Abstract
Purpose
Intracorporeal anastomosis is the most difficult procedure during pure single-incision distal gastrectomy (SIDG) that affects its generalization. We introduced unaided delta-shaped anastomosis (uDelta), a novel anastomosis technique, for gastroduodenostomy after pure SIDG, and compared the results with those of previously reported Roux-en-Y anastomosis (RY).
Materials and Methods
Between March 2014 and March 2015, SIDG with D1+ lymph node dissection was performed for early gastric cancer through a 2.5-cm transumbilical incision without any additional port. uDelta was performed by the operator alone, without any intracorporeal assistance.
Results
uDelta was performed on 11 patents, and uncut RY was performed on 5-patients without open or multiport conversion. R0 resection was performed in all cases. No significant differences were observed in mean age and body mass index between patients who underwent uDelta or RY. Mean operation times were 214.5±36.2 minutes for uDelta and 240.8±65.9 minutes for RY, which was not significantly different. Reconstruction time for uDelta was shorter than that for RY, with marginal statistical significance (26.1±8.3 minutes vs. 38.0±9.1 minutes, P=0.05). There were no intraoperative transfusions, 30-day mortality, or anastomosis-related complications in either group. Average length of hospital stay was 8.2±1.9 days in the uDelta group and 7.2±0.8 days in the RY group (P=0.320).
Conclusions
After carefully considering indications, uDelta can be a feasible and can be a reproducible reconstruction method after SIDG in early gastric cancer.
Keywords: Billroth I, Gastrectomy, Laparoscopy, Roux-en-Y anastomosis, Stomach neoplasm
Introduction
Minimally invasive laparoscopic gastrectomy offers several advantages, including less postoperative pain, better cosmesis, less inflammatory reaction, more rapid recovery of bowel function, and faster recovery compared with conventional open surgery.1,2,3 Single-incision laparoscopic surgery is expected to be the next step in minimally invasive surgery owing to the development of laparoscopic techniques and instruments, and several studies have already reported that this technique is a feasible alternative treatment for appendectomy or cholecystectomy.4,5 For gastric cancer, single-incision distal gastrectomy (SIDG) was first reported in 2011.6 However, unlike appendectomy or cholecystectomy, only limited experiences with SIDG have been published to date because of several technical difficulties, in particular, the intracorporeal anastomosis technique.7,8,9 Compared with Billroth II or Roux-en-Y anastomosis (RY), Billroth I anastomosis offers advantages such as it is simpler to perform, leads to less anatomical change after anastomosis, allows for more physiological food passage, and confers a lower incidence of internal hernia or adhesion. Consequently, Billroth I is the most common anastomosis technique used after distal gastrectomy in Korea and Japan, even among expert groups.10,11
To achieve intracorporeal Billroth I anastomosis in laparoscopic surgery, delta-shaped anastomosis for gastroduodenostomy using linear staplers was introduced. Since then, this has become the most popular technique for intracorporeal gastroduodenostomy, and has shown acceptable postoperative results.12,13 However, to perform this type of anastomosis, advanced laparoscopic cooperation between the operator and the assistant is essential. When performing pure SIDG, such advanced cooperation is nearly impossible because of the absence of additional ports, the collision of instruments, and narrow working space. To date, only two authors have reported their initial experience with or modification of Billroth I anastomosis after SIDG, and the reproducibility or generalization of gastroduodenostomy in pure SIDG has been controversial until recently.7,14,15 On the basis of our experience with SIDG, we introduced unaided delta-shaped anastomosis (uDelta), a novel technique for gastroduodenostomy, without any additional port or intracorporeal assistance for pure SIDG, and compared the results with those of previously reported RY technique.
Materials and Methods
1. Patients
We retrospectively reviewed the clinicopathological data of patients who had been treated with pure SIDG between March 2014 and March 2015. The indication was cT1N0M0 adenocarcinoma of the distal one-third of the stomach, in patients without any significant systemic morbidity or obesity. Reconstruction was performed using either uDelta or RY. For tumors located in the distal one-third stomach, uDelta was the preferred technique. If the tumor located above the angle of the stomach or the distal resection margin of the tumor was too close to the pylorus, RY was considered as an alternative technique. Morbidity and mortality was evaluated for 30 days after the operation. Statistical analyses were performed using the Mann-Whitney U-test and the χ2 test with IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). This study was approved by the Seoul National University Hospital Institutional Review Board (IRB No: 1504-063-664).
2. Unaided delta-shaped anastomosis surgical technique
The patient is placed supine, and both legs are spread. Surgeons are placed between the patient's legs, and a scopist usually stands at the right side of the patient. A vertical 2.5 cm-long transumbilical incision is made, and a commercially available 4-hole single port (Glove Port®; Nelis, Bucheon, Korea) is inserted without any additional port. Pneumoperitoneum is achieved using 12 mmHg pressure in the reverse Trendelenburg position. A flexible laparoscope (ENDOEYE FLEX HD 10 mm; Olympus Medical Systems, Tokyo, Japan) is essential because it offers unparalleled viewing among laparoscopic instruments. The operator uses a 45-cm-long energy device (HARMONIC scalpel; Ethicon Endo-Surgery Inc., Cincinnati, OH, USA or THUNDERBEAT; Olympus Medical Systems). A 43-cm-long straight Clickline Croce-Olmi grasping forcep (Karl Storz, Tuttlingen, Germany) is the main instrument, and HiQ LS curved hand instrument (5×350 mm, Olympus Medical Systems) is used as an alternative.
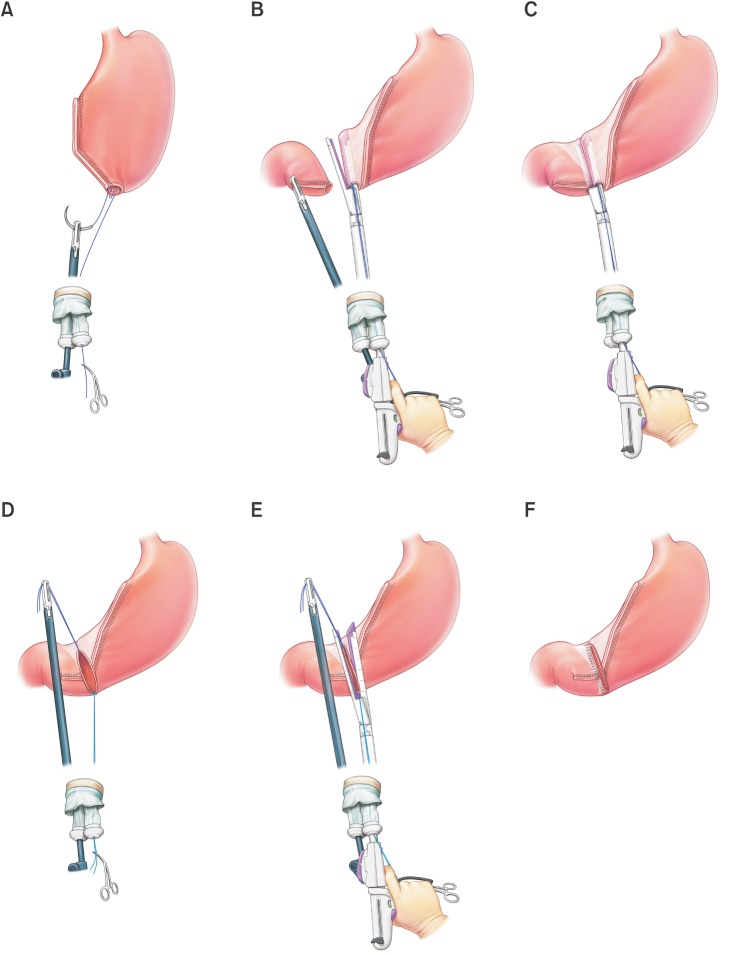
Using a single incision site without any additional port or assistance, laparoscopic distal gastrectomy with D1+ lymph node dissection is performed according to the Japanese gastric cancer treatment guidelines.16 For Billroth I anastomosis, after full mobilization around the first part of the duodenum, the duodenum is rotated 90 degrees and transected with a 60 mm linear stapler (Endo GIA™ purple or tan; Covidien, Mansfield, MA, USA), which creates a duodenal transection in the ventrodorsal direction (supplementary video). This duodenal transection was occasionally performed before ligation of the right gastric artery in totally laparoscopic distal gastrectomy (TLDG). However, in SIDG with uDelta, we performed duodenal transection after ligation of the right gastric artery to ensure sufficient mobilization and rotation of the duodenal first portion without any other assistance. After distal gastrectomy with lymph node dissection is completed, an en bloc resected specimen with lymph nodes wrapped in a plastic bag is harvested through a single umbilical incision site. To perform an intracorporeal gastroduodenostomy, a small incision is created in the previous stapled line at the corner of the greater curvature side of the remnant stomach and the dorsal side of the duodenum in order to insert a linear stapler. To achieve adequate traction without assistance inside the peritoneal cavity, a traction suture with a 3/0 monofilament thread is added at the posterior wall of the small incision hole of the remnant stomach and pulled out through a single umbilical port (Fig. 1A). After inserting a 45 mm linear stapler (Endo GIA™ purple) into the remnant stomach, an opened linear stapler with a traction suture at the remnant stomach is easily maintained by the operator's right hand, allowing for simultaneous unaided manipulation of the stapler and remnant stomach. A linear stapler gently holding the posterior wall of the remnant stomach is rotated clockwise to the duodenal side, which is then ready for gastroduodenostomy (Fig. 1B). The other jaw of the stapler is inserted at the incision site of the duodenal stump, which is simply controlled by the operator himself. The posterior wall of the stomach and cranial side of the duodenum are approximated and anastomosed (Fig. 1C). To close the common entry hole, one traction suture at the lesser curvature side of the common entry hole (which occasionally can be replaced by a previous traction suture at the remnant stomach) is simply pulled up with the operator's left hand inside the abdominal cavity. The newly added suture at the greater curvature side of the common entry hole is pulled out through the single port and simultaneously manipulated outside the abdominal cavity with a linear stapler by the operator's right hand (Fig. 1D). Under the gentle traction of 2 traction sutures inside and outside the abdominal cavity, the common entry hole can be closed up with a single application of a 60 mm linear stapler (Endo GIA™ purple) (Fig. 1E).
Fig. 1. Unaided delta-shaped anastomosis. (A) A traction suture with 3/0 monofilament thread is added at the posterior wall of the small incision hole of the remnant stomach and pulled out through a single umbilical port. (B) An opened 45-mm linear stapler inserted at the remnant stomach is manipulated by the operator with one hand. (C) The other jaw of the stapler is inserted at the incision site of the duodenal stump, which is easily controlled by the operator alone. (D) After the first stapling of the gastroduodenostomy, one traction suture at the lesser curvature side of the common entry hole is pulled up by the operator's left hand. This suture can be replaced by the previous suture at the posterior wall of the remnant stomach. An additional traction suture at the greater curvature side of the common entry hole is pulled out through the single port and simultaneously manipulated outside the abdominal cavity with a linear stapler. (E) The common entry hole can be easily closed with a single application of a 60-mm linear stapler under gentle traction with 2 traction sutures inside and outside the abdominal cavity. (F) The unaided delta-shaped gastroduodenostomy is completed.
3. Uncut Roux-en-Y anastomosis surgical technique
The proximal jejunum, 20 cm below the ligament of Treitz, is harvested extracorporeally through a single incision site. This proximal jejunum is anastomosed side to side with the distal jejunum, which is approximately 35 cm distal from the jejunojejunostomy. After anastomosis, the proximal afferent jejunum is stapled at 5 cm distal from the jejunojejunostomy, using a linear stapler without knife (TA™ 30 mm stapler, vascular; Covidien). A small hole is made 5 cm distal from the previous nonknife stapled line, and the entire jejunum is restored into the peritoneal cavity. Another small hole is then made at the corner of the greater curvature side of the remnant stomach, and antiperistaltic gastrojejunostomy is performed using two 60 mm linear staplers (Endo GIA™ purple) in the same manner as previously reported.8 After gastrojejunostomy, Peterson's space is closed using a barbed suture.
Results
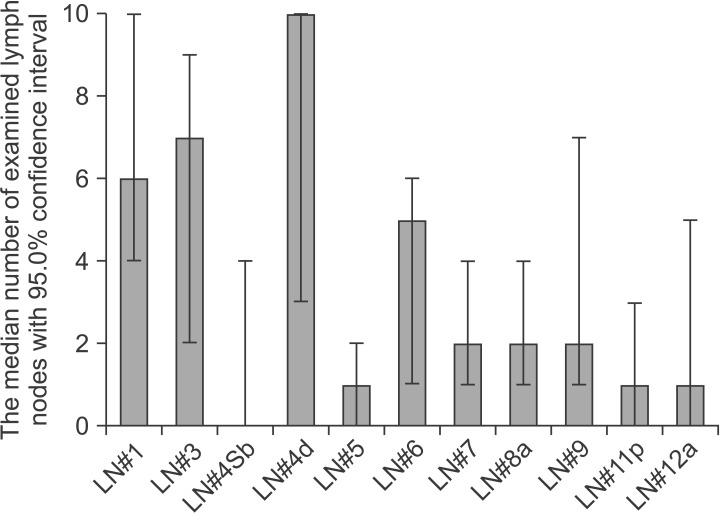
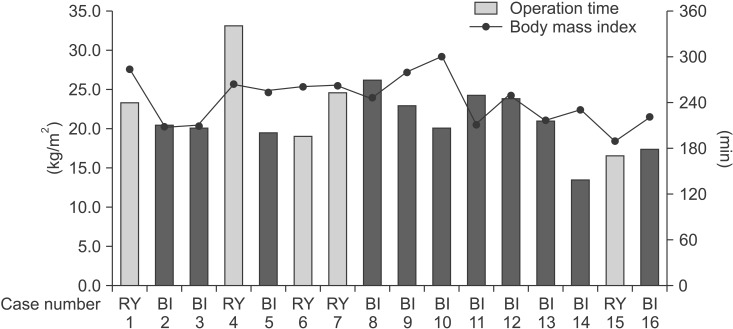
The uDelta procedure was performed in 11 patients, and RY was performed in 5 patients (Table 1). None of the patients required open or multiport conversion, and the procedure was completed without any intraoperative complications or transfusions. R0 resection with a negative resection margin was achieved in all patients. Based on the 7th AJCC TNM staging system, postoperative pathology revealed stage I gastric adenocarcinoma in 13 patients, neuroendocrine tumor without lymph node metastasis in 1 patient, and mixed adenoneuroendocrine carcinoma in 2 patients.17 There was no significant difference in age, sex, and body mass index between the uDelta and RY groups. Fig. 2 shows the number of examined lymph nodes at each lymph node station after total 16 cases of SIDG with D1+ lymph node dissection. Fig. 3 shows a series of average operation times for each SIDG case, along with each patient's body mass index. Table 2 shows that the operation time for the uDelta procedure was shorter than that for the RY procedure (214.5±36.2 minutes vs. 240.8±65.9 minutes), although the difference was not statistically significant (P=0.661). In addition, reconstruction time for uDelta was significantly shorter than that for RY (26.1±8.3 minutes vs. 38.0±9.1 minutes), and this difference was marginally significant (P=0.05).
Table 1. Patient information for single incision distal gastrectomy.
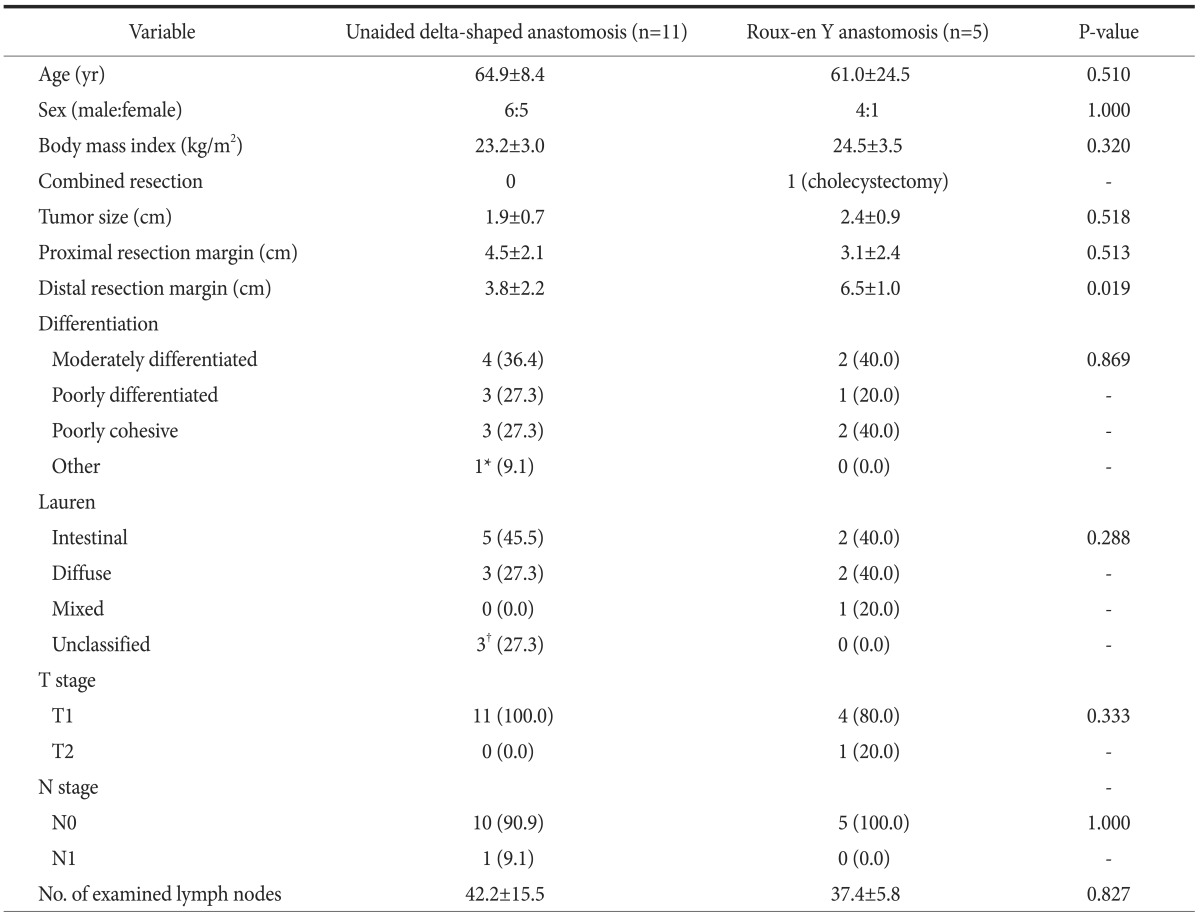
Values are presented as mean±standard deviation, number only, or number (%). *One patient with neuroendocrine tumor. †Each patient with neuroendocrine tumor, mixed adenoneuroendocrine carcinoma and no residual tumor after endoscopic biopsy, relatively.
Fig. 2. Median number of estimated lymph nodes (LNs) at each lymph node station after total cases of single-incision distal gastrectomy with D1+ lymph node dissection.
Fig. 3. Operation time of single-incision distal gastrectomy. BI indicates unaided delta-shaped anastomosis, and RY indicates Roux-en-Y anastomosis.
Table 2. Perioperative outcome after single incision distal gastrectomy.
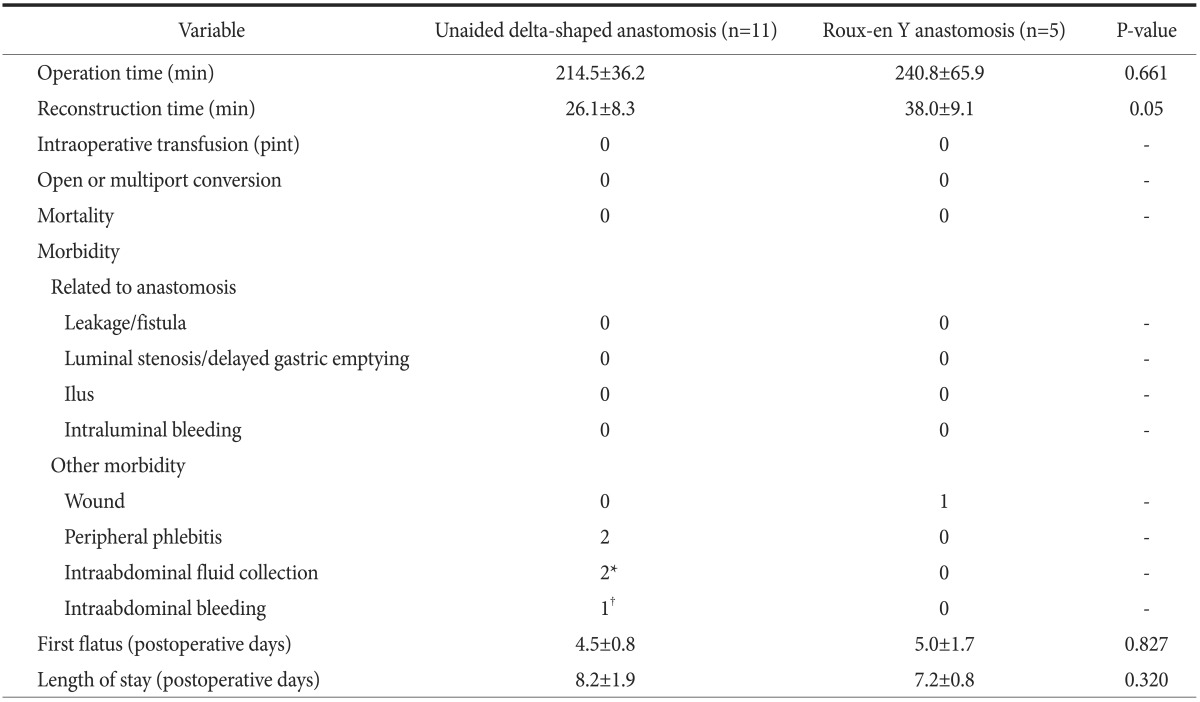
Values are presented as mean±standard deviation or number only. *One patient was managed by simple observation, and the other patient by ultrasonography-guided percutaneous aspiration (single aspiration). †Pseudoaneurysmal bleeding at splenic artery.
Regarding perioperative outcomes in both groups, no mortality or complications occurred, including leakage, stenosis, ileus, or bleeding related to the anastomosis. In terms of other morbidity, one patient had intra-abdominal fluid collection; however, it was unrelated to anastomosis leakage and was managed by 1 application of ultrasonography-guided simple aspiration. The other patient, who had been discharged at postoperative day 7 without any complications, was readmitted at postoperative day 11 owing to an intra-abdominal pseudoaneurysm. That patient recovered after radiological embolization of the pseudoaneurysm, reoperation to perform remnant total gastrectomy, and intensive care. No significant differences in postoperative recovery, including day of first flatus and length of stay, were observed between the uDelta and RY groups.
Discussion
In order to generalize a new procedure such as SIDG, the simplicity, safety, and reproducibility of the whole procedure including reconstruction should be evaluated and compared with a conventional one. Therefore, to consider procedure generalization, gastroduodenostomy or gastrojejunostomy should be evaluated equivocally, not only after conventional open surgery or TLDG but also after SIDG. Compared with gastroduodenostomy, gastrojejunostomy can be relatively easily performed, and most studies describing the initial experience with SIDG had used gastojejunostomy.8,18,19 A recent retrospective comparative study reported that SIDG with RY resulted in better short-term outcomes than TLDG in terms of postoperative pain, estimated blood loss, inflammatory reaction, and cosmetic results.9 However, unlike in open surgery, gastroduodenostomy was usually considered a more difficult technique in TLDG because of the limitations of an intracorporeal approach with a circular stapler as well as the narrow working space around the duodenal stump. To overcome these limitations, delta-shaped anastomosis has been successfully introduced for Billroth I anastomosis after TLDG.20 However, delta-shaped anastomosis still requires advanced cooperation between the operator and assistant because all manipulations related to the linear stapler and remnant stomach should be conducted by the assistant. Such an advanced assistant-dependent procedure cannot be expected for SIDG. In addition, it would be much more challenging if even one more assistance grasper were added through a single port because of the narrow port space and severe collision among surgical instruments. This limitation of reconstruction may prohibit the generalization of SIDG, especially in terms of a reconstruction such as gastroduodenostomy.
We introduced the uDelta anastomosis under the direct visualization of a flexible scope and without any additional port or intracorporeal assistance grasper. In short, our novel method is a modification of the way how delta-shaped anastomosis is performed which is optimized for SIDG, and the anatomical results after anastomosis are similar to those following the original delta-shaped anastomosis procedure. Therefore, postoperative outcomes would be easily expected based on several previous reports.13,21
Our method is characterized by 1) appropriate 'aiding' stitches as assistance through the single port, and 2) simultaneous manipulation of the linear stapler and those stitches. In single-port surgery, intracorporeal suturing and knot-tying is usually considered a more complicated technique than in converntional laparoscopic surgery. However, our technique requires only simple suturing at appropriate points without intracorporeal tying. Here, the simultaneous manipulation of the linear stapler using aiding stitches substitute the role of advanced assistance in TLDG. Finally, the operator can control the whole anastomosis procedure himself without further collision among instruments or obstacles to the laparoscopic view. This 'unaided procedure' leads to a simple and easily reproducible technique. In addition, this procedure can be effectively applied to multiport TLDG, especially when advanced assistance is not available. Whether the surgical team is expert or not, the application of our procedure to SIDG as well as TLDG can be expected to result in consistent anastomosis outcomes as well as a more standardized laparoscopic procedure.
In our early experience, one patient was readmitted to the hospital because of splenic artery pseudoaneurysm and required reoperation with intensive care. This type of unique complication might result from thermal damage by an ultrasonic energy device, and was previously reported at the same vessel (splenic artery) in a large-scale study of laparoscopic distal gastrectomy with lymph node dissection.22 In particular, the use of an energy-based device should be considered much more cautiously in SIDG because no effective assistance is available to avoid possible thermal injury to adjacent tissues, including major vessels. According to Japanese treatment guidelines for performing distal gastrectomy, dissection around the splenic artery is not mandatory.16 Therefore, if we paid strict attention to such guidelines, serious complications related to pseudoaneurysm around the splenic artery could be prevented.
This study has several limitations. First, it was retrospective and examined only a small number of cases. However, we believe that our study has introduced a new alternative and comparable reconstruction method for SIDG, and that it represents an important step toward experience with SIDG. Second, SIDG may have limited utility for obese patients because it is more difficult to create a good surgical field in these patients. Third, because no assistance is available during the operation, SIDG may have serious disadvantages when unexpected intraoperative complications, such as a major vessel injury, occur. Therefore, SIDG should be performed only by an experienced laparoscopic surgeon, and larger-scale studies are necessary to validate the clinical implications and safety of SIDG.
We introduced a novel anastomosis technique, uDelta, for gastroduodenostomy after pure SIDG. After carefully considering indications, uDelta can become a feasible and reproducible reconstruction option after SIDG for early gastric cancer. It is also expected to become a useful technique for multiport TLDG with Billroth I anastomosis.
Acknowledgments
This study was supported by a grant (1320270) from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea.
References
- 1.Yang HK, Suh YS, Lee HJ. Minimally invasive approaches for gastric cancer-Korean experience. J Surg Oncol. 2013;107:277–281. doi: 10.1002/jso.23179. [DOI] [PubMed] [Google Scholar]
- 2.Oh SY, Kwon S, Lee KG, Suh YS, Choe HN, Kong SH, et al. Outcomes of minimally invasive surgery for early gastric cancer are comparable with those for open surgery: analysis of 1,013 minimally invasive surgeries at a single institution. Surg Endosc. 2014;28:789–795. doi: 10.1007/s00464-013-3256-1. [DOI] [PubMed] [Google Scholar]
- 3.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report: a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 4.Markar SR, Karthikesalingam A, Thrumurthy S, Muirhead L, Kinross J, Paraskeva P. Single-incision laparoscopic surgery (SILS) vs. conventional multiport cholecystectomy: systematic review and meta-analysis. Surg Endosc. 2012;26:1205–1213. doi: 10.1007/s00464-011-2051-0. [DOI] [PubMed] [Google Scholar]
- 5.Frutos MD, Abrisqueta J, Lujan J, Abellan I, Parrilla P. Randomized prospective study to compare laparoscopic appendectomy versus umbilical single-incision appendectomy. Ann Surg. 2013;257:413–418. doi: 10.1097/SLA.0b013e318278d225. [DOI] [PubMed] [Google Scholar]
- 6.Omori T, Oyama T, Akamatsu H, Tori M, Ueshima S, Nishida T. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc. 2011;25:2400–2404. doi: 10.1007/s00464-010-1563-3. [DOI] [PubMed] [Google Scholar]
- 7.Omori T, Tanaka K, Tori M, Ueshima S, Akamatsu H, Nishida T. Intracorporeal circular-stapled Billroth I anastomosis in single-incision laparoscopic distal gastrectomy. Surg Endosc. 2012;26:1490–1494. doi: 10.1007/s00464-011-2034-1. [DOI] [PubMed] [Google Scholar]
- 8.Ahn SH, Son SY, Lee CM, Jung do H, Park do J, Kim HH. Intracorporeal uncut Roux-en-Y gastrojejunostomy reconstruction in pure single-incision laparoscopic distal gastrectomy for early gastric cancer: unaided stapling closure. J Am Coll Surg. 2014;218:e17–e21. doi: 10.1016/j.jamcollsurg.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SH, Son SY, Jung do H, Park do J, Kim HH. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg. 2014;219:933–943. doi: 10.1016/j.jamcollsurg.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69–77. doi: 10.5230/jgc.2011.11.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Shiraishi N, Kim HH, Hiki N, Uyama I, Choi SH, et al. Standard of practice on laparoscopic gastric cancer surgery in Korea and Japan: experts' survey. Asian J Endosc Surg. 2012;5:5–11. doi: 10.1111/j.1758-5910.2011.00111.x. [DOI] [PubMed] [Google Scholar]
- 12.Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284–287. doi: 10.1016/s1072-7515(02)01239-5. [DOI] [PubMed] [Google Scholar]
- 13.Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg. 2014;259:109–116. doi: 10.1097/SLA.0b013e31828dfa5d. [DOI] [PubMed] [Google Scholar]
- 14.Park do J, Lee JH, Ahn SH, Eng AK, Kim HH. Single-port laparoscopic distal gastrectomy with D1+β lymph node dissection for gastric cancers: report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2012;22:e214–e216. doi: 10.1097/SLE.0b013e318253df9b. [DOI] [PubMed] [Google Scholar]
- 15.Omori T, Masuzawa T, Akamatsu H, Nishida T. A simple and safe method for Billroth I reconstruction in single-incision laparoscopic gastrectomy using a novel intracorporeal triangular anastomotic technique. J Gastrointest Surg. 2014;18:613–636. doi: 10.1007/s11605-013-2419-7. [DOI] [PubMed] [Google Scholar]
- 16.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. [Google Scholar]
- 18.Ozdemir BA, Thomas RL, Soon Y. Single-port laparoscopic subtotal gastrectomy with DIα lymphadenectomy. Surg Innov. 2011;18:NP1–NP4. doi: 10.1177/1553350611400759. [DOI] [PubMed] [Google Scholar]
- 19.Kong J, Wu SD, Su Y. Translumenal single-incision laparoscopy radical gastrectomy with D2 lymph node dissection for early gastric cancer: primary experience with less invasive surgery in China. J Laparoendosc Adv Surg Tech A. 2013;23:141–145. doi: 10.1089/lap.2012.0332. [DOI] [PubMed] [Google Scholar]
- 20.Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, et al. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer. 2011;14:365–371. doi: 10.1007/s10120-011-0054-0. [DOI] [PubMed] [Google Scholar]
- 21.Kitagami H, Morimoto M, Nozawa M, Nakamura K, Tanimura S, Murakawa K, et al. Evaluation of the delta-shaped anastomosis in laparoscopic distal gastrectomy: midterm results of a comparison with Roux-en-Y anastomosis. Surg Endosc. 2014;28:2137–2144. doi: 10.1007/s00464-014-3445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale Korean multicenter study. Ann Surg Oncol. 2008;15:2692–2700. doi: 10.1245/s10434-008-0075-z. [DOI] [PubMed] [Google Scholar]