Summary
This study showed a clear dose-response relationship for the CLIP2 radiation marker in post-Chernobyl papillary thyroid carcinoma cohorts for young patients and hints to different molecular mechanisms in tumors induced at low doses compared to moderate/high doses.
Abstract
A previous study on papillary thyroid carcinomas (PTC) in young patients who were exposed to 131iodine from the Chernobyl fallout revealed an exclusive gain of chromosomal band 7q11.23 in exposed cases compared to an age-matched control cohort. CLIP2, a gene located within band 7q11.23 was shown to be differentially expressed between exposed and non-exposed cases at messenger RNA and protein level. Therefore, a standardized procedure for CLIP2 typing of PTCs has been developed in a follow-up study. Here we used CLIP2 typing data on 117 post-Chernobyl PTCs from two cohorts of exposed patients with individual dose estimates and 24 non-exposed controls to investigate a possible quantitative dose-response relationship of the CLIP2 marker. The ‘Genrisk-T’ cohort consisted of 45 PTCs and the ‘UkrAm’ cohort of 72 PTCs. Both cohorts differed in mean dose (0.59 Gy Genrisk-T, 1.2 Gy UkrAm) and mean age at exposure (AaE) (2 years Genrisk-T, 8 years UkrAm), whilst the median latency (16 years Genrisk-T, 18 years UkrAm) was comparable. We analyzed the association between the binary CLIP2 typing and continuous thyroid dose with logistic regression. A clear positive dose-response relationship was found for young PTC cases [age at operation (AaO) < 20 years, AaE < 5 years]. In the elder age group a higher proportion of sporadic tumors is assumed due to a negligible dose response, suggesting different molecular mechanisms in sporadic and radiation-induced cases. This is further supported by the association of elder patients (AaO > 20 years) with positivity for BRAF V600E mutation.
Introduction
Numerous epidemiological studies have shown a dramatic increase in papillary thyroid carcinoma (PTC) incidence among young children exposed to radiation after the Chernobyl accident. The increase is related to a radiation dose in the thyroid gland mainly from ingested 131iodine (I-131) (1–7). Among children and adolescents from the affected geographical regions, who were under the age of 18 at the time of the reactor accident in April 1986, 6848 thyroid cancer cases have been detected between 1991 and 2005 (8). To enable comprehensive biological studies on this cancer type, which appeared at first in the aftermath of the accident, the Chernobyl Tissue Bank (CTB) provides a systematic collection of thyroid tumors from residents in the contaminated regions of Ukraine and Russia (www.chernobyltissuebank.com) since 1998. A screening study of selected children and adolescents exposed to I-131 has been initiated by a Ukrainian–US consortium (9). The resulting UkrAm cohort study included only subjects with individual measurements of the thyroid activity. For each cohort member, the thyroid mass and an intake function for I-131 were estimated for reconstruction of individual thyroid doses which amounted to 0.66 Gy on average (10). Two radio-epidemiological studies on the UkrAm cohort demonstrated a significantly increased thyroid cancer risk in relation to the I-131 dose. During the first screening phase 1998–2000 45 prevalent cases (43 PTCs, two follicular carcinoma) were detected and operated at a mean age of 16 years. The dose-response for prevalence of thyroid cancer was linear with an excess relative risk of 5.3 per Gy (95% confidence interval: 1.7, 28) (4). For the 61 incidental PTCs, which were detected in three screening phases from 2001–2008, the mean age at operation (AaO) was 24 years and the dose-response analysis resulted in a markedly lower risk of 1.6 per Gy (95% confidence interval: 0.3, 5.4) (6).
The availability of tissue samples through the CTB promoted a huge number of molecular studies including investigations of specific RET/PTC gene rearrangements [reviewed in (11)], radiation-associated gene alterations as determined by global gene expression analyses (12–16) and global genomic copy number analysis (17,18). In particular, several studies demonstrated gene expressions related to I-131 exposure (14) or a differential dose-expression relationship of several genes for tumor and normal tissues from PTC of the UkrAm cohort (15,16). An in-depth genomic copy number analysis of closely matched exposed and non-exposed PTC patients revealed a radiation-specific DNA gain of chromosome band 7q11.22-11.23 and the radiation-associated messenger RNA (mRNA) overexpression of the CLIP2 gene (19). In a follow-up study the radiation-associated CLIP2 overexpression has been validated at the protein level (20).
In the present study we conducted a quantitative analysis of the relationship between I-131 dose and the binary CLIP2 marker status of PTCs provided by the CTB. We used data generated with a standardized CLIP2 marker typing approach of individual tumors based on integrated CLIP2 protein expression and genomic copy number status (20). In addition, we analyzed the expression of CLIP2 in normal tissues corresponding to the PTCs from the UkrAm cohort in existing micro-array expression data from Abend et al. (16) in order to investigate whether the observed dose-relationship for CLIP2 is tumor-specific or not.
Materials and methods
Patient cohorts
The data set of this study included 117 PTCs that have developed in young patients who were born before 26 April 1986 [age at exposure (AaE): 0.1–17.9 years, AaE: 14–35 years] after exposure to I-131 in the aftermath of the Chernobyl accident. The patients have been operated in the period between 1998 and 2009. Patients’ details are listed in Supplementary Table 1, available at Carcinogenesis Online and summarized in Table 1. Histograms of AaE, AaO, latency, and dose are visualized in Supplementary Figure 1, available at Carcinogenesis Online. The cohort of patients with I-131 exposure comprises two subcohorts consisting of 45 radiation-associated PTCs from the so-called ‘Genrisk-T’ (19) cohort and 72 PTCs from the UkrAm cohort. Additionally, we included 24 PTCs of non-exposed patients from the Genrisk-T cohort born after January 1987. All cases were typed for a positive or negative status of the CLIP2 marker (20). Fourteen patients show a BRAF V600E mutation detected by Sanger Sequencing as described previously by Powell et al. (21). These data were used for a qualitative comparison between the present study and the studies of Hamatani et al. (22) and Leeman-Neill et al. (23,24).
Table 1.
Summary of patient data
| Number of cases | Arithmetic means | CLIP2 marker | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Total | Male | Female | Age at exposure (years) | Age at operation (years) | Latency (years) | Geom./arith. mean dose (mGy) | Positive | Negative |
| Genrisk-T | 45 | 16 | 29 | 2.1 | 17.8 | 15.7 | 278/593 | 34 | 11 |
| UkrAm | 72 | 28 | 44 | 8.1 | 25.5 | 17.5 | 402/1244 | 53 | 19 |
| Both cohorts | 117 | 44 | 73 | 5.8 | 22.6 | 16.8 | 349/994 | 87 | 30 |
| non-exposeda | 24 | 6 | 18 | — | 15.0 | — | 15/15 | 6 | 18 |
aBorn after 1986, from Genrisk-T cohort with nominal background doses of 1 mGy/year.
Summary of CLIP2 marker classification approach
Immunohistochemical staining of formalin fixed paraffin embedded tumor tissue sections was performed using a primary antibody against CLIP2 in an automated staining instrument. The stained tissue sections were scanned with a digital slide scanning system. Two independent observers carried out the visual scoring classification of the staining intensity in a blinded manner. Only epithelial tumor cells were evaluated. All cases were classified into one of the four staining categories: negative staining (score 0), weak staining (score 1), intermediate staining (score 2) and strong staining (score 3). In order to obtain an individual biomarker classification for each case, scores of 0 or 1 were considered as CLIP2 marker negative, whereas cases with visual score 3 were classified as CLIP2 marker positive. For a classification of an intermediate CLIP2 staining (score 2), the genomic copy number status (gained or not gained) of chromosomal band 7q11.23 (localization of CLIP2) was taken into account. Cases with a visual score of 2 and a DNA gain of chromosomal band 7q11.23 were finally classified as CLIP2-positive, and those showing normal copy number of chromosomal band 7q11.23 were classified as CLIP2-negative. A detail description is presented by Selmansberger et al. (20).
Dosimetry
Dosimetric approaches in the UkrAm cohort have been reported previously (10,25,26). Individual I-131 thyroid doses were estimated from the combination of thyroid I-131 measurements, diet and lifestyle data and environmental transfer models. Because the dose estimates were derived from thyroid masses of typical iodine-sufficient populations, thyroid masses were adjusted by a correction coefficient. In the present study we used the same dose estimates for the UkrAm cohort as Brenner et al. (6). For the Genrisk-T cohort dose estimates were published by Likhtarov et al. (27) and provided by the CTB.
Dose-response relation of the CLIP2 marker from logistic regression
We applied logistic regression on a data set with 117 exposed PTCs from Genrisk-T and UkrAm and on an enlarged data set of 141 PTCs including additional 24 PTCs of non-exposed patients, which were born after 1 January 1987 and operated under the age 20. The regression models consisted different sets of the covariables dose, AaO, AaE, gender and oblast of residence in 1986 to investigate their effect on the probability of observing a positive CLIP2 marker. To account for the large range of dose estimates they were transformed logarithmically. The distribution of the log-transformed dose estimates is well approximated by a normal distribution. A nominal dose of 1 mGy/year was assigned to non-exposed patients accounting for the natural background radiation accumulated between birth and AaO. Oblast of residence was discarded as covariable since it did not improve the explanation of the data in a likelihood ratio test for additional parameters on a 95% level. A gender effect was tested for the groups of young patients and old patients separately. In both groups the improvement of model fits fell below the 95% level so that the gender covariable was also excluded from further analysis. However, the gender effect was slightly stronger in younger patients than in older patients. The shape of the dose response in young patients is mainly determined by female cases, which exceed male cases by some factor of two. For the remaining covariables their influence on the fit was explored at first individually. Thereafter, the impact of pairwise combinations of continuous thyroid dose with either AaO or AaE has been tested for continuous and categorical variation of the age-related covariables. Categorical boundaries were defined a priori at AaO < 20 years and ≥20 years and AaE < 5 year and ≥5 years because the sensitivity of the thyroid gland to ionizing radiation and the profile of genetic alterations in tumors differ to a large extent between children/adolescents and adults (28). Explicit forms of the tested regression models are given in Tables 2 and 3. Preferred models have been identified according to criteria of quality of fit and biological plausibility. Quality of fit was measured by the Akaike Information Criterion (AIC) = Deviance + 2 × no. of model parameters. Regression analysis was performed with the statistical software package R (R Development Core Team 2011, www.r-project.org).
Table 2.
Form of logistic regression models with number of parameters, AIC and ΔAIC (as difference to the preferred model) fitted to 141 PTC cases from cohorts Genrisk-T and UkrAm (including 24 non-exposed cases born after 1 January 1987 from Genrisk-T cohort)
| Variables included | Npar | AIC | ΔAIC |
|---|---|---|---|
| Intercept only | 1 | 182.85 | 15.28 |
| Intercept + AaO | 2 | 178.65 | 11.08 |
| Intercept + log(Dose) | 2 | 176.60 | 9.03 |
| Intercept + log(Dose) + AaO | 3 | 174.78 | 7.21 |
| Intercept + log(Dose) + AaO + log(Dose) × AaO | 4 | 163.89 | −3.68 |
| aIntercept + log(Dose) for Aa0 < 20 and AaO ≥ 20 | 4 | 167.57 | 0.00 |
Npar, number of parameters; AIC, Akaike Information Criterion.
aPreferred model 1.
Table 3.
Form of logistic regression models with number of parameters, AIC and ΔAIC (as difference to the preferred model) fitted to 117 exposed PTC cases from cohorts Genrisk-T and UkrAm
| Variables included | Npar | AIC | ΔAIC |
|---|---|---|---|
| Intercept only | 1 | 135.21 | 0.45 |
| Intercept + AaO | 2 | 137.20 | 2.44 |
| Intercept + AaE | 2 | 136.49 | 1.73 |
| Intercept + log(Dose) | 2 | 137.02 | 2.26 |
| Intercept + log(Dose) + AaO | 3 | 139.02 | 4.26 |
| Intercept + log(Dose) + AaE | 3 | 138.15 | 3.39 |
| Intercept + log(Dose) + AaO + log(Dose) × AaO | 4 | 136.85 | 2.09 |
| Intercept + log(Dose) + AaE + log(Dose) × AaE | 4 | 136.05 | 1.29 |
| Intercept + log(Dose) for AaE < 5 and AaE ≥ 5a | 4 | 134.76 | 0.00 |
| Intercept + log(Dose) for Aa0 < 20 and AaO ≥ 20 | 4 | 139.21 | 4.45 |
Npar, number of parameters; AIC, Akaike Information Criterion.
aPreferred model 2.
Interpretation of logistic regression results
The calculation of AIC value includes the deviance, which is a measure for the accuracy of the model to describe the data and a penalty for the complexity of the model (i.e. the number of parameters). It is intended to find a model with the minimum AIC. Adding parameters to a model usually increases the goodness-of-fit but also can lead to a so-called ‘over-fitting’, i.e. by adding a parameter for each observation it is possible to construct a (rather useless) model with perfect fit. Hence, in a given set of models the model with the minimum AIC optimizes the trade-off between the goodness-of-fit and the model complexity.
The minimum model, which consists of the intercept only, provides the general probability (independent of any predictive parameter) of the binary dependent variable (CLIP2 marker status) to have one certain state (in our case: CLIP2 positive).
Gene expression microarray data processing
Gene expression microarray data were generated on Agilent 44k arrays and published by Abend et al. (16). They were used to investigate the CLIP2 mRNA expression levels in tumor and normal tissue. Data quality assessment, pre-processing, normalization and data analyses were carried out using functions from the ‘limma’ package of the R software available from Bioconductor (29,30). Log2 expressions of the CLIP2 mRNA levels were calculated and compared between tumor samples and corresponding normal samples and the association of CLIP2 expression levels with dose was investigated in normal tissues.
Results
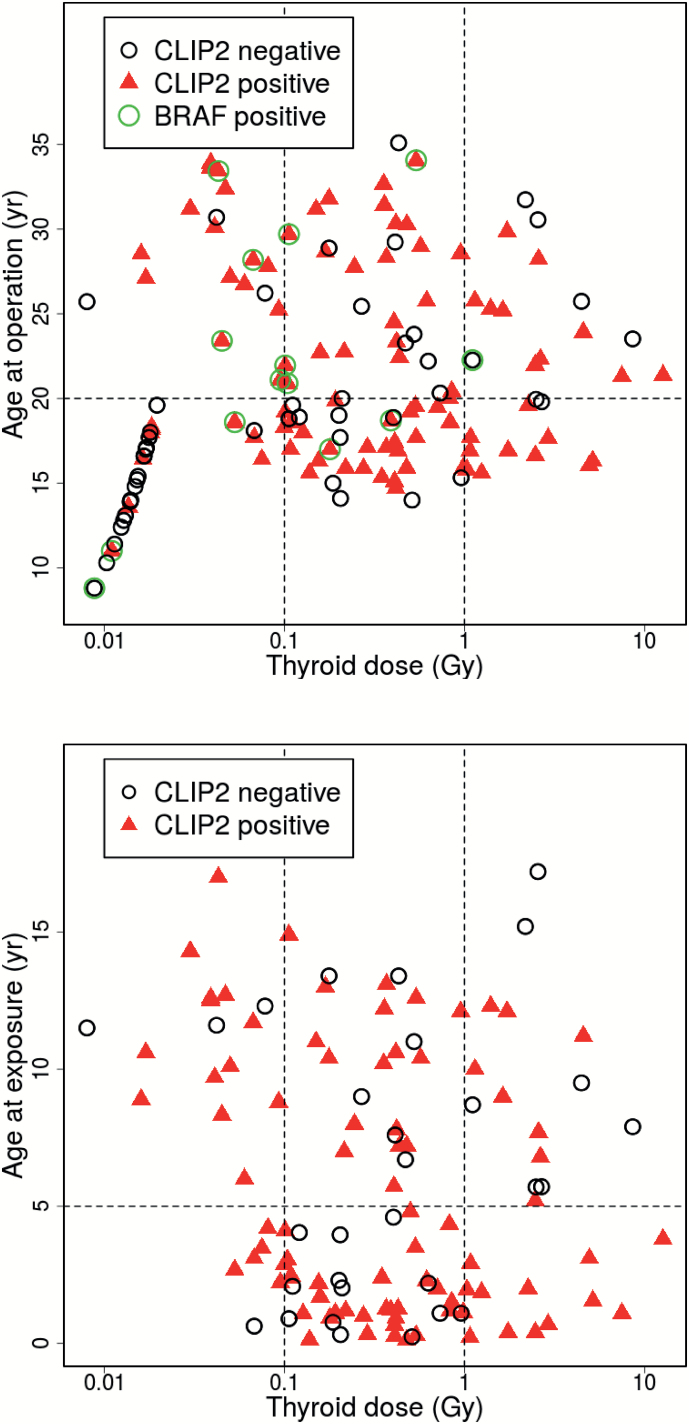
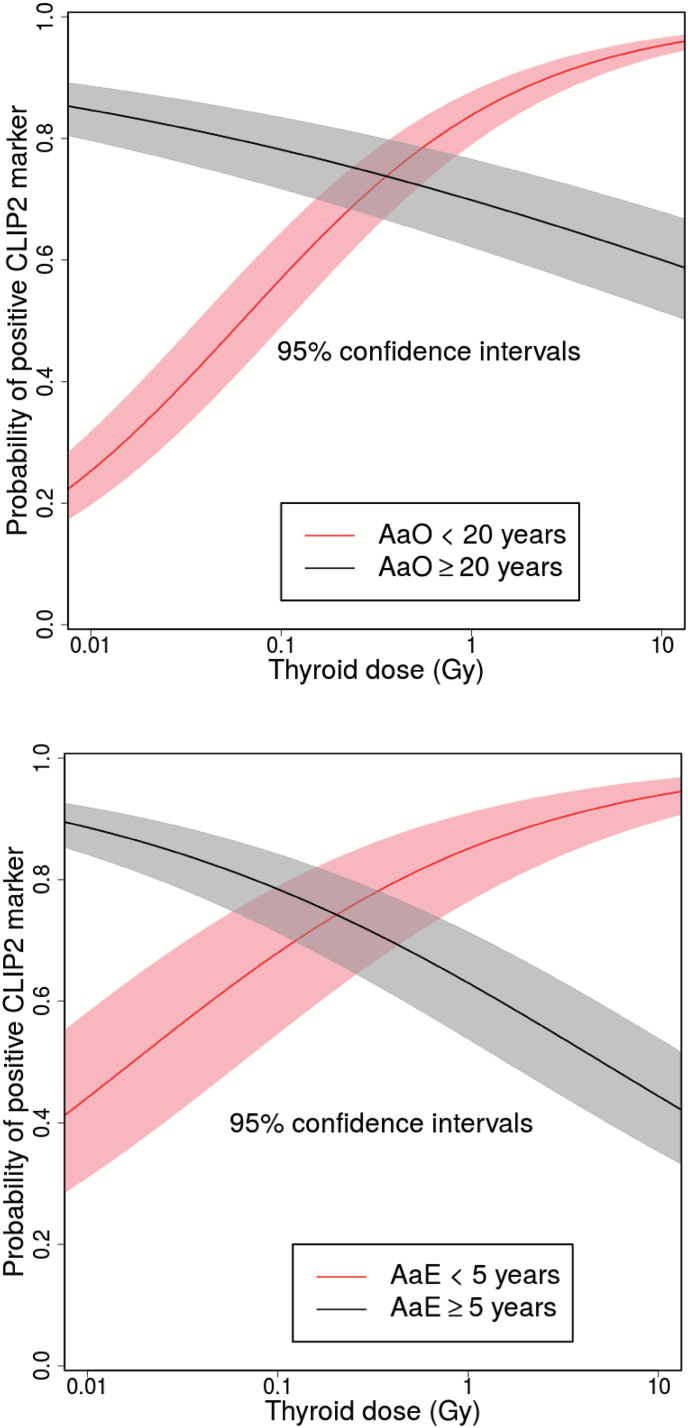
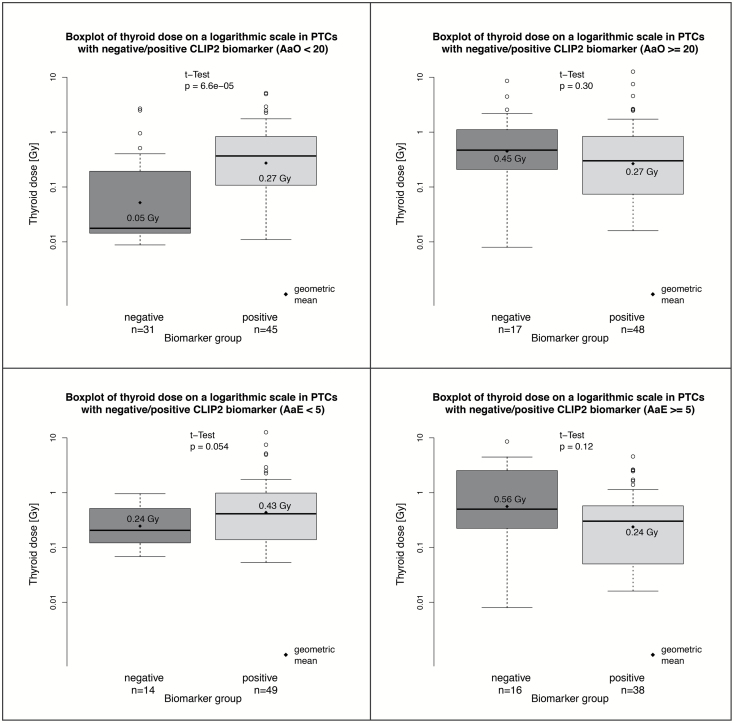
Scatter plots of binary CLIP2 data and their dependence on thyroid dose and either AaO or AaE are shown in Figure 1. AIC values of the tested models are listed in Tables 2 and 3 for the data sets that contained 141 PTCs and 117 PTCs, respectively. Our main results are derived from the larger data set since inclusion of non-exposed controls provides additional information on the age-dependence of sporadic PTC incidence. For this data set a model with four independent variables, which includes a multiplicative interaction of the continuous variables dose and AaO provides the fit with the lowest AIC (Table 2). The dose-response exhibits an inflexion point at an AaO of 23 years that separates a response with a positive slope at young AaO from a response with a negative slope at elder AaO. With this model the sensitivity of goodness-of-fit with respect to a variation of the categorical boundary was tested. The goodness-of-fit of the preferred model 1 using a categorical boundary at an AaO of 20 years, which was fixed a priori for biological reasons, decreased only slightly by 3.7 points. Parameter estimates of the preferred model 1 are given in Table 4, dose-response relations for the two categories of AaO are shown in the upper panels of Figure 2. For the reduced data set (n = 117) AaE could be additionally included as a covariable since all patients were exposed. Generally, improvements in quality of fit after introduction of additional model parameters were less pronounced in contrast to the results for the larger data set (Table 3). Using AaE as a covariable yielded a better description of the smaller data set compared to using covariable AaO. However, applying the preferred model 1 with two AaO categories to the smaller data set produced the same parameter estimates of Table 4 (left panel) in view of their uncertainties. The preferred model 2 with the lowest AIC used two categories of AaE < 5 years and ≥5 years together with thyroid dose as a continuous variable. Table 4 contains the parameter estimates and the dose-responses, which are depicted in the lower panels of Figure 2. Replacing the two categories for AaE with a continuous variation in AaE yielded a model that described the data almost equally well. For the model with continuous AaE the dose-response had a positive slope below an AaE of 6 years, whilst above AaE 6 years the slope was negative. Again, with the latter model the sensitivity of goodness-of-fit with respect to a variation of the categorical boundary was tested. All findings were consistent between UkrAm and Genrisk-T cohorts (results not shown). Figure 3 shows the thyroid doses for CLIP2 positive and negative cases in box plot representation for the larger data set (141 PTCs) with two categories AaO < 20 years and ≥20 years in the upper panels and for the reduced data set (117 PTCs) with two categories AaE < 5 years and ≥5 years in the lower panels. The hypothesis that the two arithmetic means of log-transformed dose estimates for PTCs with a positive and negative CLIP2 marker status assumed equal values has been tested with two-sample t-tests. On a 95% confidence level the hypothesis was rejected only in the larger data set for AaO < 20. The comparison of CLIP2 mRNA expression levels of 32 tumor and corresponding normal samples showed a significantly increased expression in the tumor samples (P < 0.001). An association of the dose estimates and the expression levels in normal tissues was not observed. In a group of 14 PTCs, BRAF V600E mutations were detected whilst the mean AaO was 22 years and the geometric/arithmetic dose means were 0.20/0.66 Gy. The level of CLIP2 protein abundance using the immunohistochemistry visual scoring level (Supplementary Table 1, available at Carcinogenesis Online) was analyzed. According to staining intensity, tumors were classified as having negative staining (score 0), weak staining (score 1), intermediate staining (score 2), or strong staining (score 3). The results, based on a comparison of dose distribution in tumors with intermediate and strong staining relative to tumors with negative/weak staining, were consistent with those based on a binary scale and there was a clear trend towards higher doses with higher CLIP2 protein expression for AaE < 5 years and AaO < 20 years. This trend was not visible for AaE ≥ 5 years and AaO ≥ 20 years (Supplementary Figure 2, available at Carcinogenesis Online).
Figure 1.
Scatter plots of PTC cases with CLIP2 typing (positive: red triangles, negative: black open circles) with co-variables age at operation and thyroid dose for 141 cases which include 24 non-exposed cases with a nominal background dose of 1 mGy/year (93 CLIP2 positive, 48 CLIP2 negative), 14 cases with positive BRAF mutation marked by green open circles (upper panel); and with co-variables age at exposure and thyroid dose for 117 exposed cases (87 CLIP2 positive, 30 CLIP2 negative) (lower panel).
Table 4.
Parameter estimates, standard errors and P-values of the logistic regression models; using log-transformed thyroid dose as a continuous covariable and either two categories for AaO in Model 1 fitted to 141 exposed and non-exposed PTC cases; or two categories for AaE in Model 2 fitted to 117 exposed PTC cases
| Model 1 including 34 non-exposed cases | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| With two AaO categories: | 1 (<20) | 2 (≥20) | With two AaE categories: | 1 (<5) | 2 (≥5) | ||
| n | 76 | 65 | n | 63 | 54 | ||
| Coefficient | Std. error | P-value | Coefficient | Std. error | P-value | ||
| Category 1 (<20) | Category 1 (<5) | ||||||
| Intercept | 1.46 | 0.39 | <0.001 | Intercept | 1.74 | 0.49 | <0.001 |
| Log(Dose [Gy]) | 0.36 | 0.09 | <0.001 | Log(Dose [Gy]) | 0.43 | 0.29 | 0.14 |
| Category 2 (≥20) | Category 2 (≥5) | ||||||
| Intercept | 0.84 | 0.33 | 0.011 | Intercept | 0.53 | 0.35 | 0.12 |
| −0.19 | 0.18 | 0.28 | Log(Dose [Gy]) | −0.33 | 0.19 | 0.09 | |
Figure 2.
Probability of occurrence for a positive CLIP2 marker as a continuous function of thyroid dose among 141 PTCs for categorical model 1 using AaO ≥ 20, <20 (upper panel), and among 117 PTCs for categorical model 2 using AaE ≥ 5, <5 (lower panel); 95% confidence intervals from parameter estimates pertaining to log(Dose).
Figure 3.
Thyroid doses in different groups. Thyroid doses for CLIP2 negative and positive groups in box plot representation for 141 exposed and non-exposed PTC cases (AaO < 20, left upper panel; AaO ≥ 20, right upper panel) and for 117 exposed cases (AaE < 5, left lower panel; AaE ≥ 5, right lower panel); P-values for t-tests indicate the probability that CLIP2 cases with opposite biomarker typing assume equal geometric mean values of dose estimates (or equivalently arithmetic mean values of log-transformed dose estimates).
Discussion
In previous studies a copy number gain and an overexpression of the CLIP2 gene on chromosomal band 7q11.23 has been identified as a radiation-specific marker in post-Chernobyl PTC. In the present study, we have analyzed the quantitative association between individual estimates of the post-Chernobyl thyroid dose from I-131 uptake and the occurrence of CLIP2-positive PTC patients of the UkrAm and Genrisk-T cohorts. Logistic regression analysis of the recently published CLIP2 expression data on these cohorts (20) revealed a dose-dependent increase in occurrence of a positive CLIP2 biomarker in patients with young AaO (<20 years) and young AaE (<5 years). An accumulation of CLIP2 negative cases among non-exposed patients and patients exposed to zero/low doses and an accumulation of CLIP2 positive cases at moderate/high doses points to different molecular mechanisms of tumor induction or development in the two dose categories (Figures 1 and 2). This is in line with results from epidemiologic studies on the radiation risk of post-Chernobyl thyroid cancer. A large case—control study of childhood cases from Belarus and Russia revealed a strong dose-response relationship with a linear increase of risk with doses up to 1.5 to 2 Gy but no significant risk increase below 0.2 Gy (31). More recent studies from a Belarussian cohort and a Ukrainian cohort reported no statistically significant increase of the risk below 0.1 Gy (4,7). A comprehensive review of more than 200 studies in the field of low-dose radiation conducted by Dauer et al. (32) discusses partly controversal results from studies with a focus on exposure to low doses and the subsequent cancer risk. A strong influence of AaE on the dose-dependent relationship of the CLIP2 marker reflects the much higher risk for radiation-induced carcinogenesis at young AaE as it was demonstrated in an epidemiological study (5). Models with a continuous variation of age-related covariables yielded inflexion points of the slope for the dose-response at AaO 23 years and AaE 6 years and described the data as good as a model with categorical covariables. These results emphasize that the a priori definition of categorical boundaries for biological reasons at AaO 20 years and at AaE 5 years in the preferred models is justified. Furthermore, the age values at the inflexion points for AaO and AaE are consistent with the observed median latency of PTCs of about 17 years indicating that radiation-induced tumors developed preferably in young patients. Among Japanese atomic bomb survivors the risk of radiation-induced thyroid cancer decreased sharply with increasing AaE and there was little evidence of increased thyroid cancer rates for those exposed after the age 20 (33).
It is interesting to note that the statistical evidence for a positive dose response of the CLIP2 marker in young patients is much stronger than for a negative response in adults (see e.g. P-values of Model 1 in Table 4). Nevertheless, the observation of a negative dose-relationship for patients with AaO ≥ 20 years and AaE ≥ 5 years is an interesting and surprising finding. For PTCs in UkrAm patients (23) and in atomic bomb survivors (22) an inverse association with dose for BRAF V600E mutation positive tumors was reported. In line with these observations the present study reveals that a majority of cases harboring BRAF V600E mutations were detected at doses below 0.2 Gy and were operated at AaO ≥ 20 years (Figure 1, upper panel). This is in agreement with the general assumption that BRAF mutations are mainly present in sporadic PTCs and indicate a multi-step process to cancer. Converging negative dose responses of BRAF and CLIP2 in adults suggest that for older patients (AaO ≥ 20 years) different molecular mechanisms increasingly related to sporadic cancer and with decreased CLIP2 involvement are in place compared to younger patients (AaO < 20 years) (22,23) or that radiation-related changes at older attained ages (or older exposure ages) involve different, yet to be identified events. This might explain the observed negative dose-relationship of the CLIP2 marker for patients with AaO ≥ 20 years.
Radio-epidemiologic studies have difficulties to elucidate the dose-response relationship for the cancer risk at low doses mainly because the number of expected cancer cases is too low to provide statistical evidence for a radiation effect. Therefore, it is repeatedly mentioned that an in-depth understanding of the mechanisms of radiation carcinogenesis is essential (34). In this context, it appears sensible to include the reported CLIP2 neighborhood genes BAG2, CHST3, KIF3C, NEURL1, RGS4 and PPIL3 (20) into a separate analysis of a dose dependent expression of these genes in order to clarify potential dose-dependent molecular mechanisms in radiation carcinogenesis of the thyroid gland. However, this would require additional tissue samples especially from cases with individual dose estimates.
I-131 dose-dependent gene expression has already been reported for the UkrAm cases by Abend et al. (15,16) for tumor and normal tissues and for a subset of the Genrisk-T cases in normal tissues by Dom et al. (14). In an initial global gene expression analysis of the UkrAm cases a set of 11 genes with dose-dependent expression has been observed (15) which was further specified in a second study revealing eight genes in normal tissues and six genes in tumor tissues that were significantly associated with I-131 dose (16). In a subset of the herein investigated Genrisk-T cohort, a study by Dom et al. (14) compared global gene expression profiles in PTCs and corresponding normal tissue from patients exposed and non-exposed to I-131. In this study a gene expression signature of 403 genes was identified in PTC corresponding normal tissue that was able to discriminate between exposed and non-exposed patients. Common findings between the two studies by Abend et al. (16) and Dom et al. (14) could not be observed. However, the gene LMO3, which was shown to be associated with radiation dose in the study by Abend et al. (15), is part of the second neighborhood CLIP2 gene regulatory network (20). The major limitation of the previous studies by Abend et al. (15,16) and Dom et al. (14) might be the small sample size, which is likely to have hampered a quantitative determination of the shape for the dose response. Following the previous studies on dose-dependent gene expression we also have investigated the CLIP2 expression at the mRNA level in PTC and corresponding normal tissues of the UkrAm cohort which revealed very low expression levels that were not radiation-associated indicating that CLIP2 changes are not long-lasting late effects of radiation in normal tissues. It would also be interesting to investigate additional genes of the 7q11.23 band identified in the study by Hess et al. (19) that show comparable differential expression between exposed and non-exposed cases in the same manner (e.g. LIMk1, STAG3L3 and family of PMS2-related genes). Nevertheless, PTC post-Chernobyl tissue collected and provided by the Chernobyl Tissue Bank is very limited and therefore it is not feasible to conduct such studies within a reasonable time frame.
The present study on a dose-relationship of the CLIP2 marker is based on carefully assessed molecular data of CLIP2 typing according to Selmansberger et al. (20) and has used very reliable individual dose estimates (6,35). Yet the number of cases is still limited and the tissues analyzed were taken from two heterogeneous cohorts with different mean AaE and AaO, which necessitates additional validation in another independent cohort that could be composed e.g. of Belarussian cases. For the Genrisk-T cohort CLIP2 data for only a small number of non-exposed patients were available. An increase in the number of non-exposed controls should facilitate the generation of molecular data without the radiation effect, which would substantiate the role of CLIP2 as a hallmark of radiation-induced PTC at young age.
Conclusion
In conclusion, the present study showed a clear dose-response relationship for the recently reported CLIP2 radiation marker in two Ukrainian post-Chernobyl PTC cohorts (UkrAm and Genrisk-T) for young patients with AaO less than 20 years and AaE less than 5 years. The results hint to different molecular mechanisms in tumors induced at low doses compared to moderate and high doses with a preferential occurrence of CLIP2 expression.
Funding
This study was supported by the European Commission, EpiRadBio project, FP7 grant no. 269553.
Supplementary material
Supplementary Table 1 and Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AaE
age at exposure
- AaO
age at operation
- AIC
Akaike Information Criterion
- CTB
Chernobyl Tissue Bank
- I-131
131Iodine
- mRNA
messenger RNA
- PTC
papillary thyroid carcinomas
References
- 1. Jacob P, et al. (1998) Thyroid cancer risk to children calculated. Nature, 392, 31–32. [DOI] [PubMed] [Google Scholar]
- 2. Davis S, et al. (2004) Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl Power Station accident. Radiat. Res., 162, 241–248. [DOI] [PubMed] [Google Scholar]
- 3. Cardis E, et al. (2006) Cancer consequences of the Chernobyl accident: 20 years on. J. Radiol. Prot., 26, 127–140. [DOI] [PubMed] [Google Scholar]
- 4. Tronko M.D, et al. (2006) A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine detected during first screening. J. Natl. Cancer Inst., 98, 897–903. [DOI] [PubMed] [Google Scholar]
- 5. Jacob P, et al. (2006) Thyroid cancer risk in areas of Ukraine and Belarus affected by the Chernobyl accident. Radiat. Res., 165, 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Brenner A.V, et al. (2011) I-131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ. Health Perspect., 119, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zablotska L.B, et al. (2011) Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br. J. Cancer, 104, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. UNSCEAR. (2008) Report to the General Assembly with Annexes C, D and E. In United Nations (ed) Sources and Effects of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation 2008. United Nations, New York, NY. [Google Scholar]
- 9. Stezhko V.A, et al. (2004) A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat. Res., 161, 481–492. [DOI] [PubMed] [Google Scholar]
- 10. Likhtarev I, et al. (2006) Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat. Res., 166(Pt 2), 271–286. [DOI] [PubMed] [Google Scholar]
- 11. Thomas G.A, et al. (2011) Integrating research on thyroid cancer after Chernobyl–the Chernobyl Tissue Bank. Clin. Oncol. (R. Coll. Radiol)., 23, 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Detours V, et al. (2005) Absence of a specific radiation signature in post-Chernobyl thyroid cancers. Br. J. Cancer, 92, 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Detours V, et al. (2007) Genome-wide gene expression profiling suggests distinct radiation susceptibilities in sporadic and post-Chernobyl papillary thyroid cancers. Br. J. Cancer, 97, 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dom G, et al. (2012) A gene expression signature distinguishes normal tissues of sporadic and radiation-induced papillary thyroid carcinomas. Br. J. Cancer, 107, 994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abend M, et al. (2012) Iodine-131 dose dependent gene expression in thyroid cancers and corresponding normal tissues following the Chernobyl accident. PLoS One, 7, e39103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abend M, et al. (2013) Iodine-131 dose-dependent gene expression: alterations in both normal and tumour thyroid tissues of post-Chernobyl thyroid cancers. Br. J. Cancer, 109, 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Port M, et al. (2007) A radiation-induced gene signature distinguishes post-Chernobyl from sporadic papillary thyroid cancers. Radiat. Res., 168, 639–649. [DOI] [PubMed] [Google Scholar]
- 18. Stein L, et al. (2010) Copy number and gene expression alterations in radiation-induced papillary thyroid carcinoma from chernobyl pediatric patients. Thyroid, 20, 475–487. [DOI] [PubMed] [Google Scholar]
- 19. Hess J, et al. (2011) Gain of chromosome band 7q11 in papillary thyroid carcinomas of young patients is associated with exposure to low-dose irradiation. Proc. Natl. Acad. Sci. U. S. A., 108, 9595–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selmansberger M, et al. (2014) CLIP2 as radiation biomarker in papillary thyroid carcinoma. Oncogene. doi:10.1038/onc.2014.311 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Powell N, et al. (2005) Frequency of BRAF T1796A mutation in papillary thyroid carcinoma relates to age of patient at diagnosis and not to radiation exposure. J. Pathol., 205, 558–564. [DOI] [PubMed] [Google Scholar]
- 22. Hamatani K, et al. (2008) RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res., 68, 7176–7182. [DOI] [PubMed] [Google Scholar]
- 23. Leeman-Neill R.J, et al. (2013) RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer, 119, 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leeman-Neill R.J, et al. (2014) ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer, 120, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Likhtarev I, et al. (2003) Uncertainties in thyroid dose reconstruction after Chernobyl. Radiat. Prot. Dosimetry, 105, 601–608. [DOI] [PubMed] [Google Scholar]
- 26. Likhtarov I, et al. (2005) Post-Chornobyl thyroid cancers in Ukraine. Report 1: estimation of thyroid doses. Radiat. Res., 163, 125–136. [DOI] [PubMed] [Google Scholar]
- 27. Likhtarov I, et al. (2013) Reconstruction of individual thyroid doses to the Ukrainian subjects enrolled in the Chernobyl Tissue Bank. Radiat. Prot. Dosimetry, 156, 407–423. [DOI] [PubMed] [Google Scholar]
- 28. Williams D. (2008) Radiation carcinogenesis: lessons from Chernobyl. Oncogene, 27(suppl. 2), S9–18. [DOI] [PubMed] [Google Scholar]
- 29. Smyth G.K. (2005) limma: Linear Models for Microarray Data. In Gentleman R. et al. (eds) Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer, New York, NY, pp. 397–420. [Google Scholar]
- 30. Gentleman R.C, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol., 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cardis E, et al. (2005) Risk of thyroid cancer after exposure to 131I in childhood. J. Natl. Cancer Inst., 97, 724–732. [DOI] [PubMed] [Google Scholar]
- 32. Dauer L.T, et al. (2010) Review and evaluation of updated research on the health effects associated with low-dose ionising radiation. Radiat. Prot. Dosimetry, 140, 103–136. [DOI] [PubMed] [Google Scholar]
- 33. Furukawa K, et al. (2013) Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int. J. Cancer, 132, 1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ron E, et al. (2010) Late health effects of ionizing radiation: bridging the experimental and epidemiologic divide. Radiat. Res., 174, 789–792. [DOI] [PubMed] [Google Scholar]
- 35. Likhtarov I, et al. (2013) Estimating thyroid masses for children, infants, and fetuses in Ukraine exposed to (131)I from the Chernobyl accident. Health Phys., 104, 78–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.