Abstract
Background
Although venous thromboembolism has been well studied in the pediatric trauma population, rates of VTE associated with elective pediatric orthopaedic procedures have not been addressed in current literature. The purpose of this retrospective study was to identify the incidence of venous thromboembolism (VTE) in the elective pediatric orthopaedic surgical population and delineate subsets of this population at greatest risk. This study may provide valuable data to begin the process of resolving the controversy surrounding DVT prophylaxis in the pediatric orthopaedic population.
Methods
The Pediatric Health Information System (PHIS) was queried for patients admitted on an ambulatory or inpatient basis aged <18 years from 1/2006 to 3/2011 during which an elective orthopaedic surgery was the principal procedure performed. Patients with diagnoses or procedures related to infection, trauma, malignancy, or coagulopathies were excluded. Patients admitted through the emergency department (ED) or whose orthopedic procedure was not performed on the admission date were excluded. Age, gender, ethnicity, race, admission year, and all procedures/diagnoses were recorded. The presence of VTE at the index admission or any subsequent readmission within 90 days was recorded. All criteria were coded using ICD-9-CM codes. Generalized logistic regression analyses were used to identify factors related to VTE.
Results
143,808 admissions (117,676 patients) matched the inclusion criteria. 33 had a VTE during the index admission with an additional 41 at subsequent readmissions, for a total incidence of 0.0515% by admission and 0.0629% by patient. In the multivariable model, variables significantly [p<0.05] related to VTE included increasing age, admission type, diagnosis of metabolic conditions, obesity, and/or syndromes, and complications of implanted devices and/or surgical procedures. No procedure variables were significantly related to VTE in the multivariable model.
Conclusions
The incidence of VTE in this cohort of pediatric patients undergoing elective orthopaedic surgery was 0.0515%. In children, underlying diagnosis appears to be a stronger predictor of VTE than procedures performed. Diagnosis with a metabolic condition, syndrome, and/or obesity, complications of implanted devices and/or surgical procedures, older age, and admission as an inpatient were significantly related to the development of a VTE.
Level of Evidence
Level II; Retrospective prognostic study
Introduction
Pediatric VTE has traditionally been considered to be a rare condition that has not been well studied. The first comprehensive investigation of pediatric VTE reported an incidence of 5.3 per 10,000 hospital admissions and 0.7 per 100,000 children.1 In subsequent studies, the incidence rate has been reported to be between 4 and 58 per 10,000 pediatric hospital admissions.2–7
The incidence of VTE, in particular “idiopathic” VTE, is much lower in children than adults.1–10 In contrast to adult populations in which the proportion of VTE considered to be “idiopathic” is estimated to be 30–40%, the incidence of “idiopathic” VTE in children is much lower, estimated to be between 2.0 and 12.6%.1, 6, 11–13 Approximately 90–95% of pediatric VTE are associated with risk factors that include: a central venous catheter.1, 2, 7, malignancy, underlying chronic conditions, infection, heritable prothrombotic disorders, age less than one or greater than 13 years, trauma, and operative procedures.13–15
While VTE has been previously studied in the context of pediatric trauma, VTE following elective pediatric orthopedic surgery has not been investigated.16–20 Given the high risk of VTE in the adult orthopedic surgical population,20,21, 21 and the potential for a similar link in children,3 the purpose of this study was to determine the incidence of VTE among patients undergoing elective pediatric orthopaedic surgery and to identify sub-populations at increased risk.
Materials and Methods
Data Source
The Pediatric Health Information System (PHIS) is an administrative database comprised of discharge data from 44 large pediatric hospitals across the United States (see Table 1 for a list of specific hospitals). Available data elements include age, sex, length of stay, admission type, as well as ICD-9-CM diagnosis and procedure codes.
TABLE 1.
Hospitals contributing to PHIS during data collection
| City | Hospital |
|---|---|
| Akron | Akron Children's Hospital |
| Atlanta | Children's Healthcare of Atlanta |
| Birmingham | The Children's Hospital of Alabama |
| Boston | Children's Hospital Boston |
| Buffalo | Children's Hospital of Buffalo |
| Chicago | Children's Memorial Hospital |
| Cincinnati | Children's Hospital Medical Center |
| Columbus | Nationwide Children’s Hospital |
| Corpus Christi | Driscoll Children's Hospital |
| Dallas | Children's Medical Center of Dallas |
| Dayton | The Children's Medical Center |
| Denver | Children's Hospital Colorado |
| Detroit | Children's Hospital of Michigan |
| Fort Worth | Cook Children's Medical Center |
| Hartford | Connecticut Children's Medical Center |
| Houston | Texas Children's Hospital |
| Indianapolis | Riley Hospital for Children |
| Kansas City | Children's Mercy Hospital |
| Knoxville | East Tennessee Children's Hospital |
| Little Rock | Arkansas Children's Hospital |
| Los Angeles | Children's Hospital Los Angeles |
| Madera | Children's Hospital Central California |
| Memphis | Le Bonheur Children's Medical Center |
| Miami | Miami Children's Hospital |
| Milwaukee | Children's Hospital of Wisconsin |
| Minn / St. Paul | Children's Hospitals and Clinics |
| Minneapolis | Children's Hospitals and Clinics at Minneapolis |
| Nashville | Vanderbilt Children's Hospital |
| New Orleans | Children's Hospital |
| New York | Children's Hospital of New York-Presbyterian |
| Norfolk | Children's Hospital of The King's Daughters |
| Oakland | Children's Hospital Oakland |
| Omaha | Children's Hospital |
| Orange | Children's Hospital of Orange County |
| Palo Alto | Lucile Packard Children's Hospital at Stanford |
| Philadelphia | Children's Hospital of Philadelphia |
| Phoenix | Phoenix Children's Hospital |
| Pittsburgh | Children's Hospital of Pittsburgh |
| Salt Lake City | Primary Children's Hospital |
| San Diego | Rady Children's Hospital and Health Center |
| Seattle | Children's Hospital and Medical Center |
| St. Louis | St. Louis Children's Hospital |
| St. Petersburg | All Children's Hospital |
| Washington | Children's National Medical Center |
Identification of the Elective Orthopedic Population
Following the approval of the Colorado Multiple Institutional Review Board, PHIS was queried for admissions of patients aged less than 18 years from 2006 through the first quarter of 2011 during which an orthopedic procedure was performed. Orthopedic procedures were defined as a set of ICD-9-CM procedure codes, largely within the “Operations on the Musculoskeletal System” code group. Orthopaedic procedure codes related to trauma were not included. Procedure and diagnosis codes are associated with admissions in PHIS such that there is one “principal” code, and up to 40 “secondary” codes of equal significance. Admissions were included in the initial query if any of the predefined orthopedic procedure codes appeared in either position. The initial query yielded 204,376 admissions (164,391 patients), from which all procedure and diagnosis codes, and patient demographic information were gathered.
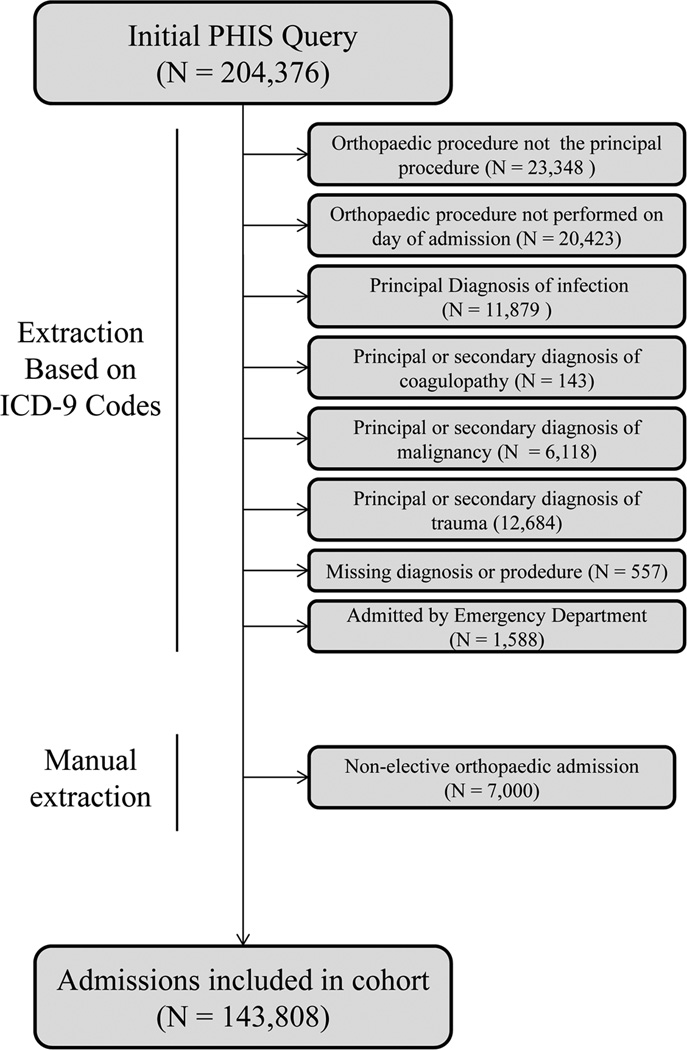
Microsoft Access was used to apply various exclusion criteria to the set of 204,376 admissions in order to isolate those which were elective in nature (see figure 1). Admissions were excluded on the basis of specific ICD-9-CM codes used, position and timing of codes within the admission, missing data, and admission type. Following the above exclusion criteria, any additional non-elective orthopedic admissions were removed upon manual review of principal diagnosis and procedure codes (7,000 admissions), resulting in a total of 60,568 excluded admissions (46,715 patients).
Figure 1. Database Extraction Techniques.
Label: Figure 1 describes the procedures used to isolate elective orthopaedic cases from all cases identified in the initial Pediatric Health Information System database query.
Following these exclusions, 143,808 elective orthopedic admissions (117,676 patients) remained. These admissions were searched for VTE during index orthopedic admission (population 1, VTE before discharge), and for any readmission within 90 days associated with a diagnosis code for VTE (population 2, VTE after discharge).
Patients with VTE before Discharge (Population 1)
Each of the 143,808 admissions was searched for a set of 21 ICD-9-CM diagnosis codes for VTE. This yielded 40 admissions (39 patients) with VTE before discharge from the index admission. Admissions meeting any of the following conditions were excluded: those with the diagnostic code V12.51 for “Personal history of venous thrombosis and embolism” (1 admission), those not admitted as inpatients and/or with a length of stay of 0 days (4 admissions). From the remaining admissions, 1 was excluded because the patient had a septic PE related to pyogenic arthritis and was not felt to be elective orthopedic in nature, and 1 was excluded because it was related to trauma. In total, 7 admissions (6 patients) were excluded, leaving 33 admissions (33 patients) with VTE prior to discharge from the elective orthopedic admission.
Patients with VTE after Discharge (Population 2)
For each of the 117,676 patients within the 143,808 elective orthopedic admissions, any re-admission within 90 days associated with a diagnosis code for VTE was identified. This yielded 93 admissions (90 patients). Admissions meeting any of the following conditions were excluded: those with the diagnostic code V12.51 for “Personal history of venous thrombosis and embolism” (N =2), those not admitted as inpatients (N=21), and those with a length of stay of zero days (N=16). Readmissions meeting any of the following conditions were also excluded: those with a principal or secondary diagnosis for coagulopathy (N=3) or trauma (N=4), and those with a principal diagnostic code for malignancy (N=1). From the remaining population, any admission already included in population 1 (N=5) and those which no longer had a preceding elective orthopedic admission within 90 days following the exclusions described above (N=7) were excluded. 2 additional admissions were excluded due to PICC line related PE. In total, 52 re-admissions with VTE were excluded, leaving 41 re-admissions (41 patients) with VTE within 90 days of the index admission.
Lists of all ICD-9-CM codes used for the inclusion and exclusion criteria described above are available from the authors upon request.
Statistical Methods
ICD-9-CM procedural and diagnostic codes were grouped for analysis. Procedure codes were categorized by location as well as by type. The Clinical Classification Software (CCS) published by the Health Care Cost and Utilization Project, 22 was utilized to help categorize the diagnosis codes. Diagnosis groups were defined as follows: 1) acquired conditions, 2) congenital conditions, 3) neuromuscular and neurological disorders, 4) osteoarthritis and rheumatologic disorders, 5) arthropathies, 6) benign neoplasms and neoplasms of unspecified nature, 7) metabolic conditions, obesity and syndromes, 8) sprains and strains, 9) complications of implants and surgical procedures, and 10) late effects of fractures, adjustment of growth rods and removal of internal fixation.
Potential correlation due to the clustering effect of hospital site and the presence of multiple admissions of the same patient was considered during the model building step of the analysis. Due to convergence issues, among those patients with multiple admissions, one was selected at random for inclusion. This resulted in a population that was slightly smaller than the one used to generate descriptive statistics. For this reason, descriptive and inferential statistics will be presented separately. A generalized logistic regression analysis was used to identify factors related to VTE. Preliminary single-predictor models were used to analyze the following variables: admission type, age, sex, ethnicity, race, admission type, principal procedure type, principal procedure location, total number of bony procedures performed and total number of soft tissue procedures performed. Variables significant at the 0.10 alpha level were considered for inclusion in the final, multi-variable model. A backwards elimination strategy was used to eliminate non-significant variables. Only those significant at the 0.05 alpha level were included in the final model. In all the logistic regression models, generalized estimating equations were used to account for the correlation due to the clustering effect of cases within the hospitals that contributed data to the PHIS database.
Source of Funding
Supported in part by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Results
Among the 143,808 elective orthopedic admissions (117,676 patients), incidence of VTE was 5.1 per 10,000 admissions and 6.3 per 10,000 patients. Of the 74 cases of VTE, 33 occurred before discharge (population 1), and 41 occurred after discharge, as re-admissions (population 2). Table 2 shows the demographic characteristics of these 74 patients, as well as those of all elective orthopedic admissions. Since a single patient may have had multiple admissions (1.22 ± 0.66 admissions per patient), incidence rates were estimated per patient as well as per admission.
TABLE 2.
Demographic Characteristics and Incidences
| Admissions |
Patientsa |
||||
|---|---|---|---|---|---|
| Variable | VTE | Orthopaedic Admissions |
VTE per 10,000 Admissions |
Orthopaedic Patients |
VTE per 10,000 Patients |
| N | 74 | 143,808 | 5.1 | 117,676 | 6.3 |
| Age | 13.04 (4.35) | 10.82 (4.98) | 11.05 (5.00) | ||
| Length of Stay | 10.20 (14.25) | 1.45 (2.93) | 1.51 (3.07) | ||
| Admissions per Patient | 1.60 (1.47) | 1.22 (0.66) | |||
| Sex | |||||
| Male | 36 (48.65%) | 76,123 (52.93%) | 4.7 | 62,611 (53.21%) | 5.7 |
| Female | 38 (51.35%) | 67,683 (47.06%) | 5.6 | 55,063 (46.79%) | 6.9 |
| Unknown | 0 (0.00%) | 2 (0.00%) | 0.0 | 2 (0.00%) | 0.0 |
| Ethnicity | |||||
| Hispanic or Latino | 11 (14.86%) | 19,661 (13.67%) | 5.6 | 16,273 (13.83%) | 6.8 |
| Not Hispanic or Latino | 31 (41.89%) | 61,575 (42.82%) | 5.0 | 48,189 (40.95%) | 6.4 |
| Unknown | 32 (43.24%) | 62,572 (43.51%) | 5.1 | 53,214 (45.22%) | 6.0 |
| Race | |||||
| Pacific Islander & Asian | 3 (4.05%) | 2,482 (1.73%) | 12.1 | 2,045 (1.74%) | 14.7 |
| American Indian | 1 (1.35%) | 934 (0.65%) | 10.7 | 774 (0.66%) | 12.9 |
| African American | 8 (10.81%) | 21,751 (15.13%) | 3.7 | 18,011 (15.31%) | 4.4 |
| White | 50 (67.57%) | 99,176 (68.96%) | 5.0 | 80,585 (68.48%) | 6.2 |
| Other | 8 (10.81%) | 14,267 (9.92%) | 5.6 | 11,802 (10.03%) | 6.8 |
| Unknown | 4 (5.41%) | 5,198 (3.61%) | 7.7 | 4,459 (3.79%) | 9.0 |
| Admission Type | |||||
| Ambulatory Surgery | 8 (10.81%) | 77,517 (53.90%) | 1.0 | 68,373 (58.10%)b | 1.2 |
| Unknown | 1 (1.35%) | 413 (0.29%) | 24.2 | 402 (0.34%)b | 24.9 |
| Inpatient | 64 (86.49%) | 55,159 (38.36%) | 11.6 | 47,342 (40.23%)b | 13.5 |
| Observation Unit | 0 (0.00%) | 10,661 (7.41%) | 0.0 | 9,853 (8.37%)b | 0.0 |
| Clinic Visit | 0 (0.00%) | 3 (0.00%) | 0.0 | 3 (0.00%)b | 0.0 |
| All other | 1 (1.35%) | 55 (0.04%) | 181.8 | 55 (0.05%)b | 181.8 |
| Admission Year | |||||
| 2006 | 11 (14.86%) | 20,797 (14.46%) | 5.3 | 19,580 (16.64%)b | 5.6 |
| 2007 | 12 (16.22%) | 25,591 (17.80%) | 4.7 | 23,918 (20.33%)b | 5.0 |
| 2008 | 17 (22.97%) | 28,392 (19.74%) | 6.0 | 26,527 (22.54%)b | 6.0 |
| 2009 | 16 (21.62%) | 30,446 (21.17%) | 5.3 | 28,358 (24.10%)b | 5.6 |
| 2010 | 17 (22.97%) | 31,236 (21.72%) | 5.4 | 29,088 (24.72%)b | 5.8 |
| 2011 (Quarter 1) | 1 (1.35%) | 7,346 (5.11%) | 1.4 | 7,237 (6.15%)b | 1.4 |
Some patients had more than one admission.
Adds to greater than 100% because some patients had admissions of more than one type, and in more than one year.
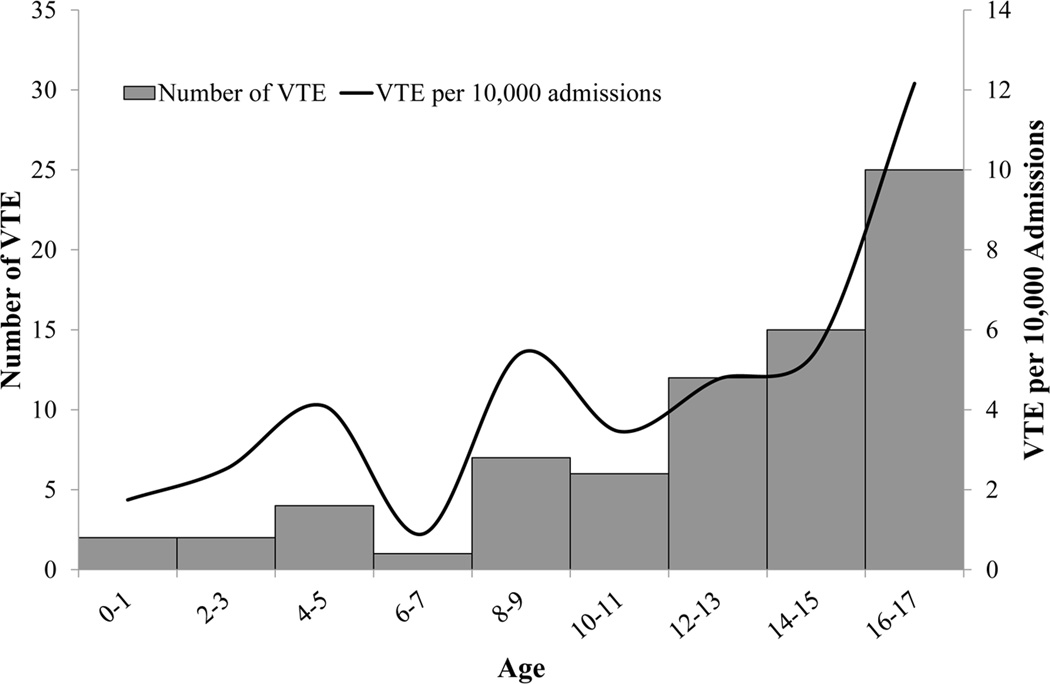
Figure 2 shows the age distribution of the 74 patients who developed VTE, as well as the resulting incidence in each age group. The rate of VTE remains stable until 14 years of age (3.5 per 10,000 admissions for ages 0 – 13 years), at which point there is roughly a twofold rise in the rate of DVT/PE (8.3 per 10,000 admissions for ages 14 – 17 years).
Figure 2. Age Distribution of Admissions with VTE.
Label: Figure 2 illustrates relationship between DVT incidence and age.
Fatality was recorded in 4 of the 74 cases of VTE (2 before discharge from the index admission and 2 after), giving rate of fatality of 5.4%. The first case of fatality was in a 12 year old male admitted for dorsal spinal fusion for idiopathic scoliosis/kyphoscoliosis, who developed post-operative septicemia due to anaerobes, acute respiratory failure, septic shock, and PE. The second was a 3 year old male with an unspecified mucopolysaccharidosis, admitted for posterior cervical spinal fusion for spinal stenosis of the cervical region, who developed post-operative pulmonary embolism. He also had diagnoses of cardiomyopathy, developmental delay, and chronic pulmonary heart disease. The third case was a 9 year old male with quadriplegic cerebral palsy admitted for a wedge osteotomy of the femur for developmental dislocation. External immobilization was utilized which was removed on day 2 of the admission, with a cast placed on day six, and discharge on day 9. He was then re-admitted one day later with a PE, and a myriad of other diagnoses including septic shock. The last fatality was a 14 year old female admitted for dorsal spinal fusion for idiopathic scoliosis/kyphoscoliosis, who was discharged after 5 days, and readmitted 22 days later with PE.
Incidence by Procedure and Diagnosis Groups
Table 3 shows the distribution of admissions by procedure group, along with the number of VTE in each group. The highest incidence rates were observed in the bony spine (18.1 per 10,000 admissions) group, with the next highest being bony hip, at only one third of that rate (6.0 per 10,000 admissions). The bony spine group accounted for 41.9% of all VTE despite representing only 11.9% of all admissions.
TABLE 3.
Incidence by Procedure Group
| Principal Procedure Only |
Principal or Secondary Procedure |
|||||
|---|---|---|---|---|---|---|
| Procedure Group | No. VTE |
No. Admit |
VTE per 10,000 Admits |
No. VTE |
No. Admit |
VTE per 10,000 Admits |
| Upper, bony | 1 | 10,499 | 0.9 | 1 | 11,402 | 0.9 |
| Upper, soft | 1 | 3,388 | 3.0 | 1 | 4,119 | 2.4 |
| Spine, bony | 31 | 17,167 | 18.1 | 31 | 17,312 | 17.9 |
| Spine, soft | 0 | 2,088 | 0.0 | 2 | 3,204 | 6.2 |
| Hip, bony | 1 | 1,655 | 6.0 | 2 | 2,374 | 8.4 |
| Hip, soft | 2 | 4,356 | 4.6 | 3 | 6,450 | 4.7 |
| Lower, bony | 20 | 39,553 | 5.1 | 27 | 44,786 | 6.0 |
| Lower, soft | 9 | 23,143 | 3.9 | 12 | 27,268 | 4.4 |
| Foot, bony | 2 | 5,297 | 3.8 | 2 | 8,546 | 2.3 |
| Foot, soft tissue | 0 | 3,656 | 0.0 | 3 | 5,408 | 5.5 |
| Other/Unspec, bony | 1 | 9,377 | 1.1 | 27 | 24,517 | 11.0 |
| Other/Unspec, soft | 6 | 23,629 | 2.5 | 15 | 32,276 | 4.6 |
| Non-orthopaedica | 0 | 0 | 52 | 34,973 | 14.9 | |
Admissions with non-orthopedic principal procedures were not included, however secondary non-orthopedic procedures were performed during admissions for which the principal procedure was orthopedic.
Table 4 shows the distribution of admissions by diagnosis, along with the number of VTE in each group, and resulting incidence rates. By far the highest incidence rates were found in the miscellaneous diagnosis group (28.7 per 10,000 admissions), followed by acquired conditions (9.1 per 10,000 admissions).
TABLE 4.
Incidence by Diagnosis Group
| Principal Diagnosis Only |
Principal or Secondary Diagnosis |
|||||
|---|---|---|---|---|---|---|
| Diagnosis Group | No. VTE |
No. Admit |
VTE per 10,000 Admits |
No. VTE |
No. Admit |
VTE per 10,000 Admits |
| Acquired Conditions | 43 | 47,122 | 9.1 | 54 | 63,804 | 8.5 |
| Arthropathy | 9 | 13,840 | 6.5 | 13 | 20.297 | 6.4 |
| Benign & Unspecified Neoplasms | 1 | 11,248 | 0.9 | 2 | 12,848 | 1.6 |
| Complications of Implants & Surgical Procedures | 2 | 6,894 | 2.9 | 11 | 8,592 | 12.8 |
| Congenital Conditions | 9 | 33,509 | 2.7 | 24 | 46,885 | 5.1 |
| Late Effects of Fx, Adj. Growth Rod, removal int. fix. | 0 | 18,390 | 0.0 | 4 | 22,021 | 1.8 |
| Metabolic Conditions, Obesity & Syndromes | 0 | 237 | 0.0 | 28 | 9,738 | 28.8 |
| Neuromuscular & Neurological Disorders | 4 | 6,226 | 6.4 | 31 | 30,022 | 10.3 |
| Osteoarthritis & Rheumatologic Disorders | 0 | 155 | 0.0 | 3 | 653 | 45.9 |
| Sprains & Strains | 1 | 4,446 | 2.2 | 1 | 4,984 | 2.0 |
| Miscellaneous | 5 | 1,741 | 28.7 | 59 | 58,153 | 10.1 |
Factors Predictive of VTE
As described previously, one admission was selected at random among subjects with multiple admissions. This reduced the number of admissions used in the logistic regression analysis from 143,808 to 117,224, and the number of VTE from 74 to 64. In the single predictor logistic models, admission type was the strongest predictor of VTE (see Table 5).
TABLE 5.
Results of Single Predictor Models
| Variable | Variable Type | P Value |
|---|---|---|
| Admission Type | Demographic/Clinical Characteristic | <0.0001* |
| Metabolic conditions obesity and syndromes | Diagnosis | <0.0001* |
| Total Number of Bony Procedures | Procedure | <0.0001* |
| Acquired Condition | Diagnosis | <0.0001* |
| Late effects of fractures, adjustment of growth rod and removal of internal fixation devices | Procedure | 0.0451* |
| Spine Procedure | Procedure | 0.0008* |
| Benign neoplasms and neoplasms of unspecified nature | Diagnosis | 0.0008* |
| Age | Demographic/Clinical Characteristic | <0.0001* |
| Neuromuscular and neurological disorders | Diagnosis | 0.0042* |
| Upper Extremity Procedure | Procedure | 0.0077* |
| Other Procedure Location | Procedure | 0.0200* |
| Complications of implanted devices and surgical procedures | Procedure | 0.0285* |
| Foot Procedure | Procedure | 0.0292* |
| Year of Admission | Demographic/Clinical Characteristic | 0.0790 |
| Sprains and strains | Diagnosis | 0.1570 |
| Osteoarthritis and rheumatologic disorders | Diagnosis | 0.2208* |
| Lower Extremity Procedure | Procedure | 0.2374 |
| Congenital conditions | Diagnosis | 0.3545 |
| Hip Procedure | Procedure | 0.4865 |
| Arthropathy | Diagnosis | 0.6353 |
| Gender | Demographic/Clinical Characteristic | 0.7883 |
| Ethnicity | Demographic/Clinical Characteristic | 0.8199 |
| Total Number of Soft Tissue Procedures | Procedure | 0.8391 |
| Race | Demographic/Clinical Characteristic | 0.8869 |
Considered for inclusion in final model
In the multivariable model, factors significantly related to the development of VTE included age [p = 0.0286], admission type [p= 0.0002], diagnosis with a metabolic condition, obesity and/or a syndrome [p = 0.0002], and the diagnosis of a complication of an implanted device and/or surgical procedure [p=0.0447]. Odds ratio estimates are displayed in Table 6.
Table 6.
Odds Ratio Estimates from the Multi-Variable Generalized Logistic Regression Analysis
| Variable | OR | 95% CI | P value* | |
|---|---|---|---|---|
| Metabolic condition, obesity and/or a syndrome | ||||
| Presence vs. Absence | 6.1 | 3.8 | 9.7 | 0.0002 |
| Complication of implanted devices and/or surgical procedures diagnosis | ||||
| Presence vs. Absence | 3.1 | 1.5 | 6.2 | 0.0447 |
| Age | ||||
| Per 5 yr increase in age | 1.6 | 1.0 | 2.5 | 0.0286 |
| Admission Type | ||||
| Inpatient vs. Ambulatory | 8.4 | 3.9 | 18.1 | 0.0002 |
| Inpatient vs. Other | 7.9 | 1.0 | 60.2 | |
Based on score test statistic for GEE analysis
Discussion
The incidence of VTE among elective pediatric orthopaedic surgical admissions in this study was 5.1 per 10,000 admissions (0.0515%). Older age, admission as an inpatient, the presence of a metabolic condition, obesity, and/or syndrome, and complications of implanted devices and/or surgical procedures increased the odds of VTE following elective orthopedic procedures.
An interesting finding in our population is the association of diagnoses within the metabolic conditions, obesity, and other syndromes group with VTE (28 VTE contained a secondary diagnosis code in this group). Diagnoses related to electrolyte and fluid disorders appear to make the largest contribution, with 20 VTE occurring in a total of 1,809 discharges with such a diagnosis (1.11%). The most frequent were dehydration (4 VTE), hyperosmolarity and/or hypernatremia (3 VTE), electrolyte disorders not elsewhere classified (2 VTE), and diabetes insipidus (2 VTE). Mixed acid/base disorders and other electrolyte disorders contributed to a lesser degree. This is likely an indication of the complex medical picture of the elective pediatric orthopedic patient who is at risk for DVT, rather than an indication of a causative relationship. That being said, some instances of cerebral vein thrombosis in dehydrated or hypernatremic neonates have been reported.23–25 A link has also been described in adults, with fluid and electrolyte disorders being significantly related to the development of subsequent VTE in those hospitalized for aneurysmal subarachnoid hemorrhage,26 as well as following acute ischemic stroke.27 Whether the observed association in our population represents, a cause, or an effect, of VTE is difficult to ascertain.
A diagnosis of being overweight or obese contributed 7 of the 28 cases of VTE to the “metabolic conditions, obesity, and other syndromes” diagnosis group. Such an association between obesity and increased risk of DVT is consistent with the well-known relationship in adults. For example, in the adult orthopedic population, the diagnosis of metabolic syndrome has been found to be associated with a 3.2 times greater risk of DVT following total knee arthroplasty, warranting increased prophylactic efforts.28 Although these findings have not been documented in elective pediatric surgical patients, the presence of obesity has been linked to an increased rate of DVT in the setting of pediatric trauma.29
In adults, it has long been recognized that the risk of VTE in the general population increases with age.9 Accordingly, the benefits of prophylaxis in older adult orthopedic surgical populations have also been consistently noted.30 Our results suggest that this effect of age on the risk of VTE also applies to the pediatric population, a finding which is also consistent with several previous reports.3, 5, 6, 10 Interestingly, the 2012 recommendations from the American College of Chest Physicians on the prevention of VTE in orthopedic surgery patients state that even though increasing age is associated with VTE, in general, surgery-specific risks outweigh patient specific factors. 21 Our results suggest that such a notion does not hold true in children, as patient specific factors, including age, were more strongly related to VTE than procedures performed. Although not significant in the multi-variable analysis, a high incidence was observed in the bony spine procedure group (18.1 per 10,000 admissions).
A relatively high incidence of 28.7 VTE per 10,000 admissions was observed in the miscellaneous diagnoses group. The next highest group, acquired conditions, had only one third of that rate (see Table 4). It is important to note that when multiple related diagnoses are present (e.g. neuromuscular conditions and deformity), the determination of the principal diagnosis code is challenging. Thus, it may be more meaningful to consider all diagnosis codes present rather than principal diagnosis alone. When all diagnosis codes are considered, the group with the highest incidence is “osteoarthritis and rheumatologic disorders” (45.9 per 10,000), followed by “metabolic conditions, obesity and syndromes” (28.8 per 10,000), with all other diagnostic categories reaching less than half of that rate.
While the incidence within the osteoarthritis and rheumatologic disorders group is high relative to the remaining diagnosis groups, it is important to acknowledge that only a small number of admissions fell into this category (3 VTE in 653 admissions, yielding a rate of 45.9 per 10,000 admissions). The first admission was a 14 year old female with scoliosis/kyphoscoliosis, admitted for dorsal spinal fusion. Her admission included a diagnosis code for “personal history of arthritis”. The second and third admissions were both of 15 year old females with aseptic necrosis of the head of the femur, the first admitted for “local excision of lesion or tissue of the femur” whose admission included a diagnosis code for dermatomyositis, and the second admitted for total hip replacement, whose admission included diagnoses of both osteoarthritis of the pelvic region and thigh, as well as polyarticular juvenile rheumatoid arthritis.
The incidence of VTE in our cohort (5.1 per 10,000 admissions) is considerably lower than previously published incidence rates in pediatric populations.2–7 However, previous studies of pediatric VTE have either focused on the incidence of pediatric VTE across all hospital admissions, or within trauma populations. Elective orthopedic surgical patients are largely free of known risk factors in such populations, and therefore a lower incidence of VTE is not surprising.
Methodologies for obtaining reliable information related to rare complications such as VTE are continually being scrutinized in relation to their ability to lead to improvements in surgical outcomes. Currently, administrative databases such as PHIS can be useful in these efforts. However, they have limitations. Of particular relevance to the current work, these databases are reliant on procedure and diagnosis codes, which are not 100% sensitive or specific. A 2010 examination by White et al. of the predictive value of many of the major VTE codes employed in the present study found that while the positive predictive value (PPV) of these codes for acute VTE was quite high when the code was in the principal diagnosis position (95%), this figure was considerably lower when in a secondary position (75%).31 The authors also note considerable variability by the specific code in question, with more highly defined codes achieving greater PPVs. Table 7 shows the distribution of the 74 admissions with VTE according to the VTE diagnosis codes present, along with the corresponding PPV for that diagnosis code as published by White et al.31 (where the particular code in question was studied by White et al.31). One explanation for this is that when a VTE diagnosis code is in a secondary position, it is not possible to distinguish directly between a history of an event and an acute event. The same 2010 examination of PPV values of VTE codes found that among patients with VTE codes in a secondary position, lower PPV values were largely due to the fact that 22% had a prior or chronic VTE and 7% had no indication of past or present VTE. The strategies employed in the present study, such as excluding admissions with a length of stay of less than one day, those not admitted as inpatients, and those which also had the diagnosis code V12.51 for “personal history of venous thrombosis and embolism”, have likely minimized the possibility that such cases were included in our analysis.
TABLE 7.
Frequency of VTE Diagnosis Codes and Positive Predictive Values as Published by White et al., 2010 33
| Code | Diagnosis Code Description | 1° | 1° PPV [95% CI]* |
2° | 2° PPV [95% CI]* |
|---|---|---|---|---|---|
| 415.11 | Iatrogenic pulmonary embolism & infarction | 5 | 96% (86–99) | 1 | 93% (88–98) |
| 415.12 | Septic pulmonary embolism § | 0 | 1 | Not studied | |
| 415.19 | Other pulmonary embolism & infarction | 10 | 96% (94–98) | 3 | 79% (74–84) |
| 451.11 | Phlebitis & thrombophlebitis, femoral vein | 0 | 3 | 33% (8–90) | |
| 451.81 | Phlebitis & thrombophlebitis, iliac vein | 0 | 1 | Not studied | |
| 451.83 | Phlebitis & thrombophlebitis, deep veins UE | 0 | 1 | Not studied | |
| 453.1 | Thrombophlebitis migrans | 0 | 1 | 100% (3–100) | |
| 453.2 | Embolism & thrombosis, vena cava | 0 | 2 | 62% (42–83) | |
| 453.40 | Acute VE & thrombosis, unspec. deep vessels LE | 0 | 4 | 61% (51–71) | |
| 453.41 | Acute VE & thrombosis, deep vessels proximal LE | 10 | 95% (92–99) | 11 | 86% (82–91) |
| 453.42 | Acute VE & thrombosis, deep vessels distal LE | 4 | 95% (86–99) | 7 | 83% (74–91) |
| 453.8 | Embolism & thrombosis, other specified veins ‡ | 1 | 94% (85–99) | 12 | 70% (62–78) |
| 453.87 | Acute VE & thrombosis, other thoracic veins † | 0 | 1 | Not studied | |
| 453.89 | Acute VE & thrombosis, other specified veins † | 0 | 1 | Not studied | |
| 453.9 | Embolism & thrombosis, unspecified site | 0 | 2 | 48% (27–68) | |
| All Codes $ | 30 | 51 |
As published by White et al., 2010 33
Code defined 10/1/07.
Code ended 9/30/2009.
Code defined 10/1/09.
Adds to 81 because 7 of the 74 patients with VTE had 2 VTE diagnosis codes simultaneously.
VE = venous embolism, LE = lower extremity.
No admissions with diagnosis codes 451.19 or 453.82 - 453.86 were identified.
Additional limitations to the present study include the inability to account for clinical variables which are known risk factors for VTE, such as smoking status, family history, BMI (beyond the presence or absence of a diagnostic code for obesity), and oral contraceptive pill use. We also did not seek to characterize what, if any, prophylactic measures were implemented to prevent VTE in this population. However, our primary intention was to comment on the frequency of such complications and the need for prophylaxis, rather than on its efficacy or comparative efficacy.
With respect to previous efforts to evaluate prophylactic guidelines for VTE in children, reports are somewhat limited. In the critically ill pediatric trauma population, a set of prophylactic guidelines implemented at one center was found to significantly reduce the rates of VTE by 65% (from 5.2% to 1.8%), without increased bleeding risk.22 However, another single-center study reported successful prevention of VTE prophylaxis in high-risk hospitalized pediatric patients, but reported major and minor bleeding complications in 2.2% and 5.6%, respectively.32 Furthermore, all of these cases of major and minor bleeding occurred in patients who underwent orthopedic surgery, highlighting the need to balance the risks of anticoagulation in orthopedic populations against any potential gains. While these results in high risk patients are not directly applicable to the elective orthopedic surgical population, they do serve as models of how future research may be designed to assess the efficacy of similar guidelines in elective orthopedic patients. In such an analysis, our results could serve as a starting point for risk stratification in prospective research specifically designed to quantify both the risk and benefit associated with anticoagulation in the elective orthopedic surgical patient.
Currently, there are no universally accepted guidelines for prophylaxis in the pediatric population. Because of the rare nature of this condition in children, it is difficult to determine which treatments provide the most effective prophylaxis. At our institution, perioperative services recommends mechanical prophylaxis for any patient over age 12 undergoing an elective orthopedic procedure greater than 1 hour in length. For longer cases, especially those about the hip and spine, chemical prophylaxis is usually administered starting on post-operative day one. Ultimately, the final decision is up to the attending surgeon. To the authors’ knowledge, this is the first report on the frequency and risk factors for VTE in elective orthopedic surgical patients. Based on our analysis, patients with an underlying metabolic condition, syndrome and/or obesity as well patients undergoing procedures related to complications of implanted devices or previous surgical procedures are at higher risk for DVT/PE. Future, multi-center collaborative studies are needed to determine whether patient populations identified as being at a higher risk for VTE in the present study would benefit from individually tailored prophylactic interventions.
Conclusions
VTE following elective pediatric orthopaedic surgical procedures is a rare but potentially devastating complication. The relative rarity of VTE has hampered investigation into its incidence in pediatric populations. Using a multi-variable logistic regression analysis of the PHIS database, our results suggest that, in contrast to adults, underlying diagnoses in children are stronger predictors of VTE than surgical procedures performed. In particular, complications of implanted devices and/or surgical procedures, and metabolic disorders, syndromes and/or obesity may place a child at an increased risk for VTE following elective orthopedic surgery. Older children admitted as inpatients may also be at increased risk. This information may be useful for future study, as well as for the pediatric surgeon when educating patients and their families about the risks associated with elective orthopaedic surgical procedures.
Acknowledgments
Source of Funding: Statistical consultation for this project was supported in part by an NIH NCRR Colorado CTSI Grant Number UL1 RR025780
References
- 1.Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83:1251–1257. [PubMed] [Google Scholar]
- 2.Sirachainan N, Chuansumrit A, Angchaisuksiri P, Pakakasama S, Hongeng S, Kadegasem P. Venous thromboembolism in Thai children. Pediatric hematology and oncology. 2007;24:245–256. doi: 10.1080/08880010701360767. [DOI] [PubMed] [Google Scholar]
- 3.Vu LT, Nobuhara KK, Lee H, Farmer DL. Determination of risk factors for deep venous thrombosis in hospitalized children. Journal of pediatric surgery. 2008;43:1095–1099. doi: 10.1016/j.jpedsurg.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval JA, Sheehan MP, Stonerock CE, Shafique S, Rescorla FJ, Dalsing MC. Incidence, risk factors, and treatment patterns for deep venous thrombosis in hospitalized children: an increasing population at risk. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2008;47:837–843. doi: 10.1016/j.jvs.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 6.Setty BA, O'Brien SH, Kerlin BA. Pediatric venous thromboembolism in the United States: A tertiary care complication of chronic diseases. Pediatric blood & cancer. 2012;59(2):258–264. doi: 10.1002/pbc.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright JM, Watts RG. Venous thromboembolism in pediatric patients: epidemiologic data from a pediatric tertiary care center in Alabama. Journal of pediatric hematology/oncology. 2011;33:261–264. doi: 10.1097/MPH.0b013e3182134111. [DOI] [PubMed] [Google Scholar]
- 8.Proctor MC, Wainess RM, Henke PK, Upchurch GR, Wakefield TW. Venous thromboembolism: regional differences in the nationwide inpatient sample, 1993 to 2000. Vascular. 2004;12:374–380. doi: 10.1258/rsmvasc.12.6.374. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Archives of internal medicine. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 10.Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. The Journal of pediatrics. 2004;145:563–565. doi: 10.1016/j.jpeds.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 11.van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. The Journal of pediatrics. 2001;139:676–681. doi: 10.1067/mpd.2001.118192. [DOI] [PubMed] [Google Scholar]
- 12.Patocka C, Nemeth J. Pulmonary Embolism in Pediatrics. The Journal of emergency medicine. 2012;42(1):105–116. doi: 10.1016/j.jemermed.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Spentzouris G, Scriven RJ, Lee TK, Labropoulos N. A review of pediatric venous thromboembolism in relation to adults. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2012;55(6):1785–1793. doi: 10.1016/j.jvs.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 14.Chan AK, Deveber G, Monagle P, Brooker LA, Massicotte PM. Venous thrombosis in children. Journal of thrombosis and haemostasis : JTH. 2003;1:1443–1455. doi: 10.1046/j.1538-7836.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 15.Molinari AC, Saracco P, Cecinati V, et al. Venous thrombosis in children: an emerging issue. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2011;22:351–361. doi: 10.1097/MBC.0b013e3283424824. [DOI] [PubMed] [Google Scholar]
- 16.Hanson SJ, Punzalan RC, Greenup RA, Liu H, Sato TT, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children after trauma. The Journal of trauma. 2010;68:52–56. doi: 10.1097/TA.0b013e3181a74652. [DOI] [PubMed] [Google Scholar]
- 17.Azu MC, McCormack JE, Scriven RJ, Brebbia JS, Shapiro MJ, Lee TK. Venous thromboembolic events in pediatric trauma patients: is prophylaxis necessary? The Journal of trauma. 2005;59:1345–1349. doi: 10.1097/01.ta.0000196008.48461.47. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien SH, Candrilli SD. In the absence of a central venous catheter, risk of venous thromboembolism is low in critically injured children, adolescents, and young adults: evidence from the National Trauma Data Bank. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2011;12:251–256. doi: 10.1097/PCC.0b013e3181f36bd9. [DOI] [PubMed] [Google Scholar]
- 19.Truitt AK, Sorrells DL, Halvorson E, et al. Pulmonary embolism: which pediatric trauma patients are at risk? Journal of pediatric surgery. 2005;40:124–127. doi: 10.1016/j.jpedsurg.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Vavilala MS, Nathens AB, Jurkovich GJ, Mackenzie E, Rivara FP. Risk factors for venous thromboembolism in pediatric trauma. The Journal of trauma. 2002;52:922–927. doi: 10.1097/00005373-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 22.Hanson SJ, Punzalan RC, Arca MJ, et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. The journal of trauma and acute care surgery. 2012;72:1292–1297. doi: 10.1097/TA.0b013e31824964d1. [DOI] [PubMed] [Google Scholar]
- 23.Duran R, Aladag N, Vatansever U, Temizoz O, Genchallac H, Acunas B. Cranial MR venography findings of severe hypernatremic dehydration in association with cerebral venous thrombosis in the neonatal period. Pediatric hematology and oncology. 2007;24:387–391. doi: 10.1080/08880010701394980. [DOI] [PubMed] [Google Scholar]
- 24.Hbibi M, Abourazzak S, Babakhouya A, et al. Severe hypernatremic dehydration associated with cerebral venous and aortic thrombosis in the neonatal period. BMJ case reports. 2012;2012 doi: 10.1136/bcr.07.2011.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staub E, Wilkins B. A fatal case of hypernatraemic dehydration in a neonate. Journal of paediatrics and child health. 2012;48:859–862. doi: 10.1111/j.1440-1754.2012.02529.x. [DOI] [PubMed] [Google Scholar]
- 26.Kshettry VR, Rosenbaum BP, Seicean A, Kelly ML, Schiltz NK, Weil RJ. Incidence and risk factors associated with in-hospital venous thromboembolism after aneurysmal subarachnoid hemorrhage. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2014;21:282–286. doi: 10.1016/j.jocn.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Kelly J, Hunt BJ, Lewis RR, et al. Dehydration and venous thromboembolism after acute stroke. QJM : monthly journal of the Association of Physicians. 2004;97:293–296. doi: 10.1093/qjmed/hch050. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi R, Razak F, Tso P, Davey JR, Mahomed NN. Metabolic syndrome and the incidence of symptomatic deep vein thrombosis following total knee arthroplasty. The Journal of rheumatology. 2009;36:2298–2301. doi: 10.3899/jrheum.090282. [DOI] [PubMed] [Google Scholar]
- 29.Rana AR, Michalsky MP, Teich S, Groner JI, Caniano DA, Schuster DP. Childhood obesity: a risk factor for injuries observed at a level-1 trauma center. Journal of pediatric surgery. 2009;44:1601–1605. doi: 10.1016/j.jpedsurg.2008.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akpinar EE, Hosgun D, Akan B, Ates C, Gulhan M. Does thromboprophylaxis prevent venous thromboembolism after major orthopedic surgery? Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2013;39:280–286. doi: 10.1590/S1806-37132013000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thrombosis research. 2010;126:61–67. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Stem J, Christensen A, Davis D, Raffini L. Safety of prophylactic anticoagulation at a pediatric hospital. Journal of pediatric hematology/oncology. 2013;35:e287–e291. doi: 10.1097/MPH.0b013e31829b7f92. [DOI] [PubMed] [Google Scholar]