Abstract
Background
The incidence and etiology of sudden cardiac death (SCD) in athletes is debated with hypertrophic cardiomyopathy (HCM) often reported as the most common etiology.
Methods and Results
A database of all NCAA deaths (2003 – 2013) was developed. Additional information and autopsy reports were obtained when possible. Cause of death was adjudicated by an expert panel. There were 4,242,519 athlete-years (AY) and 514 total student athlete deaths. Accidents were the most common cause of death (257, 50%, 1:16,508 AY) followed by medical causes (147, 29%, 1:28,861 AY). The most common medical cause of death was SCD (79, 15%, 1:53,703 AY). Males were at higher risk than females 1:37,790 AY vs. 1:121,593 AY (IRR 3.2, 95% CI, 1.9-5.5, p < .00001), and black athletes were at higher risk than white athletes 1:21,491 AY vs. 1:68,354 AY (IRR 3.2, 95% CI, 1.9-5.2, p < .00001). The incidence of SCD in Division 1 male basketball athletes was 1:5,200 AY. The most common findings at autopsy were autopsy negative sudden unexplained death (AN-SUD) in 16 (25%) and definitive evidence for HCM was seen in 5 (8%). Media reports identified more deaths in higher divisions (87%, 61%, and 44%) while percentages from the internal database did not vary (87%, 83%, and 89%). Insurance claims identified only 11% of SCDs.
Conclusions
The rate of SCD in NCAA athletes is high, with males, black athletes and basketball players at substantially higher risk. The most common finding at autopsy is AN-SUD. Media reports are more likely to capture high profile deaths, while insurance claims are not a reliable method for case identification.
Keywords: sudden death, epidemiology, athlete, pathology
Introduction
Sudden cardiac death (SCD) in an athlete is a tragic event with far-reaching impact. Those directly impacted by SCD struggle to understand why more effective screening techniques were not utilized as part of preparticipation exams required of nearly all US athletes. The rationale for the current screening model is that although SCD is shocking and often highly publicized, it is relatively rare and therefore not cost-effective to invest additional resources toward prevention.1 However, the incidence of SCD in athletes in the US is vigorously debated with much of the variation attributable to study methodology, specifically the accuracy of case identification and ascertainment of the population studied (denominator).2
Estimates of the rate of SCD in athletes range from 1:3,000 athlete-years (AY) in National Collegiate Athletic Association (NCAA) Division I male basketball athletes3 to 1:917,000 AY reported in Minnesota high school athletes;4 a difference of over 300-fold. Traditional estimates of SCD incidence in US athletes are usually around 1:200,000 AY5, 6 although studies specifically examining college athletes show higher rates of SCD with a relatively consistent estimate of 1:43,000 – 1:67,000 AY.3, 7, 8 These SCD rates in college athletes represent a pooled risk of both high and low risk groups, with higher rates identified in male, black, and basketball athletes.3
The most common cause of SCD in athletes is also questioned. Hypertrophic cardiomyopathy (HCM) is identified as the leading cause of death by the US National Registry of Sudden Death in Athletes (USNRSDA).8-11 However, studies in athletes in other countries,12-16 the US military,17, 18 and in US college athletes19 have found autopsy-negative sudden unexplained death (AN-SUD) to be the most frequent finding associated with SCD. A precise understanding of the etiology of SCD is important to devise effective screening strategies.
This study examines the incidence and etiology of forensically confirmed SCD in NCAA athletes over ten years and is a continuation of five years of previously published data.3
Methods
The NCAA tracks participation data as well as the sex and ethnicity of more than 450,000 student-athletes annually. Deaths in NCAA athletes were identified during the school years (July 1 to June 30) from 2003-2004 to 2012-2013 through: 1) the NCAA Resolutions List, 2) the Parent Heart Watch database, and 3) NCAA insurance claims.
The NCAA Resolutions List is compiled annually to honor NCAA student-athletes who have died of any cause. It is created by monitoring of national media and by institutions voluntarily providing names to the NCAA after email solicitations in November of every school year. There are no causes of death associated with the NCAA Resolutions list. Parent Heart Watch (PHW) is a national nonprofit organization dedicated to the prevention and awareness of sudden cardiac arrest (SCA) in the young. PHW maintains an ongoing database from systematic search of media reports. The database was queried for SCA/SCD among athletes 17 to 24 years of age, and each case reviewed to determine if the athlete was a member of an NCAA team. All NCAA athletes are covered by the NCAA Catastrophic Injury Insurance Plan which provides a death benefit of $25,000 for athletes who die during a competition, practice or conditioning activity organized or supervised by the institution. Claims related to SCA/SCD during the study period were retrieved.
The data sources were combined into a single data set. Missing information regarding deaths was acquired through Internet searches and media reports, or emails and telephone calls to sports information directors, head or team athletic trainers, next-of-kin, coroners, medical examiners and physicians involved in the case. SCD was defined as a sudden unexpected death due to cardiac cause, or a sudden death in a structurally normal heart with no other explanation for death and a history consistent with cardiac-related death that occurred within 1 hour of symptom onset or an unwitnessed death occurring within 24 hours of the person having been alive. Unwitnessed deaths were not included as cardiac unless additional information such as autopsy, negative toxicology screen or other information was available that could verify the death was cardiac in nature.
Deaths were categorized broadly as accident, homicide, suicide, drug/alcohol overdose, or medical. If a drug overdose appeared to be intentional it was included as a suicide; if it was unknown or accidental the death was included in the drug/alcohol overdose category. The medical causes were further broken down into cardiac, cancer, heat stroke, sickle cell trait, sport-related head injury, meningitis, and other. If the cause of death could not be reasonably determined, it was recorded as “unknown.” Activity at time of death was listed as “exertional”, “rest “, “sleep”, or “unknown”. Demographic data in NCAA athletes were obtained from the NCAA Sports Sponsorship and Participation Rates Report20 and the NCAA Student-Athlete Ethnicity Report.21
Definitions for pathological determination of cause of death were agreed upon using previously accepted definitions.22-26 (Table 1). When more than one pathologic abnormality was present, the pathology most likely related to the athlete's death was considered primary. Autopsy reports were reviewed independently by a panel of experts consisting of 4 sports medicine physicians, a cardiovascular pathologist, a cardiomyopathy specialist, an adult and pediatric electrophysiologist, and a channelopathy/genetic specialist all with expertise in SCD in athletes. Differences of opinion were adjudicated through panel discussion. This study was approved by the Division of Human Subjects, University of Washington.
Table 1.
Guidelines for Pathological Diagnosis
| Hypertrophic Cardiomyopathy | Dilated Cardiomyopathy |
|---|---|
| •Heart weight > = 50% of expected mean based on gender, age, and body size for weight (using the Mayo nomograms) plus at least one of the following: ○ Histologic myocyte disarray, ○ Septal mitral valve contact lesion (implying systolic anterior motion of the anterior mitral valve leaflet), ○ Asymmetric LV hypertrophy, particularly ventricular septal - left ventricular free wall ratio ≥ 1.3 •Significant (>75% of the area of a section) myocyte disarray in a basal or mid-ventricular section but not meeting weight criteria. Arrythmogenic Cardiomyopathy •Gross fibrofatty replacement of either ventricular free wall (excluding anterior RV in older individuals •The fatty change should appear “infiltrative” with a perpendicular pattern with respect to the epicardial surface •Variable degrees of fibrosis, vacuolization, and/or lymphocytic myocarditis Idiopathic LVH/ Possible Cardiomyopathy •Heart weight > = 50% of the expected mean based on gender, age, and body size for weight (using the Mayo nomograms) •Heart weight < = 50% of the expected mean based on sex, age, and body size for weight (using Mayo nomograms), but with: ○ Features suggestive (but not diagnostic) of CM, including: LV wall > 16 mm, interstitial fibrosis (non-replacement type), significant histologic myocyte hypertrophy. ○ No specific features of CM |
•Heart weight > = 50% expected mean based on gender, age, and body size for weight (using the Mayo nomograms) without myocyte disarray ○ LV wall < 10 mm ○ Left ventricular chamber diameter > 3.0 cm (note: agonal dilatation should be excluded by examining for cell separation and other post-mortem artifact histologically) ■ If absolute chamber diameter not measured, then comments about gross chamber dilation (without agonal dilatation from autolysis) ○ Histologically, myocyte hypertrophy with variable interstitial fibrosis (usually pericellular-type) Autopsy Negative-Sudden Unexplained Death •Normal heart pathologically •No obvious explanation for death •Presumed arrhythmia Myocarditis Related •Active lymphocytic myocarditis ○ Inflammatory infiltrates of the myocardium with associated myocyte injury/necrosis •Borderline myocarditis ○ Inflammatory infiltrates of the myocardium without associated myocyte injury/necrosis. •Healed myocarditis Coronary Artery Abnormalities •Coronary artery anomalies •Myocardial bridging •Tunneled coronary arteries •Coronary artery dissections |
| Cardiomyopathy NOS | SCD due to Coronary Artery Disease |
| •Heart weight does not meet weight criteria •There are histologic changes such as hypertrophy or fibrosis •No/ minimal myocyte disarray not meeting criteria for HCM •Does not meet criteria for DCM •No pathologic features suggestive of HCM |
•Atherosclerotic coronary arteries > 70% lumen occlusion •More likely than not that this was primary cause of death Commotio Cordis •SCD after blunt trauma to the chest •No other cardiac pathology |
Data Analysis
Data were analyzed for overall death rate and etiology, as well as death rate according to sex, ethnicity, sport, and NCAA division. Incidence rates and 95% confidence intervals (CIs) were reported as incidence of death/AY. Incidence rates were also calculated for risk over 4 years representing the risk of an athlete death over a typical 4-year athletic career by multiplying the number of annual cases by 4 and using the same denominator. The relative risk of SCD and specific disease etiology was estimated with an incidence rate ratio (IRR). A priori analysis of the relative risk between white athletes vs. black athletes, male athletes vs. female athletes, risk between divisions, males vs. female basketball athletes and basketball athletes compared to other sports was performed based on previous work suggesting a higher rate of SCD in those groups. For cause of death comparison significant results were presented. A capture-recapture analysis was performed to estimate the number of deaths that may have been missed, allow for comparison of the capture rate between divisions and estimate potential intra-divisional bias. All p-values were two sided and significance set at p<0.05. Analysis was done in STATA 11.1 software (StataCorp, Texas, USA).
Results
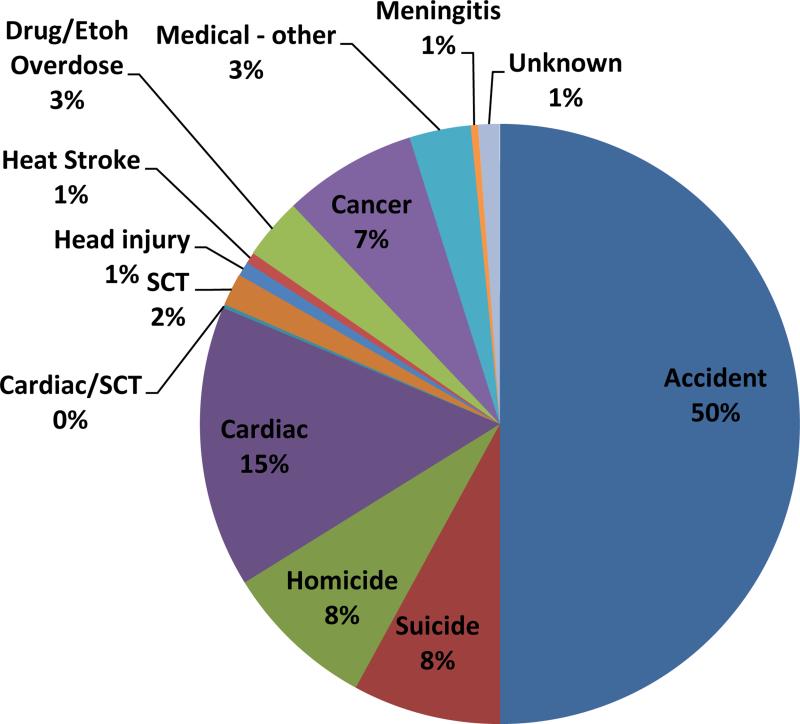
There were 4,242,519 million AY and 514 total student athlete deaths. Accidents accounted for 257 deaths (50%, 1:16,507 AY) followed by medical causes (147, 1:28,861 AY, 29%), homicide (42, 8%, 1:101,012 AY), and suicide (41, 8%, 1:103,476). The most common type of accidents were automobile (158, 62%, 1:26,851 AY), drowning (23, 9%, 1:184,457 AY), fall (18, 7%, 1:235,696 AY), and motorcycle (17, 7%, 1:249,560). The risk of death in an automobile accident for a male basketball player was 1:13,122 AY. (Table 2). The most common medical cause of death was cardiac (79, 15%, 1:53,703 AY). Sickle cell trait was associated with 10 deaths (2%, 1:424,252 AY), sport-related head injury 4 deaths (1%, 1:1,060,629 AY), and heat illness 3 deaths (0.6%, 1:1,414,173 AY). (Figure 1). Male athletes comprised 81% (64) of SCDs. 57% (45) of SCDs occurred in white athletes, 38% (30) in black athletes, and 15% (12) in other races. The majority of SCD occurred in basketball (21, 27%), football (18, 23%), and men's soccer (9, 11%).
Table 2.
Breakdown of accidental death type.
| Accident Type | Number of Cases | Percent of Accidents | Incidence per Athlete-Year* |
|---|---|---|---|
| automobile | 158 | 61.72% | 1 in 26,851 |
| drowning | 23 | 8.98% | 1 in 184,457 |
| fall | 18 | 7.03% | 1 in 235,696 |
| motorcycle | 17 | 6.64% | 1 in 249,560 |
| pedestrian | 6 | 2.34% | 1 in 707,086 |
| bus | 5 | 1.95% | 1 in 848,503 |
| head injury | 4 | 1.56% | 1 in 1,060,629 |
| plane | 4 | 1.56% | 1 in 1,060,629 |
| accidental overdose | 3 | 1.17% | 1 in 1,414,173 |
| skateboarding | 3 | 1.17% | 1 in 1,414,173 |
| fire | 2 | 0.78% | 1 in 2,121,256 |
| bike | 2 | 0.78°% | 1 in 2,121,256 |
| boating | 2 | 0.78°% | 1 in 2,121,256 |
| horse | 2 | 0.78% | 1 in 2,121,256 |
| snow mobile | 2 | 0.78% | 1 in 2,121,256 |
| electrocution | 1 | 0.39% | 1 in 4,242,519 |
| choking | 1 | 0.39% | 1 in 4,242,519 |
| jet ski | 1 | 0.39% | 1 in 4,242,519 |
| logging | 1 | 0.39% | 1 in 4,242,519 |
| other | 1 | 0.39% | 1 in 4,242,519 |
| total | 256 | 100.00% |
Total number of NCAA athlete-years is 4,242,519
Figure 1.
Causes of Death in NCAA Athletes 2003 – 2013.
Incidence of SCD
The overall incidence of SCD in NCAA athletes was 1:53,703 AY. Males were at higher risk than females (1:38,390 AY vs.1:121,593 AY; IRR 3.2, 95% CI, 1.8-6.0, p < 0.00001), black athletes at higher risk than white athletes (1:21,491,147 AY vs. 1:68,354 AY; IRR 3.2, 95% CI, 1.9-5.2, p < 0.00001), and black male athletes at higher risk than white male athletes (1:15,829 AY vs. 1:45,514 AY; IRR 2.9, 95% CI, 1.6-5.2, p =0.0001). There were 38 deaths reported in Division 1 athletes with a rate of SCD of 1:43,775 AY, compared to 22 deaths in Division II with a rate of 1:42,292 AY and 19 in Division III with a rate of 1:86,744 AY. (Table 3).
Table 3.
Incidence of sudden cardiac death in NCAA athletes.
| Characteristic | Athlete-Years | SCD | Incidence per Athlete-Year | IRR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Overall | 4,242,519 | 79 | 1 in 53,703 | - | - | - | |
| Sex | Male | 2,418,563 | 64 | 1 in 37,790 | 3.22 | 1.9 - 5.5 | >0.0001* |
| Female | 1.823,899 | 15 | 1 in 121,593 | 1.00 | Reference | ||
| Division | Division 1 | 1,663,441 | 38 | 1 in 43,775 | 1.98 | 1.1 - 3.6 | 0.0131* |
| Division 2 | 930,434 | 22 | 1 in 42,292 | 2.05 | 1.1 - 4.0 | 0.0231* | |
| Division 3 | 1,648,128 | 19 | 1 in 86,744 | 1.00 | Reference | ||
| Race | White | 3,075,942 | 45 | 1 in 68,354 | 1.00 | Reference | |
| Black | 644,715 | 30 | 1 in 21,491 | 3.18 | 1.9 - 5.2 | >0.0001* | |
| Hispanic | 168,763 | 3 | 1 in 56,254 | 1.22 | 0.2 - 3.8 | 0.6974 | |
| Other | 353,042 | 1 | 1 in 353,042 | 0.19 | 0.005 - 1.1 | 0.0491* | |
Basketball athletes (male and female) had the highest risk of SCD with a rate of 1:15,462 AY. Male basketball athletes were at significantly higher risk than female basketball athletes (1:8,978 AY vs. 1:77,061 AY; IRR 8.6, 95% CI, 2.1-76, p= 0.0003). There were 10 SCDs over 10 years in Division I male basketball athletes for a rate of 1:5,200 AY. (Table 4). Other sports at higher risk included men's soccer (1:23,689 AY), men's football (1:35,951 AY), and men's/women's cross country (1:44,973 AY). (Table 5) The incidence of SCD over an athletes’ NCAA career (considered to be 4 years) was 1:13,426 (A4Y). The risk of SCD over 4 years in a men's basketball athlete was 1:2,245 A4Y, and in a Division I male basketball player 1:1,300 A4Y. The career risk of SCD in a male soccer player was 1:5,922 A4Y. (Table 4, 5).
Table 4.
Incidence of sudden cardiac death in male basketball athletes
| Group | Black SCD | Black Athlete-Years | SCD Incidence in Blacks per Athlete-Year | Incidence in Blacks over 4 year career | White SCD | White Athlete-Years | SCD Incidence in Whites per Athlete-Year | Incidence in Whites over 4 year career | Total SCD | Total Athlete-Years | Total SCD Incidence per Athlete-Year | Incidence over 4 year career |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Division I male basketball | 7 | 30,660 | 1 in 4,380 | 1 in 1,095 | 3 | 15,689 | 1 in 5,230 | 1 in 1,307 | 10 | 51,995 | 1 in 5,200 | 1 in 1,300 |
| Division II male basketball | 3 | 24,723 | 1 in 8,241 | 1 in 2,060 | 1 | 18,016 | 1 in 18,016 | 1 in 4,504 | 4 | 47,530 | 1 in 15,843 | 1 in 3,961 |
| Division III male basketball | 4 | 19,623 | 1 in 4,906 | 1 in 1,227 | 1 | 46,368 | 1 in 46,368 | 1 in 11,592 | 5 | 71,332 | 1 in 14,266 | 1 in 3,567 |
| Overall male basketball | 14 | 74,866 | 1 in 5,348 | 1 in 1,337 | 5 | 79,972 | 1 in 15,994 | 1 in 3,999 | 19 | 170,590 | 1 in 8,978 | 1 in 2,245 |
Table 5.
Incidence of sudden cardiac death in sports.
| Sport | Athlete Deaths | Athlete-Years | Incidence per Athlete-Year | Incidence over 4-year career |
|---|---|---|---|---|
| Men's basketball | 19 | 170,590 | 1 in 8,978 | 1 in 2,245 |
| Men's soccer | 9 | 213,205 | 1 in 23,689 | 1 in 5,922 |
| Men's Football | 18 | 647,125 | 1 in 35,951 | 1 in 8,988 |
| Men's Swimming | 2 | 85,568 | 1 in 42,784 | 1 in 10,696 |
| Men's Cross-country | 3 | 128,570 | 1 in 42,857 | 1 in 10,714 |
| Men's Lacrosse | 2 | 91,699 | 1 in 45,850 | 1 in 11,463 |
| Women's Cross-country | 3 | 141,268 | 1 in 47,089 | 1 in 11,772 |
| Women's Volleyball | 3 | 147,653 | 1 in 49,217 | 1 in 12,304 |
| Men's Baseball | 6 | 300,137 | 1 in 50,023 | 1 in 12,505 |
| NCAA Athletes | 79 | 4,242,519 | 1 in 53,703 | 1 in 13,426 |
| Women's Swimming | 2 | 115,221 | 1 in 57,611 | 1 in 14,402 |
| Women's basketball | 2 | 154,121 | 1 in 77,061 | 1 in 19,265, |
| Men's track | 2 | 241,041 | 1 in 120,521 | 1 in 30,130 |
* Sports with one death: men's crew, women's golf, women's softball, women's tennis, men's tennis, women's track, wrestling, women's lacrosse
□ Other sports had no identified SCDs
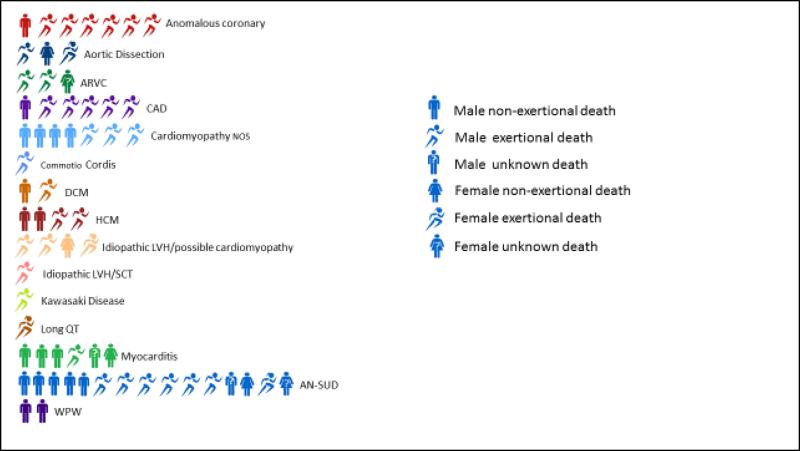
Activity at time of death could be characterized in 72 of the 79 SCDs. 56% occurred with exertion, 22% at rest, 14% during sleep, and activity in 9% was unknown. SCDs were more likely to be reported in the PHW (media) database if they occurred with exertion compared to rest or sleep (82% vs. 61%, p=0.02). The NCAA Resolutions List identified 86% of deaths, the PHW database 70%, and insurance claims 11%. Media reports identified more deaths in Division I athletes than in Division II or III (87%, 61%, 44%, p=0.003) while percentages in the NCAA Resolutions List did not vary (87%, 83%, 89%, p=0.84).
The total number of SCD cases in the aggregate dataset was 79. 23 deaths were identified only by the NCAA Resolutions List, 11 only by the PHW database, and 45 were in both. Capture-recapture analysis estimated the number of deaths at 83.1 (95% CI, 79.9 - 85.5) resulting in a SCD incidence of 1:51,046 AY. 38 SCDs were identified in Division I with 5 cases identified solely by the NCAA Resolutions List, 5 only by the PHW database, and 28 were listed in both datasets. The capture-recapture estimate for number of deaths in Division I was 38.9 (95% CI, 36.5 - 39.9). 22 SCDs occurred in Division II athletes; 8 were identified only in the NCAA Resolutions List, 3 only in the PHW database and 11 in both databases. The capture-recapture estimated number of deaths in Division II was 24.2 (95% CI, 22.7 - 25.3). In Division III there were 19 deaths identified while the capture-recapture analysis estimated 21.3 cases (95% CI, 19.4 - 24.0). 10 SCDs were identified only by the NCAA Resolutions List, 3 only by the PHW database, and 6 were identified by both lists.
Etiology of SCD
69 autopsies were initially obtained because of a history suggesting SCD or an unknown history. 11 cases were classified as non-cardiac including drowning (3), overdose (3), heat illness, SCT, pneumonia, gun shot, and a fall. Of the 514 deaths, information to assign a cause could not be ascertained in 6 (1%), and these were labeled as “unknown”.
SCD was identified by autopsy in 58 (73%) cases. All 58 cases had toxicology screens which were negative for substances which contributed to death. 21 additional cases were classified as SCD based on information in the death certificate (2), a media or legal report of the autopsy (4), a strong family or personal history of a cardiac disorder and history consistent with SCD (i.e. Long QT Syndrome or Wolff-Parkinson-White) (3), cases where the coroner, medical examiner or medical team confirmed a cardiac cause of death (8), cases where the next-of-kin provided a verbal report of the autopsy diagnosis (1), or a history consistent with commotio cordis (1). 3 additional cases were considered cardiac based on a history of exertional collapse with no other explanation. (Table 6)
Table 6.
Source of information for determination of cause of death
| Source of Information | Number of Sudden Cardiac Deaths |
|---|---|
| Autopsy confirmed | 58 |
| Coroner/medical examiner/medical team | 8 |
| Medical/legal report of autopsy | 4 |
| Personal/Family history and history consistent with SCD | 3 |
| Exertional collapse without other explanation | 3 |
| Discussion with next of kin | 1 |
| Death certificate | 2 |
| Total | 79 |
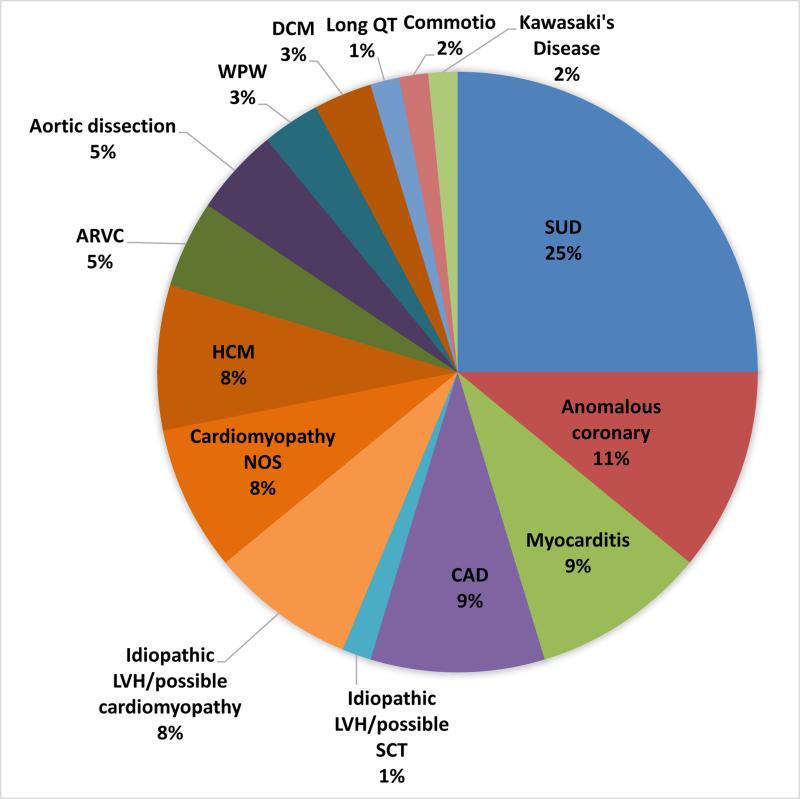
A specific cardiac etiology was assigned in 64 cases (81%) for which there was either autopsy (57) or other (7) information with which the cause could be determined. (Figure 2A, 2B). The most common finding in athletes with SCD was a structurally (gross and histologically) normal heart (AN-SUD) in 16 (25%), followed by coronary artery anomalies (CAA) (7, 11%), myocarditis (6, 10%), and coronary artery disease (CAD) (6, 10%). There were 5 cases (8%) each of HCM, idiopathic LVH/possible cardiomyopathy, and cardiomyopathy NOS. A single case in an athlete with sickle cell trait, a history of “cardiac problems”, and significant LVH could not be defined as either idiopathic LVH or exertional death due to SCT and therefore was defined as “SCT/idiopathic LVH”.
Figure 2.
A. Etiologies of sudden cardiac death in athletes. B. Etiology and activity at time of death*. *One person figure equals one death; female figures follow male figures unless no male deaths were present.
Basketball athletes were more likely to die of cardiomyopathy or possible cardiomyopathy (HCM, dilated cardiomyopathy, cardiomyopathy NOS, idiopathic left ventricular hypertrophy/possible cardiomyopathy) than other male athletes (IRR 14.82; 95% CI, 5.08 - 44.16; p <0.0001). Black athletes were more likely to die from a cardiomyopathy than white athletes (IRR 4.77; 95% CI, 2.06 - 11.01; p=0.002). Male basketball players were also more likely to die of CAA compared to other male athletes (IRR 17.57; 95% CI, 5.93 - 52.02; p=0.0008) and black athletes more likely than white athletes (IRR 9.54; 95% CI, 2.39 - 38.10; p=0.01).
Discussion
The rate of SCD in NCAA athletes is high, with males, black athletes, and male basketball players at the highest risk. HCM has long been reported to be the most common finding after SCD, however, in this study the most common finding at autopsy was AN-SUD similar to findings in the military17, 18 and other countries.13-16, 27 Media reports are more likely to capture high profile deaths in Division I or II athletes compared to Division III and using insurance claims to identify SCD in athletes missed almost 90% of cases. The methods used for identification of SCD cases significantly affects incidence.
A strength of this study was the use of three different data sources to create a comprehensive database of death from any cause in order to identify SCD and compare the relative frequency of causes of death in NCAA athletes. Automobile accidents were the leading cause of death (1:26,851 AY), although these were only twice as frequent as SCD (1:53,507 AY). It has been previously reported that automobile-associated accidents are 150 – 2500 times more frequent than SCD in NCAA athletes, however this is comparing the absolute number of automobile deaths in the entire US population to the absolute number of cardiovascular deaths in NCAA athletes.1, 28 Male basketball athletes were actually more likely to die from SCD (1:8,978 AY) than from an automobile accident (1:13,122 AY).
A prior study in college athletes has shown suicide and drug over-dose (combined) to represent a larger proportion of total athlete deaths than cardiovascular causes, however, in that study, deaths due to automobile accidents, homicide, cancer or systemic disease were not included in the denominator and the total number of deaths (denominator) was 182 compared to 514 in this study.8 The actual rate of suicide per athlete year in that study was 1:130,721 AY, and drug-overdose 1:192,970 AY which is similar to this study where the suicide rate was 1:103,476 AY and drug over-dose rate of 1:414,173 AY. In our study intentional overdoses were included in the “suicide” category and if it was clearly unintentional or unknown the cases was included in “drug-overdose” which may account for some of the difference between drug overdose rates. One of the benefits of looking at all-cause mortality in this population is the ability to make direct comparisons of risk.
This study presents incidence rates per athlete-year, however, it has been suggested that looking at the 4 year time frame of an average college athlete's career is a more accurate way to consider risk. For example the risk of SCD in an entering Division I male basketball player over the span of an average 4 year career is 1 in 1,300 AY while the rate of SCD for that same time frame in male soccer players 1 in 5,922 AY and in male football players 1 in 8,988 AY. (Table 4, 5). Not all athletes will participate 4-years, some competing a shorter time and some longer, which is a limitation when considering incidence rates in this manner.
A preparticipation physical evaluation (PPE) is currently required of all NCAA athletes prior to participating in sport29. The current AHA recommendation for cardiovascular screening consists of a 14-element personal and family history and cardiovascular physical exam.1 The European Society of Cardiology,30 International Olympic Committee,31 Fédération Internationale de Football Association, and all US professional leagues recommend a more advanced screen including a 12-lead resting electrocardiogram (ECG). No method of screening for cardiovascular disease will identify all cases, however, all of the athletes who died in this study had a PPE.
The relative infrequency of SCD compared to other causes of death has been maintained by the American Heart Association (AHA) in support of traditional screening strategies.1, 8 However, SCD was the second leading cause of death after accidents and the leading medical cause of death. SCD was much more common than other causes of death, such as sickle cell trait (SCT), heat illness and brain injury, which have received considerable attention. SCT accounts for only 2% of student athlete deaths (1:471,391 AY) about 9 times less than SCD. Yet, during the preparticipation exam, all NCAA athletes are required to provide confirmation of SCT status, and either undergo SCT testing or sign a release declining testing.29 Heat illness accounts for less than 1% of deaths in college athletes over the last ten years (1:1,060,630). Pre-season conditioning rules mandating limits on practice and gradual acclimation, heat education awareness efforts by the NCAA, and the availability of trained medical staff in the college setting at most practices where heat illness may occur may have decreased the rate of death due to heat illness. Similarly, sports-related traumatic brain injury, currently the focus of intense scrutiny, accounts for only 4 deaths (1%, 1:1,060,630) in the past decade. All deaths in young athletes are tragic and opportunities to prevent these deaths should be considered in terms of the frequency in the population and the ability to potentially change outcome.
In this study the most common finding at death was AN-SUD (16, 25%) while definitive HCM was found in only 5 athletes (8%). This differs from other reports relying on the USNRSDA for case identification.8-11 A recent report on college athletes using both the NCAA Resolutions List and the information on NCAA athletes from the USNRSDA during a similar time period (2002 – 2011) examined 47 autopsies with sufficient information for cause of death determination; 21 reported to have HCM.8 The reason for this discrepancy between studies is unclear but may be due to different criterion for pathological diagnosis or ascertainment bias of cases. In our study the cause of death was determined by an expert panel including a cardiac pathologist, whereas autopsy reports were reviewed by only one researcher from the USNRSDA and there was no description of the pathological criterion used for categorization.
A heart weight > 500 grams has been used in the past as a criteria for HCM, however, because many athletes are larger than average, it has become standard practice to use nomograms which are scaled to body weight to determine cutoffs for enlarged hearts.22, 25, 32, 33 The thickness of the ventricular septum (VS) is another measure used to make the diagnosis in living patients with HCM. However, VS measurements after death with the heart muscle contracted in rigor mortis do not directly correlate with echocardiographic measurements which are measured in diastole in the living and cannot be used at autopsy to define HCM. We used a ventricular septal to ventricular free-wall ratio of > 1.3 as one of the possible criteria for categorization as HCM. The mean heart weight in the USNRSDA study was reported to be 563 +/− 70 grams and mean ventricular septal (VS) thickness 22 +/− 4 mm. In our study, there were 57 autopsies deemed adequate to determine diagnosis and 21 hearts over 500 grams. The diagnosis of HCM in our study was given even if a heart was under the weight cutoff but had other features consistent with HCM (i.e. histology demonstrating cardiomyocyte disarray). 16 cases of some type of cardiomyopathy or possible cardiomyopathy were diagnosed in this study with a mean heart weight of 513 +/−96 grams and post-mortem IVS thickness of 17 +/− 5.7mm. Some of these cases may have represented HCM and did not fulfill formal criteria for categorization or have enough information to categorize as HCM. Although different criteria may have been used to determine cause of death between studies, the differences in mean heart weight and IVS thickness suggest that there were additional cases of HCM not included in our cohort. Given the USNRSDA is based out of the Hypertrophic Cardiomyopathy Center at the Minneapolis Heart Institute Foundation, there is the possibility of ascertainment bias with respect to the cases collected. This also suggests that there are missed cases in our cohort and the incidence of SCD and SCD due to HCM is higher than reported.
Two deaths were attributed to WPW which is often considered benign. WPW can be associated with SCD in the presence of atrial fibrillation with a short refractory period bypass tract. The risk of sudden death associated with asymptomatic WPW in most population based studies is 0.1% per year in adults.34 There is evidence to suggest a higher risk of sudden death in asymptomatic children and younger adults with WPW.35-37 In this cohort one athlete with WPW had previous SCA, had been resuscitated and had an ablation. Nine months later he was found deceased in his bed presumably secondary to repeat arrhythmia. He was not a known drinker or drug user and had a negative toxicology screen. The other case had a known clinical history of WPW and also died in his sleep. Other cases of WPW may have been present but undiagnosed in the AN-SUD group.
Athlete groups at higher risk of SCD are male athletes and black athletes, as well as basketball, football, and men's soccer athletes. Male basketball players have the highest incidence of SCD at 1:8,978 AY, and the risk in Division I male basketball athletes was 1:5,200 AY, more than 10 times the risk of the overall athlete population. Data from the USNRSDA detailed death in 23 basketball athletes due to cardiovascular causes while this study identified only 21. It is unclear why basketball players are at higher risk for SCD, however, this is consistent with earlier findings in college and high school athletes.3, 38, 39 Basketball athletes were nearly 15 times more likely to die from cardiomyopathy, suggesting many of these athletes could be identified by a preparticipation ECG.40-44 There was no notable difference in SCD rates between black and white Division I basketball players suggesting there may be something intrinsic about the demands of basketball beyond the ethnic/racial make-up of the sport that predisposes to SCD. Basketball also may select out a certain body habitus, and in fact, 2 of the 3 cases of Marfan Syndrome were in basketball players. However, Marfan Syndrome accounts for only a small fraction of deaths overall. This finding requires more study.
Many studies rely on media reports for identification of SCD cases. In this study, the media database detected 70% of the total cases. In a Denmark study only 20% of athlete deaths identified by death certificates were found by an extensive media search15 and in later Denmark study using the same methods, only 5% of sports-related sudden death were identified through media search.45 Interestingly, there was a substantially higher proportion of cases identified by the media database in Division I athletes compared to Division II and Division III while the proportion identified in the Resolutions List did not vary between division, suggesting that higher profile deaths are more likely to be reported in the media.
Insurance claims are sometimes used as a way to identify SCD.4-6 Most catastrophic injury plans only cover deaths during school sponsored practices or games. Only 11% of the deaths in this study were detected using catastrophic insurance claims, consistent with a study in Minnesota High school athletes where catastrophic insurance claims identified only 14% of deaths reported in the media.46 Prevention measures are typically aimed at preventing injury or death at any time; therefore, developing prevention programs with data from a limited scope poses inherent flaws.
Limitations
Although 3 different data sources were used, it is likely cases were missed. Data from the USNRSDA suggests that cases of HCM and basketball athletes were not identified in this cohort and the reported incidences may be higher. In addition, accurate determination of cause of death in this study relied heavily on the information provided by autopsy reports. The expertise of the examiner conducting the autopsy, the quality of autopsies and the information provided varied considerably. The pathologic search for all potential causes of SCD in a young athlete requires specific expertise and dedicated protocols, which are not usual parts of the curriculum in forensic medicine in most countries. We attempted to mitigate this effect by having an expert panel review all available information to reach a consensus diagnosis and prevent bias. In addition, we do not know the absolute number of athletes which participated as some athletes may have participated in more than one sport. At the college level this is not typical and likely incidental. We also do not have the ability to directly compare these estimated SCD rates in athletes to comparable rates in non-athletes, where reporting and detection is likely less vigilant, and methodologies differ.
Conclusions
The estimated rate of SCD in NCAA athletes has remained similar over the last 10-years and is approximately 1:50,000.3 High risk groups include males, black athletes, and basketball athletes. The most common finding in this cohort at autopsy after SCD was AN-SUD, and there were fewer cases of definitive HCM than previously described. Media reports are more likely to capture high profile deaths and lower divisions may be underrepresented in this study. Moving toward a standardized autopsy with involvement of a cardiovascular pathologist and utilization of molecular diagnostic tests will improve accuracy. Although institutional resources vary and may not be widely available to institute large scale advanced cardiovascular screening in all athletes, strong consideration should be given for additional cardiac testing beyond the traditional history and physical in higher risk groups, in particular, male basketball players.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the assistance of Parent Heart Watch and the National Collegiate Athletic Association in data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding Sources: Dr. Owens was supported by the National Center for Advancing Translational Sciences of the NIH under Award Number KL2TR000421.
Footnotes
Disclosures: No authors have any conflicts of interest other than Jordan M. Prutkin has grants from Boston Scientific and St. Jude Medical, and Michael J. Ackerman is a consultant for Boston Scientific, Gilead Sciences, Medtronic, St. Jude Medical and receives royalties from Transgenomic.
References
- 1.Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, Okin PM, Saul JP, Salberg L, Van Hare GF, Soliman EZ, Chen J, Matherne GP, Bolling SF, Mitten MJ, Caplan A, Balady GJ, Thompson PD. Assessment of the 12-lead ecg as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 years of age): A scientific statement from the american heart association and the american college of cardiology. Circulation. 2014;130:1303–1334. doi: 10.1161/CIR.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 2.Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: A state-of-the-art review. Br J Sports Med. 2014;48:1185–1192. doi: 10.1136/bjsports-2014-093872. [DOI] [PubMed] [Google Scholar]
- 3.Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in national collegiate athletic association athletes. Circulation. 2011;123:1594–1600. doi: 10.1161/CIRCULATIONAHA.110.004622. [DOI] [PubMed] [Google Scholar]
- 4.Roberts WO, Stovitz SD. Incidence of sudden cardiac death in minnesota high school athletes 1993-2012 screened with a standardized pre-participation evaluation. J Am Coll Cardiol. 2013;62:1298–1301. doi: 10.1016/j.jacc.2013.05.080. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in minnesota high school athletes. J Am Coll Cardiol. 1998;32:1881–1884. doi: 10.1016/s0735-1097(98)00491-4. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Haas TS, Ahluwalia A, Rutten-Ramos SC. Incidence of cardiovascular sudden deaths in minnesota high school athletes. Heart Rhythm. 2013;10:374–377. doi: 10.1016/j.hrthm.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Drezner JA, Rogers KJ, Zimmer RR, Sennett BJ. Use of automated external defibrillators at ncaa division i universities. Med Sci Sports Exerc. 2005;37:1487–1492. doi: 10.1249/01.mss.0000177591.30968.d4. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Haas TS, Murphy CJ, Ahluwalia A, Rutten-Ramos S. Incidence and causes of sudden death in u.S. College athletes. J Am Coll Cardiol. 2014;63:1636–1643. doi: 10.1016/j.jacc.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Profile and frequency of sudden death in 1463 young competitive athletes: From a 25 year u.S. National registry: 1980-2005. Circulation. 2006;114(II):830. [Google Scholar]
- 10.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes. Analysis of 1866 deaths in the united states, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. Jama. 1996;276:199–204. [PubMed] [Google Scholar]
- 12.Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. doi: 10.1016/s0008-6363(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 13.Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA. Sudden death in the young. Heart Rhythm. 2005;2:1277–1282. doi: 10.1016/j.hrthm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Solberg EE, Gjertsen F, Haugstad E, Kolsrud L. Sudden death in sports among young adults in norway. Eur J Cardiovasc Prev Rehabil. 2010;17:337–341. doi: 10.1097/HJR.0b013e328332f8f7. [DOI] [PubMed] [Google Scholar]
- 15.Holst AG, Winkel BG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Svendsen JH, Haunso S, Prescott E, Tfelt-Hansen J. Incidence and etiology of sports-related sudden cardiac death in denmark--implications for preparticipation screening. Heart Rhythm. 2010;7:1365–1371. doi: 10.1016/j.hrthm.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Papadakis M, Sharma S, Cox S, Sheppard MN, Panoulas VF, Behr ER. The magnitude of sudden cardiac death in the young: A death certificate-based review in england and wales. Europace. 2009;11:1353–1358. doi: 10.1093/europace/eup229. [DOI] [PubMed] [Google Scholar]
- 17.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: A 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 18.Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo-Rojas LM, Avedissian L, Pearse LA, Potter RN, Tremaine L, Gentlesk PJ, Huffer L, Reich SS, Stevenson WG. Sudden death in young adults: An autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. doi: 10.1016/j.jacc.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 19.Harmon KG, Drezner JA, Maleszewski JJ, Lopez-Anderson M, Owens D, Prutkin JM, Asif IM, Klossner D, Ackerman MJ. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ Arrhythm Electrophysiol. 2014;7:198–204. doi: 10.1161/CIRCEP.113.001376. [DOI] [PubMed] [Google Scholar]
- 20.Irick E. Ncaa sport sponsorship and participation rates. 2013 [Google Scholar]
- 21.Irick E. Ncaa student athlete ethnicity report. 2013 [Google Scholar]
- 22.Basso C, Burke M, Fornes P, Gallagher PJ, de Gouveia RH, Sheppard M, Thiene G, van der Wal A. Guidelines for autopsy investigation of sudden cardiac death. Virchows Arch. 2008;452:11–18. doi: 10.1007/s00428-007-0505-5. [DOI] [PubMed] [Google Scholar]
- 23.Basso C, Calabrese F, Corrado D, Thiene G. Postmortem diagnosis in sudden cardiac death victims: Macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001;50:290–300. doi: 10.1016/S0008-6363(01)00261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. Hrs/ehra expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the heart rhythm society (hrs) and the european heart rhythm association (ehra). Heart rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Loporcaro CG, Tester DJ, Maleszewski JJ, Kruisselbrink T, Ackerman MJ. Confirmation of cause and manner of death via a comprehensive cardiac autopsy including whole exome next- generation sequencing. Arch Pathol Lab Med. 2014;138:1083–1089. doi: 10.5858/arpa.2013-0479-SA. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard MN. Br J Sports Med. Vol. 46. Suppl 1: 2012. Aetiology of sudden cardiac death in sport: A histopathologist's perspective. pp. i15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–1963. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Maron BJ, Winkel BG, Tfelt-Hansen J. Perspectives on cardiovascular screening. JAMA. 2015;313:31–32. doi: 10.1001/jama.2014.16253. [DOI] [PubMed] [Google Scholar]
- 29.Ncaa sports medicine handbook 2013-14. 2014 [Google Scholar]
- 30.Corrado D, Pelliccia A, Bjornstad HH, Vanhees L, Biffi A, Borjesson M, Panhuyzen- Goedkoop N, Deligiannis A, Solberg E, Dugmore D, Mellwig KP, Assanelli D, Delise P, van- Buuren F, Anastasakis A, Heidbuchel H, Hoffmann E, Fagard R, Priori SG, Basso C, Arbustini E, Blomstrom-Lundqvist C, McKenna WJ, Thiene G. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: Proposal for a common european protocol. Consensus statement of the study group of sport cardiology of the working group of cardiac rehabilitation and exercise physiology and the working group of myocardial and pericardial diseases of the european society of cardiology. Eur Heart J. 2005;26:516–524. doi: 10.1093/eurheartj/ehi108. [DOI] [PubMed] [Google Scholar]
- 31.Ljungqvist A, Jenoure P, Engebretsen L, Alonso JM, Bahr R, Clough A, De Bondt G, Dvorak J, Maloley R, Matheson G, Meeuwisse W, Meijboom E, Mountjoy M, Pelliccia A, Schwellnus M, Sprumont D, Schamasch P, Gauthier JB, Dubi C, Stupp H, Thill C. The international olympic committee (ioc) consensus statement on periodic health evaluation of elite athletes march 2009. Br J Sports Med. 2009;43:631–643. doi: 10.1136/bjsm.2009.064394. [DOI] [PubMed] [Google Scholar]
- 32.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part ii (maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 33.Scholz DG, Kitzman DW, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part i (growth): A quantitative anatomic study of 200 specimens from subjects from birth to 19 years old. Mayo Clin Proc. 1988;63:126–136. doi: 10.1016/s0025-6196(12)64945-3. [DOI] [PubMed] [Google Scholar]
- 34.Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJ, Holmes DR, Jr., Gersh BJ. A population study of the natural history of wolff-parkinson-white syndrome in olmsted county, minnesota, 1953-1989. Circulation. 1993;87:866–873. doi: 10.1161/01.cir.87.3.866. [DOI] [PubMed] [Google Scholar]
- 35.Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the wolff-parkinson-white syndrome. N Engl J Med. 1979;301:1080–1085. doi: 10.1056/NEJM197911153012003. [DOI] [PubMed] [Google Scholar]
- 36.Deal BJ, Beerman L, Silka M, Walsh EP, Klitzner T, Kugler J. Cardiac arrest in young patients with wolff-parkinson-white syndrome. PACE. 1995;815(a) [Google Scholar]
- 37.Russell MW, Dick MD. Incidence of catastrophic events associated with the wolff- parkinson-white syndrome in young patients: Diagnostic and therapeutic dilemma. Circulation. 1993;484(a) [Google Scholar]
- 38.Drezner JA, Harmon KG, Marek JC. Incidence of sudden cardiac arrest in minnesota high school student athletes: The limitations of catastrophic insurance claims. J Am Coll Cardiol. 2014;63:1455–1456. doi: 10.1016/j.jacc.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Harmon KG, Asif IM, Ellenbogen R, Drezner J. The incidence of sudden cardiac arrest in united states high school athletes. Br J Sports Med. 2014;48:605. [Google Scholar]
- 40.Maron BJ, Roberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: A profile of 78 patients. Circulation. 1982;65:1388–1394. doi: 10.1161/01.cir.65.7.1388. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Papadakis M. Interpreting the athlete's ekg: Are all repolarization anomalies created equal? Circulation. 2015;131:128–130. doi: 10.1161/CIRCULATIONAHA.114.013739. [DOI] [PubMed] [Google Scholar]
- 42.Ryan MP, Cleland JG, French JA, Joshi J, Choudhury L, Chojnowska L, Michalak E, al- Mahdawi S, Nihoyannopoulos P, Oakley CM. The standard electrocardiogram as a screening test for hypertrophic cardiomyopathy. Am J Cardiol. 1995;76:689–694. doi: 10.1016/s0002-9149(99)80198-2. [DOI] [PubMed] [Google Scholar]
- 43.Drezner JA, Ashley E, Baggish AL, Borjesson M, Corrado D, Owens DS, Patel A, Pelliccia A, Vetter VL, Ackerman MJ, Anderson J, Asplund CA, Cannon BC, DiFiori J, Fischbach P, Froelicher V, Harmon KG, Heidbuchel H, Marek J, Paul S, Prutkin JM, Salerno JC, Schmied CM, Sharma S, Stein R, Wilson M. Abnormal electrocardiographic findings in athletes: Recognising changes suggestive of cardiomyopathy. Br J Sports Med. 2013;47:137–152. doi: 10.1136/bjsports-2012-092069. [DOI] [PubMed] [Google Scholar]
- 44.Corrado D, Basso C, Buja G, Nava A, Rossi L, Thiene G. Right bundle branch block, right precordial st-segment elevation, and sudden death in young people. Circulation. 2001;103:710–717. doi: 10.1161/01.cir.103.5.710. [DOI] [PubMed] [Google Scholar]
- 45.Risgaard B, Winkel BG, Jabbari R, Glinge C, Ingemann-Hansen O, Thomsen JL, Ottesen GL, Haunso S, Holst AG, Tfelt-Hansen J. Sports-related sudden cardiac death in a competitive and a noncompetitive athlete population aged 12 to 49 years: Data from an unselected nationwide study in denmark. Heart Rhythm. 2014;11:1673–1681. doi: 10.1016/j.hrthm.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Harmon KG, Drezner J. Measuring sudden cardiac arrest and death incidence in minnesota high school athletes: A comparison of methodology and implications for prevention strategies. Br J Sports Med. 2014;48:605. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.