Abstract
The dynorphin/kappa opioid receptor system (KOR) has been implicated as one potential neurobiological modulator of the abuse-related effects of cocaine and as a potential target for medications development. This study determined effects of the KOR antagonist nor-binaltorphimine (nor-BNI) on cocaine self-administration under a novel procedure that featured two daily components: (1) a 2 h “choice” component (9-11 am) when monkeys could choose between food pellets and cocaine injections (0-0.1 mg/kg/inj, IV), and (2) a 20 h “extended-access” component (noon-8 am) when cocaine (0.1 mg/kg/inj) was available under a fixed-ratio schedule to promote high daily cocaine intakes. Rhesus monkeys (n=4) were given 14 days of exposure to the choice + extended-access procedure, then treated with nor-BNI (3.2 or 10.0 mg/kg, IM), and cocaine choice and extended-access cocaine intake were evaluated for an additional 14 days. Consistent with previous studies, cocaine maintained both a dose-dependent increase in cocaine choice during choice components and a high level of cocaine intake during extended-access components. Neither 3.2 nor 10 mg/kg nor-BNI significantly altered cocaine choice or extended-access cocaine intake. In two additional monkeys, nor-BNI also had no effect on cocaine choice or extended-access cocaine intake when it was administered at the beginning of exposure to the extended-access components. Overall, these results do not support a major role for the dynorphin/KOR system in modulating cocaine self-administration under these conditions in nonhuman primates, nor do they support the clinical utility of KOR antagonists as a pharmacotherapeutic strategy for cocaine addiction.
Keywords: addiction, choice, cocaine, kappa opioid receptor, norbinaltorphimine, rhesus monkey
Introduction
Cocaine addiction remains a worldwide public health concern. In 2012, approximately 1.6 million Americans aged 12 years and older reported recent use of cocaine (SAMHSA 2012). Moreover, an estimated 22.2 million persons 12 years and older were diagnosed with substance dependence or abuse, and approximately 1.1 million of those individuals met diagnostic criteria for cocaine dependence (SAMHSA 2012). Despite both significant advances in our understanding of the neurobiological mechanisms of cocaine addiction and the prioritization of medications development by the National Institutes of Health, there remains no Food and Drug Administration-approved anti-cocaine medication.
Several lines of evidence from human (Volkow et al. 1990, 1993, 1997; Martinez et al. 2009; 2011), nonhuman primate (Nader et al. 2002; Henry et al. 2009), and rodent (Parsons et al. 1991; Weiss et al. 1992; Gerrits et al. 2002) studies have demonstrated that repeated cocaine exposure produces a hypodopaminergic state that may contribute to cocaine dependence. Furthermore, repeated cocaine exposure has also been reported to activate a central dynorphin/kappa-opioid receptor (KOR) system that has been implicated in regulating mesolimbic dopamine neurotransmission (for review, see Koob & Le Moal 2008; Wee & Koob 2010; Muschamp & Carlezon 2013). For example, humans (Hurd & Herkenham 1993; Frankel et al. 2008), nonhuman primates (Fagergren et al. 2003), and rodents (Daunais et al. 1995; Spangler et al. 1996) show upregulated prodynorphin mRNA expression in the striatum following cocaine exposure. In addition, dynorphin or exogenous kappa agonists can reduce extracellular dopamine levels in nucleus accumbens and block cocaine-induced increases in nucleus accumbens dopamine (Maisonneuve et al. 1994; Yokoo et al. 1994; Carlezon et al. 2004). Taken together, these findings suggest that increased dynorphin signaling may be a consequence of chronic cocaine exposure and this may in turn contribute to the hypodopaminergic state in cocaine addiction (Trifilieff & Martinez 2013).
Consistent with this hypothesized role of KOR stimulation in cocaine addiction, we have previously shown that continuous treatment with the exogenous KOR agonist U50,488 increased cocaine vs. food choice in rhesus monkeys, and this U50,488 effect on cocaine choice was blocked by the KOR antagonist nor-binaltorphimine (nor-BNI) (Negus 2004). However, nor-BNI did not alter cocaine choice when administered alone, suggesting little role for endogenous kappa signaling in modulating cocaine self-administration under these conditions. Recently, Wee and colleagues (2009, 2012) also found no effect on cocaine self-administration by nor-BNI or by mixtures of buprenorphine + naltrexone (also intended to produce a KOR antagonist effect) in rats given only “short access” (1 h per day) to cocaine. However, both nor-BNI and buprenorphine + naltrexone attenuated cocaine self-administration in rats given “extended or long cocaine access,” suggesting that chronic cocaine exposure is necessary for induction of the dynorphin/KOR system and sensitivity to KOR antagonism. Thus, one potential explanation for the lack of nor-BNI effect on cocaine choice in our previous study (Negus 2004) could be that cocaine was available for a relatively short duration (2 h) with limited cocaine intake (∼1.3 mg/kg/day), and this cocaine exposure was not sufficient to activate the dynorphin/KOR system and promote sensitivity to a KOR antagonist.
The aim of the present study was to determine the role of the KOR system in a nonhuman primate model of cocaine addiction that engendered higher daily cocaine intakes. Specifically, studies were conducted using a novel cocaine self-administration procedure that featured two daily components (Figure 1, Banks & Negus 2010). First, intravenous cocaine injections were concurrently available with a 1-gram banana-flavored food pellet during daily 2-h choice components. A choice procedure was chosen to provide a rate-independent measure of cocaine reinforcement (Negus 2003; Banks et al. 2008; Augier et al. 2012) and to assess nor-BNI efficacy to reallocate behavior away from cocaine and towards an alternative non-drug reinforcer. Second, cocaine injections (0.1 mg/kg/injection) were available for an additional 20 h/day under a fixed-ratio (FR) schedule to provide “extended cocaine access” and allow for high daily cocaine intakes. This procedure engenders daily cocaine intakes similar to those reported previously to increase striatal levels of prodynorphin mRNA in rhesus monkeys (Banks & Negus, 2010; Fagergren et al., 2003). We hypothesized that, under these conditions of high daily cocaine intake, nor-BNI treatment would attenuate cocaine self-administration under both components of the daily cocaine self-administration session.
Fig. 1.
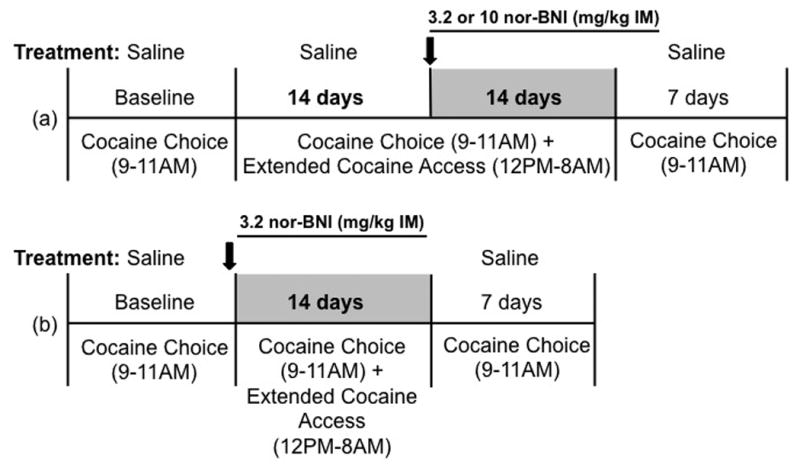
Experimental procedure. (a) Following a baseline period during which cocaine self-administration was only available during daily 2 h (0900 to 1100 h) cocaine vs food choice sessions, monkeys (N=4) were subsequently given daily access to cocaine during both “choice” sessions and “extended access” sessions lasting 20 h (noon to 0800 h). During extended-access sessions, monkeys could respond for 0.1 mg/kg/injection cocaine under a FR 10/time out 15-min schedule of reinforcement. After 14 days of extended cocaine access, 3.2 or 10 mg/kg nor-BNI was administered intramuscularly (IM). Extended-access conditions were continued for an additional 14 days following nor-BNI treatment. (b) Following a baseline period during which cocaine self-administration was only available during daily 2 h cocaine vs food choice sessions, monkeys (N=2) were administered 3.2 mg/kg nor-BNI 24 h before initiation of both “choice” sessions and “extended access” sessions. Extended-access conditions were implemented for 14 days following nor-BNI treatment.
Methods
Subjects
Six adult male rhesus monkeys (Macaca mulatta) with prior cocaine self-administration histories served as subjects (Banks et al. 2013a, 2013b, 2013c). Four monkeys had prior experience with both the choice procedure and the extended access procedure described below, and two monkeys had prior experience with the choice, but not extended access procedure. Monkeys were surgically implanted with a double-lumen catheter (Reiss Manufacturing, Blackstone, VA or STI Flow, Raleigh, NC) inserted into a major vein (femoral or jugular) as described previously (Banks et al. 2011). Monkeys weighed 9-13 kg and were maintained on a diet of fresh fruit and food biscuits (Lab Diet high Protein Monkey Biscuits no. 5045; PMI Nutrition, St. Louis, MO) provided following daily experimental sessions. Water was continuously available via an automatic watering system. A 12-h light-dark cycle was in effect (lights on from 0600 to 1800 h). Catheter patency was periodically evaluated by intravenous (IV) administration of ketamine (3 mg/kg) through the catheter lumen, and the catheter was considered to be patent if ketamine produced a loss of muscle tone within 10 s.
Animal research and maintenance were conducted according to the 8th edition of the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (National Academies Press 2011) and reported according the ARRIVE guidelines (Kilkenny et al. 2010). Animal facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved the research protocol. Monkeys had visual, auditory, and olfactory contact with other monkeys throughout the study. Operant procedures and foraging toys were provided for environmental manipulation and enrichment. Videos were played during weekdays in animal housing rooms to provide additional environmental enrichment.
Apparatus
Monkeys were housed individually in well-ventilated, stainless steel chambers (66×76×94 cm) that also served as experimental chambers. Each chamber was equipped with a custom-designed operant panel (28×28 cm) mounted on the front wall. Three square translucent response keys (5.1-cm diameter) were arranged 3.5 cm apart in a horizontal row 9 cm from the top of the panel. Each key could be transilluminated red or green. Each chamber was also equipped with a pellet dispenser (Model ENV-203-1000; Med Associates, St Albans, VT) and two syringe pumps (Model PHM-108; Med Associates), one for each lumen of the double lumen catheter. One syringe pump (the “self-administration” pump) delivered response-contingent cocaine injections. The second syringe pump (the “treatment” pump) delivered saline through the second lumen of the catheter at a programmed rate of 0.1-ml infusions every 20 min from 1200 to 1100 h. The intravenous catheter was protected by a tether and jacket system (Lomir Biomedical, Malone, NY) that allowed the monkeys to move freely.
Procedure
Initially, monkeys responded in daily 2-h choice sessions (0900 to 1100 h) that consisted of a five-component concurrent schedule of food pellet and intravenous cocaine availability as described in detail previously (Negus 2003, 2004). During each component, responses on the left key were reinforced with food (1-g banana-flavored pellets; Test Diets, Richmond, IN) according to a fixed-ratio (FR) 100 schedule, and responses on the right key were reinforced with intravenous cocaine (0–0.1 mg/kg/injection) according to an FR 10 schedule. A response on one key reset the ratio requirement on the alternative key. Each reinforcer delivery was followed by a 3-s timeout during which all stimulus lights were extinguished, and responding had no programmed consequences. During each component, the food key was transilluminated red. The stimulus lights for the cocaine key were flashed on and off in 3-s cycles, and longer flashes were associated with higher cocaine doses. Across components of the choice procedure, a different unit-cocaine dose was made available (0, 0.0032, 0.01, 0.032, and 0.1 mg/kg/injection during components 1–5, respectively) by manipulating the injection volume (0, 0.01, 0.03, 0.1, and 0.3ml/injection, respectively). Each component was in effect until 10 total reinforcers were earned or 20 min elapsed, whichever occurred first. Response allocation was deemed stable when the lowest unit-cocaine dose maintaining at least 80% preference for cocaine varied by ≤ 0.5 log units for 3 consecutive days. Experimental parameters of the choice sessions used in this study were based on extensive parametric manipulations reported previously (Negus 2003) and identical to parameters used to assess effects of kappa opioids and extended cocaine access on cocaine choice (Negus 2004; Banks & Negus 2010; Banks et al. 2013a). These parameters engender cocaine choice ED50 values that lie in approximately the middle of the cocaine dose range, and therefore permit detection of both leftward and rightward shifts in the cocaine vs. food choice dose–effect function.
Once performance was stable, a multiple schedule of reinforcement was initiated to provide “extended cocaine access” for 20 h each day (1200 to 0800 h) in addition to the 2-h choice session (Banks & Negus 2010; Banks et al. 2013a). During the extended cocaine access component, the cocaine key was transilluminated green, and completion of the FR10 response requirement extinguished the green light, activated the self-administration pump to deliver 0.1 mg/kg per injection of cocaine, and initiated a 15-min timeout. This multiple schedule of cocaine vs. food choice and extended cocaine access was implemented for a period of 14 consecutive days prior to treatment with the kappa-opioid receptor antagonist norbinaltorphimine (nor-BNI).
After the choice component on day 14, a single intramuscular (IM) dose (3.2 or 10 mg/kg) of nor-BNI was administered to groups of four monkeys, and cocaine self-administration was evaluated for an additional 14 days. We and others have previously shown 3.2 mg/kg and 10 mg/kg nor-BNI is sufficient to antagonize kappa agonist-induced behavioral effects in rhesus monkeys for at least 21 days (Butelman et al. 1993, 1998; Negus et al. 1998; Ko et al. 2003; Negus 2004). Extended cocaine access was terminated after 14 days. Nor-BNI (3.2 mg/kg) was tested before 10 mg/kg in all monkeys, and nor-BNI doses were separated by a mean of 181 days (range: 147-253 days).
An additional experiment was conducted in two monkeys that did not have prior extended cocaine access experience, but had a history of cocaine self-administration under the choice procedure. Specifically, nor-BNI (3.2 mg/kg, IM) was administered 23 h before initiating exposure to the extended cocaine access component. Extended cocaine access conditions were implemented for a period of 14 days to determine whether nor-BNI would attenuate cocaine choice and extended-access cocaine self-administration.
Data analysis
The primary dependent measures for choice sessions were (1) percent cocaine choice, defined as (number of completed ratios on the cocaine-associated key ÷ total completed ratios) × 100 per component and (2) the numbers of total, cocaine, and food reinforcers per session. The primary dependent measure for extended cocaine access sessions was the number of cocaine injections earned during each 4-h bin of the session. The mean for each dependent measure was calculated for the last 3 days of each experimental condition for each monkey. Group mean cocaine choice dose-effect functions were analyzed using two-way repeated-measures (RM) ANOVA with cocaine dose and nor-BNI treatment condition as factors. The numbers of total, cocaine, and food reinforcers earned were analyzed using one-way RM ANOVA with experimental manipulation as the factor. The mean number of injections per bin for the last 3 days of each experimental condition was calculated for each monkey. Group mean number of injections per bin was analyzed using two-way RM ANOVA with time and nor-BNI treatment condition as factors. A significant ANOVA was followed by the Holm-Sidak multiple comparisons post-hoc test. The criterion for statistical significance was set at the 95% confidence level (P<0.05). All analyses were conducted using Prism 6.0c for Mac (GraphPad Software, La Jolla, CA).
Drugs
(-)-Cocaine HCl was provided by the National Institute on Drug Abuse (Bethesda, MD) Drug Supply Program. Nor-binaltorphimine di-HCl (Drs. Kejun Cheng and Kenner Rice, NIDA and NIAAA) was dissolved in 1% lactic acid:sterile water for intramuscular injection. All drug doses are expressed as the salt forms listed above, and all drug solutions were passed through a sterile 0.2-micron filter (EMD Millipore, Billerica, MA) prior to administration.
Results
Effects of Extended Cocaine Access on Cocaine Choice
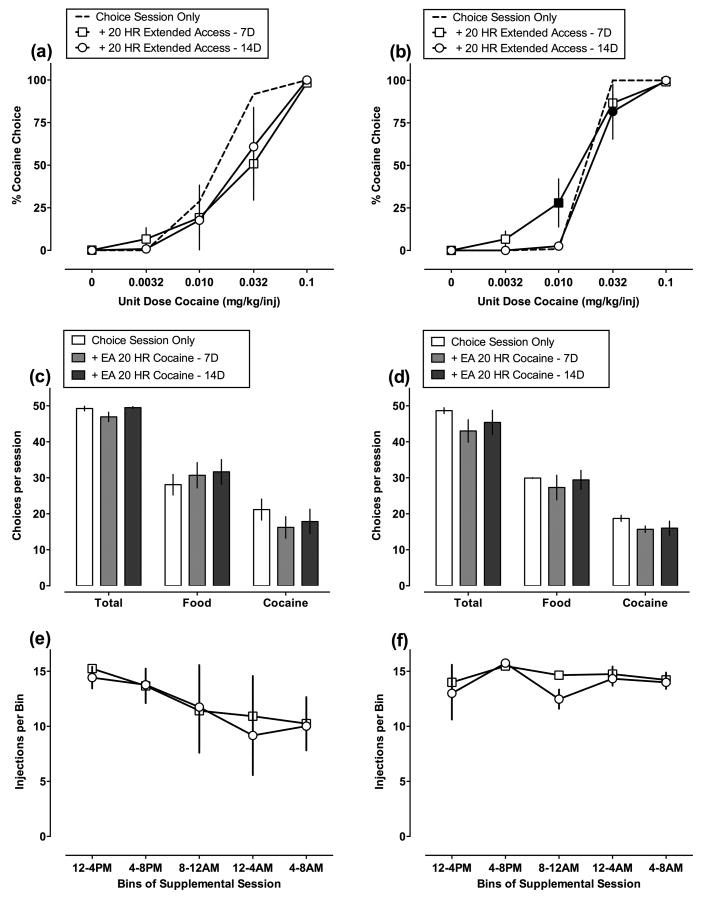
Figure 2(a-b) shows the effects of cocaine dose on choice between cocaine and food prior to and during extended cocaine access for the 3.2 mg/kg (a) and 10 mg/kg (b) nor-BNI experiment. Cocaine choice increased as a function of the unit cocaine dose (cocaine dose: (a) F4, 12 = 42.3, P<0.05; (b) F4, 12 = 162.2, P<0.05). There was no significant main effect of extended cocaine access, but there was a statistically significant interaction between cocaine dose and extended cocaine access for the 10 mg/kg nor-BNI experiment (F8, 24 = 2.38, P<0.05). Figure 2(b) shows extended cocaine access increased choice of 0.01 mg/kg/injection cocaine at the end of 7 days and decreased choice of 0.032 mg/kg/injection cocaine at the end of 14 days. Figure 2(c-d) shows that extended cocaine access did not significantly alter the number of total, food, or cocaine reinforcers. Figure 2 (e-f) shows the diurnal pattern of cocaine self-administration during successive 4-h bins of extended access components. Table 1 shows that introduction of extended cocaine access produced an approximately 6-fold increase in mean daily cocaine intake (extended access: F4, 12 = 133.7, P<0.05).
Fig. 2.
Effects of 20 h/day of extended cocaine access on cocaine vs. food choice (n=4 per group). (a-b) Abscissae: unit dose cocaine in mg/kg/injection (log scale). Ordinates: percent cocaine choice. (c-d) Abscissae: total choices, food choices, or cocaine choices. Ordinates: number of choices summed across all cocaine doses per choice session. (e-f) Abscissae: 4-h bins of the extended access cocaine self-administration session. Ordinates: number of cocaine injections per 4-h bin. All points represent mean data ± SEM obtained during the last 3 days of each 7-day treatment period (days 5-7, 12-14). Filled symbols indicate statistical significance (P<0.05) compared with choice session only condition.
Table 1.
Nor-BNI (3.2 or 10.0 mg/kg, IM) treatment effects on group mean (N=4 per group) cocaine intakes (mg/kg) during both the cocaine vs. food choice and the 20-hr extended cocaine access components of the daily session.
| Choice session cocaine (mg/kg) | Extended-access cocaine (mg/kg) | Total daily cocaine (mg/kg) | |
|---|---|---|---|
| Choice session only | 1.25 ± 0.14 | — | 1.25 ± 0.14 |
| + Extended access – 7 D | 1.10 ± 0.21 | 6.15 ± 2.22 | 7.25 ± 2.08 |
| + Extended access – 14 D | 1.20 ± 0.18 | 5.91 ± 1.89 | 7.11 ± 2.00 |
| + 3.2 nor-BNI – 7 days | 1.04 ± 0.23 | 6.41 ± 1.37 | 7.45 ± 1.31 |
| + 3.2 nor-BNI – 14 days | 1.18 ± 0.11 | 6.33 ± 1.41 | 7.51 ± 1.30 |
| Choice session only | 1.19 ± 0.16 | — | 1.19 ± 0.16 |
| + Extended access – 7 D | 1.06 ± 0.22 | 7.32 ± 0.53 | 8.38 ± 0.67 |
| + Extended access – 14 D | 1.08 ± 0.24 | 6.96 ± 0.67 | 8.04 ± 0.81 |
| + 10.0 nor-BNI – 7 days | 1.18 ± 0.09 | 7.08 ± 0.51 | 8.27 ± 0.48 |
| + 10.0 nor-BNI – 14 days | 1.21 ± 0.15 | 5.83 ± 1.48 | 7.05 ± 1.38 |
Data represent group mean ± 95% confidence limit cocaine intakes of the last 3 days means of each 7-day experimental condition for individual monkey.
Effects of nor-BNI on Cocaine Self-Administration
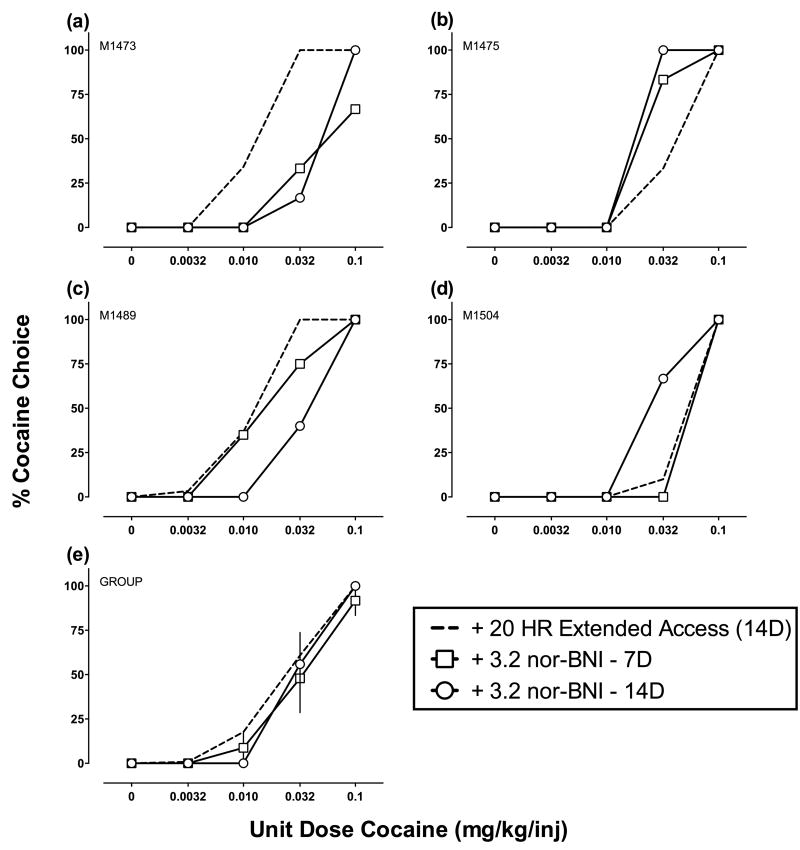
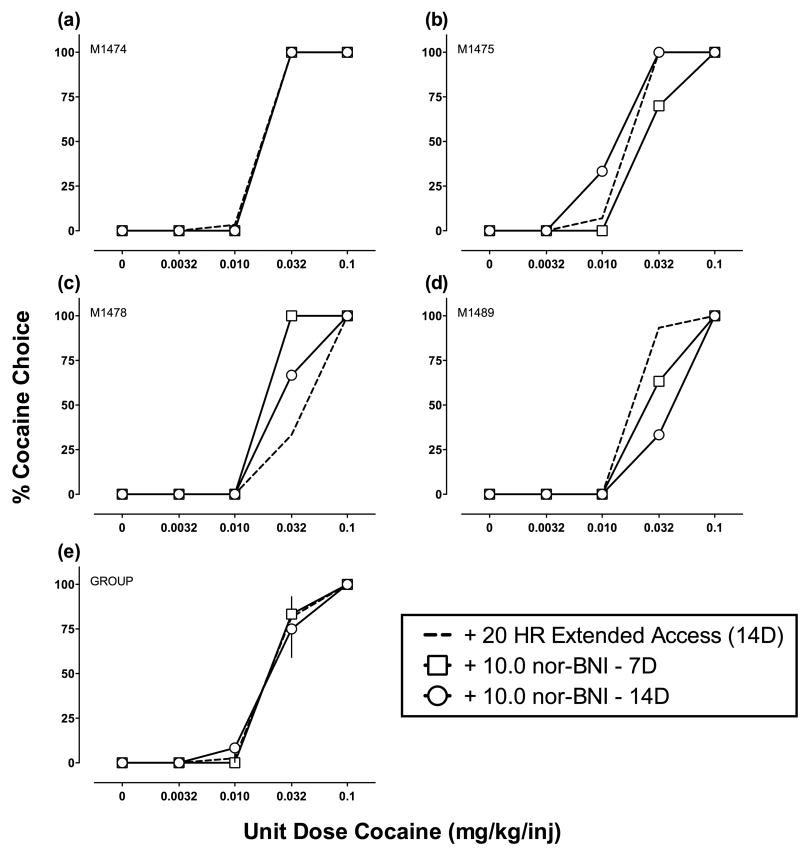
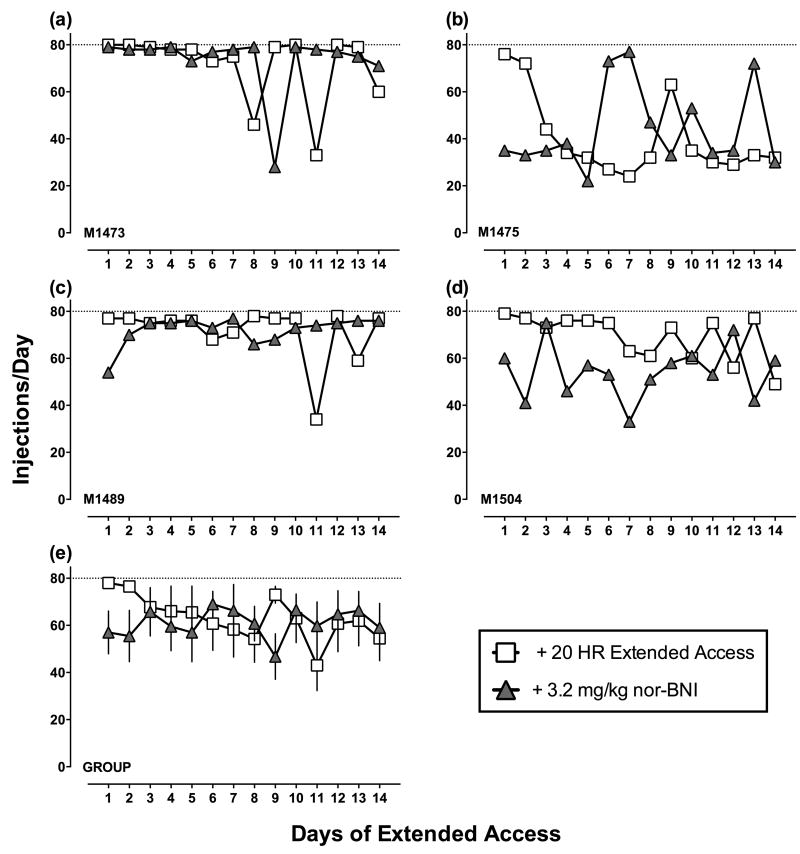
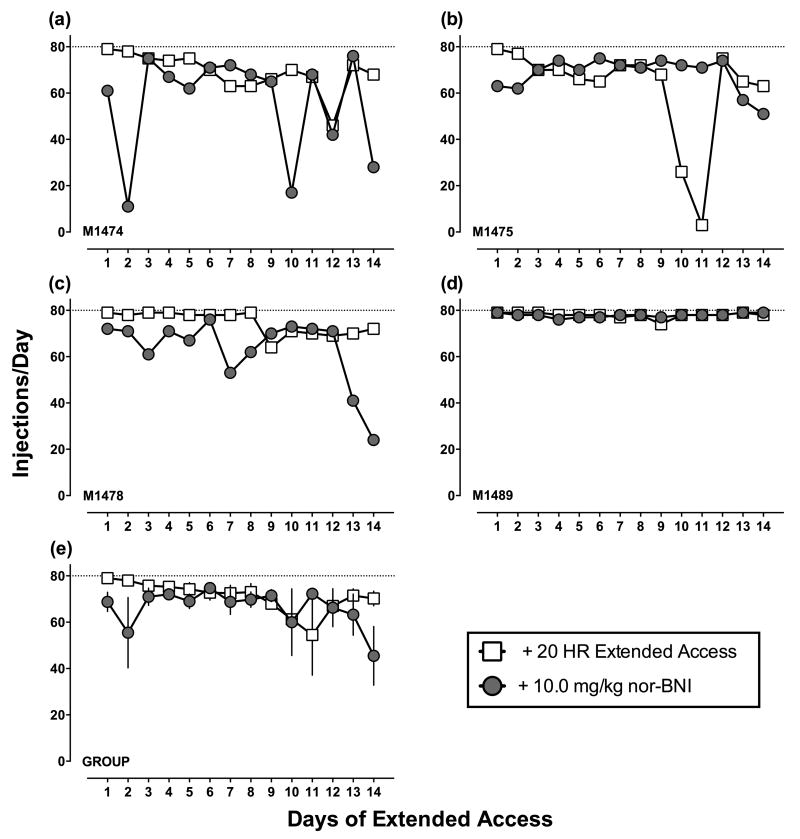
Figures 3 (3.2 mg/kg) and 4 (10 mg/kg) show nor-BNI effects on cocaine choice for individual monkeys (a-d) and group means (e). Figures 5 (3.2 mg/kg) and 6 (10 mg/kg) show nor-BNI effects on cocaine self-administration during extended access. None of the dependent measures examined during either component of the daily experimental session were significantly different following nor-BNI (see Table 1 for cocaine intakes). Supporting Figure 1 shows nor-BNI effects on total choices, food choices, and cocaine choices completed during the choice component (a-b) and diurnal pattern of cocaine self-administration (c-d). There was day-to-day variability in extended access cocaine self-administration before and after nor-BNI administration, and “binge-crash” patterns of cocaine self-administration were observed in all 6 monkeys tested. However, statistical analysis revealed no significant effect of extended cocaine access day or nor-BNI treatment.
Fig. 3.
Effects of nor-BNI (3.2 mg/kg) on cocaine vs. food choice in individual rhesus monkeys (a-d) and group means (e; each n=4). Abscissae: unit dose cocaine in mg/kg/injection (log scale). Ordinates: percent cocaine choice.
Fig. 4.
Effects of nor-BNI (10 mg/kg) on cocaine vs. food choice in individual rhesus monkeys (a-d) and group means (e; each n=4). Otherwise, details are the same as in Fig. 3.
Fig. 5.
Cocaine self-administration during extended cocaine access for 14 days before and 14 days after treatment with 3.2 mg/kg nor-BNI in individual rhesus monkeys (a-d) and group means (e). Abscissae: day of extended access cocaine self-administration session. Ordinates: number of cocaine injections (0.1 mg/kg/injection) earned during each extended cocaine access session. Each data point represents a single determination except for panel (e), which represents the group mean results.
Fig. 6.
Cocaine self-administration during extended cocaine access for 14 days before and 14 days after treatment with 10 mg/kg nor-BNI in individual rhesus monkeys (a-d) and group means (e). Otherwise, details are the same as in Fig. 5.
In the two monkeys without prior extended cocaine access experience, nor-BNI was administered at the same time as introduction of extended cocaine access rather than 14 days later as in the previous experiments (Supporting Figure 3). The cocaine choice dose-effect function was shifted to the right in both monkeys after 7 days. After 14 days, the cocaine choice dose-effect function was back to baseline for one monkey (M1478), and cocaine choice was increased in the other monkey (M1474). For both monkeys, cocaine maintained high and relatively stable rates of self-administration from day to day during the first 6-8 days, and self-administration then became more variable during the last 6-8 days. This “binge-crash” pattern of variable cocaine self-administration was also observed in most monkeys introduced to extended access cocaine without nor-BNI treatment (Figures 5 and 6) and was consistent with results obtained in our previous studies of cocaine self-administration during extended-access sessions (Banks & Negus 2010).
Discussion
The present study determined effects of the KOR antagonist nor-BNI in a nonhuman primate model of cocaine addiction using a novel procedure that incorporated two daily components: 1) a choice component to assess behavioral allocation between cocaine and a non-drug alternative reinforcer and 2) an extended-access component to permit high cocaine intake hypothesized to activate the dynorphin/KOR system (Fagergren et al., 2003; Koob & Le Moal 2008; Wee & Koob 2010). There were three main findings. First, in agreement with previous findings (Banks & Negus, 2010), cocaine maintained a dose-dependent increase in cocaine choice during choice components, and extended cocaine access produced high daily cocaine intakes. Second, neither cocaine vs. food choice nor extended-access cocaine consumption was attenuated by either nor-BNI dose. Finally, nor-BNI pretreatment did not attenuate cocaine vs. food choice or extended access cocaine self-administration in two monkeys that had no previous experience with extended cocaine access. Consequently, these results do not support the hypothesis that the dynorphin/KOR system is a significant modulator of cocaine reinforcement in this preclinical nonhuman primate model of cocaine addiction, even under conditions of high daily cocaine intake. The lack of nor-BNI effect may have implications for both the mechanisms and treatment of cocaine addiction.
Cocaine Choice Before and During Extended Cocaine Access
Under baseline conditions before introduction of extended cocaine access, cocaine maintained a dose-dependent increase in choice vs. an alternative, non-drug reinforcer. These results are in agreement with many previous findings in humans (Haney et al. 1999; Hart et al. 2000; Stoops et al. 2012), nonhuman primates (Johansen & Schuster 1975; Nader & Woolverton 1991; Woolverton & Balster 1981), and rodents (Kerstetter et al. 2012; Thomsen et al. 2013). Introduction of the extended-access component increased cocaine intake approximately six-fold and, at the end of 14 days, resulted in a small, but significant, rightward shift in the cocaine choice dose-effect function in the 10 mg/kg nor-BNI experiment. These effects of extended cocaine access on cocaine vs. food choice are consistent with previous studies in our laboratory (Banks & Negus, 2010; Banks et al. 2013a) and others (Cantin et al. 2010; Lenoir et al. 2007). Finally, in agreement with previous nonhuman primate studies in our laboratory (Banks & Negus, 2010; Banks et al. 2013a) and others (Henry & Howell, 2009; Henry et al. 2009), rates of cocaine self-administration did not significantly change across the 28 experimental days. These relatively stable rates of cocaine self-administration in nonhuman primates contrast with results from previous rodent studies demonstrating an increase in cocaine self-administration rates over time (Ahmed & Koob 1999). Although these discrepant effects may reflect procedural differences, other alternative explanations are discussed below and may be reflective of the differential results with KOR antagonists.
Nor-BNI Effects on Cocaine Self-Administration
The lack of nor-BNI effect on cocaine vs. food choice under extended cocaine access conditions was consistent with our previous study determining nor-BNI effects on cocaine choice under limited cocaine access conditions (Negus 2004). In the previous study, 3.2 mg/kg nor-BNI was sufficient to block increases in cocaine vs. food choice produced by treatment with an exogenous KOR agonist (Negus 2004), and 3.2 mg/kg nor-BNI was also sufficient to antagonize other KOR agonist-induced behavioral effects in rhesus monkeys (Butelman et al. 1993; Ko et al. 2003). However, 3.2 mg/kg nor-BNI did not alter cocaine vs. food choice in that previous study (Negus 2004). The present study expanded on this earlier work by using a procedure that incorporated daily components of extended cocaine access to increase daily cocaine intakes. However, despite an approximately 6-fold increase in total daily cocaine intake, neither 3.2 nor a higher dose of 10 mg/kg nor-BNI attenuated cocaine self-administration during either choice or extended-access components. The lack of nor-BNI effects on cocaine self-administration in these cocaine vs. food choice studies is also consistent with a lack of nor-BNI effects on cocaine self-administration in previous studies in rodents (Glick et al. 1995; Wee et al. 2009) and nonhuman primates (Negus et al. 1997) using other schedules of cocaine self-administration. Before addressing the potential implications of these results, three possible explanations for our negative nor-BNI findings should be considered.
One possible explanation for negative results with nor-BNI is that the relative reinforcing strength of cocaine assessed under this cocaine vs. food choice procedure is insensitive to pharmacological modulation. Two lines of evidence argue against this possibility. First, we have previously shown KOR agonist-induced increases in cocaine vs. food choice, and it was these effects of an exogenous KOR agonist that suggested the possibility that similar effects might be observed under conditions that activated release of the endogenous KOR agonist dynorphin (Negus 2004). Second, we (Negus 2003; Banks et al. 2011, 2013a) and others (Augier et al. 2012; Czoty & Nader 2013; Thomsen et al. 2013) have demonstrated the sensitivity of drug vs. food choice procedures to several other pharmacological and non-pharmacological manipulations (see Banks & Negus 2012 for a review of the various manipulations conducted). Overall, drug vs. food choice procedures have validated sensitivity to a multitude of experimental manipulations.
A second possible explanation for negative nor-BNI effects is that the nor-BNI dose was not sufficient to block effects produced by endogenously released dynorphin. Two lines of evidence argue against this possibility. First, 10 mg/kg nor-BNI decreased rates of food-maintained responding in two of the four monkeys during the choice component of the daily behavioral session 24 h after administration (data not shown). These results confirm that a behaviorally active nor-BNI dose was tested and that evaluation of higher doses might produce more significant undesirable effects. Second, these doses of nor-BNI have been previously shown to antagonize effects of the exogenous KOR agonist U50,488 in rhesus monkeys for up to 21 days (Butelman et al. 1993, 1998). Moreover, 10 mg/kg nor-BNI, but not 3.2 mg/kg nor-BNI, significantly attenuated the behavioral effects of the benzomorphan KOR agonist ethylketocyclazocine, and these results were interpreted to suggest that 10 mg/kg nor-BNI was sufficient to antagonize both kappa-1 and non-kappa-1 receptors, whereas 3.2 mg/kg nor-BNI was sufficient to antagonize only kappa-1 receptors (Butelman et al. 1993, 1998). Taken together, these results suggest that the nor-BNI doses tested were sufficient to produce robust kappa receptor antagonism and that evaluation of higher doses may impracticable due to untoward effects.
A third possible explanation for negative effects with nor-BNI is that the regimen of cocaine exposure was not sufficient to activate the dynorphin/KOR system. For example, one study compared prodynorphin mRNA levels in rhesus monkey striatum after 100 days of self-administration maintained by 30 daily injections of a low cocaine dose (0.03 mg/kg/inj; 0.9 mg/kg/day) or a high cocaine dose (0.3 mg/kg/inj; 9.0 mg/kg/day) (Fagergren et al. 2003). Only the high cocaine dose increased striatal prodynorphin mRNA levels. Striatal prodynorphin levels were not examined in this study, and the precise threshold for the magnitude and duration of cocaine exposure that is necessary to increase striatal prodynorphin mRNA is currently unknown. However, the maximum daily cocaine intakes in the present study were only slightly lower (∼8 vs. 9 mg/kg/day) and occurred over only a slightly shorter time span (28 vs. 30 days) than the high-dose cocaine condition in the Fagergren (2003) study. In addition, the extent of cocaine availability in the present study was sufficient to produce variable “binge-crash” patterns of daily self-administration during extended cocaine access. Furthermore, one predicted consequence of activation of the dynorphin/KOR system is enhanced cocaine self-administration similar to that produced by treatment with an exogenous KOR agonist (Negus 2004); however, we have previously studied conditions that engendered daily cocaine intakes up to 11 mg/kg/day, and neither exposure to, nor withdrawal from, these high daily cocaine intakes was sufficient to increase cocaine choice (Banks & Negus 2010; Supporting Figures 2 and 4). Finally, nor-BNI also failed to alter cocaine self-administration under a second-order schedule that maintained daily cocaine intakes of up to 2.5 mg/kg/day (Negus et al. 1997). Taken together, these studies demonstrate a range of conditions under which high and sustained rates of cocaine self-administration failed to produce effects suggestive of dynorphin/KOR system activation and failed to render cocaine self-administration sensitive to antagonism by a KOR antagonist.
Implications for the Kappa-Opioid System as a Therapeutic Target
The dynorphin/KOR system has been implicated as a potential therapeutic target in the treatment of cocaine addiction (Negus 2004; Butelman et al. 2012; Wee et al. 2009, 2012). A major goal in the treatment of cocaine addiction is not only to decrease cocaine-maintained behavior, but also to promote a reallocation of behavior toward non-drug alternative reinforcers (Vocci 2007). Drug self-administration procedures in general, and drug vs. non-drug choice procedures in particular, have been useful tools in preclinical evaluation of anti-cocaine addiction medication efficacy in both preclinical and clinical research (Comer et al. 2008; Haney & Spealman 2008; Banks & Negus 2012; Thomsen et al. 2013). The present study determined KOR antagonist effects in a preclinical nonhuman primate model of cocaine addiction that included components of the “acquisition/escalation” and “abstinence/withdrawal” addiction-like cycle (Butelman et al. 2012) in addition to a cocaine vs. food choice procedure. The failure of the KOR antagonist nor-BNI to reduce cocaine self-administration or to promote reallocation of behavior away from cocaine choice and toward choice of an alternative non-drug reinforcer does not support KOR antagonism as a pharmacotherapeutic strategy for cocaine addiction. In addition, these findings suggest that daily cocaine intakes up to approximately 8 mg/kg/day in rhesus monkeys do not engage sufficient activation of endogenous dynorphin/KOR systems to either increase the relative reinforcing efficacy of cocaine or to render cocaine self-administration sensitive to treatment with KOR antagonists.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers R01 DA026946 and T32 DA007027. A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Jennifer Gough and Crystal Reyns for technical assistance and Kevin Costa for writing the original computer program version.
Footnotes
Authors Contributions: MLB and BAH performed the behavioral experiments and analyzed the data. MLB and SSN were responsible for the study concept, design, and interpretation. KC and KCR synthesized the nor-BNI. BAH and MLB drafted the manuscript and MLB and SSN provided critical revision of the manuscript. All authors critically reviewed the content and approved the final version for publication.
References
- Ahmed SH. Imbalance between drug and non-drug reward availability: A major risk factor for addiction. Eur J Pharmacol. 2005;526:9–20. doi: 10.1016/j.ejphar.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Augier E, Vouillac C, Ahmed SH. Diazepam promotes choice of abstinence in cocaine self-administering rats. Addict Biol. 2012;17:378–391. doi: 10.1111/j.1369-1600.2011.00368.x. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Czoty PW, Nader MA. Effects of ambient temperature on the relative reinforcing strength of MDMA using a choice procedure in monkeys. Psychopharmacology. 2008;196:63–70. doi: 10.1007/s00213-007-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Effects of extended cocaine access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys. Neuropsychopharmacology. 2010;35:493. doi: 10.1038/npp.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011;22:824. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a;38:2698–2707. doi: 10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013b;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology. 2013c;38:395–404. doi: 10.1038/npp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, De Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiro K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. Kappa-opioid receptor binding populations in rhesus monkey brain: relationship to an assay of thermal antinociception. J Pharmacol Exp Ther. 1998;286:150–156. [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. κ-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS ONE. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of dopamine D2/D3 receptor ligands on food-cocaine choice in socially housed male cynomolgus monkeys. J Pharmacol Exp Ther. 2013;344:329–338. doi: 10.1124/jpet.112.201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Roberts DCS, McGinty JF. Short-term cocaine self administration alters striatal gene expression. Brain Res Bull. 1995;37:523–527. doi: 10.1016/0361-9230(95)00049-k. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Euro J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL. Assessment of the kappa opioid agonist, salvinorin A, as a punisher of drug self-administration in monkeys. Psychopharmacology. 2014;231:2751–8. doi: 10.1007/s00213-014-3436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MAFM, Petromilli P, Westenberg HGM, Chiara G Di, van Ree JM. Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res. 2002;924:141–150. doi: 10.1016/s0006-8993(01)03105-5. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Sydney A. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology. 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Henry PK, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology. 2009;205:237–247. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PK, Howell LL. Cocaine-induced reinstatement during limited and extended drug access conditions in rhesus monkeys. Psychopharmacology. 2009;204:523–529. doi: 10.1007/s00213-009-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Pharmacological and Environmental Variables Affecting Drug Preference in Rhesus Monkeys. Pharmacol Rev. 1975;27:343–355. [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37:2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Willmont KJ, Lee H, Flory GS, Woods JH. Ultra-long antagonism of kappa opioid agonist-induced diuresis by intracisternal nor-binaltorphimine in monkeys. Brain Res. 2003;982:38–44. doi: 10.1016/s0006-8993(03)02938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS ONE. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D2/D3 receptors following acute dopamine depletion. Am J Psychiatry. 2009;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging Dopamine Transmission in Cocaine Dependence: Link Between Neurochemistry and Response to Treatment. Am J Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Stevens Negus S. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Carlezon WA. Roles of Nucleus Accumbens CREB and Dynorphin in Dysregulation of Motivation. Cold Spring Harb Perspect Med. 2013;3:a012005. doi: 10.1101/cshperspect.a012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals, Eighth Edition. The National Academies Press; Washington, DC: 2011. [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: Effects of environment manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. J Pharmacol Exp Ther. 1993;267:896–903. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB. Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2011 National Survery on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. pp. 12–4713. [Google Scholar]
- Spangler R, Ho A, Zhou Y, Maggos CE, Yuferov V, Kreek MJ. Regulation of kappa opioid receptor mRNA in the rat brain by “binge”pattern cocaine administration and correlation with preprodynorphin mRNA. Brain Res Mol Brain Res. 1996;38:71–76. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Hays LR, Rush CR. Alternative reinforcer response cost impacts cocaine choice in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:189–193. doi: 10.1016/j.pnpbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–233. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Kappa-Opioid Receptor Signaling in the Striatum as a Potential Modulator of Dopamine Transmission in Cocaine Dependence. Front Psychiatry. 2013;4:44. doi: 10.3389/fpsyt.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ. Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction. 2007;102:1888–1889. doi: 10.1111/j.1360-0443.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzemann R, Henn F. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin–κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med. 2012;4:146ra110–146ra110. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. Effects of antipsychotic compounds in rhesus monkeys given a choice between cocaine and food. Drug Alcohol Depend. 1981;8:69–78. doi: 10.1016/0376-8716(81)90088-0. [DOI] [PubMed] [Google Scholar]
- Yokoo H, Yamada S, Yoshida M, Tanaka T, Mizoguchi K, Emoto H, et al. Effect of opioid peptides on dopamine release from nucleus accumbens after repeated treatment with methamphetamine. Eur J Pharmacol. 1994;256:335–338. doi: 10.1016/0014-2999(94)90560-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology. 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.