In response to appropriate stimuli in their microenvironment, naïve CD4+ T cells differentiate into one of several T helper cell lineages, such as Th1, Th2, Th17, and Treg cells, as defined by their transcription factor expression, cytokine secretion, and effector functions. Although T helper subsets are known to be regulated by specific transcription factors and cytokines, the molecular basis of T helper cell differentiation, especially the role of microRNA (miRNA) pathways, is incompletely understood. MicroRNAs are short non-coding RNAs that post-transcriptionally modulate the expression of multiple target genes and are implicated in a wide array of cellular and developmental processes, including immune system development.
MicroRNA-21 (miR-21) was one of the earliest identified cancer-promoting “oncomirs,” and therefore much of the research involving this miRNA has focused on its role in tumor promotion [1]; however, recent data also suggest a crucial role for miR-21 in immune system function. MiR-21 is expressed at low levels in T cells and antigen-presenting cells (APCs), but is upregulated in these cells following activation. Although miR-21 seems to have some anti-inflammatory properties at the APC level [2], emerging studies indicate that miR-21 promotes tissue inflammation and autoimmunity by altering the balance of T helper differentiation and function.
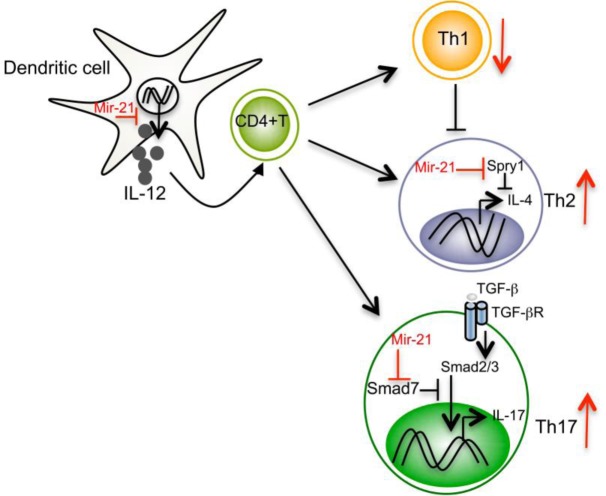
Proinflammatory Th1 and anti-inflammatory Th2 cells exist in a balanced state by counter-regulating each other's differentiation and function. MiR-21 has been shown to regulate this balance via multiple pathways [3]. For example, miR-21 is induced in activated DCs and directly targets the mRNA that encodes the p35 subunit of Th1-promoting IL-12, causing miR-21 deficient (miR-21−/−) mice to present with increased DC-secreted IL-12 and enhanced Th1 development. Because IL-12 is highly potent at inducing Th1 differentiation and inhibiting Th2 cells, IL-12 inhibition may represent a major mechanism by which miR-21 affects the Th1/Th2 balance. Moreover, by inhibiting Th1 differentiation, miR-21 might indirectly relieve the suppressive effect of Th1-secreted IFN-γ on Th2 cells, further promoting Th2 development (Figure 1). In addition to DC-intrinsic miR-21, T-cell intrinsic miR-21 has been shown to promote Th2 differentiation by inhibiting expression of the Spry1 transcript, a MAP kinase pathway inhibitor. Mice with a deletion of miR-21 show defects in Th2 development and resistance to Th2 mediated allergic airway inflammation. (In contrast to Th2-mediated immediate hypersensitive responses, miR-21 deficiency enhances Th1 development and more delayed type hypersensitivity responses in vivo.) In fact, miR-21 has been shown to be one of the most highly upregulated miRNAs in multiple models of experimental asthma and human eosinophilic esophagitis [4]. These data suggest that miR-21 may modulate asthma pathogenesis by shifting T helper differentiation towards the Th2 subtype. However, since the IL-12-Th1 cell response is crucial for protective immunity to various intracellular pathogens, miR-21-mediated shifts in Th1/Th2 balance may also have profound effects on host susceptibility to infection and disease course. To our knowledge, this possibility has yet to be formally investigated.
Figure 1. Mir-21 regulation of T helper subset differentiation.
In addition to regulating Th1 and Th2 cells, miR-21 also affects Th17 differentiation. Most recently, we found that miR-21 expression is specifically elevated in Th17 cells and that T cell-intrinsic expression of miR-21 is important for effective Th17 differentiation [5]. MiR-21 promotes Th17 differentiation by targeting Smad-7, a negative regulator of TGF-β signaling. MiR-21−/− mice show a defect in Th17 differentiation and are resistant to Th17-mediated experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). In support of the EAE model, increased expression of miR-21 has been observed in peripheral blood mononuclear cells from MS patients. Although Th1 and Th17 cells are considered to be key participants in EAE and MS, the Th1-signature cytokine IFN-γ and Th17-associated cytokines such as IL-17A, IL-17F, IL-21, and IL-22 have all been shown to be dispensable for the development of EAE. Rather, it is granulocyte macrophage colony-stimulating factor (GM-CSF) which is crucial for the pathogenic functions of Th1 and Th17 cells. Autoreactive Th1 and Th17 cells specifically lacking GM-CSF do not transfer EAE. Interestingly, the almost complete EAE resistance we observed in miR-21−/− mice is associated with reduced GM-CSF in both Th1 and Th17 cells. Consistent with our study, others have found that overexpression of miR-21 in vivo leads to elevated levels of multiple proinflammatory cytokines, including IL-17 [6], and that in vivo silencing of miR-21 is associated with a reduction in Th17 cells and the transcription factor RAR-related orphan receptor gamma (RORγt) during myocarditis [7].
These and other miR-21-mediated mechanisms seem to affect fundamental immune system functions and are likely involved in a number of other inflammatory diseases (reviewed in [8]). For example, miR-21 has been found to be overexpressed in CD4+ T cells derived both from patients with lupus and from lupus-prone MRL/lpr mice, while silencing miR-21 in vivo has been shown to ameliorate splenomegaly in lupus mice. Increased expression of miR-21 has also been observed in patients with ulcerative colitis, and most recently, miR-21−/− mice have been shown to be resistant to dextran sulfate sodium (DSS)-induced colitis. Elevated miR-21 levels in T cells from patients with psoriasis have even been shown to correlate with disease activity. In addition, miR-21 expression has been reported in other diseases fueled by chronic inflammation, including atherosclerosis, type 2 diabetes, and cancer.
Together, these studies highlight a stronger role for miR-21 in the pathogenesis of multiple autoimmune and chronic inflammatory disorders. However, the complete molecular mechanisms by which miR-21 regulates these pathologies remains to be investigated. Thus, a better understanding of the role of miR-21 in the innate and adaptive immune systems, during healthy states as well as during infection, chronic inflammation, and autoimmunity, is required before we design any therapeutic strategies aimed at targeting miR-21.
REFERENCES
- 1.Esquela-Kerscher A, et al. Nat Rev Cancer. 2006;6(4):259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 2.Sheedy FJ, et al. Nat Immunol. 2010;11(2):141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 3.Lu TX, et al. J Immunol. 2009;182(8):4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu TX, et al. J Immunol. 2011;187(6):3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murugaiyan G, et al. J Clin Invest. 2015;125(3):1069–80. doi: 10.1172/JCI74347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando Y, et al. J Autoimmun. 2013;41:111–9. doi: 10.1016/j.jaut.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YL, et al. Arch Virol. 2013;158(9):1953–63. doi: 10.1007/s00705-013-1695-6. [DOI] [PubMed] [Google Scholar]
- 8.Xu WD, et al. Expert Opin Ther Targets. 2013;17(6):659–65. doi: 10.1517/14728222.2013.773311. [DOI] [PubMed] [Google Scholar]