Abstract
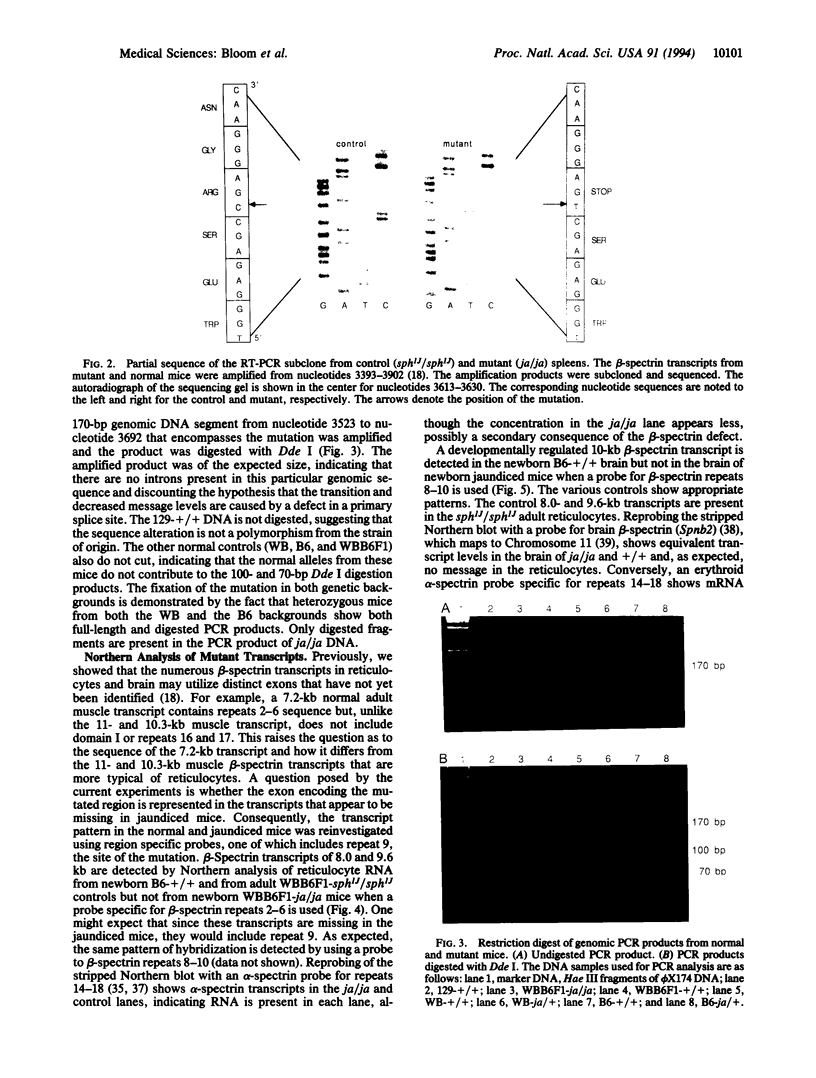
The jaundiced, ja/ja, mouse mutant has a severe hemolytic anemia associated with a deficiency of beta-spectrin in erythrocyte ghosts. Genes for the disease phenotype and beta-spectrin colocalize on Chromosome 12. beta-Spectrin mRNA is not detected in reticulocytes or in brain from newborn mutant mice. To locate the nucleotide sequence alteration, the erythroid beta-spectrin transcript from mutant spleen was amplified by reverse transcription PCR and sequenced. A C-to-T alteration is present in the mutant transcript and produces a premature stop codon from an arginine codon in mRNA encoding repeat 9 of beta-spectrin at amino acid position 1160. The point mutation introduces a Dde I site that is present in PCR-amplified DNA of ja/ja and ja/+ mice but not of +/+ control mice from the strain of origin, 129/Sv, or from the two strains, WB/Re and C57BL/6J, in which the mutation has been fixed by over 53 generations of backcrossing. The genetic data confirm that the point mutation is responsible for the severe reductions in beta-spectrin mRNA of jaundiced mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Asimos A., Casella J. F., McMillan C. Inheritance pattern and clinical response to splenectomy as a reflection of erythrocyte spectrin deficiency in hereditary spherocytosis. N Engl J Med. 1986 Dec 18;315(25):1579–1583. doi: 10.1056/NEJM198612183152504. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Lovrien R. E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984 Feb 16;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. S., Morrow J. S., Lux S. E. Abnormal oxidant sensitivity and beta-chain structure of spectrin in hereditary spherocytosis associated with defective spectrin-protein 4.1 binding. J Clin Invest. 1987 Aug;80(2):557–565. doi: 10.1172/JCI113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. S., Tse W. T., Lux S. E., Forget B. G. Beta spectrin kissimmee: a spectrin variant associated with autosomal dominant hereditary spherocytosis and defective binding to protein 4.1. J Clin Invest. 1993 Aug;92(2):612–616. doi: 10.1172/JCI116628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Bernstein S. E., Deveau S. A. Bone marrow replacement in the treatment of hemolytic disease in mice. Exp Hematol. 1989 Nov;17(10):1004–1010. [PubMed] [Google Scholar]
- Bernstein S. E. Inherited hemolytic disease in mice: a review and update. Lab Anim Sci. 1980 Apr;30(2 Pt 1):197–205. [PubMed] [Google Scholar]
- Birkenmeier C. S., McFarland-Starr E. C., Barker J. E. Chromosomal location of three spectrin genes: relationship to the inherited hemolytic anemias of mouse and man. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8121–8125. doi: 10.1073/pnas.85.21.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Davisson M. T., Beamer W. G., Ganschow R. E., Vogler C. A., Gwynn B., Lyford K. A., Maltais L. M., Wawrzyniak C. J. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989 Apr;83(4):1258–1266. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. L., Birkenmeier C. S., Barker J. E. Complete nucleotide sequence of the murine erythroid beta-spectrin cDNA and tissue-specific expression in normal and jaundiced mice. Blood. 1993 Nov 1;82(9):2906–2914. [PubMed] [Google Scholar]
- Bloom M. L., Lee B. K., Birkenmeier C. S., Ma Y., Zimmer W. E., Goodman S. R., Eicher E. M., Barker J. E. Brain beta spectrin isoform 235 (Spnb-2) maps to mouse chromosome 11. Mamm Genome. 1992;3(5):293–295. doi: 10.1007/BF00292159. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. Sequence similarity of the amino-terminal domain of Drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol. 1989 Oct;109(4 Pt 1):1633–1641. doi: 10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert R., Bennett P., Gratzer W. Properties and structural role of the subunits of human spectrin. Eur J Biochem. 1980 Jun;107(2):355–361. doi: 10.1111/j.1432-1033.1980.tb06036.x. [DOI] [PubMed] [Google Scholar]
- Cioe L., Curtis P. Detection and characterization of a mouse alpha-spectrin cDNA clone by its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1367–1371. doi: 10.1073/pnas.82.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioè L., Laurila P., Meo P., Krebs K., Goodman S., Curtis P. J. Cloning and nucleotide sequence of a mouse erythrocyte beta-spectrin cDNA. Blood. 1987 Oct;70(4):915–920. [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F. Biochemical characterization of complex formation by human erythrocyte spectrin, protein 4.1, and actin. Biochemistry. 1984 Dec 4;23(25):6091–6098. doi: 10.1021/bi00320a029. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Langley R. C., Jr Functional characterization of human erythrocyte spectrin alpha and beta chains: association with actin and erythrocyte protein 4.1. Biochemistry. 1984 Sep 11;23(19):4488–4495. doi: 10.1021/bi00314a039. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Palumbo A., Ming J., Fraser P., Cioe L., Meo P., Shane S., Rovera G. Sequence comparison of human and murine erythrocyte alpha-spectrin cDNA. Gene. 1985;36(3):357–362. doi: 10.1016/0378-1119(85)90191-x. [DOI] [PubMed] [Google Scholar]
- Gallagher P. G., Tse W. T., Forget B. G. Clinical and molecular aspects of disorders of the erythrocyte membrane skeleton. Semin Perinatol. 1990 Oct;14(5):351–367. [PubMed] [Google Scholar]
- Garbarz M., Boulanger L., Pedroni S., Lecomte M. C., Gautero H., Galand C., Boivin P., Feldman L., Dhermy D. Spectrin beta Tandil, a novel shortened beta-chain variant associated with hereditary elliptocytosis is due to a deletional frameshift mutation in the beta-spectrin gene. Blood. 1992 Aug 15;80(4):1066–1073. [PubMed] [Google Scholar]
- Harris H. W., Jr, Lux S. E. Structural characterization of the phosphorylation sites of human erythrocyte spectrin. J Biol Chem. 1980 Dec 10;255(23):11512–11520. [PubMed] [Google Scholar]
- Kam Z., Josephs R., Eisenberg H., Gratzer W. B. Structural study of spectrin from human erythrocyte membranes. Biochemistry. 1977 Dec 13;16(25):5568–5572. doi: 10.1021/bi00644a028. [DOI] [PubMed] [Google Scholar]
- Kanzaki A., Rabodonirina M., Yawata Y., Wilmotte R., Wada H., Ata K., Yamada O., Akatsuka J., Iyori H., Horiguchi M. A deletional frameshift mutation of the beta-spectrin gene associated with elliptocytosis in spectrin Tokyo (beta 220/216). Blood. 1992 Oct 15;80(8):2115–2121. [PubMed] [Google Scholar]
- Karinch A. M., Zimmer W. E., Goodman S. R. The identification and sequence of the actin-binding domain of human red blood cell beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11833–11840. [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. P., Warren S. L., Forget B. G., Morrow J. S. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991 Oct;115(1):267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula L., Laury-Kleintop L. D., Showe L., Sahr K., Linnenbach A. J., Forget B., Curtis P. J. The exon-intron organization of the human erythrocyte alpha-spectrin gene. Genomics. 1991 Jan;9(1):131–140. doi: 10.1016/0888-7543(91)90230-c. [DOI] [PubMed] [Google Scholar]
- Kozak L. P., Birkenmeier E. H. Mouse sn-glycerol-3-phosphate dehydrogenase: molecular cloning and genetic mapping of a cDNA sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3020–3024. doi: 10.1073/pnas.80.10.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zimmer W. E., Riederer B. M., Bloom M. L., Barker J. E., Goodman S. M., Goodman S. R. The complete amino acid sequence for brain beta spectrin (beta fodrin): relationship to globin sequences. Brain Res Mol Brain Res. 1993 Apr;18(1-2):87–99. doi: 10.1016/0169-328x(93)90176-p. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A. A., Rapp S., Lottspeich F., Schleicher M., Stewart M. The Dictyostelium gelation factor shares a putative actin binding site with alpha-actinins and dystrophin and also has a rod domain containing six 100-residue motifs that appear to have a cross-beta conformation. J Cell Biol. 1989 Aug;109(2):607–618. doi: 10.1083/jcb.109.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J., Liu S. C., Liu P. Y., Prchal J., Castleberry R. P. Altered assembly of spectrin in red cell membranes in hereditary pyropoikilocytosis. Blood. 1981 Jan;57(1):130–139. [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Sahr K. E., Coetzer T. L., Moy L. S., Derick L. H., Chishti A. H., Jarolim P., Lorenzo F., Miraglia del Giudice E., Iolascon A., Gallanello R. Spectrin cagliari. an Ala-->Gly substitution in helix 1 of beta spectrin repeat 17 that severely disrupts the structure and self-association of the erythrocyte spectrin heterodimer. J Biol Chem. 1993 Oct 25;268(30):22656–22662. [PubMed] [Google Scholar]
- Sands M. S., Birkenmeier E. H. A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6567–6571. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher D. W., DeSilva T. M., Speicher K. D., Ursitti J. A., Hembach P., Weglarz L. Location of the human red cell spectrin tetramer binding site and detection of a related "closed" hairpin loop dimer using proteolytic footprinting. J Biol Chem. 1993 Feb 25;268(6):4227–4235. [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Weglarz L., DeSilva T. M. Properties of human red cell spectrin heterodimer (side-to-side) assembly and identification of an essential nucleation site. J Biol Chem. 1992 Jul 25;267(21):14775–14782. [PubMed] [Google Scholar]
- Tse W. T., Gallagher P. G., Pothier B., Costa F. F., Scarpa A., Delaunay J., Forget B. G. An insertional frameshift mutation of the beta-spectrin gene associated with elliptocytosis in spectrin nice (beta 220/216). Blood. 1991 Jul 15;78(2):517–523. [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. A., Birkenmeier C. S., Lux S. E., Barker J. E. Ankyrin and the hemolytic anemia mutation, nb, map to mouse chromosome 8: presence of the nb allele is associated with a truncated erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3117–3121. doi: 10.1073/pnas.87.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11827–11832. [PubMed] [Google Scholar]
- Woods C. M., Lazarides E. Degradation of unassembled alpha- and beta-spectrin by distinct intracellular pathways: regulation of spectrin topogenesis by beta-spectrin degradation. Cell. 1985 Apr;40(4):959–969. doi: 10.1016/0092-8674(85)90356-3. [DOI] [PubMed] [Google Scholar]
- Zimmer W. E., Ma Y. P., Goodman S. R. Identification of a mouse brain beta-spectrin cDNA and distribution of its mRNA in adult tissues. Brain Res Bull. 1991 Aug;27(2):187–193. doi: 10.1016/0361-9230(91)90066-s. [DOI] [PubMed] [Google Scholar]