Abstract
Purpose
To explore genetic counselors’ information preferences on reports of variant of uncertain significance (VUS) results from cancer genetic testing.
Methods
This mixed methods report (quantitative and qualitative approaches) utilized a survey of genetic counselors containing closed- and open-ended questions to explore genetic counselors’ information needs and perceptions of the industry’s current information sharing practices. Descriptive statistics were calculated for responses to the closed-ended questions and thematic analysis guided the interpretation of the open-ended questions.
Results
Of the 267 participants (28.6% response rate), the majority indicated a perceived lack of information on VUS laboratory reports, were concerned about the perceived practice of withholding information, and stated the information they wanted to see. Although most did not indicate how additional information would be used, some reported they would provide information directly to patients, and others reported the information would be used to contextualize the VUS result when counseling patients.
Conclusion
This analysis identified information genetic counselors believe is needed on VUS reports indicating what they believe are best practices in lieu of guidelines for laboratories currently providing genetic testing services, and implies needed guidelines for reporting VUS. Future studies should explore how genetic counselors use additional information contained on VUS reports.
Keywords: genetic counseling, variant of uncertain significance (VUS), information needs, genetic testing, hereditary breast and ovarian cancer
INTRODUCTION
Approximately 5-10% of women with breast cancer1 and 15% of women with ovarian cancer2 have a mutation in the BRCA1 or BRCA 2 (BRCA) genes. By age 70, women with a BRCA1 mutation have a 60% average risk of developing breast cancer and a 59% average risk of developing ovarian cancer, and women with a BRCA2 mutation have a 55% average risk of developing breast cancer and a 16.5% average risk of developing ovarian cancer.3 Definitive genetic test results (i.e., true positive or true negative) can guide prevention, diagnosis, and treatment to reduce cancer incidence and mortality.4-6 Unfortunately, genetic test results are not always straightforward. For example, a variant of uncertain significance (VUS) result indicates a change in a BRCA gene was detected, yet the conferred cancer risk resulting from that change is unknown. This result is among the most difficult to discuss with patients.7,8
Ongoing efforts to reclassify VUS rely on laboratory-based and/or epidemiological studies to generate sufficient data to determine the likelihood a gene mutation is harmful (i.e., pathogenic or deleterious).9 Until these studies yield clinically relevant findings, genetic counselors (GCs) will face challenges associated with disclosing VUS results to patients. Yet, to our knowledge, only one prior U.S.-based study examined GC practices related to VUS,7 but none examined GCs’ information needs, for example, the amount and type of information included on laboratory reports, and preferences for receiving updates when VUS are reclassified.
As part of a larger study to optimize the integration of VUS reclassification data into clinical practice, a national survey of GCs was used to explore attitudes, practice patterns, and information preferences related to BRCA VUS reporting and counseling. The purpose of the current study was to analyze responses from closed- and open-ended questions using a mixed methods approach to identify GCs’ information needs related to VUS reports and updates.
MATERIALS AND METHODS
Recruitment and data collection
Mailing lists from the National Society of Genetic Counselors (NSGC) and the American Board of Genetic Counseling (ABGC) were used to identify GCs who practiced cancer genetic counseling, reported clinical practice as their primary activity, and had a U.S. mailing address. NSGC provided a spreadsheet of all members (n = 2,451) containing information about practice, specialization, and work setting. This list was refined to only include members who listed “cancer” or “personalized genomic medicine” as their primary or other specialization; “cancer” as an area of practice including “cancer genetics,” “adult cancer genetics,” “cancer risk counseling;” and/or who worked at an organization with “cancer” or “oncology” in the name, resulting in a list of 751 GCs. ABGC provided a pre-sorted mailing list of 621 members practicing cancer genetic counseling in the U.S. The lists were merged and duplicates (i.e., individuals listed on both) were removed resulting in a list of 975 GCs. An additional 43 GCs were removed including eleven who piloted the survey for face and content validity and those identified as duplicates during the mailing process, resulting in a total sample of 932 GCs.
Following institutional review board approval from Mayo Clinic and the University of South Florida, a multiphase recruitment approach10 was utilized. GCs were mailed a postcard to alert them to the upcoming survey and to verify the accuracy of mailing addresses. Seven weeks later, a packet containing a cover letter, survey, prepaid return envelope, prepaid response card, and two articles about VUS reclassification11,12 was mailed. The prepaid response card allowed GCs to indicate survey completion and enter a drawing for one of four $50 bills. Surveys were mailed in February 2013, completed surveys were accepted through May 15, 2013.
Materials
Surveys can be an efficient and cost-effective approach to gather data among a national sample of providers; however, respondents are limited by the set of predetermined fixed responses. Including free text areas on surveys may allow other attitudes and beliefs to emerge, providing additional insight into important factors to address health care providers’ needs.13
Quantitative data collection and analysis
To capture GCs’ perceptions about the amount of information included on BRCA VUS reports, participants selected one of three response options to the following statement: “In your opinion, the amount of information currently provided by Myriad Genetic Laboratories1 about BRCA VUS results is: too much, just right, or too little.” GCs were asked to indicate which resources they used and how often when VUS results were received, and which guidelines they referred to and how often when counseling patients with VUS results (see Table 2 for response options). GCs were asked to indicate the clinical screening they recommend for patients based on receiving a VUS test result. Response options included: counsel as if positive result (full high-risk surveillance), counsel based on family history and other risk factors, or other. Finally, GCs were asked to indicate the preferred source for receiving professional education and information on VUS reclassification (see Table 3 for response options) and preferred channel of communication (see Figure 1 for response options).
Table 2.
Resources and sources currently used to counsel patient about VUS (N = 267).
| Resource | Never/Rarelya n (%)b |
Sometimes n (%)a |
Often/Always n (%)a |
|---|---|---|---|
| Myriad supplemental reports | 43 (16.1) | 40 (15.0) | 181 (67.8) |
| Contact Myriad Genetics | 25 (9.3) | 62 (23.2) | 179 (67.1) |
| Contact researcher | 163 (61) | 59 (22.1) | 44 (16.5) |
| Search scientific literature | 60 (22.5) | 58 (21.7) | 147(55) |
| Search BIC website | 145 (54.3) | 41 (15.4) | 78 (29.2) |
| Discuss with a colleague | 29 (10.9) | 51 (19.1) | 181 (67.8) |
|
| |||
|
Source
| |||
| NSGCc | 43(16.1) | 62 (23.2) | 157 (58.8) |
| NCCNd | 20 (7.5) | 23 (8.6) | 219 (82.1) |
| IARCe | 196 (73.4) | 43 (16.1) | 19 (7.1) |
Response options were based on a 5-point Likert-type scale from never to always
Percentages may not sum 100 due to missing data.
National Society of Genetic Counselors (NSGC) www.nsgc.org
National Comprehensive Cancer Network (NCCN) www.nccn.org
International Agency for Research on Cancer (IARC) www.iarc.fr
Table 3.
Preferences for professional education (N = 267).
| Do not prefer |
Somewhat prefer |
Neutral | Moderately prefer |
Strongly prefer |
|
|---|---|---|---|---|---|
| Source | n (%)a | n (%)a | n (%)a | n (%)a | n (%)a |
| Direct communication from | 167 | ||||
| Myriad | 7 (2.6) | 8 (3.0) | 17 (6.4) | 68 (25.5) | (62.6) |
| Updated reclassification lists mailed |
47 (17.6) | 20 (7.5) | 38 (14.2) | 80 (30.0) | 79 (29.6) |
| Updated reclassification lists posted on FORCEb website |
110 (41.2) | 23 (8.6) | 57 (21.4) | 53 (19.9) | 21 (7.9) |
| Updated reclassification lists posted on NSGC website |
55 (20.6) | 20 (7.5) | 67 (25.1) | 69 (25.8) | 55 (20.6) |
Percentages may not sum 100 due to missing data.
Facing our Risk of Cancer Empowered www.facingourrisk.org
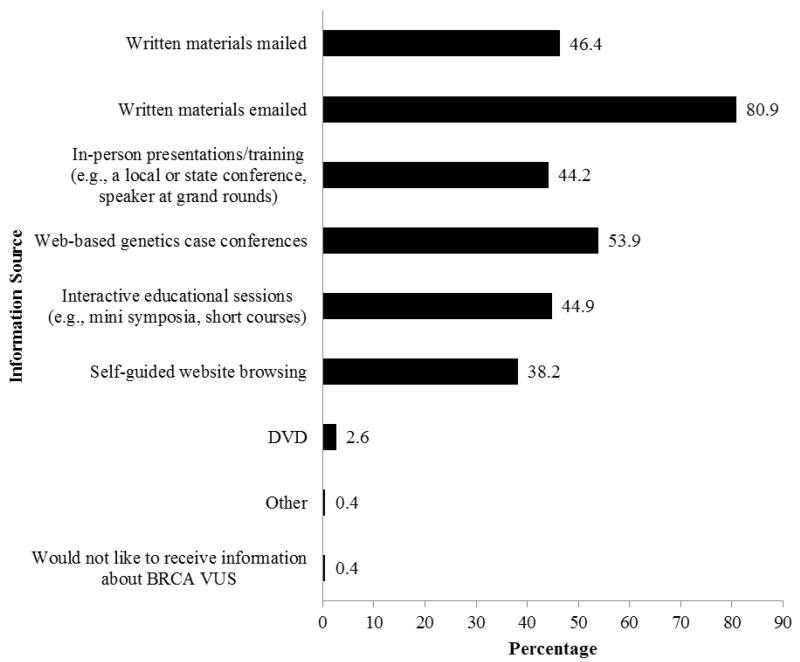
Figure 1.
Preferred format for professional education/information (N = 267).
Frequencies and percentages for participant demographics and responses to the aforementioned questions were calculated using SAS 9.3.
Qualitative data collection and analysis
At three points in the survey, respondents were asked to provide written comments. The first open-ended question (Q1) was a follow-up to the closed-ended question about the amount of information currently included on BRCA VUS reports, and asked, “If you answered too much or too little to the question, please explain.” The other qualitative questions upon which this study draws appeared at the end of the survey: “What additional information would you like to have available on BRCA VUS to help you counsel your patients” (Q2); and “If you have any additional comments you would like to share with our research team, please feel free to share them here”(Q3). Thematic analysis was used to identify themes in responses. Three coders independently reviewed all responses to each question and completed rounds of open coding, creating categories, and abstraction.14 Coders met after each coding phase to discuss and compare results and resolve differences in coding, categorization, and abstraction.
Initially, responses to each question were coded independently; for example, coding was completed on all responses to Q1 separately from Q2 and Q3. However, significant overlap in responses and themes to each question was identified; therefore, responses from all three questions were analyzed together to achieve a holistic understanding of GCs’ views on VUS results. The resulting themes were drawn from responses to all three questions.
RESULTS
Participants
A total of 410 completed surveys were received. As the survey was designed for individuals with experience providing genetic counseling for HBOC, 12 participants were excluded because they reported no experience providing genetic counseling for HBOC or had missing data. Of the 398 GCs who completed the survey (42.7% total response rate) and were considered for analysis, approximately 267 (28.6% response rate) provided written comments to at least one of the three open-ended questions. Only those who provided responses to the written comments were included due to the inter-related nature of this analysis. Upon comparing those who responded to the open-ended questions with those who did not respond, no statistically significant differences between groups were found for age, sex, race, ethnicity, and board certification (Table 1). A Pearson chi-square test showed those who reported cancer as their primary specialty, x2(1, N = 396) = 16.16, p < .0001, and those who reported providing direct patient care as their primary role, x2(1, N = 397) = 11.99, p = .0005, were more likely to respond to the open-ended questions.
Table 1.
Comparison of samples, stratified by participants who did vs. did not provide comments.
| Variable | ≥1 Comment N = 267 n (%) |
No Comments N = 131 n (%) |
p a |
|---|---|---|---|
| Age (mean, [range]) | 36.8 [24-71] | 37.0 [24-69] | .8516 |
| Sex | |||
| Male | 14 (60.9) | 9 (39.1) | .5134 |
| Female | 253 (67.5) | 122 (32.5) | |
| Race | |||
| White/Caucasian | 245 (67.1) | 120 (32.9) | .9574 |
| Other | 22 (66.7) | 11 (33.3) | |
| Ethnicity | |||
| Hispanic or Latino | 7 (53.9) | 6 (46.2) | .3693b |
| Not Hispanic or Latino | 260 (67.5) | 125 (32.5) | |
| Primary Specialty Area (missing = 2) | |||
| Cancer | 217 (72.6) | 82 (27.4) | >.0001* |
| Other | 49 (50.5) | 48 (49.5) | |
| Primary Role (missing = 1) | |||
| Direct patient care | 247 (70.2) | 105 (29.8) | .0005* |
| Other | 20 (44.4) | 25 (55.6) | |
| Board Certified in GC | |||
| Yes | 250 (67.2) | 122 (32.8) | .8486 |
| No, board eligible and plan to take/ not board eligible |
17 (65.4) | 9 (34.6) |
Independent samples t-test for age; Pearson chi-square tests for all other comparisons, except where noted
Fisher’s Exact Test
p < .05
Note: Row percentages are reported
After removing responses including “n/a,” “none,” “no,” or “0,” 239 participant comments were included in the qualitative analysis from Q1, 109 from Q2, and 46 from Q3. Participants provided genetic counseling for hereditary breast and ovarian cancer for an average of 7.4 years, were predominately female (94.8%; n = 253), Caucasian (91.8%; n = 245), and non-Hispanic (97.4%; n = 260). The primary specialty for the majority was cancer (81.3%; n = 217), the majority indicated their primary role was direct patient care (92.5%; n = 247), and was board certified in genetic counseling (93.6%; n = 250).
Quantitative results
Of respondents, 243 (91%) reported the amount of information provided on BRCA VUS laboratory reports was too little, 1 (0.4%) reported too much, and 23 (8.6%) reported just enough. When VUS results were received, the majority reported often or always using supplemental reports provided by the laboratory (67.8%), or discussing results with a colleague (67.8%), followed closely by contacting the laboratory directly (67.1%), and searching the scientific literature (55%; Table 2). When faced with a VUS, guidelines from the National Comprehensive Cancer Network (NCCN) (55.1%) guided practice most frequently followed by guidelines from NSGC (22.5%), and the International Agency for Research on Cancer (IARC) (7.1%; Table 2). When counseling patients with a VUS, 254 (95.1%) reported providing clinical guidelines based on family history whereas 6 (2.3%) reported counseling as if the result was positive and 5 (1.9%) reported “other.”
The majority strongly preferred to receive reclassification information about BRCA VUS through direct communication from the laboratory about their patient only (62.6%), followed by comprehensive lists of reclassified VUS mailed to them directly (29.6%), posted on NSGC website (20.6%), or posted on the Facing our Risk of Cancer Empowered (FORCE) website (7.9%; Table 3). The preferred delivery of information was written materials sent via email (80.9%) followed by a web-based genetics case conference (53.9%), mailed written materials (46.4%), an interactive educational session (44.9%), in-person presentations/trainings (44.2%), or a self-guided website (38.2%). A minority preferred a DVD (2.6%) and one participant did not want to receive information about BRCA VUS (0.4%; Figure 1).
Qualitative results
GCs would like more information about VUS
Consistent with quantitative findings, participants’ comments reflected the desire for more information on BRCA VUS laboratory reports. For example, one respondent wrote, “I would like more information about why it is a VUS; they provide almost no information other than ethnicity.” In addition, participants listed specific information they preferred on VUS reports, including: “prediction models” or “probability models” such as the “polyphen score,” “in silico analysis,” “functional data;” “conservation of the sequence;” “cosegregation studies;” “frequency of tracking in families;” “evolutionary conservation data;” “estimated time to reclassification;” and “references to any published literature available on VUS.” Some mentioned one or two of the aforementioned items, whereas others provided a comprehensive list.
In addition to listing specific desired information, there were references to BRCA VUS reports previously provided by the laboratory providing BRCA testing at the time of this study, and examples of non-BRCA VUS reports from other laboratories. For example:
“I do understand their reasoning, but I wish Myriad still provided all the info they previously did regarding the number of times the VUS had been seen and how it tracked in families. I appreciated having that info and find that I do miss it. Also, other labs (doing testing for other genes) provide the in silico model results which could also be helpful in counseling.”
GCs proactively sought additional information
In response to the absence of information, participants described proactive methods for acquiring more information including: “calling the laboratory directly,” “seeking help from others,” and “researching.” However, limitations to seeking additional information also were noted, including a “lack of time” and “not much luck.” Participants reported complete information was difficult to obtain even when calling the laboratory directly; for example: “Many times it [VUS report] only includes very vague descriptions and I need to call a genetic counselor at Myriad to get the complete information they have. They often reference “internal data” on families which is so very vague and unfortunate.” Some suggested consulting with other providers, as one counselor noted:
“Seeking help from other providers is essential for providing proper risk assessment for individuals with a VUS. You can bounce ideas off them, use their past experience, and learn tricks of the trade for dealing with these patients. I highly recommend that anyone who deals often with VUS find some way to get themselves involved with other cancer counselors to discuss these patients, especially as panel tests become more mainstream.”
As noted, the anticipated increasing frequency of VUS results arising from the testing of even rarer genes on newly available multigene-panels will require GCs to develop strategies to deal with VUS results.
A minority did not want additional information
Although the majority desired more information on the laboratory reports, it is worth noting a minority reported the information was just right, as: “Mutation classification (interpretation) should always be the responsibility of the lab doing the test. No genetic counselor or other clinician should ever determine for themselves the clinical impact of the mutation.” There was concern additional information could lead GCs to alter medical management based on personal interpretation. It was suggested the amount of information provided could be tailored according to professional training and clinical setting. For example, “For non-genetics providers, the current amount is just right. However, academic medical centers with genetics/research expertise should have access to additional info.”
Transparency
GCs believed testing laboratories had additional information, yet did not provide it: “I feel that Myriad does not provide enough information about their classification system and about the data they have about their VUSs.” Participants reported the testing laboratory cited “internal data” when additional information was requested, and the lack of information created a negative feeling towards the organization, “The information is just not helpful because it’s not enough detail which is frustrating because Myriad has the info.” In addition, the lack of data led GCs to question the laboratory’s decision to classify a mutation as a VUS, “We do not have any proof to their classification if nothing has been previously published.”
Lack of publicly available shared data
In response to perceived lack of transparency, participants suggested using a publicly available database for information sharing: “It would be nice to have a world-wide collaboration of VUS and not be directly dependent on only the information that Myriad is able to collect.” Some cited the Breast Information Core (BIC) as a publicly available resource for information on VUS, but indicated an absence of information sharing from the laboratory to BIC; for example: “My understanding is that Myriad does not report to BIC – I think this is a huge disservice to the genetics community in understanding these mutations/VUS/polymorphisms.”
Communication of results to patients, their family, and other providers
Although participants were not directly asked about how they would use additional information, some specified the information would help them when communicating results with patients, patients’ family members, and other providers. Some suggested additional information would not be shared directly with patients, but it could be used to contextualize the result; for example:
“I preferred when they used to provide data about whether the VUS had been seen with a known deleterious mutation. While we never used it to change the clinical interpretation or shared it with patients it was helpful for discussion of the “gestalt” of the VUS.”
In addition to providing more information, some requested patient education materials; for example: “A visual aid regarding the possibility of a VUS for pre-test counseling as well as a visual aid for post-test counseling when a VUS result is received.” Despite not asking directly about patient education materials in the open-ended questions, requests were made for this type of information.
The absence of information about VUS on laboratory reports challenged participants’ ability to discuss clinical management (i.e., screening and prevention behaviors), and the possibility for family members to participate in research (i.e., Myriad’s Variant Classification Program (VCP)) during the posttest counseling session. For example:
“In most cases, there is not enough detailed information about the variant to help guide clinical management. It is also often difficult to encourage the family to participate in the VCP because in the first appointment [with] the genetic counselor I am often unsure who it will be offered to and/or how it may help.”
Finally, a minority of participants wanted information about VUS for other health care professionals due to concerns about awareness of VUS and appropriate medical recommendations:
“There is a HUGE lack of understanding of VUS with many physicians. I have had many patients who were wrongly counseled/advised based upon a VUS result (both counseled as deleterious as well as a negative result). This seems to be happening more and more frequently as more physicians without genetic training are ordering BRCA testing by themselves. And as Next-Gen panels become more frequently ordered, I am very concerned about what will happen with our patients when they go back and discuss with their physicians (even if I have provided accurate counseling).”
Open-ended questions on the survey did not ask about other providers, nor does this concern relate specifically to additional information, but the concern about other providers’ interpretation of VUS was raised by participants, and highlighted the unease about the use of panel testing and the potential for negative consequences related to VUS results.
DISCUSSION
Cancer GCs in the U.S. were surveyed to explore perceptions about information they receive on VUS reports and to identify needs and mechanisms for providing additional information. As a significant number of respondents provided written responses to open-ended questions, a mixed methods approach to data analysis was utilized.
Most participants believed laboratory reports containing VUS results lacked information, and described information they wanted on VUS reports; however, it was unclear how additional information might be used. Some indicated additional information would help contextualize the VUS result for patients. For example, a GC might be able to say something like: “we know very little about whether this variant is present in other families” or “there are several studies currently underway to learn more about cancer risk in families who carry this variant.” Additionally, previous studies reported GCs perceived a lack of patient understanding about VUS,7 and documented negative patient outcomes when receiving VUS results including higher levels of distress about cancer risk,15 interpreting the result as a genetic predisposition to cancer,16 and engaging in prevention measures not indicated by their result (e.g., prophylactic surgeries).17 These potential negative outcomes and GCs’ frustrations with their inability to provide the patient with a clear result7 may motivate the desire for additional information on laboratory reports. For clarification, future studies should ask GCs how they would use additional information, and identify what information would be the most useful to share with patients.
Although most wanted additional information, it is worth noting a minority of respondents did not. Participants believed it could be problematic if additional information is interpreted by GCs to guide patient medical management. Interpretation, they believed, was the responsibility of the laboratory conducting the test, and medical management should be based solely on personal and family history. These beliefs are consistent with current NCCN and NSGC medical management guidelines for patients with a VUS.18,19 The potential for GCs to use additional information for interpretation was viewed as concerning if the information was incomplete or the interpretation incorrect.
In response to the lack of information provided on VUS test results, GCs reported proactively seeking additional information, including consulting with other colleagues, calling the laboratory for more information, and searching the scientific literature. Interestingly, some requested a publicly available database, but only ~30% reported often or always using the BIC database. In addition to BIC, participants infrequently mentioned other websites offering publicly available data on VUS (not specific to BRCA), including MAPP-MMR, Align GVGD, PolyPhen, and ClinVar. These databases provide available evidence and results from mathematical models12 that synthesize existing evidence to provide likelihood scores that may guide clinical interpretation. However, they are not regularly updated, provide limited data, and do not explicitly state whether this information is intended to guide clinical interpretation and medical management. Therefore, requests for public databases, compounded by the low use of BIC, may either indicate GCs are unaware of these resources, have uncertainty about how to use them or interpret the information, or the resources are not meeting GCs’ clinical needs. Future research should examine cancer GCs’ awareness of these databases, barriers to use, and identify strategies or education for GCs to integrate these resources into clinical practice.
Additionally, in 2004, the sole laboratory providing BRCA testing stopped contributing their findings to the BIC database,20 and in 2010, the laboratory stopped including additional information about VUS such as variant frequency, ethnic groups in which the variant was observed, co-segregation with disease, and whether the variant was identified in a person with a known deleterious mutation as part of their report. It may be, in anticipation of the loss of their patent, the company stopped providing detailed VUS reports.20 These practices likely contributed to the perceived lack of transparency noted by participants and created mistrust of the laboratory and the results. Additionally, the lack of information sharing adds to the paucity of publically available information about VUS. Several Free the Data campaigns21 are currently underway to encourage sharing of de-identified information about VUS. Laboratories who do share their data may have a competitive advantage over those who do not, as their contribution provides open access to the information used to classify a mutation as a VUS. Additionally, the majority preferred previously provided information, updates provided by the laboratory on their specific patients rather than comprehensive lists provided through other organizations, and wanted updates to be provided via email. Therefore, in the future, providing additional information on VUS reports and providing available updates directly to GCs on their patients with VUS may offer another competitive advantage to laboratory companies who do so. Whether this is feasible within the current business models of most laboratories remains to be seen, and the legal obligation of testing laboratories is undefined regarding reanalysis or re-contact when new information becomes available.
In addition to the impact on the patient, GCs were sensitive to the impact VUS results may have on the family. Testing other family members is one means to gain information about a VUS, by seeing if it cosegregates with disease in the family.22 The American College of Medical Genetics and NCCN guidelines recommend follow-up studies to test a limited number of family members for research purposes only to gather additional data towards reclassifying a VUS.18,23,24 However, if such studies are unavailable, very careful discussions and counseling are necessary to clarify the costs and goals of testing additional family members for a VUS.
These results were based on six closed-ended questions and three open-ended questions, thereby limiting the depth of analysis to the amount of information provided in participants’ written comments; however, information in this report will be useful for future research as a number of questions were raised when analyzing the responses. The population in this study included only GCs who provide cancer genetic counseling, and not all GCs provided written comments. Those who provided comments were more likely to indicate their primary role was direct patient care and their primary specialty area was cancer. These findings may reflect resources needed to deliver optimal patient care in the context of BRCA VUS results, but study results cannot be generalized to all cancer GCs.
Although the purpose of the larger study was not focused on the open-ended responses, the high rate of written comments prompted our team to examine their responses to identify other important factors regarding VUS results reporting.13 Nearly 70% of respondents provided additional comments about their preferences and experiences about VUS on this survey, indicating VUS results reporting is a highly charged issue among cancer GCs. In the future, the increased use of multi-gene panel testing will dramatically increase the rate of VUS results.25 GCs will progressively face challenges associated with communicating VUS results to patients, their family members, and other providers. As most counselors turn to NCCN for guidance when receiving VUS results, it may be useful for NCCN to provide additional guidelines for medical management of patients with VUS results. Future studies exploring how cancer GCs handle VUS results in terms of interpretation, providing medical management recommendations, and sharing results with the patient and their family remains a critical area for future study.
ACKNOWLEDGEMENTS
This work was supported by the Breast Cancer Spore CA 116201. Dr. Courtney L. Scherr is supported by the R25 CA 090314 Behavioral Oncology Education and Career Development grant. This work was supported in part by the Survey Methods Core Facility at the H. Lee Moffitt Cancer Center & Research Institute; an NCI designated Comprehensive Cancer Center (P30-CA76292). We wish to thank Mary Lou Smith for providing feedback on an earlier draft of this manuscript. We also would like to thank members of the Research Advocacy Network for their input on the development of this project.
Footnotes
The authors declare they have no conflict of interests.
This survey was conducted prior to the court decision that lead to multiple laboratories offering BRCA testing, at the time of this survey, Myriad Genetics was the sole provider for BRCA testing in the United States.
References
- 1.Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Human genetics. 2008;124(1):31–42. doi: 10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 2.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 3.Mavaddat N, Peock S, Frost D, et al. Cancer Risks for BRCA1 and BRCA2 Mutation Carriers: Results From Prospective Analysis of EMBRACE. Journal of the National Cancer Institute. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 4.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA: the Journal of the American Medical Association. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gage M, Wattendorf D, Henry L. Translational advances regarding hereditary breast cancer syndromes. Journal of surgical oncology. 2012;105(5):444–451. doi: 10.1002/jso.21856. [DOI] [PubMed] [Google Scholar]
- 6.Grann V, Patel P, Jacobson J, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011;125(3):837–847. doi: 10.1007/s10549-010-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrucelli N, Lazebnik N, Huelsman KM, Lazebnik RS. Clinical interpretation and recommendations for patients with a variant of uncertain significance in BRCA1 or BRCA2: a survey of genetic counseling practice. Genetic testing. 2002;6(2):107–113. doi: 10.1089/10906570260199357. [DOI] [PubMed] [Google Scholar]
- 8.Miller-Samuel S, MacDonald DJ, Weitzel JN, et al. Variants of Uncertain Significance in Breast Cancer–Related Genes: Real-World Implications for a Clinical Conundrum. Part One: Clinical Genetics Recommendations. Seminars in Oncology. 2011;38(4):469–480. doi: 10.1053/j.seminoncol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Iversen ES, Jr., Couch FJ, Goldgar DE, Tavtigian SV, Monteiro AN. A computational method to classify variants of uncertain significance using functional assay data with application to BRCA1. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(6):1078–1088. doi: 10.1158/1055-9965.EPI-10-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillman DA. Mail and internet surveys: The tailored design method. Vol. 2. Wiley; New York: 2000. [Google Scholar]
- 11.Lindor NM, Goldgar DE, Tavtigian SV, Plon SE, Couch FJ. BRCA1/2 sequence variants of uncertain significance: a primer for providers to assist in discussions and in medical management. Oncologist. 2013;18(5):518–524. doi: 10.1634/theoncologist.2012-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindor NM, Guidugli L, Wang X, et al. A review of a multifactorial probability-based model for classification of BRCA1 and BRCA2 variants of uncertain significance (VUS) Human mutation. 2012;33(1):8–21. doi: 10.1002/humu.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esses VM, Maio GR. European Review of Social Psychology. John Wiley & Sons, Ltd; 2005. Expanding the assessment of attitude components and structure: The benefits of open-ended measures; pp. 71–101. [Google Scholar]
- 14.Elo S, Kyngäs H. The qualitative content analysis process. Journal of Advanced Nursing. 2008;62(1):107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 15.Vos J, Gómez-García E, Oosterwijk JC, et al. Opening the psychological black box in genetic counseling. The psychological impact of DNA testing is predicted by the counselees’ perception, the medical impact by the pathogenic or uninformative BRCA1/2-result. Psycho-Oncology. 2012;21(1):29–42. doi: 10.1002/pon.1864. [DOI] [PubMed] [Google Scholar]
- 16.Vos J, Otten W, van Asperen C, Jansen A, Menko F, Tibben A. The counsellees’ view of an unclassified variant in BRCA1/2: recall, interpretation, and impact on life. Psycho-Oncology. 2008;17(8):822–830. doi: 10.1002/pon.1311. [DOI] [PubMed] [Google Scholar]
- 17.Murray ML, Cerrato F, Bennett RL, Jarvik GP. Follow-up of carriers of BRCA1 and BRCA2 variants of unknown significance: Variant reclassification and surgical decisions. Genetics in Medicine. 2011;13(12):998–1005. doi: 10.1097/GIM.0b013e318226fc15. [DOI] [PubMed] [Google Scholar]
- 18.Daly MB, Pilarski R, Axilbund JE, et al. NCCN Guidelines Version 1.2014 - Genetic/Familial High-Risk Assessment: Breast and Ovarian. 2014. [Google Scholar]
- 19.Berliner J, Fay A, Cummings S, Burnett B, Tillmanns T. NSGC Practice Guideline: Risk Assessment and Genetic Counseling for Hereditary Breast and Ovarian Cancer. Journal of Genetic Counseling. 2013;22(2):155–163. doi: 10.1007/s10897-012-9547-1. [DOI] [PubMed] [Google Scholar]
- 20.Cook-Deegan R, Conley JM, Evans JP, Vorhaus D. The next controversy in genetic testing: clinical data as trade secrets? European journal of human genetics: EJHG. 2013;21(6):585–588. doi: 10.1038/ejhg.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genetic Alliance Free the Data. 2013. 2014 http://www.free-the-data.org/ [Google Scholar]
- 22.Cummings S. The Genetic Testing Process: How Much Counseling Is Needed? Journal of Clinical Oncology. 2000;18(suppl 1):60–64. [PubMed] [Google Scholar]
- 23.Group ALPCW ACMG recommendations for standards for interpretation of sequence variations. Genetics in Medicine. 2000;2(5):302–303. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 24.Richards CS, Bale S, Bellissimo DB, et al. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genetics in Medicine. 2008;10(4):294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 25.Robson M. Multigene Panel Testing: Planning the Next Generation of Research Studies in Clinical Cancer Genetics. Journal of Clinical Oncology. 2014 doi: 10.1200/JCO.2014.56.0474. [DOI] [PubMed] [Google Scholar]