Abstract
BACKGROUND & AIMS
Clinical studies have shown similar rapid improvements in body mass and glycemic control after Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG). Evidence suggests that adaptive intestinal tissue growth and reprogramming of intestinal glucose disposal plays a key role in the beneficial effects on glucose homeostasis after RYGB, but it is not known whether such adaptive changes also occur after sleeve gastrectomy.
METHODS
High-fat diet-induced obese rats were subjected to either VSG or RYGB and intestinal growth and functional adaptations were assessed by using morphometric, immunohistochemical and immuno-blot techniques, 3 months after surgery or sham surgery.
RESULTS
The cross-sectional areas of the Roux and common limbs are significantly increased after RYGB compared with sham surgery (Roux limb: 17.1 ± 4.0 vs. 5.5 ± 0.1 mm2; common limb: 11.7 ± 0.6 vs. 5.1 ± 0.5 mm2; p < 0.01), but the cross-sectional area of the corresponding jejunum is not different from controls after VSG. Similarly, mucosal thickness and the number of GLP-1 cells are not increased after VSG. Protein expression of hexokinase II is increased four-fold (p <0.01) in the Roux limb after RYGB, but not in the jejunum after VSG.
CONCLUSIONS
Adaptive hypertrophy and reprogramming of glucose metabolism in the small intestine are not necessary for VSG to improve body composition and glycemic control. The similar beneficial effects of VSG and RYGB on glucose homeostasis might be mediated by different mechanisms.
Keywords: Bariatric surgery, RYGB, obesity, intestinal adaptation, GLP-1, Hexokinase II
Introduction
Roux-en-Y gastric bypass surgery (RYGB) and vertical sleeve gastrectomy (VSG) have quite similar beneficial effects on body weight and diabetes resolution [1], and VSG is increasingly considered as a less invasive and expensive alternative. One explanation for the similar effectiveness is that both surgeries share a common mechanism of action even though they are fundamentally different. While the flow of nutrients is drastically changed in RYGB, it is essentially left intact after VSG. However, one commonality is the rapid delivery of ingested nutrients to the small intestine [2]. After RYGB and biliopancreatic bypass, these rapidly delivered nutrients are in the form of largely undigested food, thought to be responsible for major structural and functional changes in the Roux limb and common limb [3–6]. Specifically, reprogramming of glucose metabolism towards growth-promotion, leading to increased glucose utilization of the enlarged Roux and common limbs, has been suggested as a major mechanism for improved glucose homeostasis after RYGB [7]. Studies in rats, 5–10 months after RYGB, have demonstrated significant growth of all layers of the Roux and common limbs, including the mucosa [8, 9], and an increase in the total number of enteroendocrine cells producing a variety of hormones including GLP-1, GLP-2, PYY, CCK, neurotensin, and 5-HT [8, 9]. Earlier research in children with short bowel syndrome and various animal models with small bowel resection suggested a role for GLP-2 in the adaptive growth response of the remaining small bowel [10–15]. The significantly increased postprandial GLP-2 levels in rats [5, 6] and humans [16] suggests that GLP-2 is also involved in the adaptive hypertrophic response after RYGB. Because elevated GLP-2 levels have also been reported after sleeve gastrectomy in rats [17], we wanted to see if this surgical procedure would also lead to adaptive hypertrophy and reprogramming of glucose utilization in the small intestine and whether this could represent the common mechanism for improvement in glycemic control after the two bariatric surgeries.
Materials and Methods
Animals and diets
Male Sprague-Dawley rats weighing ~200 g (Harlan Industries, Indianapolis, IN) were housed individually in wire-mesh cages at a constant temperature of 21–23°C with a 12h light-dark cycle (lights on at 07:00, off at 19:00). Food and water were provided ad libitum unless otherwise indicated. Animals were made obese by putting them on a two-choice diet for 14–16 weeks consisting of normal laboratory chow (Kcal%: Carb, 58; Fat, 13.5; Prot, 28.5, # 5001, Purina LabDiet, Richmond IN ) and high-fat diet (Kcal%: Carb, 35; Fat, 45; Prot, 20; D12451, Research Diets, New Brunswick, NJ), with each of the diets containing sufficient vitamins and minerals. They were then randomly assigned to either VSG or sham surgery. A lean control group without surgery was placed on a regular chow diet throughout the experiment.
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Surgeries and postoperative care
To remove all solid food particles from the stomach, rats undergoing VSG or RYGB were fed liquid Ensure (Kcal%: Carb, 64; Fat, 21.6; Prot, 14.4, Abbott Laboratories, Columbus, OH) for 3–5 days. Ensure was removed the night before surgery. Rats were anesthetized with isoflurane and administered atropine (1 mg/kg, s.c.).
For VSG, laparotomy was followed by removal of the gastric omentum, the stomach was placed on the open tongue of a cutting stapler (Model ATW35 with two straight triple-staple lines; Ethicon, Ithaca, NY), making sure that most of the fundus was below the staple line. The tongues of the stapler were then slowly closed starting from fundus to antrum and the knife activated, resulting in the removal of about 80% of the total stomach volume. Any epiploic blood vessels not included in the staple line were ligated and the severed portion of the stomach was removed. The rest of the stomach was put back in place before suturing muscle and skin separately. Sham surgery consisted of laparotomy and removal of the gastric omentum. RYGB and its sham surgery have been described in detail earlier [8]
RYGB was carried out according to a protocol described in detail earlier {Mumphrey, 2013 #6007}. Briefly, a gastric pouch of about 20% of the total gastric volume was anastomosed with a 15 cm-long Roux limb and the 25 cm-long common limb with a roughly 40 cm-long biliopancreatic limb. Sham-surgery consisted of the same basic procedure as for RYGB, except that the transected jejunum was re-anastomosed and a small incision in the jejunum 25 cm from ileocecal valve and one in the gastric fundus were sutured closed.
After surgery, only liquid Ensure was provided for the first 10 days, before giving back increasing amounts of regular chow and HF diet.
Measurement of body weight, body composition, and food intake
Body weight was monitored daily for the first two weeks, and then was recorded weekly. Body composition was measured before introduction of the high-fat diet (16 weeks before surgery), and monthly after surgery, by using a Minispec LF 90 NMR Analyzer (Bruker Corporation, The Woodlands, TX). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility [18].
Immunohistochemical and morphometric analysis
3 months after surgery, rats were euthanized after overnight food deprivation, and the gastrointestinal tract was harvested. Half the tissue samples from each location were immersion-fixed in 10% buffered formalin for later immunohistochemical processing, and the other half was rapidly frozen in liquid nitrogen for later extraction of protein. Immunohistochemical processing and analysis was carried out as previously reported [8]. Briefly, GLP-1 mouse monoclonal antibody 1:5000 (ImmunDiagnostik, Bensheim, Germany, distributed by ALPCO, Windham, NH) was used as primary antibody. Sections were viewed using a Zeiss Axioplan fluorescence microscope. GLP-1 immunoreactive enteroendocrine cells were counted only if the nucleus was clearly visible. Counts were averages from 2–4 intestinal cross-sections from each limb for each animal.
Cross-sectional areas were measured based on darkfield images generated in a Leitz microscope with a 1x macro lens and image analysis software (ImageJ, NIH, Bethesda, MD). Average cross-sectional areas were calculated from at least 3 representative sections per rat and limb.
Measurements of mucosal thickness have been described earlier [8]. Briefly, average thickness of the mucosa (combined thickness of crypts and villi) was calculated for each limb of each animal, and these averages were used for statistical analysis.
Western blotting
Frozen intestinal tissues were crushed to powder using liquid nitrogen cooled mortar and pestles. Frozen powder was transferred to conical tubes and 1ml of ice cold T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific Inc., Waltham, MA, Cat # 78510) containing 1X Halt Protease Inhibitor Cocktail (complete EDTA-free, Thermo Fisher Sceintific Inc., Cat # 87785) was added. Samples were homogenized for 30 s on ice, and then sonicated using a Branson Sonifier S-450 Digital Ultrasonic Cell Disruptor/Homogenizer (Branson Ultrasonics Americas, Danbury, CT). Samples were centrifuged at 12,000 rpm for 20 min. Protein concentration of each lysate was determined using BCA Protein Assay Kit (Thermo Fisher). Protein lysates were heated at 95°C for 5 min with Laemmli buffer and separated by polyacrylamide gel electrophoresis through 12% SDS gels. Proteins were transferred to PVDF membranes (Bio-Rad Laboratories, Inc., Hercules, CA, Cat. # 162-0177) at 100V for 70 min at 4°C, using a wet transfer system (Mini Trans-Blot, Bio-Rad Laboratories, Inc.). Membranes were blocked in 5% milk dissolved in PBS-T (1X PBS + 0.1% Tween20) for 45 min at room temperature. Primary antibodies were diluted in 5% milk or 5% bovine serum albumin in PBS-T according to manufacturer’s recommendations. Anti-beta 2 Microglobulin antibody (B2M, rabbit monoclonal [EP2978Y], ab75835, Abcam, Cambridge, MA) was used at 1:20,000 dilution. Hexokinase II antibody (rabbit monoclonal [C64G5], Cat. # 2867, Cell Signaling Technology, Danvers, MA) was used at 1:1,000 dilution. Blots were incubated with primary antibodies overnight at 4°C. After washing with PBS-T, blots were incubated with goat anti-rabbit IgG HRP-linked (Cat # 7074, Cell Signaling Technology) in 5% milk dissolved in PBS-T for 1 h at room temperature. Membranes were developed using Western Lightning Plus-ECL, Enhanced Chemiluminescence Substrate (PerkinElmer Inc., Waltham, MA, Cat # NEL104001EA).
Statistical analysis
Body weight, body composition, and food intake across time were analyzed by two-way repeated measures ANOVA, with treatment as a between-subject factor and days as a repeated within-subject factor, followed by Bonferroni’s post-hoc multiple comparison test. Cross-sectional area, mucosal thickness, and the number of GLP-1-IR cells per section were analyzed by two-way ANOVA, and hexokinase II expression by t-test.
Results
VSG in high-fat diet-induced obese rats reduces body weight and fat mass to levels of chow-fed controls
Exposure to the high-fat diet for 14 weeks resulted in significantly increased body weight (Fig. 1a) and fat mass (Fig. 1b) compared with age-matched chow-fed controls. Subsequent VSG in the obese rats led to a rapid decrease, followed by a slow and steady regain of body weight, whereas sham surgery resulted in only a small transient decrease (Fig. 1a). At the end of the 3-month observation period, VSG rats weighed 521 ± 11 g, significantly less than sham-operated (~609 ± 11 g), but not significantly different from chow-fed control rats (537 ± 16 g). Body weight loss at 54 days after VSG was almost entirely due to fat mass loss (−19 g), with only a small loss of lean mass (−4 g). Body weight gain in sham-operated rats was due to both fat mass (+13 g) and lean mass gain (+30 g) (data not shown). Just before the end of the 3-month observation period, total fat mass and percent fat mass (adiposity) were significantly lower in VSG rats compared with sham-operated rats, but not significantly different from chow-fed controls (Fig. 1b,c). Both epididymal and retroperitoneal fat pads weighed significantly less in VSG compared with sham-operated rats and were only slightly higher than in chow-fed controls (Fig. 1d). Lean mass was slightly, but not significantly lower 80 days after VSG compared with both sham-operated and chow controls (Fig. 1e).
Fig. 1.
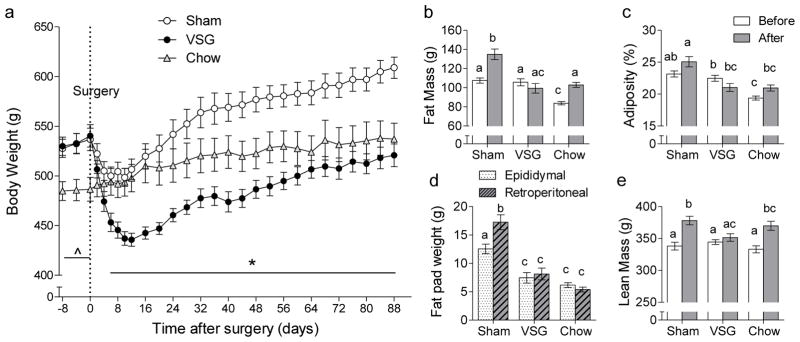
Effect of VSG in diet-induced obese rats on body weight and composition. Rats exposed to a two-choice high/low fat diet were either subjected to VSG (closed circles, n = 8) or sham surgery (open circles, n = 9) and compared to rats exposed to regular low-fat chow (open triangles, n = 4). a: Body weight monitored before and for 3 months after surgery. * p < 0.05, VSG vs. Sham; ^ p<0.05, VSG and Sham vs. Chow. b, c: Total fat mass and relative fat mass (adiposity) measured by NMR whole body imaging, 80 days after surgery. d: Epididymal and retroperitoneal fat pad weights measured at termination, 90 days after surgery. e: Lean mass at 80 days after surgery. Bars that do not share the same letters are significantly different from each other (based on ANOVA followed by Bonferroni-corrected multiple comparisons).
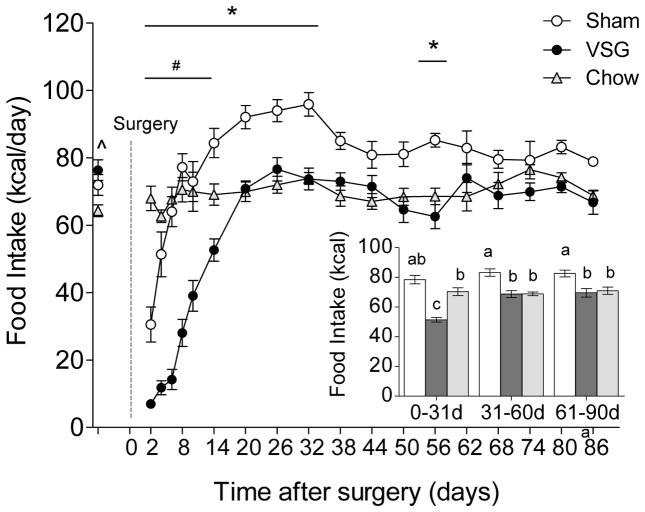
VSG transiently reduces energy intake
Before surgery, high-fat-fed rats ingested slightly (~10 %) but significantly more calories than chow-fed controls (Fig. 2). VSG initially drastically reduced total energy intake but it returned to pre-surgical levels after about 20 days and remained at this level for the rest of the study. Sham operation reduced energy intake only briefly, followed by an overshoot and subsequent stabilization at a level about 15% higher than VSG animals. Average daily energy intake in VSG rats was significantly reduced by about 40% during the first month and by about 15% during the second and third month, compared to sham-operated rats (Fig. 2 inset).
Fig. 2.
Effect of VSG in diet-induced obese rats on food intake. Total intake of high and low-fat offered as two-choice diet monitored before and 3 months after surgery in rats with VSG (closed circles, n = 8) or sham surgery (open circles, n = 9), compared with non-surgical rats fed low-fat regular chow (open triangles, n = 4). * p< 0.05, VSG vs. Sham; # p< 0.05, VSG vs. Chow, ^ p<0.05, VSG and Sham vs. Chow. The inset shows average daily total (from high and low-fat diet) food intake during the first, second, and third month post-surgery. Bars that do not share the same letters are significantly different from each other (based on ANOVA followed by Bonferroni-corrected multiple comparisons).
VSG does not increase cross-sectional area of small intestine and thickness of mucosa
The small intestine of VSG rats does not show any hypertrophic response as seen after RYGB (Fig. 3). Cross-sectional areas (Fig. 3a, b) and mucosal thickness (Fig. 3c) of duodenum, jejunum and ileum, were similar in VSG and sham-operated rats. In contrast, cross-sectional areas and mucosal thickness for the corresponding intestinal locations ( Roux and common limbs) are significantly larger after RYGB.
Fig. 3.

Effect of VSG and RYGB in rats on cross-sectional area and mucosal thickness at 3 locations of the small intestine. a: Representative histological cross sections of the Duodenum, Jejunum, and Ileum after VSG (n = 6) or sham surgery (n = 4) and of the corresponding biliopancreatic (BP)), Roux (RX), and common (CM) limbs after Roux-en-Y gastric bypass (n = 4). Note the absence of any enlargement of small intestinal diameter and surface area after VSG. Scale bar = 1 mm. b: Quantitative assessment of cross-sectional area of corresponding intestinal segments after VSG and RYGB and their respective sham surgeries. c: Mucosal thickness is not significantly different between VSG and sham rats, but as reported earlier {Mumphrey, 2013 #6007}, is significantly higher after RYGB compared to all other groups. * p < 0.01, RYGB vs. sham.
VSG does not change number of GLP-1 immunoreactive L-cells
Immunohistochemical staining of GLP-1-producing L-cells showed no difference of number and distribution between VSG and sham-operated rats (Fig. 4). As expected, the number of L-cells per section was much lower in the duodenum than in the jejunum and ileum. This contrasts with our earlier data [8] and observations by others [9], showing more than doubling of the number of L-cells and other enteroendocrine cells in the Roux and common limbs but not the biliopancreatic limb after RYGB.
Fig. 4.
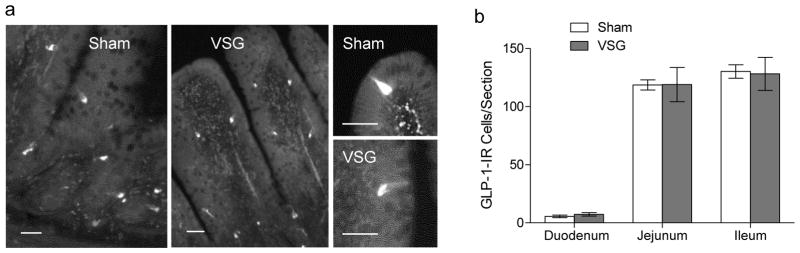
Effect of VSG on number of GLP-1 immunoreactive enteroendocrine cells at 3 locations of the small intestine. a: Representative images of GLP-1 cells in the jejunal mucosa after VSG and sham surgery. Scale bars = 50 μm. b: Quantitative analysis of the number of GLP-1 cells per section in the duodenum, jejunum, and ileum of rats after VSG (n =6) and sham surgery (n =7).
RYGB, but not VSG increases hexokinase-II protein expression in small intestine
Protein expression of hexokinase II was significantly elevated in the Roux limb of rats 10 months after RYGB (Fig. 5b), confirming earlier observations by Saeidi et al. [7]. However, expression of this key glycolytic enzyme was not increased in the upper small intestine 3 months after VSG (Fig. 5a).
Fig. 5.
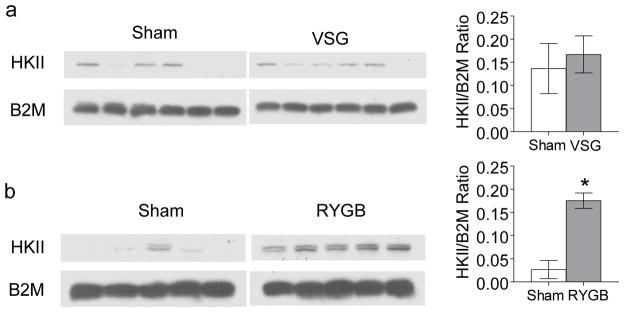
Effect of VSG (a) or RYGB (b) in rats on protein expression of hexokinase II (HK II) in the jejunum and corresponding Roux limb. Original Western Blots are shown on the left and quantitative densitometric analysis on the right for VSG (n = 6) and sham VSG (n = 6) as well as for RYGB (n = 5) and sham RYGB (n =5). There was no significant difference between VSG and sham-operated rats, but significantly increased hexokinase II after RYGB compared with sham. * p <0.01, RYGB vs. sham. B2M, Beta-2-microglobulin.
Discussion
Reprogramming of glucose utilization allowing for growth of the adapting gut [7] is one of the many hypotheses put forward to explain the remarkable beneficial metabolic effects of bariatric surgeries on glucose homeostasis. Studies in rats have shown increased expression of key glycolytic enzymes diverting its products from oxidation towards tissue growth-related pathways. The significantly increased glucose utilization by the hypertrophied intestine was suggested to be important for lowering plasma glucose and improve the diabetic state associated with obesity [7]. Because glycemic control is much improved [1, 19] and because circulating levels of the main hypertrophic hormone GLP-2 are greatly increased [20] after both RYGB and VSG, the question arose whether increased intestinal glucose disposal may be common to both surgical procedures. Here we show that this is not likely, as there was no intestinal growth and no reprogramming of the glycolytic pathway after VSG in rats. If this mechanism is important for improved glycemic control after RYGB, another mechanism has to be working after VSG. Because we examined these responses only at one time point after surgery, we cannot completely rule out that relevant changes occur at an earlier time point. However, after RYGB, up-regulation of hexokinase II was not different at 1 and 6 months [7] and postprandial GLP-2 levels remain elevated for extended periods of time either after RYGB in rats and humans [5] or after VSG in rats [17]. Also, because we only examined hexokinase II, we cannot rule out that other steps in the glycolytic pathway are changed (reprogrammed) after VSG. This is rather unlikely, given the key proximal position of hexokinase II within the pathway.
Another limitation of our study is that we have not directly assessed gut hormone levels and improvements of glycemic control. However, as indicated by the similar effectiveness on body weight, body composition, and food intake, it is unlikely that our VSG rat model is different from other published studies which assessed effects on glycemic control [21]. Specifically, Chambers et al. demonstrated that VSG and RYGB in male Long-Evans rats produced similar decreases in body weight, fat mass, and food intake as we report here. While insulin sensitivity and ip glucose tolerance 4–5 weeks after surgery as well as whole body insulin sensitivity as measured by hyperinsulinemic hyperglycemic clamp 2 weeks after surgery were greatly improved after both sleeve gastrectomy and RYGB in a more or less weight loss-dependent manner, there was a significant weight loss-independent contribution (~50%) to hepatic insulin sensitivity as manifested in greater suppression of hepatic glucose production after VSG and RYGB vs. pair-feeding [21]. Our present demonstration that there was small intestinal hypertrophy and hexokinase II upregulation after RYGB but not after VSG strongly suggests that the critical mechanism for the similar improvements in insulin sensitivity and glycemic control after these two surgeries cannot be the hypertrophied small intestine. Rather, the major mechanism for both surgeries appears to be the hypocaloric state and body weight loss.
Acknowledgments
Funded by National Institutes of Health grant DK047348 (HRB).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Informed consent: Does not apply.
Animal rights: All applicable institutional and/or national guidelines for the care and use of animals were followed.”
References
- 1.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306:E424–432. doi: 10.1152/ajpendo.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evrard S, Aprahamian M, Hoeltzel A, Vasilescu M, Marescaux J, Damge C. Trophic and enzymatic adaptation of the intestine to biliopancreatic bypass in the rat. Int J Obes Relat Metab Disord. 1993;17:541–547. [PubMed] [Google Scholar]
- 4.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG. Biliopancreatic diversion in rats is associated with intestinal hypertrophy and with increased GLP-1, GLP-2 and PYY levels. Obes Surg. 2007;17:1193–1198. doi: 10.1007/s11695-007-9211-2. [DOI] [PubMed] [Google Scholar]
- 5.le Roux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252:50–56. doi: 10.1097/SLA.0b013e3181d3d21f. [DOI] [PubMed] [Google Scholar]
- 6.Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45:987–995. doi: 10.1016/j.jpedsurg.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–410. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25:e70–79. doi: 10.1111/nmo.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen CF, Bueter M, Theis N, et al. Hypertrophy Dependent Doubling of L-Cells in Roux-en-Y Gastric Bypass Operated Rats. PLoS ONE. 2013;8:e65696. doi: 10.1371/journal.pone.0065696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin GR, Beck PL, Sigalet DL. Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation. World J Gastroenterol. 2006;12:4117–4129. doi: 10.3748/wjg.v12.i26.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964–972. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 12.Sigalet DL, Bawazir O, Martin GR, et al. Glucagon-like peptide-2 induces a specific pattern of adaptation in remnant jejunum. Dig Dis Sci. 2006;51:1557–1566. doi: 10.1007/s10620-006-9077-5. [DOI] [PubMed] [Google Scholar]
- 13.Kaji T, Tanaka H, Redstone H, Wallace LE, Holst JJ, Sigalet DL. Temporal changes in the intestinal growth promoting effects of glucagon-like peptide 2 following intestinal resection. J Surg Res. 2009;152:271–280. doi: 10.1016/j.jss.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Hua Z, Turner JM, Sigalet DL, et al. Role of glucagon-like peptide-2 deficiency in neonatal short-bowel syndrome using neonatal piglets. Pediatr Res. 2013;73:742–749. doi: 10.1038/pr.2013.44. [DOI] [PubMed] [Google Scholar]
- 15.Ljungmann K, Hartmann B, Kissmeyer-Nielsen P, Flyvbjerg A, Holst JJ, Laurberg S. Time-dependent intestinal adaptation and GLP-2 alterations after small bowel resection in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G779–785. doi: 10.1152/ajpgi.2001.281.3.G779. [DOI] [PubMed] [Google Scholar]
- 16.Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26:2231–2239. doi: 10.1007/s00464-012-2166-y. [DOI] [PubMed] [Google Scholar]
- 17.Cummings BP, Bettaieb A, Graham JL, et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620–3632. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res. 2004;12:1604–1615. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- 19.Helmio M, Victorzon M, Ovaska J, et al. Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: A prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-up. Scand J Surg. 2014;103:175–181. doi: 10.1177/1457496913509984. [DOI] [PubMed] [Google Scholar]
- 20.Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]