cases, is linked to citrus consumption (22); 4-vinylphenol sulfate, lower in cases, is a styrene metabolite found in wine, cider, berries and peanuts (22); and tryptophan betaine, reduced in cases, is abundant in legumes. Moreover, pyridoxate, a vitamin B6 metabolite reduced in cases, is linked to multivitamin consumption, and maltose, reduced in cases, is a disaccharide abundant in many foods. Another metabolite with lower levels in cases, 3-carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF), is a uremic toxin, but also correlates with dietary intake of green vegetables, garlic, onions, and fish (22). CMPF has been described to compete with penicillin for binding to the renal tubule transporter rOat3; it is unclear whether a trend for higher penicillin G levels in cases vs. controls (fold-change: 2.83, P=0.22, not shown) might have contributed to observed reductions in CMPF in cases (23). Interestingly, elevations in CMPF have recently been described in the setting of impaired glucose tolerance, GDM, and T2DM; in mice, CMPF directly inhibits beta cell insulin secretion (24). Such data contrast with our observation of decreased CMPF levels in cord blood from cases despite a similar prevalence of GDM in both groups. Future studies will be required to assess transplacental passage and metabolism of this potentially important metabolite. Taken together, the numerous food and plant components among the most differentially regulated metabolites between cases and controls are largely consistent with the literature linking maternal dietary patterns (both during and after pregnancy) with offspring obesity (25).
A unique feature of our data is that it implicates specific nutrients and metabolites as potential contributors to early childhood obesity risk. To what degree such signatures might stem from differences in maternal BMI, which differed in cases and controls, or from differences in feeding/fasting in the hours leading up to delivery, vs. long-term dietary patterns, is not clear.
Our results point to an association between cord blood levels of metabolites related to tryptophan and one-carbon metabolism, and accelerated postnatal weight gain. Three of the 16 significantly altered metabolites in cases vs. controls are structurally related to or metabolized from tryptophan. Moreover, 2 of the 16 metabolites that differed in cases and controls were methyl donors, and metabolite set enrichment analysis revealed “Betaine Metabolism” as one of the top-ranking pathways altered between cases vs. controls (betaine is diet-derived methyl donor). Associations of biochemicals related to tryptophan and obesity trajectories in our analysis are intriguing in light of the multiple lines of evidence linking serotonin to appetite regulation, beta cell development, and brown adipose tissue thermogenesis, as well as links between diet-derived tryptophan betaine and sleep regulation (26-30). Metabolites involved in one-carbon metabolism (e.g. dimethylglycine, methionine, betaine) have also recently been implicated in the pathogenesis of obesity, insulin resistance, and fatty liver (15)(31), in addition to an established role in the regulation of epigenetic marks (e.g. DNA and histone methylation). However, it would be premature to conclude, from our analysis of cord blood, that these metabolite patterns are causally linked to obesity pathogenesis. Rather, these relationships may result from differences in dietary patterns among the mothers of cases, or from other differences between cases and controls that were not accounted for in our adjusted analyses. It will be important for future studies to further explore whether tryptophan or one-carbon metabolism are linked to childhood obesity risk.
Because our categorical and pathway analyses highlighted differences in the levels of several amino acids and their derivatives, we examined whether the glutamine:glutamate ratio – a marker for the activity of glutamine synthetase – differed in cases vs. controls. Cases had a reduced glutamine:glutamate ratio. Other groups have described reductions in cord blood glutamine levels and in the glutamine:glutamate ratio in LBW infants (8;32), but this has not, to our knowledge, previously been assessed in the setting of early childhood obesity risk. Plasma glutamine levels fluctuate in response to stress, and are reduced during acute infection or trauma (33); the reduction in cord glutamine:glutamate ratio we observed in cases vs. controls might therefore reflect greater stress around the time of delivery. These associations are intriguing in light of an emerging literature linking reductions in plasma glutamine, and in the glutamine:glutamate ratio, to increased risk of diabetes, insulin resistance, and hypertension (15), as well as a recent study linking genetic polymorphisms in glutamate metabolism to cardiovascular risk in individuals with T2D (34). Because we did not measure cord blood insulin or c-peptide levels, we could not directly examine associations between infant hyperinsulinemia or cord HOMA-IR and glutamine:glutamate ratio, or other metabolites. This will be an important area for future analyses.
Our study has several limitations. First, observational nature of the analysis precludes causal inferences. Second, relatively few metabolites were significantly altered by case vs. control status – only 16 out of 415 detected metabolites were altered at P<0.05 in our unadjusted analysis, and only 17 were significantly altered (P<0.05) after adjustment for covariates. The small sample size likely limited our power to detect differences. Clinical heterogeneity between groups, such as differences in maternal BMI, may have contributed to differences in the cord blood metabolome. Future studies using propensity-matched cases and controls could address this possibility. We recognize the possibility that the cord blood metabolome may have limited “predictive” significance, instead providing a snapshot of the immediate developmental and metabolic status of the infant. This possibility is supported by the observation that relatively few metabolites (n=13) were significantly correlated with the change in weight from 0-6 months, yet large numbers of cord blood metabolites were significantly associated with infant adiposity (n=42) and newborn T4 (n=34) (not shown). It is also unclear to what extent the cord blood metabolome is influenced by maternal diet immediately prior to delivery as opposed to maternal, fetal or placental metabolism. Despite these limitations, pathway analysis allowed us to identify concordant regulation of both methyl donor and tryptophan-related metabolites. Some of the strengths include the detailed assessments of mothers during pregnancy, and the extensive information captured about clinical and environmental factors that may influence childhood obesity risk.
In summary, we examined the cord blood metabolome in infants with and without accelerated early childhood weight gain to better understand the nutritional and metabolic determinants of early-onset obesity risk. While we found few overall differences in the cord metabolome of cases vs. controls, several metabolites linked to tryptophan and one-carbon metabolism were significantly reduced in cord blood from cases with accelerated postnatal weight gain. Moreover, the ratio of glutamine to glutamate, which has recently been linked to insulin resistance and cardiometabolic risk, was lower in cases vs. controls. Our data raise the possibility that perturbed metabolite patterns may already be present in cord blood of infants at risk for rapid postnatal weight gain, prior to obesity onset. Elucidating the mechanistic basis for these metabolite alterations and their potential role in obesity pathogenesis will require future study.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the Joslin Bioinformatics Core, funded by Diabetes Research Center grant DK036836. Grant support was provided by K99/R00 (HD064793), the Pediatric Endocrine Society, the Canadian Institutes of Health Research, and the Graetz Foundation (to E.I.); by K24 HD069408 (to E.O.), and by the American Diabetes Association and the Graetz Foundation (to M.-E.P.).
Footnotes
Conflict of interest
W.G. formerly had a professional relationship with Metabolon, Inc., where he was employed as Program Manager for Diagnostic Development, and where the metabolomic analyses reported in this article were performed. There are no other potential conflicts of interest to disclose.
Supplementary information is available at International Journal of Obesity’s website.
Reference List
- 1.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. New England Journal of Medicine. 2005;353/17:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 2.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301/21:2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 3.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123/4:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isganaitis E, Jimenez-Chillaron J, Woo M, Chow A, Decoste J, Vokes M, et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes. 2009;58/5:1192–1200. doi: 10.2337/db08-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, et al. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49/8:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 6.Michaliszyn SF, Sjaarda LA, Mihalik SJ, Lee S, Bacha F, Chace DH, et al. Metabolomic profiling of amino acids and beta-cell function relative to insulin sensitivity in youth. J Clin Endocrinol Metab. 2012;97/11:E2119–E2124. doi: 10.1210/jc.2012-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17/4:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivorra C, Garcia-Vicent C, Chaves FJ, Monleon D, Morales JM, Lurbe E. Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J Transl Med. 2012;10:142. doi: 10.1186/1479-5876-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La TD, Seppanen-Laakso T, Larsson HE, Hyotylainen T, Ivarsson SA, Lernmark A, et al. Decreased cord-blood phospholipids in young age-at-onset type 1 diabetes. Diabetes. 2013;62/11:3951–3956. doi: 10.2337/db13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De MD, et al. Cohort Profile: Project Viva. Int J Epidemiol. 2014 doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46/6:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE. 2010;5/5:e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100/16:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. doi: 10.1093/nar/gkp356. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Cirulation. 2012;125/18:2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis GD, Wei R, Liu E, Yang E, Shi X, Martinovic M, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest. 2008;118/10:3503–3512. doi: 10.1172/JCI35111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihalik SJ, Michaliszyn SF, de las HJ, Bacha F, Lee S, Chace DH, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care. 2012;35/3:605–611. doi: 10.2337/DC11-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2012 doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tea I, Le GG, Kuster A, Guignard N, Alexandre-Gouabau MC, Darmaun D, et al. 1H-NMR-based metabolic profiling of maternal and umbilical cord blood indicates altered materno-foetal nutrient exchange in preterm infants. PLoS One. 2012;7/1:e29947. doi: 10.1371/journal.pone.0029947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh BH, Broadhurst DI, Mandal R, Wishart DS, Boylan GB, Kenny LC, et al. The metabolomic profile of umbilical cord blood in neonatal hypoxic ischaemic encephalopathy. PLoS One. 2012;7/12:e50520. doi: 10.1371/journal.pone.0050520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Servillo L, Giovane A, Balestrieri ML, Ferrari G, Cautela D, Castaldo D. Occurrence of pipecolic acid and pipecolic acid betaine (homostachydrine) in Citrus genus plants. J Agric Food Chem. 2012;60/1:315–321. doi: 10.1021/jf204286r. [DOI] [PubMed] [Google Scholar]
- 22.Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr. 2014 doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deguchi T, Kouno Y, Terasaki T, Takadate A, Otagiri M. Differential contributions of rOat1 (Slc22a6) and rOat3 (Slc22a8) to the in vivo renal uptake of uremic toxins in rats. Pharm Res. 2005;22/4:619–627. doi: 10.1007/s11095-005-2486-x. [DOI] [PubMed] [Google Scholar]
- 24.Prentice KJ, Luu L, Allister EM, Liu Y, Jun LS, Sloop KW, et al. The Furan Fatty Acid Metabolite CMPF Is Elevated in Diabetes and Induces beta Cell Dysfunction. Cell Metab. 2014;19/4:653–666. doi: 10.1016/j.cmet.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Murrin C, Shrivastava A, Kelleher CC. Maternal macronutrient intake during pregnancy and 5 years postpartum and associations with child weight status aged five. Eur J Clin Nutr. 2013;67/6:670–679. doi: 10.1038/ejcn.2013.76. [DOI] [PubMed] [Google Scholar]
- 26.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51/2:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Ozawa M, Honda K, Nakai I, Kishida A, Ohsaki A. Hypaphorine, an indole alkaloid from Erythrina velutina, induced sleep on normal mice. Bioorg Med Chem Lett. 2008;18/14:3992–3994. doi: 10.1016/j.bmcl.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Keller BO, Wu BT, Li SS, Monga V, Innis SM. Hypaphorine is present in human milk in association with consumption of legumes. J Agric Food Chem. 2013;61/31:7654–7660. doi: 10.1021/jf401758f. [DOI] [PubMed] [Google Scholar]
- 29.Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2014 doi: 10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohara-Imaizumi M, Kim H, Yoshida M, Fujiwara T, Aoyagi K, Toyofuku Y, et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic beta cells during pregnancy. Proc Natl Acad Sci U S A. 2013;110/48:19420–19425. doi: 10.1073/pnas.1310953110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J. 2003;370(Pt 3):987–993. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexandre-Gouabau MC, Courant F, Moyon T, Kuster A, Le GG, Tea I, et al. Maternal and cord blood LC-HRMS metabolomics reveal alterations in energy and polyamine metabolism, and oxidative stress in very-low birth weight infants. J Proteome Res. 2013;12/6:2764–2778. doi: 10.1021/pr400122v. [DOI] [PubMed] [Google Scholar]
- 33.Askanazi J, Carpentier YA, Michelsen CB, Elwyn DH, Furst P, Kantrowitz LR, et al. Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg. 1980;192/1:78–85. doi: 10.1097/00000658-198007000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi L, Qi Q, Prudente S, Mendonca C, Andreozzi F, di PN, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA. 2013;310/8:821–828. doi: 10.1001/jama.2013.276305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.