Abstract
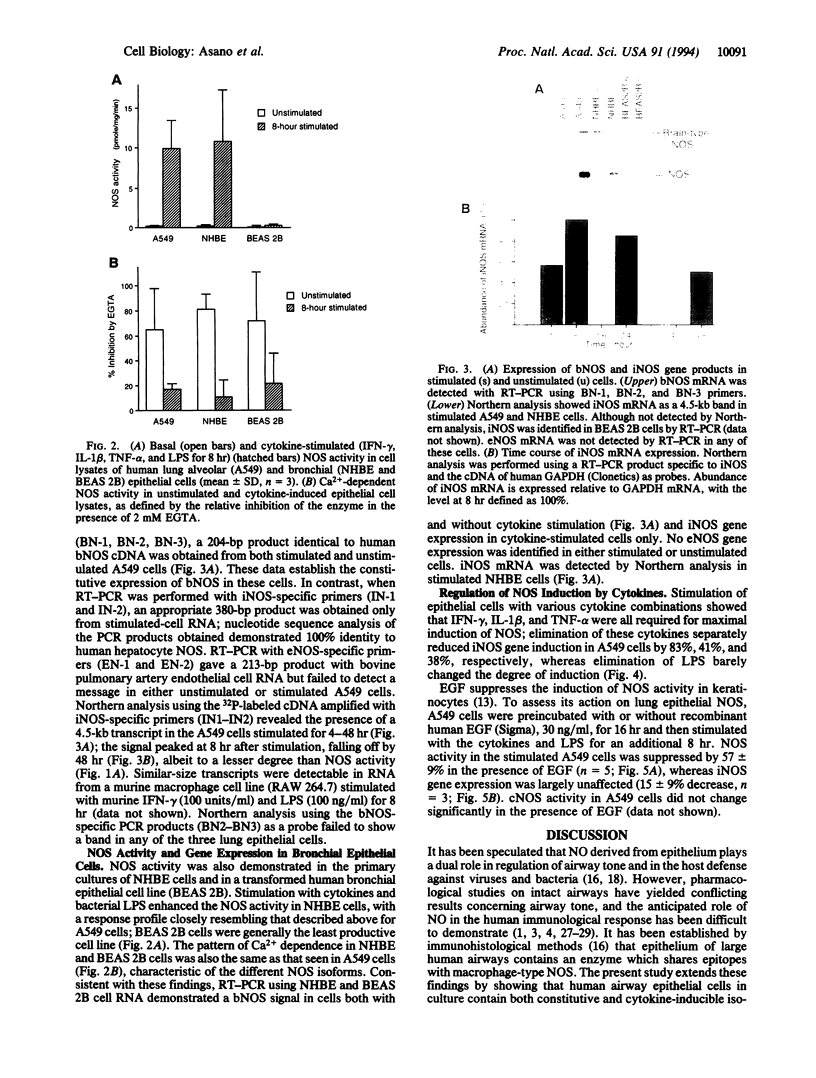
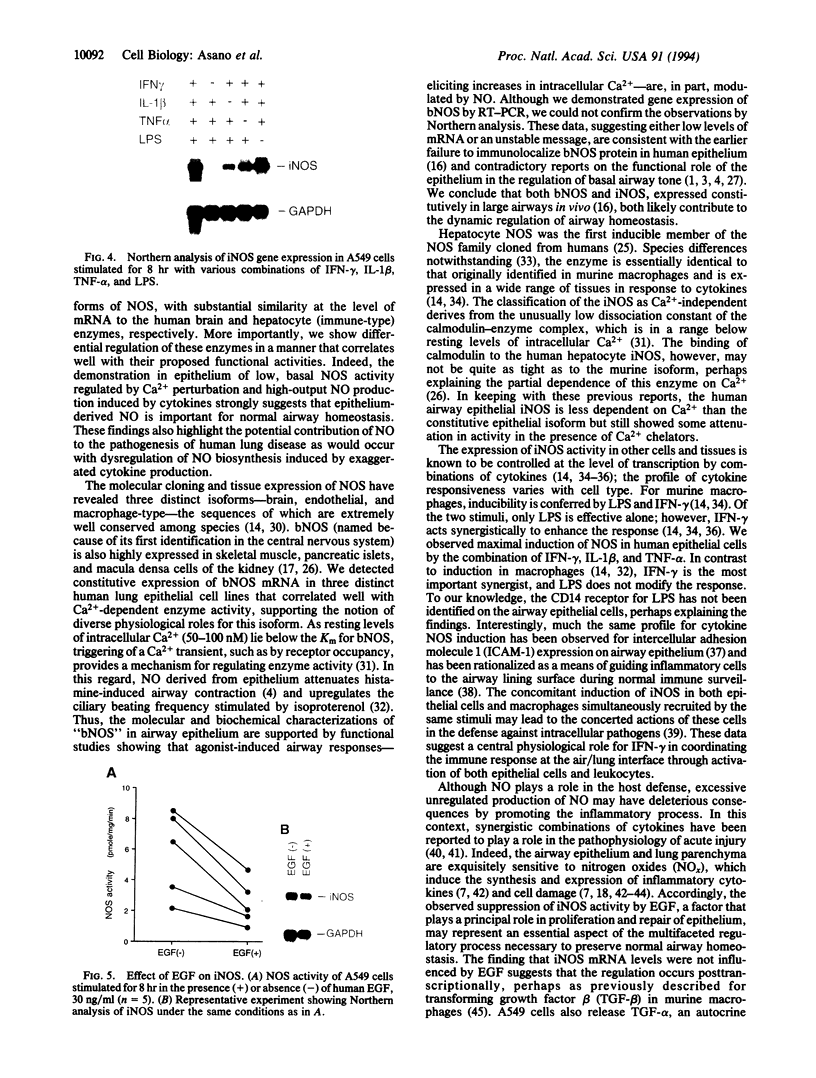
Histochemical activity and immunoreactivity of nitric oxide synthase (NOS, EC 1.14.13.39) have been recently demonstrated in human lung epithelium. However, the molecular nature of NOS and the regulation and function of the enzyme(s) in the airway is not known. A549 cells (human alveolar type II epithelium-like), BEAS 2B cells (transformed human bronchial epithelial cells), and primary cultures of human bronchial epithelial cells all exhibited constitutive NOS activity that was calcium dependent and inhibitable by the NOS inhibitor NG-monomethyl-L-arginine. Nitric oxide production by epithelial cells was enhanced by culture in the presence of interferon gamma, interleukin 1 beta, tumor necrosis factor alpha, and lipopolysaccharide; the NOS activity expressed under these conditions showed less dependence on calcium, reminiscent of other inducible forms of NOS. Two distinct NOS mRNA species, homologous to previously identified constitutive brain (type I) and inducible hepatic (type II) NOS, were demonstrated by reverse transcription-polymerase chain reaction in all cell lines. Northern analysis confirmed the expression of inducible NOS mRNA. Cell culture with epidermal growth factor, a principal regulator of epithelial cell function, decreased inducible NOS activity by posttranscriptional action but did not affect constitutive NOS activity. The coexistence of constitutive and inducible NOS in human alveolar and bronchial epithelial cells is consistent with a complex mechanism evolved by epithelial cells to protect the host from microbial assault at the air/surface interface while shielding the host from the induction of airway hyperreactivity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Hu S. C., Delvin R. B. Effect of nitrogen dioxide on human nasal epithelium. Am J Respir Cell Mol Biol. 1993 Sep;9(3):264–270. doi: 10.1165/ajrcmb/9.3.264. [DOI] [PubMed] [Google Scholar]
- Castranova V., Rabovsky J., Tucker J. H., Miles P. R. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol. 1988 May;93(3):472–483. doi: 10.1016/0041-008x(88)90051-8. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Xie Q. W., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992 Aug 1;176(2):599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Stamler J., Andon N., Davies P. F., McKinley G., Loscalzo J. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol. 1990 Sep;259(3 Pt 2):H804–H812. doi: 10.1152/ajpheart.1990.259.3.H804. [DOI] [PubMed] [Google Scholar]
- Cromwell O., Hamid Q., Corrigan C. J., Barkans J., Meng Q., Collins P. D., Kay A. B. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992 Nov;77(3):330–337. [PMC free article] [PubMed] [Google Scholar]
- Devalia J. L., Campbell A. M., Sapsford R. J., Rusznak C., Quint D., Godard P., Bousquet J., Davies R. J. Effect of nitrogen dioxide on synthesis of inflammatory cytokines expressed by human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol. 1993 Sep;9(3):271–278. doi: 10.1165/ajrcmb/9.3.271. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Freundlich B., Kern J. A., Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990 Jun;97(6):1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furfaro S., Berman J. S. The relation between cell migration and activation in inflammation: beyond adherence. Am J Respir Cell Mol Biol. 1992 Sep;7(3):248–250. doi: 10.1165/ajrcmb/7.3.248. [DOI] [PubMed] [Google Scholar]
- Gaston B., Drazen J. M., Loscalzo J., Stamler J. S. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- Gaston B., Reilly J., Drazen J. M., Fackler J., Ramdev P., Arnelle D., Mullins M. E., Sugarbaker D. J., Chee C., Singel D. J. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goureau O., Lepoivre M., Becquet F., Courtois Y. Differential regulation of inducible nitric oxide synthase by fibroblast growth factors and transforming growth factor beta in bovine retinal pigmented epithelial cells: inverse correlation with cellular proliferation. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4276–4280. doi: 10.1073/pnas.90.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck D. E., Laskin D. L., Gardner C. R., Laskin J. D. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing. J Biol Chem. 1992 Oct 25;267(30):21277–21280. [PubMed] [Google Scholar]
- Holtzman M. J., Hansbrough J. R., Rosen G. D., Turk J. Uptake, release and novel species-dependent oxygenation of arachidonic acid in human and animal airway epithelial cells. Biochim Biophys Acta. 1988 Dec 16;963(3):401–413. doi: 10.1016/0005-2760(88)90308-6. [DOI] [PubMed] [Google Scholar]
- Holtzman M. J., Look D. C. Cell adhesion molecules as targets for unraveling the genetic regulation of airway inflammation. Am J Respir Cell Mol Biol. 1992 Sep;7(3):246–247. doi: 10.1165/ajrcmb/7.3.246. [DOI] [PubMed] [Google Scholar]
- Hunter J. A., Finkbeiner W. E., Nadel J. A., Goetzl E. J., Holtzman M. J. Predominant generation of 15-lipoxygenase metabolites of arachidonic acid by epithelial cells from human trachea. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4633–4637. doi: 10.1073/pnas.82.14.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi K., Yamaguchi K., Kuranami M., Kyo E., Hozumi T., Abe K. Inhibition of growth of human lung adenocarcinoma cell lines by anti-transforming growth factor-alpha monoclonal antibody. J Natl Cancer Inst. 1989 Feb 1;81(3):220–223. doi: 10.1093/jnci/81.3.220. [DOI] [PubMed] [Google Scholar]
- Jain B., Rubinstein I., Robbins R. A., Leise K. L., Sisson J. H. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993 Feb 26;191(1):83–88. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- Janssens S. P., Shimouchi A., Quertermous T., Bloch D. B., Bloch K. D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992 Jul 25;267(21):14519–14522. [PubMed] [Google Scholar]
- Karupiah G., Xie Q. W., Buller R. M., Nathan C., Duarte C., MacMicking J. D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993 Sep 10;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993 Oct;9(4):371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Teitelbaum R. F. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992 Mar;165(3):513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- Nakane M., Schmidt H. H., Pollock J. S., Förstermann U., Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993 Jan 25;316(2):175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nijkamp F. P., van der Linde H. J., Folkerts G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro. Role of the epithelium. Am Rev Respir Dis. 1993 Sep;148(3):727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993 Aug;54(2):171–178. [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett K. A., Awburn M. M., Cowden W. B., Clark I. A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991 Sep;59(9):3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N., Milstien S. Availability of tetrahydrobiopterin is not a factor in the inability to detect nitric oxide production by human macrophages. Biochem Biophys Res Commun. 1993 May 28;193(1):378–383. doi: 10.1006/bbrc.1993.1634. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Shoji S., Ertl R. F., Linder J., Romberger D. J., Rennard S. I. Bronchial epithelial cells produce chemotactic activity for bronchial epithelial cells. Possible role for fibronectin in airway repair. Am Rev Respir Dis. 1990 Jan;141(1):218–225. doi: 10.1164/ajrccm/141.1.218. [DOI] [PubMed] [Google Scholar]
- Siegfried J. M. Detection of human lung epithelial cell growth factors produced by a lung carcinoma cell line: use in culture of primary solid lung tumors. Cancer Res. 1987 Jun 1;47(11):2903–2910. [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Strek M. E., White S. R., Ndukwu I. M., Munoz N. M., Williams F. S., Vita A. J., Leff A. R., Mitchell R. W. Physiologic significance of epithelial removal on guinea pig tracheal smooth muscle response to acetylcholine and serotonin. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1477–1482. doi: 10.1164/ajrccm/147.6_Pt_1.1477. [DOI] [PubMed] [Google Scholar]
- Tepperman B. L., Brown J. F., Whittle B. J. Nitric oxide synthase induction and intestinal epithelial cell viability in rats. Am J Physiol. 1993 Aug;265(2 Pt 1):G214–G218. doi: 10.1152/ajpgi.1993.265.2.G214. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Del Vecchio P. J., Shaffer J. B. IL-6 enhances TNF-alpha- and IL-1-induced increase of Mn superoxide dismutase mRNA and O2 tolerance. Am J Physiol. 1992 Jul;263(1 Pt 1):L22–L26. doi: 10.1152/ajplung.1992.263.1.L22. [DOI] [PubMed] [Google Scholar]
- Werner E. R., Werner-Felmayer G., Fuchs D., Hausen A., Reibnegger G., Wachter H. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J. 1989 Sep 15;262(3):861–866. doi: 10.1042/bj2620861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E. R., Werner-Felmayer G., Fuchs D., Hausen A., Reibnegger G., Yim J. J., Pfleiderer W., Wachter H. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem. 1990 Feb 25;265(6):3189–3192. [PubMed] [Google Scholar]
- Wood E. R., Berger H., Jr, Sherman P. A., Lapetina E. G. Hepatocytes and macrophages express an identical cytokine inducible nitric oxide synthase gene. Biochem Biophys Res Commun. 1993 Mar 31;191(3):767–774. doi: 10.1006/bbrc.1993.1283. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]