Abstract
Exercise at regular intervals is assumed to have a positive effect on immune functions. Conversely, after spaceflight and under simulated weightlessness (e.g., bed rest), immune functions can be suppressed. We aimed to assess the effects of simulated weightlessness (Second Berlin BedRest Study; BBR2-2) on immunological parameters and to investigate the effect of exercise (resistive exercise with and without vibration) on these changes. Twenty-four physically and mentally healthy male volunteers (20–45 years) performed resistive vibration exercise (n=7), resistance exercise without vibration (n=8) or no exercise (n=9) within 60 days of bed rest. Blood samples were taken 2 days before bed rest, on days 19 and 60 of bed rest. Composition of immune cells was analyzed by flow cytometry. Cytokines and neuroendocrine parameters were analyzed by Luminex technology and ELISA/RIA in plasma. General changes over time were identified by paired t-test, and exercise-dependent effects by pairwise repeated measurements (analysis of variance (ANOVA)). With all subjects pooled, the number of granulocytes, natural killer T cells, hematopoietic stem cells and CD45RA and CD25 co-expressing T cells increased and the number of monocytes decreased significantly during the study; the concentration of eotaxin decreased significantly. Different impacts of exercise were seen for lymphocytes, B cells, especially the IgD+ subpopulation of B cells and the concentrations of IP-10, RANTES and DHEA-S. We conclude that prolonged bed rest significantly impacts immune cell populations and cytokine concentrations. Exercise was able to specifically influence different immunological parameters. In summary, our data fit the hypothesis of immunoprotection by exercise and may point toward even superior effects by resistive vibration exercise.
Keywords: immune function, monitoring of immunological parameters, physical inactivity, spaceflight
Introduction
Physical activity and inactivity can have an impact on the immune system.1,2,3,4 However, it is necessary to perform controlled studies to understand the potential mechanisms of impact on the immune system. Prolonged bed rest in healthy individuals is a methodology used by space agencies to simulate the effects of spaceflight on the human body and to trial exercise countermeasures against these changes in a controlled fashion.5,6,7 Prolonged bed rest also represents strict, controlled, physical inactivity and presents a novel methodology to investigate the impact of inactivity and exercise on the immune system.
There are some findings available from previous work on the immune system in bed rest and related environments, such as spaceflight.8 Earlier studies have shown a change in cytokine production/secretion like increases in soluble tumor necrosis factor-α-receptor levels in an untrained control group during bed rest, or the increased secretion of cytokines such as IL-1, IL-2, IL-10 and elevated C reactive protein concentrations.9,10,11,12 Shearer and co-workers9 found an increased IL-1-receptor antagonist concentration in the human exercise group, which is an indicator for anti-inflammatory effects of training. Several other bed rest studies in fact did not find any changes in the concentrations of IL-6 or IL-1β.13,14,15,16,17
Moreover, bed rest alters neuroendocrine-immune factors in human individuals. Several studies showed an increased or decreased plasma cortisol level depending on the duration of bed rest.18,19 The levels of epinephrine and norepinephrine were increased in control and intervention groups in a study of Kanikowska and colleagues.20 Yet another bed rest study showed an increase in noradrenalin, dopamine, adrenocorticotropic hormone, growth hormone, prolactin levels and decreased expression of interferon (IFN)-γ during and after bed rest.19 Further bed rest studies did not show any change or a decrease in serum growth hormone levels.21,22,23,24 Prior work has shown conflicting results with increases,25 decreases26,27 or no change19,28 in testosterone levels during bed rest. A better understanding of the interplay between nervous, endocrine and immune systems under bed rest conditions is needed, since these systems are critically involved not only in the pathophysiology of chronic inflammatory diseases, but also in regulating energy metabolism and long-term disease sequelae.29
With respect to cellular immunity, bed rest does affect subpopulations of white blood cells. Previous studies have demonstrated that natural killer (NK) cells were seen to decrease in number.10,12,19 The number and proliferation of lymphocytes has been shown to decrease in bed rest.19,30 CD3+ T cells and monocytes indicated a decreased level in bed rest.12 The number of stimulated CD4+ T cells decreased and the number of stimulated CD8+ T cells increased in a study of Uchakin and co-workers.12,30 This might contribute to virus reactivation.30,31,32
In this regard, it is important to find out more about numbers and functions of subpopulations of leukocytes in order to better understand immune processes during bed rest. This was the main motivation behind the current work. The Second Berlin Bed Rest Study (BBR2-2) provided the opportunity to carry out this analysis.33 Furthermore, this study also evaluated two types of exercise during bed rest in comparison to the inactive control group. Previous work has shown that white blood cell counts remained stable in a group that underwent a short-arm centrifuge exercise 30 min each day during bed rest but increased in the inactive control group.20 Therefore, as a secondary aim, we examined the impact of exercises conducted in the current study on sub-populations of leukocytes and the concentrations of cytokines, chemokines and neuroendocrine factors known to impact immune system functions.
In summary, we hypothesize that bed rest (simulating weightlessness) has an impact on numbers of subpopulations of leukocytes and concentrations of immune and neuroendocrine factors. Additionally, we consider that regular exercise during bed rest/immobilization/space flights positively influences immune function. Furthermore, we also hypothesize that the resistance vibration exercise should be more effective also in regard to an improvement of immune functions, as it is known that this is superior when compared to conventional resistance exercise, e.g., in terms of more effective muscle building and bone density.6 The BBR2-2 study was designed for the investigation of effects primarily on bone and muscles. In this connection, we were able to analyze immune parameters. The small numbers of each group of participants are unfortunately not in favor of making a really clear point about the effects of the two kinds of exercise. This is reason enough to define this study as a data mining approach; we consider it is a matter of utmost importance to carry out especially careful observations in respect to confounders in our statistical analysis.
Materials and methods
Bed rest protocol, countermeasure exercise protocol and subject characteristics
Twenty-four medically and psychologically healthy males participated in the BBR2-2 (Table 1). The study protocol is discussed in detail elsewhere.33 In brief, subjects attended the facility for the baseline data collection (BDC) from nine days prior to a 60-day 6° head-down tilt (HDT) bed rest period and remained in the facility for a 7-day post-bed rest observation period. For logistical reasons, six subjects completed the 60-day bed rest phase at a time, resulting in four ‘cohorts'. The first cohort started in September 2007 and the last cohort ended in August 2008. During the HDT phase, subjects performed all hygiene activities in the HDT position. A detailed list of exclusion criteria is given elsewhere.33 However, exclusion criteria specifically relevant to the current investigations were current smokers (more than 10 cigarettes per day before bed rest) or those not willing to cease smoking for the duration of the study (the status as a former smoker was considered to be a confounder in our statistical analysis (Supplementary Table 1), regular medication use, current or chronic illness, any metabolic, hormone, cardiovascular disorder, any addictions, any allergies and current high-level sport participation. Body weight, urine production, intake of fluids and body temperature were monitored. For successful completion of all aspects of the study, subjects received a royalty of 8000 EUR. For completion of only a part of the tests, remuneration was reduced according to a fixed schedule.33 The study was approved by the ethical committee of the Charité Universitätsmedizin Berlin. All subjects gave their informed written consent prior to participation in the study.
Table 1. Baseline anthropometric characteristics.
| Subject group | Age (years) | Weight (kg) | Height (cm) | Body mass index (kg/m2) |
|---|---|---|---|---|
| Inactive control (n=9) | 33.1 (7.8) | 80.6 (5.2) | 181.3 (6.0) | 24.6 (2.2) |
| Resistive exercise only (n=8) | 31.1 (5.1) | 75.0 (12.8) | 179.3 (7.7) | 23.2 (2.3) |
| Resistive exercise with whole-body vibration (n=7) | 32.2 (10.4) | 81.5 (6.2) | 179.6 (5.8) | 25.3 (1.6) |
Values are mean (SD). No significant differences between groups were found (P≥0.11).
Subjects were randomized into three different groups: one that performed resistive exercises with whole-body vibration during bed rest (RVE; n=7), one that performed resistive exercise only (RE; n=8), and one that performed no exercise and thus served as a control group (CTR; n=9).
Furthermore, an additional non-bed rest group was tested as a control for selected parameters at the same intervals such as those of the subjects within the BBR2-2 study. In this non-bed rest group, ten men followed their normal daily activities without any immobilization (please see subject characteristics in Supplementary Table 2).
The countermeasure exercise protocol is discussed in detail elsewhere.33 In brief, exercise maneuvers targeted those load-bearing regions of the body where most bone and muscle activities are lost during bed rest (i.e., lower-quadrant and lumbar region). The training program was designed as a high load resistive exercise training program to achieve muscle hypertrophy.34 A single set regime was chosen to minimize exercise time. Training was performed 3 days a week during the HDT phase. The subject lay in the HDT posture on a sliding back rest with padded shoulder restraints. The feet were positioned on the foot plate and force was also transmitted via the shoulder restraint to the shoulders. A pneumatic system generated the required pressure. The force levels were monitored via sensors in the foot plate. In the vibration group, additional force was generated via side-alternating movement of the foot plate. After a short warm-up, the following four exercises were performed on the Galileo Space exercise device (Novotec Medical GmbH, Pforzheim, Germany): (i) bilateral leg press (about 75%–80% of pre-bed rest maximum voluntary contraction; in the RVE group, vibration frequency=24 Hz, amplitude 3.5–4 mm, peak acceleration ∼8.7g where g=9.81 m/s); (ii) single leg heel raises (∼1.3 times body weight; in the RVE group, vibration frequency=26 Hz, amplitude 3.5–4 mm, peak acceleration ∼10.2g); (iii) double leg heel raises (∼1.8 times body weight; in the RVE group, vibration frequency=26 Hz, amplitude 3.5–4 mm, peak acceleration ∼10.2g); and (iv) back and forefoot raise (performing hip and lumbar spine extension against gravity with ankle dorsiflexion but with ∼1.5 times body weight applied at the shoulders; in the RVE group: vibration frequency=16 Hz, amplitude 3.5–4 mm, acceleration ∼3.9g). The RVE group performed the same exercises as the RE group did except that whole body vibration was applied (see Ref. 33 for more details on the countermeasure protocol). Loading levels were increased by 5% when the subject could perform 12 repetitions of the exercise in two consecutive sessions. Note that that acceleration stated refers to the acceleration of the platform itself; effective accelerations on the subject are much lower and depend on subject position and muscle stiffness. The maximum ground reaction forces thereby transmitted to the feet of the subjects result in an effective acceleration at the feet in the order of 0.7g (unpublished observations).
The samples incorporated in of the BBR2-2 study were also intended to yield information on bone mineral content.35 As a priority in this investigation, we looked at immune cell populations. Since there appears to be only limited data available on the variables examined here with countermeasures during bed rest, it is difficult to carry out a definite analysis for the whole current study. Thus, we consider the current work to be an exploratory study concerning the effect of the exercise countermeasures on immune system changes during bed rest.
Group dropouts33
One subject (RVE) decided to leave the study on HDT1 and was replaced with a stand-by subject. This stand-by subject underwent the same duration of bed rest, but his bed rest phase began 4 days after that of the other subjects. Also, another subject was randomized to the RVE group, but felt unable to perform the exercise programs in HDT due to exercise-induced headaches.33 A medical consultant assessed the subject and decided that the subject could not take part in the RVE group, but could still continue as a CTR subject. Another subject from the RE group withdrew from the study at HDT30 for medical reasons.33 Thus, the CTR group comprised nine subjects, the RE group eight (up to HDT30, then seven afterwards) and the RVE group seven.
Blood sample collection and handling
Peripheral blood samples for various analyses were collected at multiple time points during the study. As the current work is an exploratory study, and not only the flow cytometry but also multiplex suspension arrays are expensive analyses, we have chosen to analyze only three time points: one time point before bed rest (BDC-2), one time point during bed rest (HDT19) and one time point at the end of bed rest (HDT60). Blood was collected in Lithium Heparin tubes (BD Biosciences, Heidelberg, Germany) at the same time of day (7:00–8:00 a.m.) to avoid circadian influences. Subsequently, the blood samples were centrifuged and plasma and cell pellets were separated. Plasma samples were frozen and stored at −80°C prior to a batch cytokine analysis. The cells (containing leukocytes and erythrocytes) were incubated in erythrocyte lysis buffer (0.01 M KHCO3, 0.155 M NH4Cl, 0.1 mM EDTA, pH 7.5) for 6 min at 4 °C and washed with PBS/BSA. The leukocytes obtained were filtered through a MACS pre-separation filter (30 µm; Miltenyi Biotech, Bergisch Gladbach, Germany). Additionally, a whole fresh blood count was conducted in a standardized laboratory fashion (standard values: leukocytes 3.9–10.5/nl, neutrophils 1.5–7.7/nl, lymphocytes 1.1–4.5/nl and monocytes 0.1–0.9/nl).
Flow cytometric analysis
To block unspecific bindings, the cell suspension was treated with human IgG (Grifols, Los Angeles, CA, USA). Flebogamma contains IgGs of different specifities: it is a mix of average IgG1 66.6%, IgG2 28.5%, IgG3 2.7% and. IgG4 2.2%. Stainings (incubation for ten min at four °C followed by washing with PBS/BSA) were performed with the following anti-human antibodies: anti-CD3-Pacific blue (UCHT1), anti-CD4-APC-Cy7 (RPA-T4), anti-CD25-FITC (M-A251), anti-CD56-PE-Cy7 (B159), anti-CD19-APC (HIB19), anti-CD34-FITC (581), anti-CD14-PE (M5E2), anti-CD16-PE-Cy5 (3G8), anti-IgD-PE (IA6-2) (all from BD Biosciences), anti-CD45RA-APC (MEM-56) and anti-CD8-Pacific-Orange (3B5) (all from Caltag Laboratories, Hamburg, Germany). Acquisition and analysis were performed using a LSR II cytometer (BD Biosciences, Heidelberg, Germany) and FlowJo software (Tree Star, Ashland, Oregon, USA). The following gating strategy is additionally explained in the Supplementary Figure 1. Debris and dead cells were excluded from the analysis in order to ensure the analysis of only live cells. Then the granulocyte and lymphocyte gates were defined via size and granularity. The monocyte gate was additionally defined by the expression of CD14. The CD34+ cells were gated within the mononuclear gate. The following gates were defined within the lymphocyte gate according to the expression of antigens: CD3+ cells, CD3+CD4+ cells, CD3+CD8+ cells, CD3−CD19+ cells, CD3−CD56+ cells and CD3+CD56+ cells. Within the CD3+CD4+ population, the following subpopulations were gated: CD45RA+, CD45RA−CD25+ and CD45RA+CD25+. Within the CD3+CD8+ population, the CD45RA+ subpopulation was gated. After the percentage analysis, the cell counts were calculated on the basis of the leukocyte count conducted in the routine hospital laboratory undergoing regular quality controls.
Quantification of cytokine concentrations
The concentrations of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, IFN-γ, IFN-γ-induced protein 10 kD (CXCL10, IP-10), tumor necrosis factor-α, IL-1 receptor antagonist, monocyte chemotactic protein-1, macrophage inflammatory protein 1α (CCL3), MIP-1β (CCL4), eotaxin-1 (CCL11), basic fibroblast growth factor, platelet-derived growth factor, vascular endothelial growth factor, granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor and RANTES (regulated upon activation normal T-cell expressed and secreted, CCL5) were all quantified in plasma samples by use of a multiplex suspension array (Bio-Rad Laboratories, Munich, Germany) according to the manufacturer's instructions. Data acquisition was conducted using the Bio-Plex suspension system.
Quantification of neuroendocrine variables
The concentrations of 17-OH-progesterone, 17-β-estradiol, free testosterone, adrenocorticotropic hormone, dehydroepiandrosterone (DHEA), DHEA sulphate (DHEA-S), neuropeptide Y (all from IBL, Hamburg, Germany) and cortisol (DSL, Webster, Texas, USA) were quantified in plasma samples by ELISA/RIA according to the manufacturer's instructions as described previously.36
Statistical analyses
Due to the mass of variables and data, a data-mining approach was used:
For each dependent variable, the data were analyzed for all subjects pooled as to whether or not there was a significant change during bed rest (simple paired t-tests HDT19 versus BDC-2, HDT60 versus BDC-2 and HDT19 versus HDT60). The data are shown as box plots.
A repeated measure analysis of variance (ANOVA) was carried out in order to identify the impact of ‘exercise' (CTR vs. RE+RVE and CTR vs. RE vs. RVE) on change during bed rest controlling for confounding by subject age, body mass index (BMI), ‘cohort' (first, second, third or fourth), smoker before bed rest and interactions between these parameters.
Where ‘exercise' or the interaction of ‘exercise' with other confounders were significant on repeated measures (ANOVA) (CTR vs. RE vs. RVE), additional repeated measures (ANOVA) comparing individual groups (i.e., CTR versus RE, CTR versus RVE and RE versus RVE) were performed to determine which exercise group(s) generated differing effects. The data are shown as box plots.
An alpha level of 0.05 was considered to be statistically significant. For analysis 1, for each variable alpha level adjustment was done for the three parallel tests by making use of simple Bonferroni correction. Statistical tests were performed using IBM SPSS Statistics (version 19; IBM, Armonk, NY, USA).
Results
Time-dependent impact of immobilization (all training groups pooled)
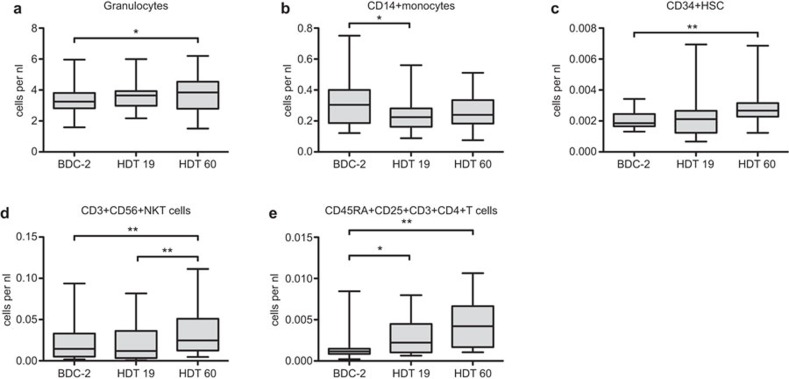
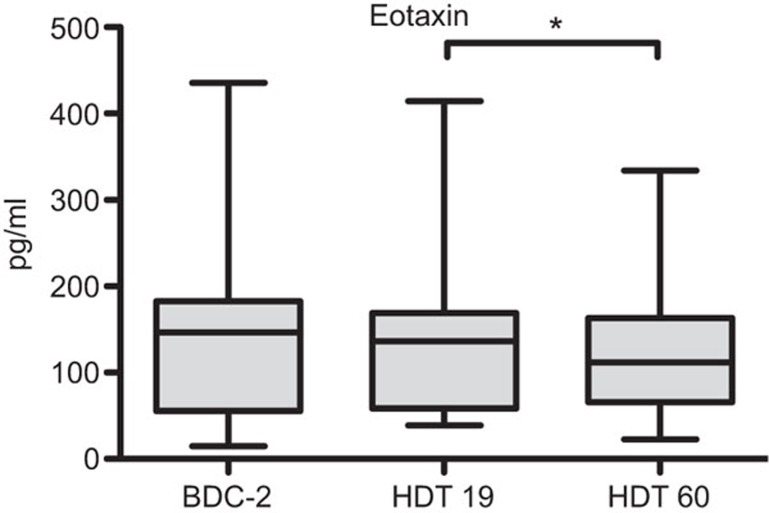
In order to increase the sample size to evaluate the effects of immobilization, we first pooled the data of all subjects analyzed. Several immune parameters showed changes between the time points analyzed in the pooled group of all subjects (Figures 1 and 2). Granulocyte numbers increased during bed rest from two days before baseline data collection to 60 days of HDT (P<0.05, Figure 1a). CD14+ monocyte numbers decreased initially from BDC-2 to HDT19 (P<0.05, Figure 1b). The number of CD34+ human stem cells increased significantly from BDC-2 to HDT60 (P<0.01, Figure 1c). CD3+CD56+ NK T cells rose from HDT19 to HDT60 thus being also significantly increased when compared to BDC-2 and HDT60 (P<0.01, respectively, Figure 1d). The number of CD45RA+CD25+CD3+CD4+ T cells increased from 2 days before baseline data collection to day 19 (P<0.05) and to day 60 (BDC-2 vs. day 60, P<0.01, Figure 1e). The concentration of eotaxin decreased from day 19 to day 60 (P<0.05, Figure 2). All other immune parameters analyzed did not show statistical differences between the time points with all subjects pooled.
Figure 1.
Time-dependent effects in leukocyte subpopulations for all subjects pooled (n=24). Blood samples were drawn on BDC-2, HDT19 and HDT60. Leukocytes were analyzed flow cytometrically according to their morphology and different surface markers. Then the cell counts were calculated on the basis of the leukocyte count conducted in a standardized laboratory manner. Data are shown as box and whisker plots (box: median and the 25th and 75th percentiles; whiskers: minimum+maximum). Time-dependent differences were found in (a) granulocytes, (b) CD14+ monocytes, (c) CD34+ HSC, (d) CD3+CD56+ NKT cells and (e) CD45RA+CD25+CD3+CD4+ T cells. Paired t-tests were performed and P values corrected for multiple testing within each subpopulation, *P<0.05, **P<0.01. BDC, baseline data collection; HDT, head-down tilt; NKT, natural killer T.
Figure 2.
Time-dependent effects in Eotaxin for all subjects pooled (n=24). Blood samples were drawn on BDC-2, HDT19 and HDT60. Plasma was analyzed by a multiplex suspension array. Data are shown as box and whisker plots (box: median and the 25th and 75th percentiles; whiskers: minimum+maximum). Paired t-tests were performed and P values corrected for multiple testing, *P<0.05. BDC, baseline data collection; HDT, head-down tilt.
Furthermore, we performed an additional analysis of selected parameters within a non-bed rest group. In regard to all parameters analyzed, there were absolutely no significant changes obvious among granulocytes, monocytes, lymphocytes, NK cells, CD19+ B cells, CD3+ T cells, CD4+ T helper cells or CD8+ cytotoxic T cells. The results are shown in the Supplementary Figure 2.
Time-dependent impact of training (RE+RVE pooled vs. CTR)
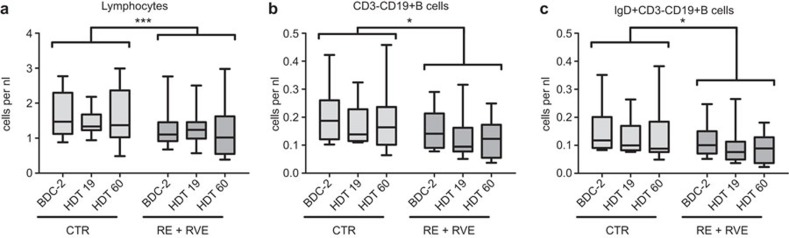
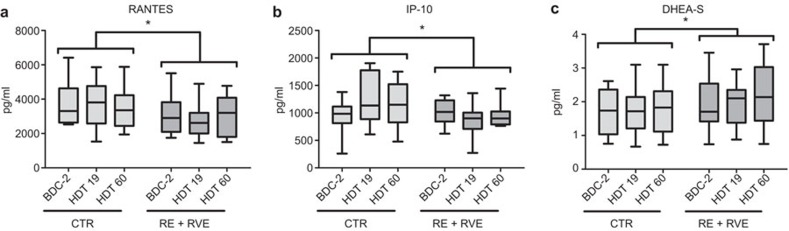
In order to evaluate the effect of training, we pooled the RE and RVE group (both training groups) and compared these with the CTR group. To identify the impact of exercise (RE+RVE) vs. no exercise (CTR) on time-dependent changes (from BDC-2 to HDT 19 to HDT60) of immunological parameters during bed rest, a repeated measure ANOVA was conducted. The ANOVA was controlled additionally for confounding by looking at subject age, BMI, ‘cohort' (first, second, third or fourth), smoker before bed rest and interactions between these parameters. The interactions are marked via *. Results of this basic analysis for identification of interesting time-dependently changed parameters are listed in Table 2. An impact of exercise over time was seen for the numbers of lymphocytes, CD3−CD19+ B cells, IgD+CD3−CD19+ B cells and the concentrations of RANTES, IP-10 and DHEA-S. Lymphocytes exhibited a difference in their time-dependent course between the CTR group and the training groups (P<0.001, Figure 3a). There was a difference in the time-dependent progress of CD3−CD19+ B cells (P<0.05, Figure 3b) and IgD+CD3−CD19+ B cells (P<0.05, Figure 3c) between the CTR group and the training groups. Concerning cytokines, chemokines and neuroendocrine factors, the CTR group and the training groups (RE+RVE) differed in the time-wise progress of the concentrations of RANTES (P<0.05, Figure 4a), IP-10 (P<0.05, Figure 4b) and DHEA-S (P<0.05, Figure 4c).
Table 2. Immune cells, cytokines, chemokines and neuroendocrine factors which show time-dependent effects (from BDC-2 to HDT 19 to HDT60) in repeated measurement analyses. The ANOVA was controlled for confounding by subject exercise, age, body mass index, cohort (first, second, third or fourth), smoker before study and interactions between these parameters. The interactions are marked with *. Confounding factors are listed. Significant P values (P<0.05) are shown. Where ‘exercise' values (bold) or the ‘exercise'בother confounder' interaction values were significant, results are given in Figures 3 and 4.
| Parameters | ||
|---|---|---|
| Confounding factors | P value | |
| Immune cell subpopulations | ||
| Granulocytes | 0.006 | |
| Age | 0.017 | |
| Cohort | <0.001 | |
| Smoker | <0.001 | |
| Cohort*smoker | <0.001 | |
| Lymphocytes | Cohort | <0.001 |
| Exercise*cohort | <0.001 | |
| Exercise*smoker | 0.014 | |
| Cohort*smoker | <0.001 | |
| CD14+ monocytes | Cohort | 0.005 |
| Lymphocyte subpopulations | ||
| T cells | ||
| CD3+ | Cohort | 0.004 |
| T-cell subpopulations | ||
| CD3+CD4+ T helper cells | Cohort | <0.001 |
| CD3+CD8+ cytotoxic T cells | Cohort | 0.044 |
| B cells | ||
| CD3−CD19+ B cells | Cohort | 0.003 |
| Exercise*cohort | 0.036 | |
| B-cell subpopulations | ||
| IgD+CD3−CD19+ B cells | Cohort | 0.010 |
| Exercise*cohort | 0.038 | |
| NK cells | ||
| CD3−CD56+ NK cells | Cohort | 0.003 |
| Cohort*smoker | 0.030 | |
| Hematopoietic stem cells | ||
| CD34+ HSC | 0.019 | |
| BMI | 0.004 | |
| Age | 0.040 | |
| Cohort | 0.033 | |
| Cohort*smoker | 0.007 | |
| Cytokines and chemokines | ||
| IP-10 | Exercise | 0.025 |
| PDGF | Cohort | <0.001 |
| RANTES | Exercise | 0.016 |
| Neuroendocrine factors | ||
| ACTH | 0.030 | |
| DHEA-S | 0.049 | |
| Smoker | 0.020 | |
| Exercise*cohort | 0.019 | |
| Cohort*smoker | 0.024 | |
| Age | 0.024 | |
| Progesterone | Age | 0.031 |
Abbreviations: ACTH, adrenocorticotropic hormone; BMI, body mass index; DHEA, dehydroepiandrosterone, PDGF, platelet-derived growth factor; RANTES, regulated upon activation normal T-cell expressed and secreted.
Figure 3.
Exercise (RE+RVE pooled versus CTR) dependent time effects in leukocyte subpopulations. Blood samples were drawn on BDC-2, HDT19 and HDT60. Leukocytes were analyzed flow cytometrically according to their morphology and different surface markers. Then the cell counts were calculated on the basis of the leukocyte count conducted in the routine hospital laboratory undergoing regular quality controls. Data are shown as box and whisker plots (box: median and the 25th and 75th percentiles; whiskers: minimum+maximum). Exercise-dependent time effects were found in (a) lymphocytes, (b) CD3−CD19+ B cells and (c) IgD+CD3−CD19+ B cells. Repeated measurement analyses were performed; the statistical analysis refers to differences in the time course, *P<0.05, ***P<0.001. BDC, baseline data collection; HDT, head-down tilt; RE, resistive exercise; RVE, resistive exercise with whole-body vibration.
Figure 4.
Exercise (RE+RVE pooled versus CTR) dependent time-effects in plasma factors. Blood samples were drawn on BDC-2, HDT19 and HDT60. Plasma was analyzed by a multiplex suspension array or ELISA/RIA. Data are shown as box and whisker plots (box: median and the 25th and 75th percentiles; whiskers: minimum+maximum). Exercise-dependent time-effects were found in (a) RANTES, (b) IP-10 and (c) DHEA-S. Repeated measurement analyses were performed; the statistical analysis refers to the difference in time course, *P<0.05. BDC, baseline data collection; HDT, head-down tilt; RE, resistive exercise; RVE, resistive exercise with whole-body vibration.
Time-dependent impact of the specific kind of training (RE vs. RVE vs. CTR)
To identify the impact of the different kinds of exercise (RE or RVE) vs. no exercise (CTR) on time-dependent changes (from BDC-2 to HDT 19 to HDT60) of immunological parameters during bed rest, a repeated measure ANOVA was conducted. The ANOVA was controlled additionally for confounding by subject age, BMI, ‘cohort' (first, second, third or fourth), smoker before bed rest and interactions between these parameters. The interactions are marked via *. Results of this basic analysis for identification of interesting time-dependently changed parameters are listed in the Supplementary Table 3. In this analysis of all groups versus each other (CTR vs. RE vs. RVE), the sample size per group was low (n=7–9). Nonetheless, statistical differences could be found. But an interpretation still remains difficult due to the small sample size. An impact of exercise type over time was seen for the numbers of lymphocytes, CD45RA+CD3+CD4+ T helper cells and the concentrations of RANTES and DHEA-S.
In order to clarify varying impacts of the specific types of exercise (CTR vs. RE vs. RVE) on the change over time for the immune parameters listed in the Supplementary Table 3, separate pairwise repeated measurements were performed. In these analyses, the lymphocytes exhibited a difference in their time-dependent course between the CTR group and the RE group (P<0.001, Supplementary Figure 3a). There was a difference in the time-dependent progress of the CD45RA+CD3+CD4+ T cells between the following groups: CTR vs. RE (P<0.01) and RE vs. RVE (P<0.001, Supplementary Figure 3b). Concerning cytokines, chemokines and neuroendocrine factors, the CTR group versus the RVE group and the RE versus the RVE groups differed in the time-wise progress of the concentrations of DHEA-S (P<0.01, Supplementary Figure 4b).
Discussion
We examined a number of neuroendocrine and immune parameters over 60 days of bed rest with and without the effects of exercise. Head down bed rest studies turned out to simulate weightlessness in a reliable manner. It mimics the most physiological effects of space flight: the elimination of gravitation stimuli (no posture change, no work against gravity, and reduction of body sensor stimuli). Such immobilization and inactivity result in an upward fluid shift and a reduction of energy requirements. This also results in a reduction of plasma volume by 10%–15%, consequently leading to cardiovascular changes.37 Cardiac performance and baroreflex sensitivity under bed rest are identical to those observed in space travel. In summary, bed rest studies mimic space flight conditions and are a very useful tool for scientific investigations of space flight effects.7 Astronauts are very well selected physically and mentally healthy subjects not exhibiting any chronic immune diseases. But—and this is one of the reasons for our study—although they may have been selected very carefully, during spaceflight, infectious diseases and hyperreactivity reactions can nonetheless occur (despite isolation from terrestrial pathogens). The depression of immune functions during immobilization or spaceflight is especially reflected in the occurrence of common colds and virus reactivations.30,31,32,38,39 Especially the reactivation of herpes viruses plagues many astronauts.40 Thus, the existence of a dysregulation of immune reactions during space flight is long known and has been discussed for a long time, but not yet characterized in detail.41 Despite the relatively low subject numbers in the BBR2-2 study, we were able to demonstrate significant changes of some immune parameters in the course of bed rest. Our results show a decrease in the numbers of monocytes which could partly explain the depression of immune functions. The subpopulation of CD45RA and CD25 co-expressing T helper cells most likely represents regulatory T cells: these cells co-express naïve and activation-induced molecules. This population increases during the study as a possible regulation of immune activation. The numerical rise of monocytes at the end of bed rest could point toward an adaptation of the immune system or even a counter regulation which could also explain the increase of the NK T cells. However, the findings do not completely fit to this pattern as we did not find any significant effects on the numbers of CD8+ cytotoxic T cells or their activation status, which we would expect in the case of virus reactivation. Overall, the findings do not clearly fit to either a pro- or an anti-inflammatory pattern. Specifically, we have not found any significant change in the level of pro-inflammatory cytokines (e.g., IL-1, IL-6 or IFN-γ) or anti-inflammatory cytokines (e.g. IL-10 or IL-1 receptor antagonist). It is very difficult to discuss all parameters which did not change during bed rest. It is not clear if there are really no changes which occur or if we were just not able to detect them. It is, however, known that during space flight the hypothalamo–pituitary–adrenal axis is stimulated with a consequent hypercortisolism.42 We were able to demonstrate an increase of granulocytes after 60 days of immobilization. Increase of granulocytes is a common glucocorticoid-induced effect which explains our finding. We assume the same effect to be responsible for the decreased concentration of eotaxin. It is known that the secretion of eotaxin is suppressed by glucocorticoids.43,44 Furthermore, we demonstrated an increase of CD34+ HSC at HDT 60. We assume this increase to be based on the bone marrow adiposity observed earlier in bed rest study.45 We hypothesize that bone marrow adiposity induces alterations within the stem cell niche, leading to increased release in peripheral blood. Furthermore, the concentration of eotaxin (CCL11) decreased in our study during bed rest. Eotaxin is a chemoattractant for eosinophils, basophils and type 2 T helper cells.46,47 A lack of the chemoattraction of type 2 T helper cells could lead to a dominance of type 1 T helper cells and thus, a pro-inflammatory situation explaining the immunodysregulation during bed rest/weightlessness at least in part.
Exercise-dependent time effects on the immune cell numbers were found. In the light of our evaluation of the time-dependent effects of training (RE+RVE pooled vs. CTR), we were able to demonstrate effects on lymphocytes, CD3−CD19+ B cells and the IgD+CD3−CD19+ B cell subpopulation. Within the CTR group, the number of lymphocytes remained stable while it decreased after 60 days of bed rest within the training groups. The number of CD3−CD19+ B cells and IgD+CD3−CD19+ B cells decreased slightly from BDC-2 to HDT19 among the CTR and the training groups. But while the IgD+CD3−CD19+ B cells further decreased within the CTR group, the CD3−CD19+ B cells and especially the IgD+ subpopulation increased within the training groups. This points towards a better reconstitution of especially the IgD+ naive B cells during training and might lead to improved cellular immunocompetence within the training groups. Furthermore, the concentrations of RANTES, IP-10 and DHEA-S were dependent on exercise. While the concentrations of RANTES (CCL5) within the CTR group increased at HDT19, on HDT 60, no difference to BDC-2 was seen. In contrast, within the training group (RE+RVE), the concentration of RANTES was increased at HDT 60 when compared to BDC-2. RANTES is chemotactic for T cells, basophils and eosinophils.48,49 It recruits leukocytes to the site of inflammation.48,49 It has also been shown that CD8+ T cells do release RANTES which suppresses viruses like HIV.50,51 Thus, the increase of the concentration of RANTES within the training groups (RE+RVE) indicates a superior defense and confirms our hypothesis concerning improvement of immunofunction by regular exercise. Moreover, this increase of the RANTES concentration is even more prominent in the RVE group than it is in the RE group. Thus, we can speculate that the RVE is even more effective in terms of immunoprotection. The IP-10 (CXCL10) concentration increased during bed rest in the CTR group, while it remained stable in the exercise groups (RE+RVE). IP-10 is an inflammatory cytokine induced by IFN-γ.52,53 It is a chemoattractant of monocytes, T cells, NK cells and dendritic cells; it inhibits angiogenesis and mediates T-cell adhesion to endothelial cells.52,53,54,55 IP-10 exhibits a clear pro-inflammatory role, pointing towards pro-inflammatory conditions in the CTR group and stable conditions in the training groups (RE+RVE). This again confirms our hypothesis of a protective role of training on immune functions. The concentration of DHEA-S is stable over time in the CTR group, but increases during bed rest with the training groups (RE+RVE). DHEA is metabolized in the liver to DHEA-S for the stable transport in blood as a metabolic intermediate in the production of steroid hormones.56,57 DHEA and DHEA-S decrease in concentrations upon aging and are suspected to be at least in part responsible for the effects of immunosenescence in terms of declined immune function.56,58,59 Thus, positive effects of DHEA on immune functions are assumed.56,58,59 The observed increase of the concentration of DHEA-S during bed rest in the exercise groups (RE+RVE) points toward increased immunocompetence, again fitting our hypothesis of immunoprotection induced by exercise. This increase in the concentration of DHEA-S is mainly based on the increase within the RVE group confirming our assumption of the superiority of vibration training (RVE).
In summary, our data fit the hypothesis of immunoprotection by exercise and may point toward even superior effects offered by RVE. In a next study, we will evaluate especially the cell function of the altered subpopulations.
It is appropriate to discuss the limitations of the current study: the subject numbers here are limited in bed rest studies for logistical and financial reasons. This limits our ability to generalize to a wider population with the current data. With the current study, we cannot directly comment on the mechanisms of action underlying the changes we observed. This would have to be evaluated in future work.
In conclusion, we conducted a data mining study and despite small subject numbers we could in our opinion still find important effects of prolonged bed rest on immune cell populations and cytokine concentrations. The impact of exercise was seen to involve several immunological parameters.
Acknowledgments
We would like to thank Manuela Jakstadt and Tina Bongrazio for technical assistance.
Dieter Felsenberg acts as a consultant to the European Space Agency and Novotec Medical for the exploitation of the results of this study. Apart from that, the authors declare no conflict of interest.
The BBR2-2 was supported by grant 14431/02/NL/SH2 from the European Space Agency and grant 50WB0720 from the German Aerospace Center (DLR). The 2nd Berlin Bed rest Study was also sponsored by Novotec Medical, Charité Universitätsmedizin Berlin, Siemens, Osteomedical Group, Wyeth Pharma, Servier Deutschland, P&G, Kubivent, Seca, Astra-Zeneka and General Electric. Daniel L. Belavý was supported by a post-doctoral fellowship from the Alexander von Humboldt Foundation.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- Kruger K, Mooren FC. Exercise-induced leukocyte apoptosis. Exerc Immunol Rev. 2014;20:117–134. [PubMed] [Google Scholar]
- Radom-Aizik S, Zaldivar FP, Jr, Haddad F, Cooper DM. Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain Behav Immun. 2014;39:121–129. doi: 10.1016/j.bbi.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitlic A, Lord JM, Phillips AC. Stress, ageing and their influence on functional, cellular and molecular aspects of the immune system. Age (Dordr) 2014;36:9631. doi: 10.1007/s11357-014-9631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicogossian AE, Dietlein LF.Microgravity simulation and analoguesNicogossian AE (ed.) Space Physiology and Medicine. Philadelphia, PA: Lea & Febiger; 1982240–248. [Google Scholar]
- Belavy DL, Beller G, Armbrecht G, Perschel FH, Fitzner R, Bock O, et al. Evidence for an additional effect of whole-body vibration above resistive exercise alone in preventing bone loss during prolonged bed rest. Osteoporos Int. 2011;22:1581–1591. doi: 10.1007/s00198-010-1371-6. [DOI] [PubMed] [Google Scholar]
- Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006) Eur J Appl Physiol. 2007;101:143–194. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- Stein TP, Schluter MD, Moldawer LL. Endocrine relationships during human spaceflight. Am J Physiol. 1999;276:E155–E162. doi: 10.1152/ajpendo.1999.276.1.e155. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Ochs HD, Lee BN, Cohen EN, Reuben JM, Cheng I, et al. Immune responses in adult female volunteers during the bed-rest model of spaceflight: antibodies and cytokines. J Allergy Clin Immunol. 2009;123:900–905. doi: 10.1016/j.jaci.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schmitt DA, Schaffar L, Taylor GR, Loftin KC, Schneider VS, Koebel A, et al. Use of bed rest and head-down tilt to simulate spaceflight-induced immune system changes. J Interferon Cytokine Res. 1996;16:151–157. doi: 10.1089/jir.1996.16.151. [DOI] [PubMed] [Google Scholar]
- Zwart SR, Crawford GE, Gillman PL, Kala G, Rodgers AS, Rogers A, et al. Effects of 21 days of bed rest, with or without artificial gravity, on nutritional status of humans. J Appl Physiol. 2009;107:54–62. doi: 10.1152/japplphysiol.91136.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchakin PN, Tobin BW, Morukov BV, Larina IV, Cubbage ML. Type 1 vs. type 2 cytokine secretion in vitro and its regulation by hydrocortisone in humans subjected to 120-day anti-orthostatic bed-rest regime. J Gravit Physiol. 2002;9:71–82. [PubMed] [Google Scholar]
- Feuerecker M, Feuerecker B, Matzel S, Long M, Strewe C, Kaufmann I, et al. Five days of head-down-tilt bed rest induces noninflammatory shedding of L-selectin. J Appl Physiol (1985) 2013;115:235–242. doi: 10.1152/japplphysiol.00381.2013. [DOI] [PubMed] [Google Scholar]
- Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–2656. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojbjerre L, Sonne MP, Alibegovic AC, Nielsen NB, Dela F, Vaag A, et al. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34:2265–2272. doi: 10.2337/dc11-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt DA, Schwarzenberg M, Tkaczuk J, Hebrard S, Brandenberger G, Mauco G, et al. Head-down tilt bed rest and immune responses. Pflugers Arch. 2000;441:R79–R84. doi: 10.1007/s004240000349. [DOI] [PubMed] [Google Scholar]
- Xu X, Tan C, Li P, Zhang S, Pang X, Liu H, et al. Changes of cytokines during a spaceflight analog—a 45-day head-down bed rest. PLoS One. 2013;8:e77401. doi: 10.1371/journal.pone.0077401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Yetman DL, Storm WF, Sams CF, Pierson DL. Neuroendocrine and immune responses to 16-day bed rest with realistic launch and landing G profiles. Aviat Space Environ Med. 2008;79:117–122. doi: 10.3357/asem.2205.2008. [DOI] [PubMed] [Google Scholar]
- Murdaca G, Setti M, Brenci S, Fenoglio D, Lantieri P, Indiveri F, et al. Modifications of immunological and neuro-endocrine parameters induced by antiorthostatic bed-rest in human healthy volunteers. Minerva Med. 2003;94:363–378. [PubMed] [Google Scholar]
- Kanikowska D, Sato M, Iwase S, Shimizu Y, Inukai Y, Nishimura N, et al. Immune and neuroendocrine responses to head-down rest and countermeasures. Aviat Space Environ Med. 2008;79:1091–1095. doi: 10.3357/asem.2301.2008. [DOI] [PubMed] [Google Scholar]
- Blanc S, Normand S, Pachiaudi C, Duvareille M, Gharib C. Leptin responses to physical inactivity induced by simulated weightlessness. Am J Physiol Regul Integr Comp Physiol. 2000;279:R891–R898. doi: 10.1152/ajpregu.2000.279.3.R891. [DOI] [PubMed] [Google Scholar]
- Ksinantova L, Koska J, Kvetnansky R, Marko M, Hamar D, Vigas M. Effect of simulated microgravity on endocrine response to insulin-induced hypoglycemia in physically fit men. Horm Metab Res. 2002;34:155–159. doi: 10.1055/s-2002-23200. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Smith SR, Zachwieja JJ, Bray GA, Windhauser MM, Wickersham PJ, et al. Low-dose T3 improves the bed rest model of simulated weightlessness in men and women. Am J Physiol. 1999;277:E370–E379. doi: 10.1152/ajpendo.1999.277.2.E370. [DOI] [PubMed] [Google Scholar]
- Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37:802–806. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- Belavý DL, Seibel MJ, Roth HJ, Armbrecht G, Rittweger J, Felsenberg D. The effects of bed-rest and countermeasure exercise on the endocrine system in male adults: evidence for immobilization-induced reduction in sex hormone-binding globulin levels. J Endocrinol Invest. 2012;35:54–62. doi: 10.3275/7606. [DOI] [PubMed] [Google Scholar]
- Vernikos J, Dallman MF, Keil LC, O'Hara D, Convertino VA. Gender differences in endocrine responses to posture and 7 days of -6 degrees head-down bed rest. Am J Physiol. 1993;265:E153–E161. doi: 10.1152/ajpendo.1993.265.1.E153. [DOI] [PubMed] [Google Scholar]
- Zorbas YG, Naexu KA, Federenko YF. Blood serum biochemical changes in physically conditioned and unconditioned subjects during bed rest and chronic hyperhydration. Clin Exp Pharmacol Physiol. 1992;19:137–145. doi: 10.1111/j.1440-1681.1992.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E233. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine—immune control in chronic inflammatory diseases. J Intern Med. 2010;267:543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- Uchakin PN, Stowe RP, Paddon-Jones D, Tobin BW, Ferrando AA, Wolfe RR. Cytokine secretion and latent herpes virus reactivation with 28 days of horizontal hypokinesia. Aviat Space Environ Med. 2007;78:608–612. [PubMed] [Google Scholar]
- Crucian BE, Stowe RP, Mehta SK, Yetman DL, Leal MJ, Quiriarte HD, et al. Immune status, latent viral reactivation, and stress during long-duration head-down bed rest. Aviat Space Environ Med. 2009;80:A37–A44. doi: 10.3357/asem.br05.2009. [DOI] [PubMed] [Google Scholar]
- Kelsen J, Bartels LE, Dige A, Hvas CL, Frings-Meuthen P, Boehme G, et al. 21 Days head-down bed rest induces weakening of cell-mediated immunity—some spaceflight findings confirmed in a ground-based analog. Cytokine. 2012;59:403–409. doi: 10.1016/j.cyto.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Belavý DL, Bock O, Börst H, Armbrecht G, Gast U, Degner C, et al. The 2nd Berlin BedRest Study: protocol and implementation. J Musculoskelet Neuron Interact. 2010;10:207–219. [PubMed] [Google Scholar]
- Wolfe BL, LeMura LM, Cole PJ. Quantitative analysis of single- vs. multiple-set programs in resistance training. J Strength Condition Res. 2004;18:35–47. doi: 10.1519/1533-4287(2004)018<0035:qaosvm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Belavý DL, Beller G, Armbrecht G, Perschel FH, Fitzner R, Bock O, et al. Evidence for an additional effect of whole-body vibration above resistive exercise alone in preventing bone loss during prolonged bed-rest. Osteoporosis Int. 2011;22:1581–1591. doi: 10.1007/s00198-010-1371-6. [DOI] [PubMed] [Google Scholar]
- Harle P, Straub RH, Wiest R, Mayer A, Scholmerich J, Atzeni F, et al. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann Rheum Dis. 2006;65:51–66. doi: 10.1136/ard.2005.038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider T, Gunga HC, Matteucci-Gothe R, Sottara E, Griesmacher A, Belavy DL, et al. Effects of long-term head-down-tilt bed rest and different training regimes on the coagulation system of healthy men. Physiol Rep. 2013;1:e00135. doi: 10.1002/phy2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbiato G, Vago T, Battocchio L. Microbial and fungal contamination contributes to physical stress in space flight: studies in the Euromir-95 mission. J Gravit Physiol. 1998;5:P145–P146. [PubMed] [Google Scholar]
- Pierson DL, Stowe RP, Phillips TM, Lugg DJ, Mehta SK. Epstein–Barr virus shedding by astronauts during space flight. Brain Behav Immun. 2005;19:235–242. doi: 10.1016/j.bbi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Reactivation of herpes simplex virus type 1 is associated with cytomegalovirus and age. J Med Virol. 2012;84:1797–1802. doi: 10.1002/jmv.23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt DA, Schaffar L. Isolation and confinement as a model for spaceflight immune changes. J Leukoc Biol. 1993;54:209–213. doi: 10.1002/jlb.54.3.209. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182:1761–1764. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- Errahali YJ, Thomas LD, Keller TC 3 rd, Lee HJ. Inhibition by new glucocorticoid antedrugs [16alpha, 17alpha-d] isoxazoline and [16alpha, 17alpha-d]-3′-hydroxy-iminoformyl isoxazoline derivatives of chemotaxis and CCL26, CCL11, IL-8, and RANTES secretion. J Interferon Cytokine Res. 2013;33:493–507. doi: 10.1089/jir.2012.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly CM, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda EA, et al. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel G, Payne M, Madler B, Ramachandran N, Lecompte M, Wade C, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol. 2009;107:540–548. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- Dent G, Hadjicharalambous C, Yoshikawa T, Handy RL, Powell J, Anderson IK, et al. Contribution of eotaxin-1 to eosinophil chemotactic activity of moderate and severe asthmatic sputum. Am J Respir Crit Care Med. 2004;169:1110–1117. doi: 10.1164/rccm.200306-855OC. [DOI] [PubMed] [Google Scholar]
- Polzer K, Karonitsch T, Neumann T, Eger G, Haberler C, Soleiman A, et al. Eotaxin-3 is involved in Churg–Strauss syndrome—a serum marker closely correlating with disease activity. Rheumatology (Oxford) 2008;47:804–808. doi: 10.1093/rheumatology/ken033. [DOI] [PubMed] [Google Scholar]
- Marques RE, Guabiraba R, Russo RC, Teixeira MM. Targeting CCL5 in inflammation. Expert Opin Ther Targets. 2013;17:1439–1460. doi: 10.1517/14728222.2013.837886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffee N, Richard B, Hlawaty H, Oudar O, Charnaux N, Sutton A. Angiogenic properties of the chemokine RANTES/CCL5. Biochem Soc Trans. 2011;39:1649–1653. doi: 10.1042/BST20110651. [DOI] [PubMed] [Google Scholar]
- Isgro A, Aiuti A, Leti W, Gramiccioni C, Esposito A, Mezzaroma I, et al. Immunodysregulation of HIV disease at bone marrow level. Autoimmun Rev. 2005;4:486–490. doi: 10.1016/j.autrev.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. . J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Booth V, Keizer DW, Kamphuis MB, Clark-Lewis I, Sykes BD. The CXCR3 binding chemokine IP-10/CXCL10: structure and receptor interactions. Biochemistry. 2002;41:10418–10425. doi: 10.1021/bi026020q. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Buford TW, Willoughby DS. Impact of DHEA(S) and cortisol on immune function in aging: a brief review. Appl Physiol Nutr Metab. 2008;33:429–433. doi: 10.1139/H08-013. [DOI] [PubMed] [Google Scholar]
- Dillon JS. Dehydroepiandrosterone, dehydroepiandrosterone sulfate and related steroids: their role in inflammatory, allergic and immunological disorders. Curr Drug Targets Inflamm Allergy. 2005;4:377–385. doi: 10.2174/1568010054022079. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Vandenput L, Tivesten A.DHEA and mortality: What is the nature of the association J Steroid Biochem Mol Biol 2014. in press. [DOI] [PubMed]
- Pluchino N, Drakopoulos P, Bianchi-Demicheli F, Wenger JM, Petignat P, Genazzani AR.Neurobiology of DHEA and effects on sexuality, mood and cognition. J Steroid Biochem Mol Biol 2014. in press. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.