Abstract
Background
Our previous studies have demonstrated that targeting FVIII expression to platelets results in FVIII storage together with VWF in platelet α-granules and that platelet-derived FVIII (2bF8) corrects the murine hemophilia A phenotype even in the presence of high-titer anti-FVIII inhibitory antibodies (inhibitors).
Objective
To explore how VWF impacts platelet gene therapy of hemophilia A with inhibitors.
Methods
2bF8 transgenic mice in the F8−/− background (2bF8tg+/−F8−/−) with varying VWF phenotypes were used in this study. Animals were analyzed by VWF ELISA, FVIII activity assay, Bethesda assay, and tail clip survival test.
Results
Only 18% of 2bF8tg+/−F8−/−VWF−/− animals, in which VWF was deficient, survived the tail clip challenge with inhibitor titers of 3 – 8000 BU mL−1. In contrast, 82% of 2bF8tg+/−F8−/−VWF+/+ mice, which had normal VWF levels, survived tail clipping with inhibitor titers of 10 – 50,000 BU mL−1. All 2bF8tg+/−F8−/−VWF−/− mice without inhibitors survived tail clipping and no VWF−/−F8−/− mice survived this challenge. Since VWF is synthesized by endothelial cells and megakaryocytes and distributes in both plasma and platelets in peripheral blood, we further investigated the effect of each compartment of VWF in platelet-FVIII gene therapy of hemophilia A with inhibitors. In the presence of inhibitors, 42% of animals survived tail clipping in the group with plasma-VWF and 50% survived in the platelet-VWF group.
Conclusion
VWF is essential for platelet gene therapy of hemophilia A with inhibitors. Both platelet-VWF and plasma-VWF are required for optimal platelet-derived FVIII gene therapy of hemophilia A in the presence of inhibitors.
Keywords: Hemophilia A, Neutralizing Antibody, Gene therapy, Platelet, von Willebrand Factor
Introduction
Our previous studies with a platelet-derived FVIII (2bF8) using transgenic or lentivirus-mediated hematopoietic stem cell (HSC) gene delivery models have demonstrated that FVIII can be speci[1]fically expressed and stored together with endogenous von Willebrand factor, VWF, in platelet α-granules when FVIII expression is driven by the platelet-specific αIIb promoter.[2;3] This platelet-derived FVIII can correct the murine or the canine hemophilia A phenotype.[2;4;5] More importantly, this platelet-derived FVIII can still function even in the presence of high-titer anti-FVIII inhibitory antibodies (inhibitors).[2;6;7] We found that pre-existing anti-FVIII immunity does not negate 2bF8 engraftment, and even as little as one to five percent of platelets containing FVIII can still significantly improve hemostasis in hemophilia A mice with inhibitors.[6] The highly efficient clinical efficacy of the platelet-derived FVIII has been substantiated in our study using human HSCs.[8]
VWF is a multimeric glycoprotein that plays a major role in blood coagulation.[9] VWF has three distinct and important functional properties in hemostasis. Firstly, VWF chaperones FVIII in a non-covalently bound complex in blood circulation, protecting FVIII from protease degradation.[10] Secondly, at the site of vascular injury, VWF serves as a bridging molecule that promotes platelet binding to sub-endothelium and other platelets (platelet adherence and aggregation).[11;12] Thirdly, VWF binds to collagens to facilitate clot formation at sites of injury.[13] Our hypothesis that platelet-targeted FVIII gene therapy of hemophilia A can be effective in the presence of inhibitors is built upon the association between VWF and FVIII plus the ability of VWF to delay the time-dependent inactivation of FVIII by inhibitors. It is known that inhibitory antibodies against FVIII are time dependent.[14;15] The association-disassociation interaction occurs between VWF and FVIII and inhibitors. Evidence suggests that VWF has a protective effect on FVIII from inhibitor inactivation.[16–20] VWF binds to FVIII forming a complex, which prevents the binding of inhibitory antibodies to FVIII. Without VWF, anti-FVIII inhibitors can freely bind to and inactivate functional FVIII.[1] Our studies have demonstrated that a pre-formed complex of VWF with FVIII provides 2.9- and 6.7-fold, respectively, more effective protection of FVIII from inhibitor inactivation versus unbound VWF in the Bethesda assay with or without 2-hour incubation. [1] These support the premise of platelet-FVIII gene therapy of hemophilia A with inhibitors in which the FVIII transgene protein is stored together with VWF forming a VWF/FVIII complex in platelet α-granules before exposure to inhibitors.
VWF is synthesized in two cell types within the body (megakaryocytes and endothelial cells) and is stored in α-granules in megakaryocytes and platelets and in Weibel-Palade bodies in endothelial cells.[21–23]. The bulk of plasma VWF comes from endothelial synthesis and not from megakaryocytes.[24] While studies from our group[2] and another[25] have demonstrated that albeit FVIII can be stored within platelet α-granules even without VWF, the level of platelet-FVIII significantly decreases, suggesting that VWF is required for optimizing platelet-FVIII expression. How VWF impacts the efficacy of platelet gene therapy of hemophilia A with inhibitors has not been explored. In the current study, we investigated: 1) the role of VWF, 2) the effect of plasma-derived VWF, and 3) the effect of platelet-derived VWF on 2bF8 gene therapy of hemophilia A with inhibitors. Our results demonstrate that VWF is necessary for optimal platelet-derived FVIII gene therapy of hemophilia A with inhibitors.
Materials and methods
Mice
All mice used in this study were on a 129/SV x C57BL/6 mixed genetic background. FVIII knockout with normal VWF expression (F8−/−VWF+/+) (FVIIInull) mice, which have a disruption of exon 17 of the FVIII gene,[26] were a kind gift from Haig Kazazian at the University of Pennsylvania School of Medicine. F8−/− VWF−/− mice, which have undetectable levels of FVIII and VWF, were generated by our laboratory by crossing VWF−/− (The Jackson Laboratory, Bar Harbor, Maine) with F8−/− mice. Heterozygous transgenic (2bF8tg+/−) mice, which express platelet-specific FVIII, in the F8−/− (2bF8tg+/−F8−/−VWF+/+) or in the F8−/− and VWF−/− background (2bF8tg+/−F8−/−VWF−/−) were generated by our laboratory.[2] Homozygous T2F8 mice, which express endothelial cell-specific FVIII, in the F8−/− (T2F8tg+/+F8−/−VWF+/+) or in the F8−/− and VWF−/− double knockout (T2F8tg+/+F8−/−VWF−/−) background were generated by our laboratory.[27] All mice were maintained in pathogen-free microisolator cages at the animal facilities operated by the Medical College of Wisconsin. Animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Isoflurane or ketamine was used for anesthesia.
Mouse immunization and inhibitor models
Six to ten-week-old F8−/−VWF+/+ or F8−/− VWF−/− mice were immunized with recombinant human B-domain deleted FVIII (rhF8, Xyntha, Pfizer Inc, New York, NY, USA) at a dose of 50 IU kg−1 via the retro-orbital vein injection weekly for 4 weeks total. One week after the last immunization, plasmas were collected and inhibitor titers were determined by Bethesda assay as described in our previous report.[2] For 2bF8tg+/− mice, six to ten-week-old animals were immunized with rhF8 at a dose of 600 U kg−1 in the presence of adjuvant (Sigma, St. Louis, MO, USA) twice with a 3-week interval. One week after the second immunization, blood samples were collected and inhibitor titers were determined.
For inhibitor model studies, both chronic and acute models were used. In the chronic inhibitor model, rhF8-immunized F8−/− mice received bone marrow transplantation (BMT) from 2bF8tg+/− mice or rhF8-immunized 2bF8tg+/− mice were used. In the acute model, inhibitory plasmas from rhF8-immunized F8−/−VWF−/− mice with high-titer of inhibitors were infused into unimmunized 2bF8 mice with varying VWF conditions to a level of inhibitor titers of 2.5, 25, or 250 BU mL−1 followed by phenotypic correction assessment.
Bone marrow transplantation (BMT)
To explore how plasma- or platelet-VWF affects the efficacy of platelet gene therapy of hemophilia A with inhibitors, BMT was carried out to establish the plasma- and the platelet-VWF models. Recipient mice were conditioned for cellular transplantation with a lethal total body irradiation (TBI) dose of 1100 cGy using a cesium irradiator (Gammacell 40 Exactor, Best Theratronics Ltd, Ottawa, Canada). BM isolation and transplantation were performed as described in our previous report.[2] Briefly, BM cells were collected from femurs and tibias of donor mice and BM mononuclear cells were isolated using Fico/Lite-LM (mouse) (ATLANTA Biological, Lawrenceville, GA, USA) and transplanted into irradiated recipients at a cell dose of 10 × 106 in 300 μL of Phosphate-Buffered Saline by retro-orbital plexus venous injection at 24 hours after irradiation. Four months after BMT, some recipients were irradiated with 1100 cGy TBI again and a second BMT was performed. Animals were analyzed starting one month after BMT. Blood samples were collected from eye bleeds or tail bleeds. Plasma and platelets were isolated as previously described.[6]
FVIII assays
Functional FVIII activity (FVIII:C) levels in platelet lysates were quantified by a modified chromogenic assay using the Coatest SP4 FVIII Kit (DiaPharma, Franklin, OH) as previously described.[2;7] rhF8 was used as the standard. Platelet lysates from F8−/− mice were used as negative controls. The titers of anti-FVIII inhibitors were determined by a chromogenic-based modified Bethesda assay as described in our previous report.[2]
VWF assay
VWF antigen levels in plasma and platelet lysates were determined by enzyme-linked immunosorbant assay (ELISA). Briefly, a 96-well plate was coated with 50 μL of the mouse monoclonal antibody 344.2 (5 μg mL−1), which was produced by our laboratory, at 4ºC overnight. Fifty μL serial dilutions of plasmas or platelet lysates were added and incubated for one hour at room temperature. A biotin conjugated rabbit anti-human VWF polyclonal antibody (5 μg mL−1) that cross-reacts with murine VWF (Dako, Carpinteria, CA, USA), was used as the detection antibody. Pooled plasma from normal wild type C57BL6 mice was used as the standard. Plasma from VWF−/− mice was used as a negative control.
Phenotypic correction analysis
Phenotypic correction of the FVIIInull coagulation defect was assessed by the tail clip survival test as described in our previous reports.[3;4] For the BMT recipients, the tail clip was performed after at least 12 weeks of BM reconstitution in the chronic inhibitor model. For acute inhibitor model, tail clip was performed following immediately inhibitory plasma infusion.
Statistical analysis
The levels of FVIII:C or VWF:Ag are expressed as mean ± SD and the significance of differences between groups of mice was evaluated by 2-tailed Student t-test. The survival rates between different groups of recipients were evaluated by Fisher’s Exact Test. A value of P < 0.05 was considered statistically significant.
Results
Platelet-FVIII expression in F8−/− mice with varying VWF phenotypes
To address how VWF affects the clinical efficacy of platelet-derived FVIII in the inhibitor model, 2bF8 mice in the F8−/− background with varying VWF phenotypes were used in this study (see Table 1). 2bF8tg+/−F8−/−VWF+/+ or F8−/−VWF+/+ mice that received BMT from 2bF8tg+/−F8−/−VWF+/+ mice were used in the normal-VWF model. F8−/−VWF+/+ mice that received BMT from 2bF8tg+/−F8−/−VWF−/− mice were used in the plasma-VWF model. F8−/−VWF−/− mice that received BMT 2bF8tg+/−F8−/−VWF+/+ mice were used in the platelet-VWF model. 2bF8tg+/−F8−/−VWF−/− mice were used in the no-VWF model.
Table 1.
2bF8 mice with varying VWF phenotypes used in this study
| Type of VWF | Mice |
|---|---|
| Normal-VWF model |
|
| Plasma-VWF model | F8−/−VWF+/+ received BMT from 2bF8tg+/− F8−/−VWF−/− |
| Platelet-VWF model | F8−/−VWF−/− received BMT from 2bF8tg+/− F8−/−VWF+/+ |
| No-VWF model | 2bF8tg+/− F8−/−VWF−/− |
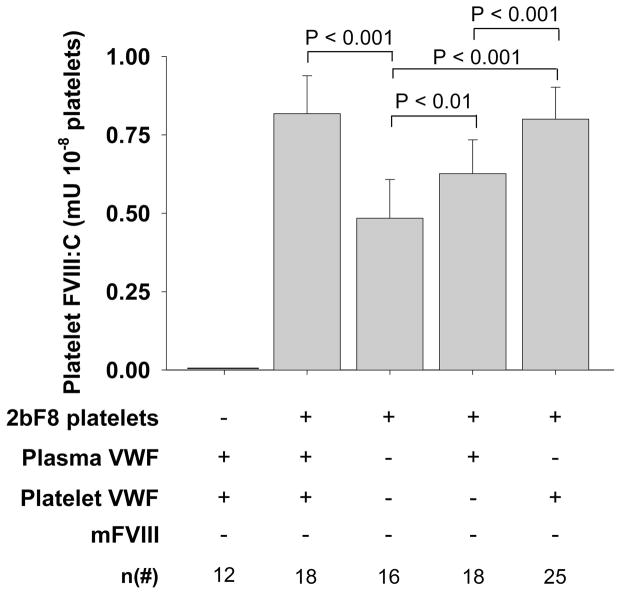
The level of platelet-FVIII in the normal-VWF model was 0.8 ± 0.1 mU per 108 platelets, which was not different from the platelet-VWF group, but significantly higher than the plasma-VWF group or the no-VWF group (Fig. 1). The level of platelet-FVIII expression in the platelet group was also significantly higher than the plasma group or the group with no VWF (Fig. 1). The presence of plasma-VWF results in significantly higher platelet-FVIII even in the absence of platelet-VWF (Fig. 1).
Fig. 1. Platelet FVIII expression in various 2bF8 mice with varying VWF phenotypes.
The levels of platelet-FVIII activity (FVIII:C) were quantitated by a chromogenic assay on platelet lysates. Platelets were collected from eye bleeds, washed, and lysed in 0.5% CHAPS. Recombinant human B-domain deleted FVIII (rhF8) was used as the standard. Data are expressed as mean ± SD. The results demonstrate that the level of platelet-FVIII is significantly decreased in 2bF8 mice when platelets lack VWF.
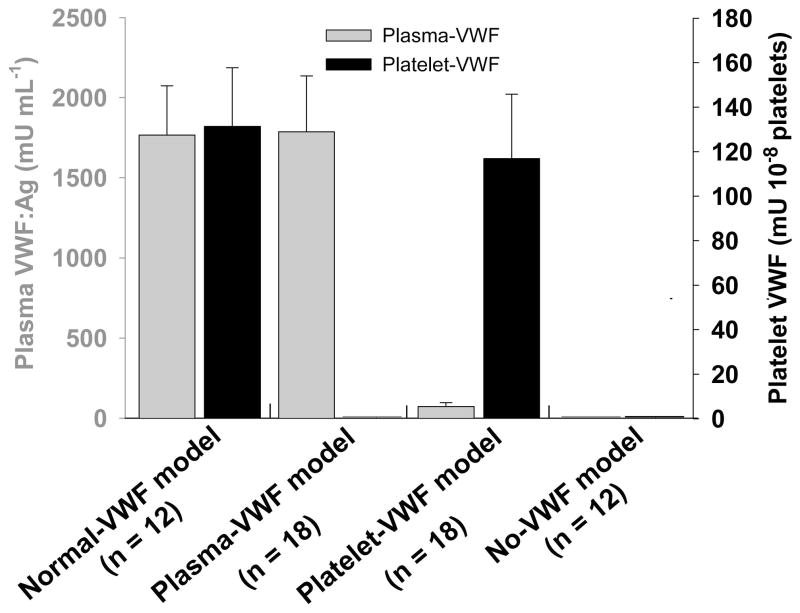
The level of plasma-VWF in the normal-VWF model was 1767 ± 307 mU mL−1, which was not significantly different from the plasma-VWF model. The level of platelet-VWF in the normal-VWF model was 131 ± 26 mU per 108 platelets, which was not significantly different from the platelet-VWF model. This level of platelet-VWF composes up to 40% of total VWF in whole blood in the normal-VWF model in which a mean platelet number was 4.36 ×108 mL−1. No VWF were detected in platelets from the plasma-VWF model, demonstrating that platelets do not take up plasma VWF. As expected, no VWF was detected in plasma or platelet lysates from the no-VWF model. However, 73 ± 24 mU mL−1 VWF was detected in plasma from the platelet-VWF model (Fig. 2). This level of VWF corresponds to 4% of plasma-VWF in the normal-VWF model.
Fig. 2. VWF expression in various VWF models.
The plasma- and platelet-VWF antigen levels were determined by ELISA assay. Pooled plasma from C57BL6 was used as the standard. Data are expressed as mean ± SD. Animals in the normal-VWF model had normal levels of VWF in both plasma and platelets. Animals in the plasma-VWF model had normal levels of plasma-VWF. Animals in the platelet-VWF model had normal levels of platelet-VWF. Animals in the no-VWF model had neither plasma- nor platelet-VWF. All the results were expected with the exception of approximately 4% plasma-VWF detected in the platelet-VWF group.
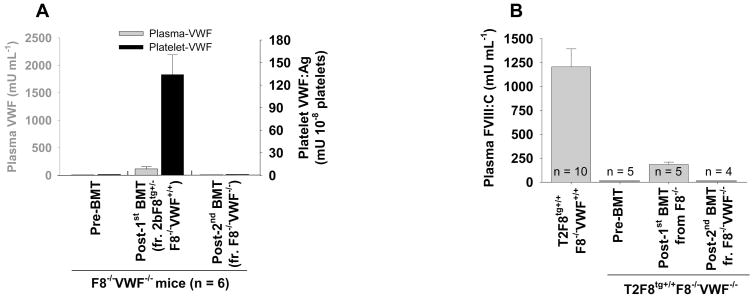
To explore whether the small amount of plasma-VWF in the platelet-VWF model was produced by endothelial cells derived from donor BM stem cells, we did a second BMT from F8−/−VWF−/− mice into the platelet-VWF model. As shown in Fig. 3A, both platelet-VWF and plasma-VWF were undetectable in recipients after the second BMT. To confirm that small amount of plasma-VWF in the platelet-VWF model was present in vivo, we transplanted BM from F8−/−VWF+/+ into T2F8tg+/+ F8−/− VWF−/−mice, which do not have detectable level of plasma-FVIII before transplantation.[27] As shown in Fig. 3B, plasma-FVIII was restored to 184 ± 25 mU mL−1, which corresponds to 15% of the levels in T2F8tg+/+ F8−/−VWF+/+ mice. This level of FVIII dropped to undetectable when a second BMT from F8−/− VWF−/− mice was performed (Fig. 3B). Taken together, these data suggest that small amount of plasma-VWF deriving from hematopoietic cells persists in vivo in the platelet-VWF model.
Fig. 3. The potential source(s) of the small amount of plasma-VWF in the platelet-VWF model.
To investigate whether this small amount of plasma-VWF was produced by donor BM-derived seeding endothelial cells, a second transplant was carried out using BM from F8−/−VWF−/− mice. After the second BMT, plasma-VWF dropped to undetectable (A). To confirm that the small amount of plasma-VWF was present in vivo, we transplanted BM from F8−/−VWF+/+ into T2F8tg+/+F8−/−VWF−/− mice. After BMT, a small percentage of FVIII in plasma was rescued in all recipients (B). These results demonstrate that the small amount of plasma-VWF in the platelet-VWF model is derived from platelet lineage cells and exists in vivo.
VWF affects the clinical efficacy of platelet-FVIII in inhibitor models
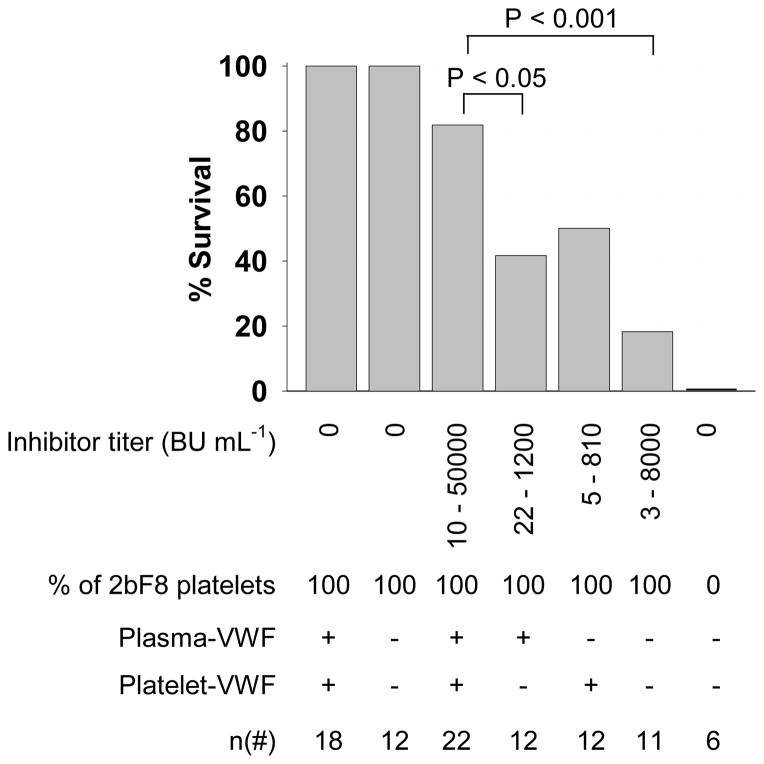
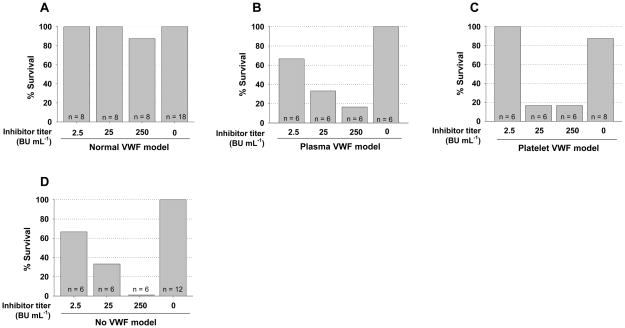
To investigate how VWF influences the clinical efficacy of platelet-derived FVIII in hemophilia A mice in the presence of inhibitors, two strategies for inhibitor model studies were used: 1) a chronic model generated by active immunization of animals with rhF8 and 2) an acute model established by infusion of plasma from highly immunized F8−/−VWF−/− mice. The tail clip survival test was used to assess the phenotypic correction of various 2bF8 mice with varying VWF phenotypes in the presence of inhibitors. As shown in Fig. 4, the results from the chronic model show that all 2bF8 transgenic mice survived tail clipping regardless of VWF in the absence of inhibitory antibodies. When both plasma- and platelet-VWF are present, 82% of animals with 10–50000 BU mL−1 inhibitor titer survived tail clipping. Forty-two percent of 2bF8 mice with plasma-VWF and 50% of mice with platelet-VWF survived tail clipping in the presence of inhibitors. Without VWF, only 18% of 2bF8 mice survived tail clipping with 3 to 8000 BU mL−1 inhibitors. None survived under the same challenge in F8−/−VWF−/− mice without platelet-FVIII. The tail clip survival rate in the normal-VWF model is significantly higher than the model without VWF (P < 0.01) or the plasma-VWF model (P < 0.05). The tail clip survival rate in the platelet-VWF model appears lower than the normal-VWF model, but there is no significant difference between two groups. These results demonstrate that VWF is essential for optimal platelet-FVIII gene therapy of hemophilia A with inhibitors.
Fig. 4. Phenotypic correction analysis of various 2bF8 mice with inhibitors (a chronic model).
The tail clip survival challenge was used to assess phenotypic correction of FVIIInull coagulation defect in various 2bF8 mice with varying VWF (plasma- and/or platelet-VWF) phenotypes in the presence of anti-FVIII inhibitory antibodies induced by the chronic model of active immunization with rhF8. These results indicate that VWF is essential in platelet-FVIII gene therapy of hemophilia A mice with inhibitors.
To investigate the dose effect of inhibitors on platelet-FVIII gene therapy of animals that have varying VWF distributions, we used an acute model with infusion of inhibitory plasma from immunized VWF and FVIII double knockout mice into 2bF8 mice with varying VWF phenotypes to various inhibitor levels followed by tail clip test. As shown in Fig 5A, all mice with normal VWF (normal platelet- and plasma-VWF) survived tail clipping with inhibitor titers of 2.5 and 25 BU/ml and 7 of 8 survived with inhibitor titers of 250 BU/ml. All control mice, which did not received infusion of inhibitory plasma, survived under the same tail clipping challenge. When inhibitory plasma was infused into 2bF8 mice with only plasma-VWF followed by tail clipping, as shown in Fig 5B, 4 of 6 mice with 2.5 BU/ml inhibitors survived tail clipping; 2 of 6 mice survived tail clipping with an inhibitor titer of 25 BU/ml; and 1 of 6 mice survived with an inhibitor titer of 250 BU/ml. As controls, all animals without infusion of inhibitory plasma survived tail clipping. When inhibitory plasma was infused into 2bF8 mice with only platelet-VWF followed by tail clipping, as shown in (Fig. 5C), all mice with 2.5 BU/ml inhibitors survived tail clipping; 1 of 6 mice survived tail clipping with inhibitor titers of 25 and 250 BU/ml. In contrast, 7 of 8 mice without inhibitors survived tail clipping. When inhibitory plasma was infused into 2bF8 mice with neither plasma- nor platelet-VWF followed by tail clipping, as shown in Fig. 5D, 4 of 6 mice with 2.5 BU/ml inhibitors survived tail clipping; 2 of 6 mice survived tail clipping with 25 BU/ml inhibitors. None survived with inhibitor titers of 250 BU/ml. All mice without inhibitors survived tail clipping.
Fig. 5. The dose effect of inhibitors on the clinical efficacy of platelet-derived FVIII in hemophilia A mice with varying VWF distributions (acute model).
Inhibitory plasma from rhF8-immunized F8−/− mice was infused into various 2bF8 mice with varying VWF phenotypes to 2.5, 25, or 250 BU mL−1 followed by the tail clip survival challenge. (A) Tail clip survival challenge of 2bF8 mice with normal-VWF in the presence of inhibitors. (B) Tail clip survival challenge of 2bF8 mice with plasma-VWF in the face of inhibitors. (C) Tail clip survival challenge of 2bF8 mice with platelet-VWF in the presence of inhibitors. (D) Tail clip survival challenge of 2bF8 mice with neither plasma- nor platelet-VWF in the presence of inhibitors. These data demonstrate that both plasma- and platelet-VWF are important for the optimal clinical efficacy of platelet-derived FVIII in hemophilia A with anti-FVIII inhibitors, especially high-titer inhibitors.
These acute dose effect model studies demonstrate that platelet-FVIII with either plasma- or platelet-VWF or even without VWF can still partially improve hemostasis in hemophilic mice in the presence of low titer inhibitors, but the efficacy is limited in the presence of higher titers of inhibitors.
Discussion
FVIII is associated with VWF in a tightly bound, non-covalently linked complex while inactive in blood circulation.[28] FVIII is dissociated from VWF when it is activated.[29–31] Without VWF, circulating FVIII is degraded rapidly by proteases.[32] The immune response to FVIII is highly heterogeneous.[33–35] While how antibodies inhibit FVIII coagulation activities is not fully understood, one pathway that has been well characterized is the interference of antibodies with the association between VWF and FVIII.[16;20;36] Our previous studies have demonstrated that VWF has a protective effect on FVIII from inhibitor inactivation both in vitro in a chromogenic-based Bethesda assay and in vivo in hemophilia A mouse models.[1] Our previous studies have also demonstrated that when FVIII expression is targeted to platelets, it stores together with endogenous VWF and that platelet-derived FVIII can maintain its clinical efficacy even in the presence of inhibitors.[2;6;7] In the current study, we show that VWF plays fundamental roles in platelet gene therapy of murine hemophilia A in the presence of inhibitors.
VWF, including both platelet-VWF and plasma-VWF, is required for optimal platelet-derived FVIII gene therapy of hemophilia A with inhibitors although our studies may under estimate the benefit of platelet FVIII because of platelet adherence differences in these models. Without VWF, the level of platelet-FVIII expression was significantly lower than the group with normal VWF, which is consistent with our previous findings.[2] Our data demonstrate that platelet-FVIII expression was optimized when platelet-derived VWF was present even without endothelial cell-derived VWF, suggesting that platelet-VWF is critical for optimal platelet-FVIII expression and storage in platelet α-granules. Interestingly, we found that platelet-FVIII expression was significantly higher even when there was only plasma-VWF, but no platelet-VWF, compared to the group of mice with neither platelet- nor plasma-VWF. The reason why the plasma-VWF appears to improve platelet-FVIII expression is unclear. While there was no detectable level of VWF in platelet lysates when VWF was solely derived from endothelial cells in the plasma-VWF model, it is likely that there was a trace amount of VWF, under the limit of detection but enough to stabilize the platelet-derived FVIII during sample processing, was present in the platelet samples.
A low level of VWF detected in the plasma of the platelet-VWF model mice, which is consistent with the findings in our previous report.[37] It has been shown that hematopoietic stem cells can give rise to endothelial cells in both in vitro and in vivo seeding in somatic tissues.[38–41] We explored whether the small amount of plasma-VWF in our platelet-VWF model mice was produced by donor derived endothelial cells after BMT. To this end, a second BMT from F8−/−VWF−/− mice was carried out on some of the platelet-VWF model mice. In theory, if the small amount of VWF was produced by donor BM-derived endothelial cells, VWF production should be maintained even after the second transplantation because donor BM-derived endothelial cells would already seeded in somatic tissues. However, plasma-VWF dropped to undetectable in the platelet-VWF model mice after the second transplantation, indicating that small amount of plasma-VWF in the platelet-VWF model was not produced by endothelial cells.
To further explore the source of this small amount of plasma VWF, we performed more extensive BMT experiments. We used T2F8 transgenic mice in the VWF and FVIII double knockout background as a model, in which FVIII is undetectable in plasma.[27] Our previous studies have shown that infusion of VWF into T2F8tg+/+F8−/−VWF−/− mice can rescue plasma FVIII in these animals.[27] We hypothesized that plasma FVIII in T2F8tg+/+F8−/−VWF−/− mice would be rescued if a small amount of plasma-VWF was present at all time in vivo in the platelet-VWF model. Indeed, when BM cells from F8−/−VWF+/+ were transplanted into T2F8tg+/+F8−/−VWF−/− mice, establishing a platelet-VWF model with endothelial cell-specific FVIII expression, FVIII levels in plasma were restored from undetectable to 15% of the levels in T2F8tg+/+F8−/−VWF+/+ mice (Fig. 3B). These data strongly suggest that, in the platelet-VWF model, the small amount of VWF detected in plasma was present in vivo and was not the artifactual result of platelet activation during sample processing. Thus, we conclude that this small amount of plasma-VWF in the platelet-VWF model is from hematopoietic cells. Whether it is secreted by megakaryocytes or platelets is still unclear.
Previous studies done by Schick and co-workers[42] have demonstrated that VWF is primarily synthesized in mature pig megakaryocytes, 7.5 times more than in immature megakaryocytes. 14.5% and 4.6% of total synthesized VWF was secreted into culture media by mature and immature pig megakaryocytes, respectively. In their tissue culture system, approximately 13% of synthesized VWF was secreted into culture media from megakaryocytes (both mature and immature). In contrast, 7% of total whole blood VWF was distributed in plasma and 93% in platelets in our in vivo platelet-VWF model. This difference could reflect species differences or in our platelet-VWF model mice, small amount of plasma VWF is secreted by platelets, rather than megakaryocytes.
Our studies show that both platelet- and plasma-VWF are important for optimal clinical efficacy of platelet-targeted FVIII gene therapy in hemophilia A with inhibitors. Without any VWF, platelet-FVIII can still restore hemostasis in hemophilia A mice in the absence of inhibitors. However, the clinical efficacy is limited in the presence of inhibitors. This is because there is no VWF available to associate with FVIII to form a protective complex. Thus, inhibitory antibodies can freely inactivate functional FVIII activity once it is released from platelets, with the exception of excessive amounts of FVIII released from accumulated activated platelets at the site of injury which can overcome a small amount of neutralizing antibodies. Indeed, in our acute inhibitor infusion study, 67% of 2bF8 mice in the no-VWF model survived tail clipping when a low dose of inhibitors were infused (Fig. 5D). This could be due to a limited amount of neutralizing antibodies in the circulation after antibody infusion. In contrast, in the chronic model, in which antibodies were constitutively produced in vivo by plasma cells, only 18% of 2bF8 mice with no VWF survived in the tail clip challenge.
When there was only plasma-VWF present, no platelet-VWF, the clinical efficacy of platelet-FVIII in the presence of inhibitors was significantly reduced even with a normal level of plasma-VWF. In the plasma-VWF model mice, albeit VWF is available to form a complex with FVIII when FVIII is released from accumulated activated platelets, inhibitory antibodies will compete with VWF to bind to newly released FVIII. While platelet-FVIII expression was only 0.63 mU per 108 platelets in the 2bF8 with plasma VWF model mice, 42% of animals survived tail clipping with inhibitor titers of 22–1200 BU mL−1. Our previous studies showed that none of the F8−/−VWF+/+ mice with a inhibitor titer of 25 BU mL−1 survived tail clipping even though animals were infused with rhF8 up to 2% in plasma.[2] Our data suggest that, in addition to the important role the association between VWF and FVIII plays in platelet-FVIII gene therapy of hemophilia A with inhibitors, the unique characteristic of platelet delivery, recruitment, and accumulation at sites of injury, releasing FVIII locally, is also crucial.
In the platelet-VWF model, the clinical efficacy of platelet-FVIII was attenuated from 82% to 50% in the chronic inhibitor model. In this model, although platelet-targeted FVIII expression is optimal and transgene protein FVIII is stored together with endogenous VWF in α-granules forming a VWF/FVIII complex before exposure to neutralizing antibodies against FVIII at sites of injury, plasma-VWF appears necessary for optimal clinical efficacy of platelet-FVIII in the inhibitor model, not from its interaction with FVIII but for its role in improving platelet adhesion. Through binding to the sub-endothelial matrix and linking to platelets, VWF is not only essential for initiating platelet adhesion, but also critical for platelet accumulation and aggregation in hemostasis at the site of vascular injury.[43] Although there was small amount of plasma-VWF present in the platelet VWF model mice, the properties of this plasma-VWF, which appears to derive from platelets or megakaryocytes, may be different from endothelial cell-derived VWF. Evidence suggests that the glycosylation profile and the functional properties of the platelet-derived VWF differ from those of the endothelial cell-derived plasma VWF.[44–46] Platelet-derived VWF demonstrates significantly lower binding affinity to platelet GPIbα compared to plasma VWF. Thus, in the platelet-VWF model, platelet adhesion and accumulation may be attenuated in the hemostasis, leading to less effectiveness of platelet-FVIII in hemophilia A mice in the presence of a high-titer of inhibitors. However, platelet-VWF does appear to improve the clinical efficacy of platelet-FVIII in the inhibitor model when the inhibitor titer is low (Fig. 5C).
In conclusion, our studies demonstrate that VWF is essential in platelet-derived FVIII gene therapy of hemophilia A in the presence of inhibitors. For optimal platelet-derived FVIII gene therapy of hemophilia A with inhibitors, both platelet- and plasma-VWF are required.
Acknowledgments
This work was supported by the National Institutes of Health grants HL-102035 (QS), HL-44612 (RRM), HL-33721 (RRM), and HL-81588 (RRM), American Heart Association National Center SDG 0730183N (QS), National Hemophilia Foundation CDA (QS), Hemophilia Association of New York grant (QS), the Children’s Hospital Foundation (QS), and the MACC Fund (QS).
Footnotes
Addendum
Q. Shi designed research, analyzed data, and wrote the manuscript; J. A. Schroeder and performed research, analyzed data and made comments to manuscript; E. L. Kuether performed research; R. A. Montgomery helped in research design and made comments to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Shi Q, Kuether EL, Schroeder JA, Perry CL, Fahs SA, Cox GJ, Montgomery RR. Factor VIII inhibitors: von willebrand factor makes a difference in vitro and in vivo. J Thromb Haemost. 2012 doi: 10.1111/j.1538-7836.2012.04902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, Desai D, Morateck PA, Gorski J, Montgomery RR. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116:1974–82. doi: 10.1172/JCI28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haberichter SL, Shi Q, Montgomery RR. Regulated release of VWF and FVIII and the biologic implications. Pediatr Blood Cancer. 2006;46:547–53. doi: 10.1002/pbc.20658. [DOI] [PubMed] [Google Scholar]
- 4.Shi Q, Wilcox DA, Fahs SA, Fang J, Johnson BD, DULM, Desai D, Montgomery RR. Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A. J Thromb Haemost. 2007;5:352–61. doi: 10.1111/j.1538-7836.2007.02346.x. [DOI] [PubMed] [Google Scholar]
- 5.DULM, Nurden P, Nurden AT, Nichols TC, Bellinger DA, Jensen ES, Haberichter SL, Merricks E, Raymer RA, Fang J, Koukouritaki SB, Jacobi PM, Hawkins TB, Cornetta K, Shi Q, Wilcox DA. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat Commun. 2013;4:2773. doi: 10.1038/ncomms3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Q, Fahs SA, Wilcox DA, Kuether EL, Morateck PA, Mareno N, Weiler H, Montgomery RR. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112:2713–21. doi: 10.1182/blood-2008-02-138214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuether EL, Schroeder JA, Fahs SA, Cooley BC, Chen Y, Montgomery RR, Wilcox DA, Shi Q. Lentivirus-mediated platelet gene therapy of murine hemophilia A with pre-existing anti-factor VIII immunity. J Thromb Haemost. 2012;10:1570–80. doi: 10.1111/j.1538-7836.2012.04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q, Kuether EL, Chen Y, Schroeder JA, Fahs SA, Montgomery RR. Platelet gene therapy corrects the hemophilic phenotype in immunocompromised hemophilia A mice transplanted with genetically manipulated human cord blood stem cells. Blood. 2014;123:395–403. doi: 10.1182/blood-2013-08-520478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 10.Terraube V, O’Donnell JS, Jenkins PV. Factor VIII and von Willebrand factor interaction: biological, clinical and therapeutic importance. Haemophilia. 2010;16:3–13. doi: 10.1111/j.1365-2516.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- 11.Peyvandi F, Garagiola I, Baronciani L. Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011;9 (Suppl 2):s3–s8. doi: 10.2450/2011.002S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428–37. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
- 13.Flood VH, Schlauderaff AC, Haberichter SL, Slobodianuk TL, Jacobi PM, Bellissimo DB, Christopherson PA, Friedman KD, Gill JC, Hoffmann RG, Montgomery RR. Crucial role for the VWF A1 domain in binding to type IV collagen. Blood. 2015 doi: 10.1182/blood-2014-11-610824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briet E, Reisner HM, Roberts HR. Inhibitors in Christmas disease. Prog Clin Biol Res. 1984;150:123–39. [PubMed] [Google Scholar]
- 15.Gadarowski JJ, Jr, Czapek EE, Ontiveros JD, Pedraza JL. Modification of the Bethesda assay for factor VIII or IX inhibitors to improve efficiency. Acta Haematol. 1988;80:134–8. doi: 10.1159/000205619. [DOI] [PubMed] [Google Scholar]
- 16.Gawryl MS, Hoyer LW. Inactivation of factor VIII coagulant activity by two different types of human antibodies. Blood. 1982;60:1103–9. [PubMed] [Google Scholar]
- 17.Jacquemin M, Benhida A, Peerlinck K, Desqueper B, Vander EL, Lavend’homme R, d’Oiron R, Schwaab R, Bakkus M, Thielemans K, Gilles JG, Vermylen J, Saint-Remy JM. A human antibody directed to the factor VIII C1 domain inhibits factor VIII cofactor activity and binding to von Willebrand factor. Blood. 2000;95:156–63. [PubMed] [Google Scholar]
- 18.Shima M, Scandella D, Yoshioka A, Nakai H, Tanaka I, Kamisue S, Terada S, Fukui H. A factor VIII neutralizing monoclonal antibody and a human inhibitor alloantibody recognizing epitopes in the C2 domain inhibit factor VIII binding to von Willebrand factor and to phosphatidylserine. Thromb Haemost. 1993;69:240–6. [PubMed] [Google Scholar]
- 19.Saenko EL, Shima M, Rajalakshmi KJ, Scandella D. A role for the C2 domain of factor VIII in binding to von Willebrand factor. J Biol Chem. 1994;269:11601–5. [PubMed] [Google Scholar]
- 20.Jacquemin M. Variable region heavy chain glycosylation determines the anticoagulant activity of a factor VIII antibody. Haemophilia. 2010;16:16–9. doi: 10.1111/j.1365-2516.2010.02233.x. [DOI] [PubMed] [Google Scholar]
- 21.Nachman R, Levine R, Jaffe EA. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977;60:914–21. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe EA, Hoyer LW, Nachman RL. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973;52:2757–64. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner DD. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993;70:105–10. [PubMed] [Google Scholar]
- 24.Bowie EJ, Solberg LA, Jr, Fass DN, Johnson CM, Knutson GJ, Stewart ML, Zoecklein LJ. Transplantation of normal bone marrow into a pig with severe von Willebrand’s disease. J Clin Invest. 1986;78:26–30. doi: 10.1172/JCI112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarovoi H, Nurden AT, Montgomery RR, Nurden P, Poncz M. Intracellular interaction of von Willebrand factor and factor VIII depends on cellular context: lessons from platelet-expressed factor VIII. Blood. 2005;105:4674–6. doi: 10.1182/blood-2004-12-4701. [DOI] [PubMed] [Google Scholar]
- 26.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–21. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 27.Shi Q, Fahs SA, Kuether EL, Cooley BC, Weiler H, Montgomery RR. Targeting FVIII expression to endothelial cells regenerates a releasable pool of FVIII and restores hemostasis in a mouse model of hemophilia A. Blood. 2010;116:3049–57. doi: 10.1182/blood-2010-03-272419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer LW. The factor VIII complex: structure and function. Blood. 1981;58:1–13. [PubMed] [Google Scholar]
- 29.Andersson LO, Brown JE. Interaction of factor VIII-von Willebrand Factor with phospholipid vesicles. Biochem J. 1981;200:161–7. doi: 10.1042/bj2000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogami K, Shima M, Nishiya K, Hosokawa K, Saenko EL, Sakurai Y, Shibata M, Suzuki H, Tanaka I, Yoshioka A. A novel mechanism of factor VIII protection by von Willebrand factor from activated protein C-catalyzed inactivation. Blood. 2002;99:3993–8. doi: 10.1182/blood.v99.11.3993. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman RJ, Wasley LC, Davies MV, Wise RJ, Israel DI, Dorner AJ. Effect of von Willebrand factor coexpression on the synthesis and secretion of factor VIII in Chinese hamster ovary cells. Mol Cell Biol. 1989;9:1233–42. doi: 10.1128/mcb.9.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss HJ, Sussman II, Hoyer LW. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s disease. J Clin Invest. 1977;60:390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulcher CA, de Graaf MS, Roberts JR, Kasper CK, Zimmerman TS. Localization of human factor FVIII inhibitor epitopes to two polypeptide fragments. Proc Natl Acad Sci U S A. 1985;82:7728–32. doi: 10.1073/pnas.82.22.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scandella D, DeGraaf MS, Mattingly M, Roeder D, Timmons L, Fulcher CA. Epitope mapping of human factor VIII inhibitor antibodies by deletion analysis of factor VIII fragments expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:6152–6. doi: 10.1073/pnas.85.16.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Healey JF, Parker ET, Barrow RT, Langley TJ, Church WR, Lollar P. The humoral response to human factor VIII in hemophilia A mice. J Thromb Haemost. 2007;5:512–9. doi: 10.1111/j.1538-7836.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 36.Jacquemin MG, Desqueper BG, Benhida A, Vander EL, Hoylaerts MF, Bakkus M, Thielemans K, Arnout J, Peerlinck K, Gilles JG, Vermylen J, Saint-Remy JM. Mechanism and kinetics of factor VIII inactivation: study with an IgG4 monoclonal antibody derived from a hemophilia A patient with inhibitor. Blood. 1998;92:496–506. [PubMed] [Google Scholar]
- 37.Kanaji S, Fahs SA, Shi Q, Haberichter SL, Montgomery RR. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J Thromb Haemost. 2012;10:1646–52. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 39.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 40.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 41.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–9. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 42.Schick PK, Walker J, Profeta B, Denisova L, Bennett V. Synthesis and secretion of von Willebrand factor and fibronectin in megakaryocytes at different phases of maturation. Arterioscler Thromb Vasc Biol. 1997;17:797–801. doi: 10.1161/01.atv.17.4.797. [DOI] [PubMed] [Google Scholar]
- 43.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120 (Suppl 1):S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams SB, McKeown LP, Krutzsch H, Hansmann K, Gralnick HR. Purification and characterization of human platelet von Willebrand factor. Br J Haematol. 1994;88:582–91. doi: 10.1111/j.1365-2141.1994.tb05077.x. [DOI] [PubMed] [Google Scholar]
- 45.Kagami K, Williams S, Horne M, Gralnick H. A preliminary analysis of platelet von Willebrand factor oligosaccharides. Nagoya J Med Sci. 2000;63:51–6. [PubMed] [Google Scholar]
- 46.McGrath RT, McRae E, Smith OP, O’Donnell JS. Platelet von Willebrand factor--structure, function and biological importance. Br J Haematol. 2010;148:834–43. doi: 10.1111/j.1365-2141.2009.08052.x. [DOI] [PubMed] [Google Scholar]