Abstract
Due to their hypoimmunogenicity and unique immunosuppressive properties, mesenchymal stem cells (MSCs) are considered one of the most promising adult stem cell types for cell therapy. Although many studies have shown that MSCs exert therapeutic effects on several acute and subacute conditions, their long-term effects are not confirmed in chronic diseases. Immunogenicity is a major limitation for cell replacement therapy, and it is not well understood in vivo. We evaluated the immunogenicity of allogeneic MSCs in vivo by transplanting MSCs into normal and diabetic rats via the tail vein or pancreas and found that MSCs exhibited low immunogenicity in normal recipients and even exerted some immunosuppressive effects in diabetic rats during the initial phase. However, during the later stage in the pancreas group, MSCs expressed insulin and MHC II, eliciting a strong immune response in the pancreas. Simultaneously, the peripheral blood mononuclear cells in the recipients in the pancreas group were activated, and alloantibodies developed in vivo. Conversely, in the tail vein group, MSCs remained immunoprivileged and displayed immunosuppressive effects in vivo. These data indicate that different transplanting routes and microenvironments can lead to divergent immunogenicity of MSCs.
Keywords: allogeneic mesenchymal stem cells, diabetes, immunogenicity, transplantation routes
Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic, multipotent stromal cells that can differentiate into a variety of cell types and have been investigated as a treatment for a wide range of diseases such as diabetes, myocardial infarction, stroke and various autoimmune diseases because of their self-renewal capacity, as well as their unique immunosuppressive and paracrine properties.1,2,3,4 Historically, MSCs have been considered hypoimmunogenic in vitro because of their limited expression of MHC I, lack of expression of MHC II and costimulatory molecules, inability to stimulate T-cell proliferation and even resistance against the cytotoxicity of cytotoxic lymphocytes.5,6,7 However, MSCs are no longer considered immunologically silent in vivo. Zangi et al.8 demonstrated that MSCs can induce memory against allogeneic MHC I and MHC II molecules in the CD8+ and CD4+ T cells of T-cell antigen receptor transgenic mice. Similarly, Badillo et al.9 showed that the introduction of allogeneic MSCs into an immunologically competent mouse elicits both cellular and humoral host immune responses rather than the induction of tolerance. Schu et al.10 showed that costimulation with IFN-γ and IL-1β induces MSCs to express elevated levels of MHC I, MHC II and VCAM-1 and to significantly increase cytotoxic lysis. Additionally, a recent report11 suggests that the route of delivery may affect MSC immunogenicity in vivo to some extent. The transplantation routes through which allogeneic MSCs appear to be non-immunogenic or very weakly immunogenic include the intra-cranial, intra-cerebral and intra-articular routes, and implantation into skin wounds. In contrast, intravenous, intraperitoneal, subcutaneous and intra-myocardial administration are sometimes associated with detectable anti-donor immunity. The immunosuppressive effects of MSCs are well established, but these findings demonstrate the complexity and variability of MSC immunogenicity in vivo. In the present study, the immunogenicity of allogeneic MSCs in vivo was analyzed by transplantation into normal and diabetic rats via the tail vein or pancreas. The results revealed that MSCs exhibit low immunogenicity and even exert some immunosuppressive effects in diabetic rats during the initial phase (day 1 to day 7). However, during the later stage (day 14 to day 28), MSCs transplanted via the pancreas differentiate into insulin-producing cells and upregulate expression of MHC II, leading to the activation of peripheral blood mononuclear cells (PBMCs) and development of alloantibodies, which leads to the immune rejection of MSCs. In contrast, MSCs transplanted via the tail vein exhibited low immunogenicity in vivo. All of these data suggest that the changes in MSCs immunogenicity in vivo are associated with the transplantation route.
Materials and methods
Animals
Rats were purchased from the Experimental Animal Center of Southern Medical University and housed under specific pathogen-free conditions. All animal procedures were approved by the Institutional Animal Care and Use Committee at Shenzhen PKU-HKUST Medical Center. Four-week-old male Sprague-Dawley (SD) rats were used as MSC donors. Eight-week-old male Wistar rats were used as MSC recipients. Male Wistar rats with an initial body weight of 200–250 g were fasted for 12 h and then intraperitoneally injected with streptozotocin (Sigma, St Louis, MO, USA) at a dose of 45 mg/kg. After 48 h, tail vein blood samples were obtained for blood glucose (BG) measurements using a BG device (Abbott Diabetes Care INC, Abbott Park, Illinois, USA). Rats with a non-fasting BG of ≥300 mg/dl for 2 consecutive days, which was stable for 1 week, were considered diabetic.
Isolation and culture of rat MSCs and GFP labeling
Bone marrow cells were flushed from the cavities of tibias and femurs from male SD rats as described previously.12 The cells were cultured in Iscove's modified Dulbecco's medium (Gibco, Carlsbad, California, USA) with 10% FBS, 100 U/ml penicillin G and 100 µg/ml streptomycin and maintained for 3–5 days in a humidified incubator at 37 °C with 5% CO2. To purify the MSCs, non-adherent cells were eliminated by replacing the medium at 48 h after cell seeding. Flow cytometry on a FACSCanto II (BD Pharmingen, San Diego, CA, USA) was performed to analyze characteristic MSC markers including CD29, CD34, CD44, CD45 and CD11b/c. MSCs were also characterized by differentiation toward adipogenic and osteogenic lineages using previously described protocols.13 The purified MSCs at passage (P)3 were transduced with a lentiviral vector carrying a GFP reporter gene as described previously.14,15
Immune antigen expression in MSCs
The immunophenotype (MHC I, MHC II, CD40, CD80, CD86) of MSCs was examined by flow cytometry on a FACSCanto II (BD Pharmingen, San Diego, CA, USA) with specific phycoerythrin-conjugated monoclonal antibodies (eBioscience, San Diego, CA, USA) and performed as previously described.16 CellQuest software (BD Pharmingen, San Diego, CA, USA) was used for data analysis. Results are expressed as percentage of positive cells or as mean relative fluorescence intensity, obtained as a ratio between the mean fluorescence intensity of cells stained with specific mAb and the mean fluorescence intensity obtained with isotype control.
Lymphocyte proliferation assay
PBMCs as effector cells were isolated from normal and diabetic rats. MSCs pretreated with 25 µg/ml mitomycin C (Roche, Basel, Switzerland) were used as stimulator cells. A total of 1×105 effector cells were cocultured with 1×104 stimulator cells in 96-well U-bottom plates. Effector cells treated with ConA (5 µg/ml; Sigma) were used as a positive control. Autoproliferation of effector cells was used as a negative control. After coculture for 3 days, the proliferation of effector cells was assayed with a cell counting kit (Dojindo, Xiongben, Japan) and the OD at 450 nm was measured with a Bio-Rad 550 microplate reader (Bio-Rad, Hercules, California, USA). The following formula was used to calculate the SI: SI=sample OD/negative control OD.
Cell transplantation
All animals were randomly assigned based on the transplanting route and animal model.
Tail vein transplantation (TVT) groups: (i) Normal+PBS (n=15); (ii) Normal+MSCs (n=15); (iii) Diabetes+PBS (n=15); and (iv) Diabetes+MSCs (n=15); normal/diabetic rats received an injection of PBS (1 ml) or P7 GFP-MSCs (5×106) via the tail vein. Pancreas subcapsular transplantation (PST) groups: (i) Normal+PBS (n=15); (2) Normal+MSCs (n=15); (iii) Diabetes+PBS (n=15); and (iv) Diabetes+MSCs (n=15); normal/diabetic rats received an injection of PBS (1 ml) or P7 GFP-MSCs (5×106) into five distinct sites of the pancreas subcapsule by celiotomy.
Histopathological analyses
The lungs and pancreas were removed from rats in the TVT groups (n=3) and PST groups (n=3), respectively, on days 1, 3, 7, 14 and 28 post-transplantation, and then fixed in 10% buffered formalin. Paraffin-embedded 5-µm sections were routinely stained with hematoxylin and eosin. The hematoxylin and eosin-stained lung sections were evaluated under a light microscope by a histopathological inflammatory scoring system based on the inflammatory infiltration around the bronchioles, bronchi, blood vessels and interstitial pneumonia, as described previously (specific scoring criteria is detailed in Supplementary Table 1).17 The final score per sample was on a scale from 0 to 26 (least to most severe). The hematoxylin and eosin-stained pancreas sections were semiquantitatively analyzed by counting the number of inflammatory cells infiltrating into the pancreas in five high power (×400) fields per sample, as described previously.18
Gene expression analysis of inflammatory cytokines
The lungs and pancreas were obtained from rats in the TVT groups (n=3) and PST groups (n=3), respectively, on days 1, 3, 7, 14 and 28 post-transplantation. The lung and pancreas tissues were frozen in liquid nitrogen, ground and then the total RNA was isolated using TRIzol reagent (TaKaRa, Tokyo Japan). Total RNA was reverse transcribed into cDNA using a PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa) according to the manufacturer's instructions. Quantitative gene expression of IFN-γ, IL-10 and TGF-β was determined using SYBR Green Premix EX Taq (TaKaRa) and performed using standard methods described previously.19 The primer sets were as follows: IFN-γ, forward 5′-cgaggtgaacaacccacagat-3′ and reverse 5′-gactccttttccgctccttag-3′ TGF-β, forward 5′-ctgctgacccccactgatac-3′ and reverse 5′-ctgtattccgtctccttggttc-3′ IL-10, forward 5′-gctatgttgcctgctcttactg-3′ and reverse 5′-tctggctgactgggaagtg-3′ IL-4, forward 5′-caccttgctgtcaccctgt-3′ and reverse 5′-ctttcagtgttgtgagctt-3′ GAPDH, forward 5′-cccatctatgagggttacgc-3′ and reverse 5′-tttaatgtcaccacgatttc-3′.
Cell-mediated lysis assay
At 1, 7, 14 and 28 days post-transplantation, 1×106 PBMCs harvested from the recipients in each group (n=3) were cocultured with 1×105 MSCs for 72 h, and then apoptosis was assessed using an Annexin V-APC Cell Apoptosis Analysis Kit (including 7-AAD staining; Keygen Biotech, Guangzhou, China) according to the manufacturer's instructions. Stained cells were analyzed by a FACSCanto II (BD Pharmingen, San Diego, CA, USA). MSCs cultured alone were used as controls.
Alloantibody analysis
At 7, 14 and 28 days post-transplantation, blood was collected from the rats in each group (n=3) via a cardiac puncture. The blood was coagulated and centrifuged to obtain the serum. Cultured allogeneic MSCs at P7 were fixed with 4% paraformaldehyde, washed, blocked and then incubated with the serum samples (diluted at 1∶2 in PBS) at 4 °C overnight. The cells were carefully washed and then immunostained with a RPE-conjugated anti-rat IgG1 antibody (1∶200; SoutherBiotech, Birmingham, Alabama, USA) for 1 h at room temperature. The nuclei were counterstained with DAPI (1∶800; Sigma).
Immunofluorescence staining for PCNA, CD86, MHC II and insulin
Rats were euthanized at 7, 14 and 28 days post-transplantation to remove their lungs and pancreas. Frozen tissue blocks were embedded in O.C.T. compound (SAKURA Tissue-Tek, Pennsylvania, USA). Sections (5 µm) were then prepared for immunofluorescence staining. Frozen tissue sections were fixed in 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. The sections were then incubated with a rabbit anti-GFP antibody (1∶800; Abcam, Cambridge, UK) either alone or with mouse anti-rat PCNA (GeneTex, Alton PkwyIrvine, CA, USA), mouse anti-rat MHC II (1∶200; eBioscience), mouse anti-rat CD86 (1∶200; eBioscience) or mouse anti-rat insulin (1∶400; Abcam) antibodies at 4 °C overnight. Then, the sections were incubated with RPE-conjugated goat anti-mouse IgG (1∶200; SoutherBiotech) and/or FITC-conjugated goat anti-rabbit IgG (1∶200; SoutherBiotech). The nuclei were counterstained with DAPI (1∶800), and the cells were visualized under a fluorescence microscope.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
Paraffin-embedded tissue sections of the lungs and the pancreas were subjected to a TUNEL assay. An in situ apoptotic cell death detection kit (Roche, Basel, Switzerland) was used to detect apoptotic cells following the manufacturer's instructions. The sections were also stained with a rabbit anti-GFP (1∶800; Abcam) primary antibody and Cy3-labeled goat anti-rabbit IgG (H+L) (1∶100; Earthox LLC, San Francisco, USA) secondary antibody. Nuclei were counterstained with DAPI. The fluorescence signals of the transplanted MSCs, including apoptotic MSCs, were observed under a fluorescence microscope. The average number of apoptotic MSCs were counted in five high power (×400) fields per sample.
Assessment of the curative effect of MSC transplantation
Non-fasting BG levels were measured by a BG device at 4:00 p.m. every 3 days post-transplantation until day 30, and the weight of each recipient was monitored at the same time. On days 3, 7, 14 and 28 post-transplantation, the serum of each recipient was collected to measure the insulin and C-peptide levels using Rat Insulin and Rat C-peptide ELISA kits (Mercodia, Uppsala, Sweden), respectively, according to the manufacturer's instructions. On days 14 and 28 post-transplantation, recipients in each group underwent fasting for 6–8 h and then were intraperitoneally injected with a 10% glucose solution (2 g/kg). Tail vein blood samples were collected for BG measurements at 0, 15, 30, 60, 120 and 180 min.
Statistical analysis
All data are expressed as the mean±s.d. Statistical analysis was performed using one-way ANOVA to compare the difference among different groups. Independent sample t-tests were used to statistically compare the number of cells between the tail vein and pancreas groups. BG levels were compared using repeated-measures analysis of variance. Values of P<0.05 were considered to be significant.
Results
Isolation, differentiation and immunophenotyping of MSCs
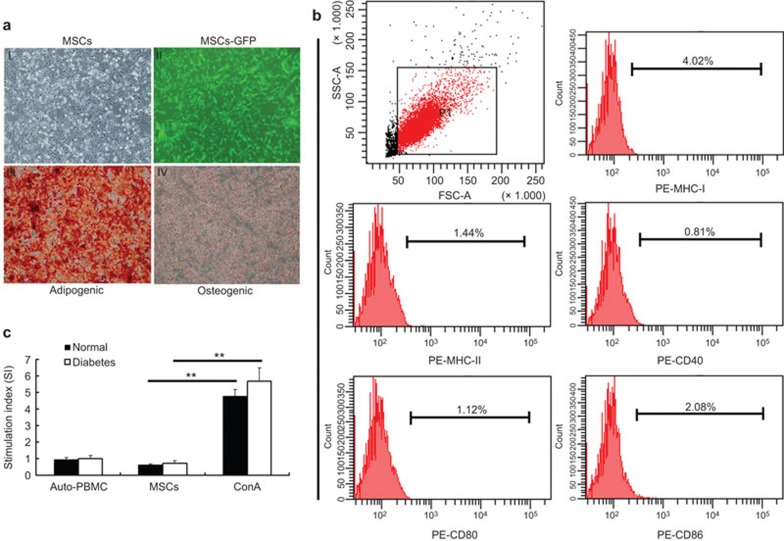
After purification, MSCs were spindle-shaped with a fibroblast-like morphology and attached to the plate during cell culture. Flow cytometric analysis for the immunophenotypic characterization of MSCs demonstrated that these cells were positive for CD29 (99.88%) and CD44 (99.83%), but negative for CD11b/c (0.60%), CD34 (1.06%) and CD45 (1.77%) (data not shown). After Ad-GFP transduction for 48 h, flow cytometric analysis showed that GFP-positive cells accounted for 92.6% of the cell population (data not shown). After 21 days of adipogenic induction, the cells were stained positive for Oil Red O, showing lipid-filled vesicles. After 21 days of osteogenic induction, these cells displayed osteogenesis, as shown by Alizarin red staining of calcium deposits (Figure 1a). These results demonstrated that the MSCs had multipotency.
Figure 1.
Adipogenesis and osteogenesis of MSCs and immunogenicity testing in vitro. Cells were spindle-shaped after three passages and transduction with a GFP expression vector (aI and II). MSCs were then cultured in appropriate induction media for 3 weeks. Adipogenesis was confirmed by Oil Red O staining and osteogenesis was confirmed by Alizarin red staining (aIII and IV, respectively). FACS showed that P7 MSCs expressed a low level of MHC I and did not express MHC II, CD40, CD80 or CD86 (b). PBMCs from normal and diabetic rats were cocultured with MSCs for 72 h. No proliferation was observed in the MSC group compared with the autoproliferation and ConA (positive) group (c). **P<0.01. MSC, mesenchymal stem cell; PBMC, peripheral blood mononuclear cell.
Immune antigen expression on MSCs and lymphocyte proliferation assay in vitro
FACS revealed that the MSCs expressed low levels of MHC I and no MHC II, CD40, CD80 or CD86 in vitro (Figure 1b). Under coculture conditions, the stimulation index of PBMCs from normal and diabetic rats after coculture with MSCs was 0.61±0.08 and 0.71±0.16, respectively (Figure 1c), which was significantly different compared with the positive control group (ConA) (P<0.01), while no difference was observed with the negative control group (auto-PBMC, P>0.05). These data indicated that the MSCs used in this study had low immunogenicity in vitro.
Histopathological analysis of lungs and pancreas
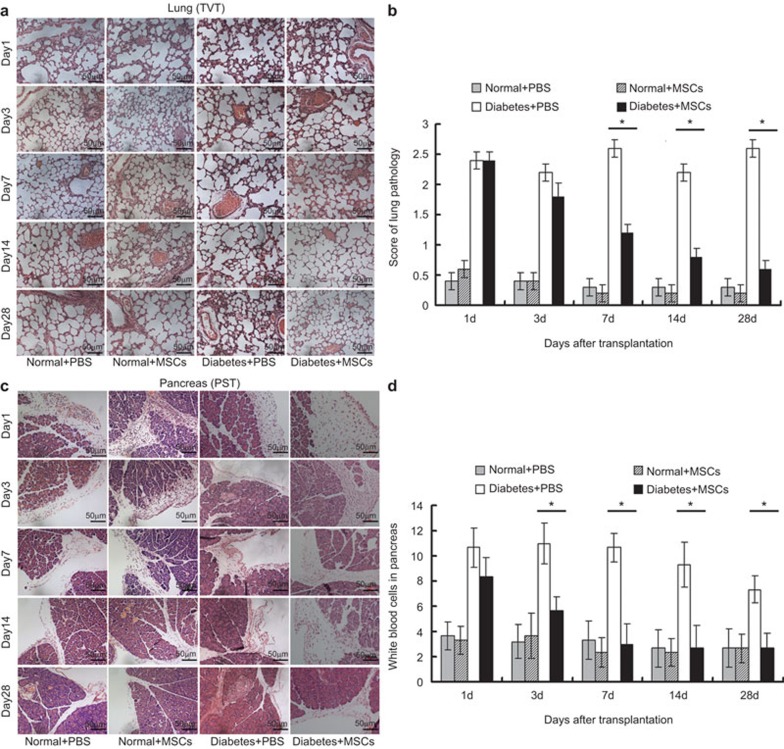
In TVT groups, histopathological examination of the lungs showed no changes in normal rats (Normal+PBS and Normal+MSCs), but obvious changes were observed in diabetic rats (Diabetes+PBS and Diabetes+MSCs). The interstitial spaces of the alveoli became thick with polymorphonuclear leukocyte infiltration, and edematous changes of the alveolar walls were observed in the Diabetes+PBS group (Figure 2a). All of these changes returned to normal after MSC administration, especially on day 14 post-transplantation. The histopathological inflammatory scoring system (Supplementary Table 1) shows that the scores for the lungs in the Normal+PBS and Normal+MSCs groups were relatively low. In contrast, the scores for the lungs in the Diabetes+PBS and Diabetes+MSCs groups were higher. Thereafter, the scores decreased markedly at 7, 14 and 28 days post-transplantation in the Diabetes+MSCs group due to a significant reduction of inflammatory cell infiltration around the pulmonary perivascular region (Figure 2b). In the PST groups, the histopathological features of the pancreas were evaluated using a semiquantitative method, where the number of inflammatory cells infiltrating the pancreas was counted (Figure 2c). We found that there was no significant difference between the Normal+PBS and Normal+MSCs groups, while the number of inflammatory cells was significantly decreased on days 3, 7, 14 and 28 in the Diabetes+MSCs group compared to the Diabetes+PBS group (Figure 2d). These data suggest that the inflammatory events in the pancreas were downregulated by MSC transplantation.
Figure 2.
Histopathological analyses of the lung in the TVT groups and the pancreas in the PST groups (hematoxylin and eosin stain, ×200 magnification). Representative images of the lung (a) and histopathological inflammatory scores (b) at various time points in the TVT groups. Representative images of the transplant site and white blood cell counts in the pancreas subcapsular region in five high power fields (×400) per sample at different time points in the PST groups (c, d). *P<0.05. MSC, mesenchymal stem cell; PST, pancreas subcapsular transplantation; TVT, tail vein transplantation.
Quantitative real-time PCR analysis of inflammatory cytokine mRNA levels
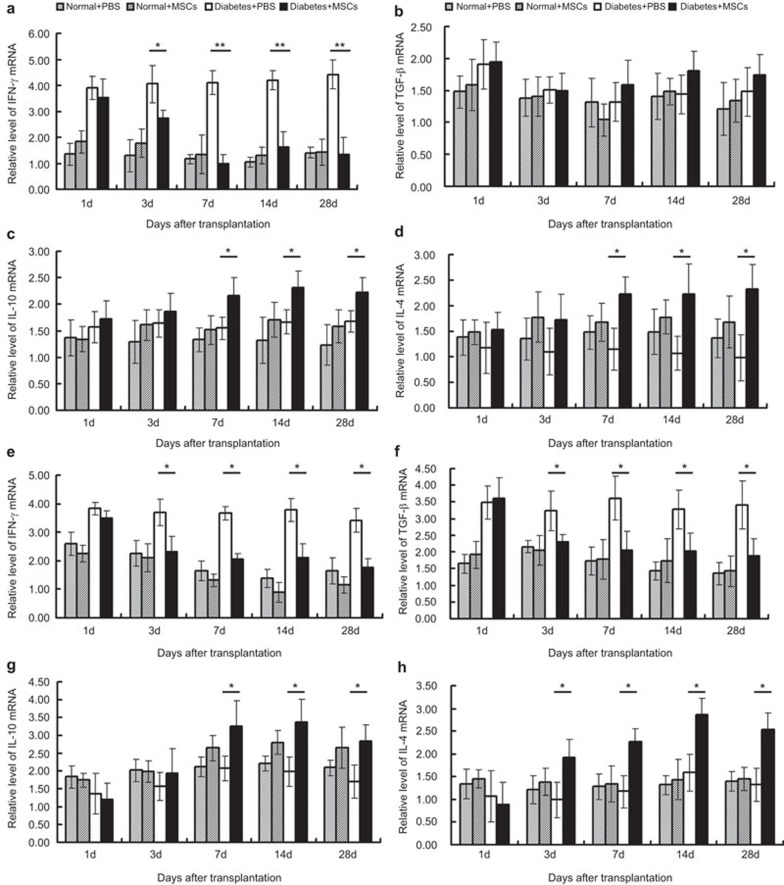
To further investigate the inflammatory responses in the lungs and pancreas, quantitative real-time PCR was performed to analyze the expression of inflammatory cytokine (IFN-γ, TGF-β, IL-10 and IL-4) mRNA. In the TVT groups, no significant difference on the expression of four cytokines mRNA was found between the Normal+PBS and Normal+MSCs groups. In the Diabetes+ MSCs group compared with the Diabetes+PBS group, IFN-γ mRNA expression was downregulated on day 3, and the trend lasted to the end of the study period. Unexpectedly, TGF-β mRNA expression was not changed significantly. IL-10 and IL-4 mRNA expression was upregulated on days 7, 14 and 28 (Figure 3a–d).
Figure 3.
mRNA levels of inflammatory cytokines in the lung and pancreas. The lungs (a–d) and pancreas (e–h) were obtained from rats in the TVT and PST groups, respectively, at 1, 3, 7, 14 and 28 days post-transplantation. Total mRNA was extracted to evaluate the expression of inflammatory cytokines (IFN-γ, TGF-β, IL-10 and IL-4) by qRT-PCR. *P<0.05; **P<0.01. MSC, mesenchymal stem cell; PST, pancreas subcapsular transplantation; qRT-PCR, quantitative real-time PCR; TVT, tail vein transplantation.
In the PST groups, similarly, the expression of the mRNA for the four cytokines was not changed significantly in the Normal+PBS and Normal+MSCs groups at any time point of the study. In the Diabetes+MSCs group, IFN-γ and TGF-β mRNA expression was downregulated on day 3 compared with the Diabetes+PBS group, and the downward trend was maintained to day 28. The expression of IL-4 mRNA increased significantly at the same time, while the expression of IL-10 mRNA was upregulated on days 7, 14 and 28. These data indicated that the MSCs did not elicit an inflammatory response in normal rats, but displayed certain immunosuppressive effects in diabetic rats (Figure 3e–h).
Cell-mediated lysis
To determine the cytotoxicity of PBMCs isolated from rats treated with MSCs at various time points, we cocultured PBMCs with MSCs and evaluated the cytolytic capacity of MSCs by flow cytometry. In the TVT groups, the degree of cytolysis elicited by PBMCs from the Normal+MSCs and Diabetes+MSCs groups was similarly low (P>0.05) throughout the study and not significantly different from the control group (P>0.05). However, on days 14 and 28, a much higher level of MSC cytolysis was observed in the PST (Normal+MSCs and Diabetes+MSCs) groups compared with the control group (P<0.01). This result indicates the activation of the PBMCs in the PST groups at 14 and 28 days post-transplantation (Figure 4a).
Figure 4.
Cytotoxicity of PBMCs toward MSCs and alloantibody production in the serum at various time points post-transplantation. PBMCs collected from recipients of each group on days 1, 7, 14 and 28 post-transplantation were considered as effector cells, and then cocultured with MSCs (target cell to effector cell ratio: 1∶10) for 72 h. PBMCs from the TVT group (Normal+PBS) as control. Percentage of apoptotic MSCs were evaluated by annexin V-APC/7-AAD staining and flow cytometry (a). **P<0.01. Peripheral blood serum was collected from recipients on days 7, 14 and 28 post-transplantation, and then incubated with MSCs. Incubated samples were stained with a RPE-conjugated secondary antibody against rat IgG1. Micrographs illustrate the IgG1 antibody in the MSC-treated pancreas group on days 14 and 28 (b). MSC: mesenchymal stem cell; PBMC: peripheral blood mononuclear cell; TVT: tail vein transplantation.
The production of alloantibodies after MSC transplantation
To investigate whether allogeneic MSCs elicit an antibody response in vivo, we collected serum from the MSC-treated rats on days 7, 14 and 28. Alloantibodies against the donor MSCs in the circulation of the recipient were detected by immunostaining. In the TVT groups, no detectable alloantibodies were found in the serum of recipients. In the PST groups, a specific anti-donor alloantibody (IgG1) against MSCs appeared in the serum on days 14 and 28, while it was negative on day 7 (Figure 4b). These results suggest that an antibody response occurred at 14 and 28 days post-transplantation in the PST groups.
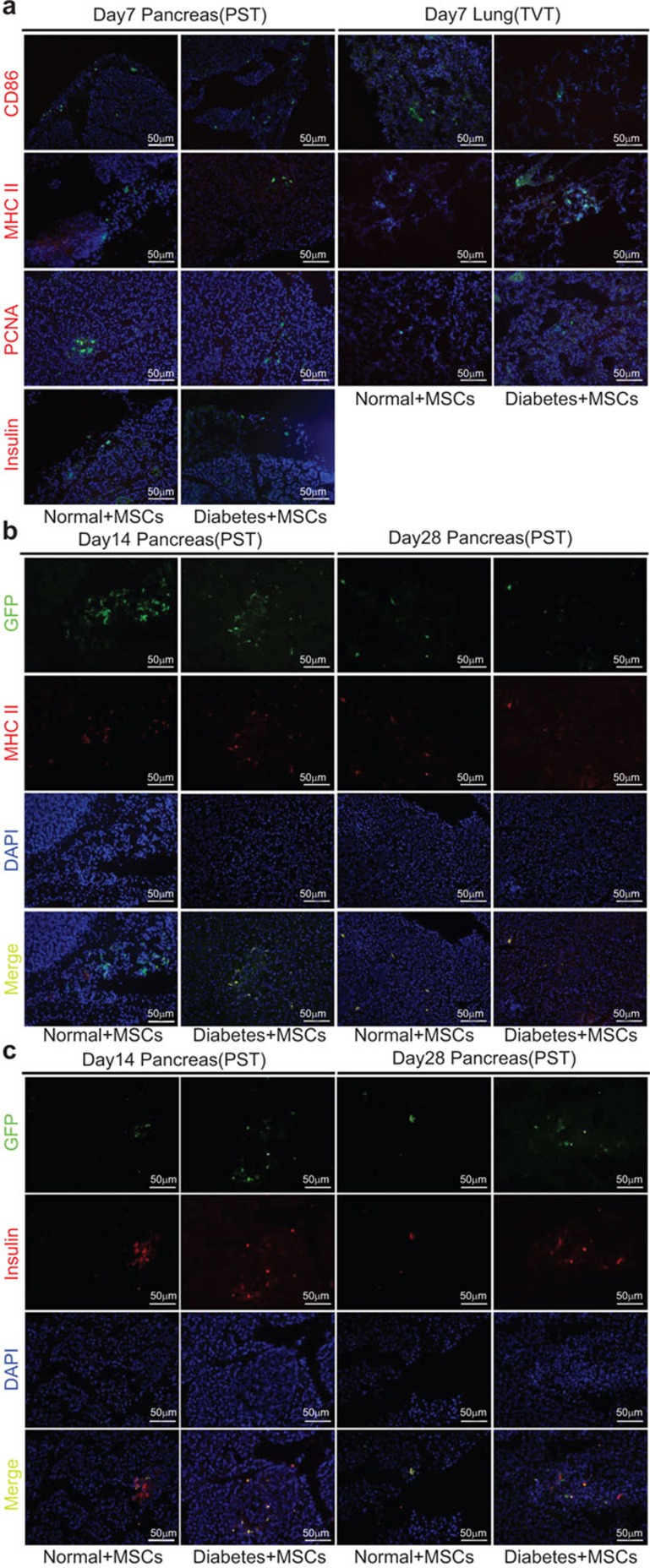
Immunofluorescence staining for PCNA, insulin, MHC II and CD86
The fate of MSCs in vivo was analyzed by double immunofluorescence with antibodies against GFP and proliferation (PCNA), differentiation (insulin), as well as immune antigen markers (MHC II and CD86) in the lungs and pancreas at 7, 14 and 28 days post-transplantation. On day 7, in both the TVT group and the PST group, GFP+ MSCs did not express any markers, indicating a lack of differentiation and proliferation, and appeared to exhibit low immunogenicity at this time point, as there was no observed immune antigen expression (Figure 5a). On days 14 and 28, GFP+ MSCs were positive for MHC II and insulin (Figure 5b and c), but negative for PCNA and CD86 (data not shown) in the pancreas of the PST groups, but not in the lungs of the TVT groups, suggesting that MSCs can differentiate into insulin-producing cells and upregulate MHC II expression in the pancreas.
Figure 5.
Immunofluorescence staining of differentiation, proliferation, and immune antigen marker expression of transplanted MSCs. Rats were euthanized at 7, 14 and 28 days post-transplantation and their lungs and pancreas were obtained in the TVT and PST groups, respectively. Representative micrographs illustrate the expression of GFP (green, transplanted cells), immune antigens (red, CD86 and MHC II), a proliferation marker (red, PCNA) and a differentiation marker (red, insulin) in the lung and pancreas tissues of recipients. GFP+ MSCs were visible in all samples. By day 7, the expression of CD86, MHC II, PCNA and insulin was negative (a). However, expression of MHC II (b) and insulin (c) was observed at 14 and 28 days post-transplantation. Blue indicates nuclear staining with DAPI. MSC, mesenchymal stem cell; PST, pancreas subcapsular transplantation; TVT, tail vein transplantation.
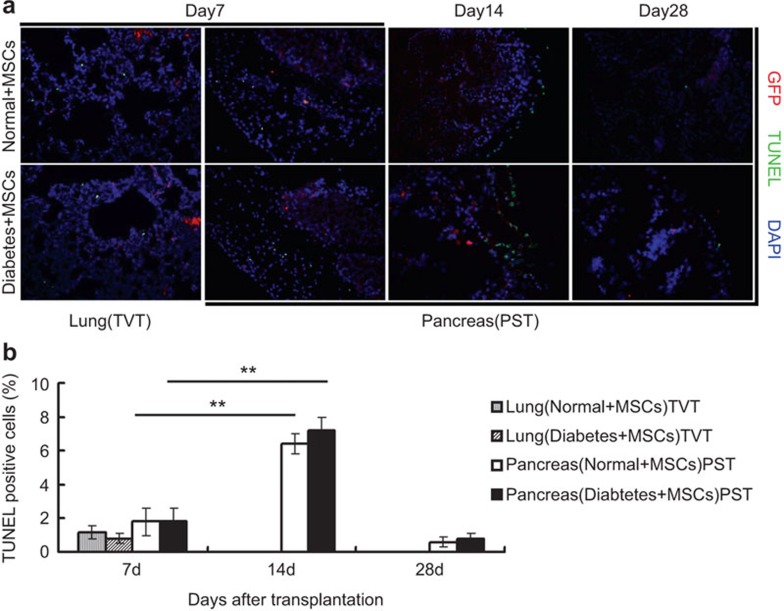
Apoptosis of MSCs in vivo
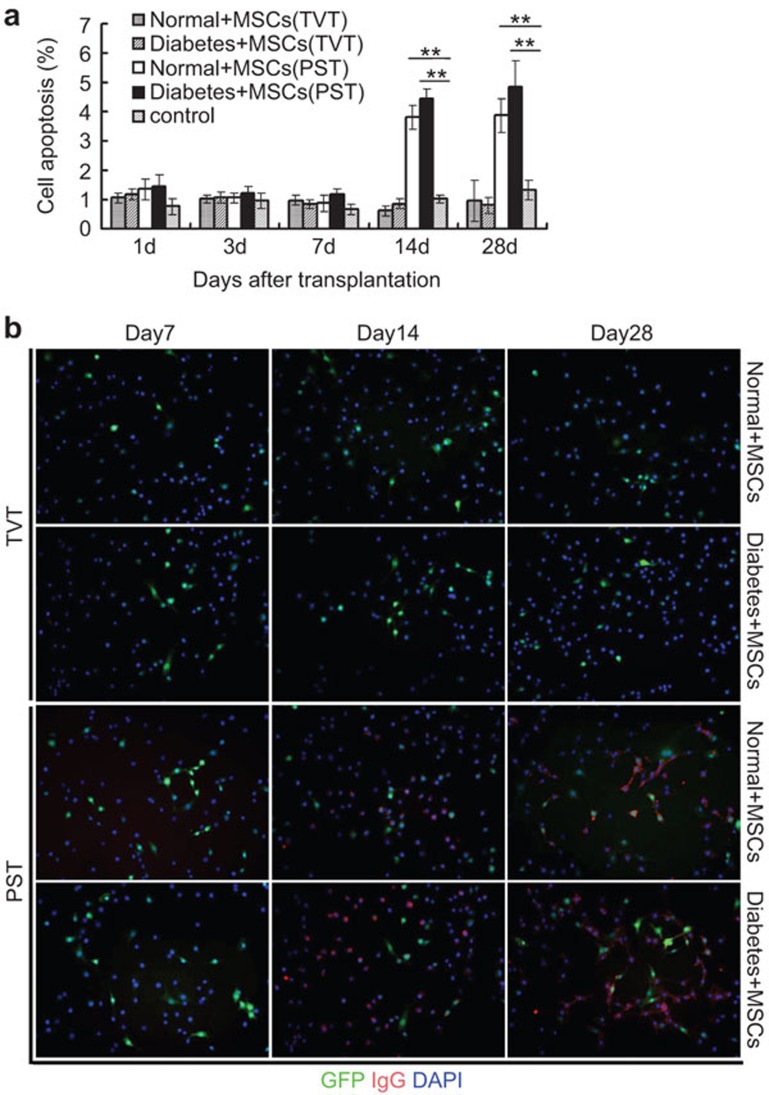
On days 7, 14 and 28 post-transplantation, TUNEL assays were performed to evaluate MSC apoptosis in vivo. In the TVT groups, we observed only a few GFP+ cells with TUNEL staining by day 7 (Figure 6a), while no detectable GFP+ MSCs were observed in the lungs at 14 and 28 days post-transplantation (data not shown). In PST groups, similarly, only a few GFP+ cells with TUNEL staining were found on day 7. However, the number of GFP and TUNEL double-positive cells significantly increased by day 14 compared with that on day 7 (P<0.01), but fewer GFP+ cells were detectable by day 28 in the pancreas (Figure 6a and b).
Figure 6.
Apoptosis of MSCs in the lung and pancreas. On days 7, 14 and 28 post-transplantation, the lungs and pancreas were obtained from rats in the TVT and PST groups. Numerous MSCs (red) were positive for DNA fragmentation (green, TUNEL staining), indicating apoptotic death of the transplanted cells (a). GFP+ cells stained with TUNEL were counted in five high-power fields (×400) (b). **P<0.01. MSC, mesenchymal stem cell; PST, pancreas subcapsular transplantation; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; TVT, tail vein transplantation.
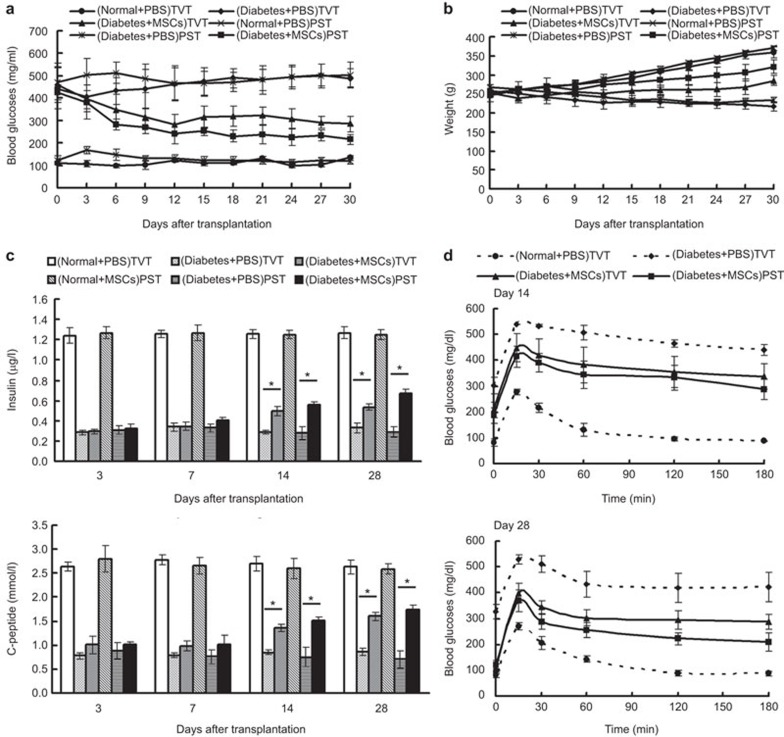
Reversal of hyperglycemia in streptozotocin-induced diabetic rats
After MSC administration, the BG levels in the TVT (Diabetes+MSCs) and PST (Diabetes+MSCs) groups declined gradually compared to the Diabetes+PBS group. In the TVT group, the BG levels dropped rapidly and decreased to 280.8±45.4 mg/dl by day 12, followed by a BG fluctuation, and reached 285.4±33.8 mg/dl by day 30. In the PST group, BG levels dropped relatively steadily and declined until reaching almost euglycemic values (215.8±22.4 mg/dl) by day 30 (Figure 7a). The BG in the PST group was obviously lower than that in the TVT groups by day 18 (P<0.05). The weight of diabetic rats was improved to some extent in both MSC-treated groups by the end of the study (Figure 7b). In addition, the levels of insulin and C-peptide in the serum gradually increased post-transplantation. On day 14, the levels of insulin and C-peptide in the TVT group and PST group were significantly increased compared with those in the Diabetes+PBS group (P<0.05), but no significant difference was observed between the TVT and PST group (P>0.05). On day 28, the levels of insulin and C-peptide were increased further. In addition, the insulin level in the PST group (0.67±0.04 µg/l) increased significantly compared with that in the TVT group (0.54±0.03 µg/l) (P<0.05) (Figure 7c). In the intraperitoneal glucose tolerance test on day 14, the BG levels were slightly decreased after glucose stimulation in both MSC-treated groups. On day 28, compared with the Diabetes+PBS rats, the BG levels dropped rapidly after 15 min of glucose stimulation, and the degree of BG decline was much more obvious in the PST group (Figure 7d). All of these data show that the MSCs improved the conditions of diabetes, and it appears that the therapeutic effect in the PST group was more obvious than that in TVT group.
Figure 7.
Therapeutic effects of MSCs on diabetic rats. Diabetic rats received 5×106 MSCs via tail vein injection or pancreas subcapsular infusion. The BG levels (a) and weight (b) were assessed every 3 days. The levels of insulin and C-peptide in the serum were determined on days 3, 7, 14 and 28 post-transplantation (c). Glucose tolerance testing was performed on days 14 and 28 post-transplantation (d). BG, blood glucose; MSC, mesenchymal stem cell.
Discussion
MSCs are considered to be immunoprivileged and may escape the attack of the immune system. Due to their hypoimmunogenicity, immunosuppressive and paracrine properties, MSCs are used as a ‘universal donor' in cell replacement therapy. Although many studies have shown that MSCs exert therapeutic benefits in several acute and subacute conditions, the long-term effects have not been confirmed in chronic disease. The reason for this knowledge gap might be the change in the immunoprivileged state in vivo, which leads to the failure of MSCs to survive in the host. As multipotent stem cells, the immunogenicity of MSCs may be altered in different microenvironments (such as hypoxia, low nutrition, hyperglycemia, inflammation, acidic pH, mechanical loading, and osmotic conditions).20,21,22 In the present study, we analyzed the immunogenicity of allogeneic MSCs in vivo by transplanting MSCs into normal and diabetic rats via the tail vein or pancreas and investigated the possibilities of these modalities for the therapeutic treatment of diabetes.
As a precursor stem cell, the phenotype of MSCs is immature, and they express low levels of MHC I and lack expression of MHC II and costimulatory molecules (CD40, CD80, CD86) in the quiescent state. Our data showed that the cultured MSCs expressed low MHC I and expressed no MHC II, CD40, CD80 and CD86, implying that MSCs lack the ability to present alloantigens directly to recipient CD4+ T cells. Lymphocyte proliferation would be the expected outcome after successful presentation. However, no lymphocyte proliferation was observed in our study. Thus, even with the assistance of antigen presenting cells, MSCs did not activate allogeneic CD4+ T cells to elicit an immune response in vitro. These data are consistent with previous reports showing that MSCs lack immunogenic antigens in vitro.23,24
It has been demonstrated that the structure and function of the lungs may change in diabetic rats induced by Streptozotocin (STZ); a decline of pulmonary diffusion function has been observed, and these rats are prone to pulmonary fibrosis and concurrent infection.25 Additionally, macrophages first invade the pancreatic parenchyma and destroy islet β cells, supposedly by the release of IL-1, which induces free radical formation, leading to a shortage of insulin.26,27 In our study, we found that the inflammatory score of lung and inflammatory cell infiltration into the pancreas of diabetes rats was much higher than that in normal rats and rapidly decreased after MSC administration. Similarly, the expression of pro-inflammatory factors (IFN-γ, TGF-β) was downregulated, while anti-inflammatory factors (IL-4, IL-10) were upregulated both in the lung (TVT groups) and in the pancreas (PST groups) after MSC transplantation. These results suggest that MSCs may shift the balance of T-cell subsets from pro-inflammatory to anti-inflammatory, as described previously.28 According to Liu et al.,29 these immunomodulatory effects may be due to MSC secretion of various cytokines, especially KGF, to modulate the thymic microenvironment. These immunological changes may contribute to the reduction of inflammatory injury of β cells and restored BG homeostasis, although other mechanisms may be involved in the process, such as the secretion of bioactive factors (IL-6, HGF, TGF-β1, SDF-1, VEGF, IL-1β, PGE) and reduction of systemic oxidative stress.30,31
A previous study demonstrated that allogeneic MSCs can escape host immune surveillance in vivo.32 In our study, in the TVT (Normal+MSCs and Diabetes+MSCs) groups, the cell-mediated lysis assay showed that PBMCs have minimal cytotoxicity against MSCs, and no alloantibody that reacted with MSCs was observed in the serum by immunostaining. These data indicate that MSCs may not activate the recipients' immune system. While we cannot find MSCs in the lungs at day 14 post-transplantation in the TVT groups, this phenomenon may explained by the ‘first-pass effect' theory, which has been demonstrated by previous research;33,34,35 namely, a large number of MSCs are entrapped in the lung and fail to survive, and only a few cells can pass the endovascular barrier to play a therapeutic role. In the PST groups (Normal+MSCs and Diabetes+MSCs), we discovered insulin expression on MSCs by days 14 and 28. This observation suggests that the MSCs differentiated into insulin-producing cells in the pancreas, which is supported by previous studies.36,37 However, another mechanism, whereby MSCs injected into the pancreatic tissue fuse to pancreatic cells, may also be involved in the process, as reported by Ferrand et al.38 Our immunostaining results showed that MHC II expression was positive on MSCs. These data suggest that MSCs transition from an immunoprivileged state to an immunogenic phenotype that may trigger cellular cytotoxicity or immune rejection. As expected, PBMCs in the PST groups had a much higher level of cytotoxicity toward MSCs, and alloantibodies were detected in the serum compared with that in the TVT groups. Moreover, TUNEL staining showed that MSCs undergo extensive apoptosis at day 14. These data are in accordance with previous reports that differentiation initiates an immune ‘switch' that alters the immune characteristics of MSCs.39
The pancreas islet function was obviously improved after MSC transplantation via two routes, which was evidenced by the corrected hyperglycemia, increased secretion of insulin and C-peptide in the serum, and improvement in the glucose tolerance test. As our previous study showed the existence of new islets in the pancreas and reversal of hyperglycemia after MSCs were transplanted into the pancreatic subcapsular region,40 the therapeutic effects in our study in the PST groups was much better than those in the TVT groups. This distinction may be explained by the different quantity of MSCs residing in the pancreas. In the TVT groups, a large number of MSCs were entrapped in the lung, and limited cells were able to bypass the endovascular barrier and reach the pancreas. In the PST groups, more MSCs were located in the pancreas, and the pancreas microenvironment can directly stimulate MSCs to secrete trophic factors exerting repair effects, promoting differentiation and immunomodulatory effects,41,42 although immune privilege is lost by the expression of MHC II.
In conclusion, we analyzed the immunogenicity of allogeneic MSCs in vivo by transplanting MSCs into normal and diabetic rats via the tail vein or pancreas. We found that MSCs had low immunogenicity in normal recipients and even exerted some immunosuppressive functions in diabetic rats during the initial phase. However, during the later stage, with the expression of insulin, MSCs upregulated the expression of MHC II to elicit a strong antigen antibody reaction that resulted in immunological rejection in the PST groups. In contrast, MSCs transplanted via the tail vein remained immunoprivileged. These data indicate that different transplantation routes and microenvironments can lead to changes in the immunogenicity of MSCs.
Acknowledgments
We thank our colleagues Ya-Ying Zhou, Chun-Yan Deng and Li-Li Ren for their technical assistance. This work was supported by the National Natural Science Foundation of China (No. 81270857), the Natural Science Foundation of Guangdong (No. S2013010014832), and the Science and Technology Project of Shenzhen (JCYJ20120618153743791, GJHZ20120618153934353).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- DelaRosa O, Dalemans W, Lombardo E. Mesenchymal stem cells as therapeutic agents of inflammatory and autoimmune diseases. Curr Opin Biotechnol. 2012;23:978–983. doi: 10.1016/j.copbio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Liu N, Zhang Y, Fan L, Yuan MZ, Du HW, Cheng RH, et al. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med. 2011;9:105. doi: 10.1186/1479-5876-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transpl. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Vassalli G, Moccetti T. Cardiac repair with allogeneic mesenchymal stem cells after myocardial infarction. Swiss Med Wkly. 2011;141:w13209. doi: 10.4414/smw.2011.13209. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783–790. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation. Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Zangi L, Margalit R, Reich-Zeliger S, Carpio D, Yañez AJ, Conget PA. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27:2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- Badillo AT, Beggs KJ, Javazon EH, Tebbets JC, Flake AW. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol Blood Marrow Transpl. 2007;13:412–422. doi: 10.1016/j.bbmt.2006.12.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu S, Nosov M, O'Flynn L, Shaw G, Treacy O, Barry F, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21:1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- Zhang H, Fazel S, Tian H, Mickle DA, Weisel RD, Fujii T, et al. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005;289:H2089–H2096. doi: 10.1152/ajpheart.00019.2005. [DOI] [PubMed] [Google Scholar]
- Guo Y, Su L, Wu J, Zhang D, Zhang X, Zhang G, et al. Assessment of the green florescence protein labeling method for tracking implanted mesenchymal stem cells. Cytotechnology. 2012;64:391–401. doi: 10.1007/s10616-011-9417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M, Soleimani M, Taghvaie NM, Sheikhfatollahi M, Azadmanesh K, Lotfi AS, et al. Mesenchymal stem cells as vehicles for targeted delivery of anti-angiogenic protein to solid tumors. J Gene Med. 2011;13:171–180. doi: 10.1002/jgm.1552. [DOI] [PubMed] [Google Scholar]
- Bai X, Pinkernell K, Song YH, Nabzdyk C, Reiser J, Alt E. Genetically selected stem cells from human adipose tissue express cardiac markers. Biochem Biophys Res Commun. 2007;353:665–671. doi: 10.1016/j.bbrc.2006.12.103. [DOI] [PubMed] [Google Scholar]
- Morandi F, Levreri I, Bocca P, Galleni B, Raffaghello L, Ferrone S, et al. Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release. Cancer Res. 2007;67:6433–6441. doi: 10.1158/0008-5472.CAN-06-4588. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol. 2001;24:577–582. doi: 10.1165/ajrcmb.24.5.4315. [DOI] [PubMed] [Google Scholar]
- Ye J, Liao YT, Jian YQ, Zhang XD, Wei P, Qi H, et al. Alpha-1-antitrypsin for the improvement of autoimmunity and allograft rejection in beta cell transplantation. Immunol Lett. 2013;150:61–68. doi: 10.1016/j.imlet.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WL, Jia ZQ, Wang WP, Zhang JY, Fu X, Duan XN, et al. Proliferation and apoptosis property of mesenchymal stem cells derived from peripheral blood under the culture conditions of hypoxia and serum deprivation. Chin Med J. 2011;124:3959–3967. [PubMed] [Google Scholar]
- Dhanasekaran M, Indumathi S, Rajkumar JS, Sudarsanam D. Effect of high glucose on extensive culturing of mesenchymal stem cells derived from subcutaneous fat, omentum fat and bone marrow. Cell Biochem Funct. 2013;31:20–29. doi: 10.1002/cbf.2851. [DOI] [PubMed] [Google Scholar]
- Huang YC, Leung VY, Lu WW, Luk KD. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J. 2013;13:352–362. doi: 10.1016/j.spinee.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Yuan T, Li K, Guo L, Fan H, Zhang X. Modulation of immunological properties of allogeneic mesenchymal stem cells by collagen scaffolds in cartilage tissue engineering. J Biomed Mater Res Part A. 2011;98:332–341. doi: 10.1002/jbm.a.33121. [DOI] [PubMed] [Google Scholar]
- Jia Z, Jiao C, Zhao S, Li X, Ren X, Zhang L, et al. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res. 2012;102:44–49. doi: 10.1016/j.exer.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Forgiarini LA, Jr, Kretzmann NA, Porawski M, Dias AS, Marroni NA. Experimental diabetes mellitus: oxidative stress and changes in lung structure. J Bras Pneumol. 2009;35:788–791. doi: 10.1590/s1806-37132009000800011. [DOI] [PubMed] [Google Scholar]
- Sassy-Prigent C, Heudes D, Mandet C, Bélair MF, Michel O, Perdereau B, et al. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- Papaccio G, Frascatore S, Esposito V, Pisanti FA. Early macrophage infiltration in mice treated with low-dose streptozocin decreases islet superoxide dismutase levels: prevention by silica pretreatment. Acta anatomica. 1991;142:141–146. doi: 10.1159/000147179. [DOI] [PubMed] [Google Scholar]
- Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, Conget P. MSC anti-diabetic effect is unrelated to their trans-differentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 2012;30:1664–1674. doi: 10.1002/stem.1132. [DOI] [PubMed] [Google Scholar]
- Liu G, Wang L, Pang T, Zhu D, Xu Y, Wang H, et al. Umbilical cord-derived mesenchymal stem cells regulate thymic epithelial cell development and function in Foxn1−/− mice. Cell Mol Immunol. 2014;11:275–284. doi: 10.1038/cmi.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AH, et al. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 2010;89:509–517. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- Ho JH, Tseng TC, Ma WH, Ong WK, Chen YF, Chen MH, et al. Multiple intravenous transplantations of mesenchymal stem cells effectively restore long-term blood glucose homeostasis by hepatic engraftment and beta-cell differentiation in streptozocin-induced diabetic mice. Cell Transplant. 2012;21:997–1009. doi: 10.3727/096368911X603611. [DOI] [PubMed] [Google Scholar]
- Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Lee J, Han DJ, Kim SC. In vitro differentiation of human adipose tissue-derived stem cells into cells with pancreatic phenotype by regenerating pancreas extract. Biochem Biophys Res Commun. 2008;375:547–551. doi: 10.1016/j.bbrc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- Xu YX, Chen L, Hou WK, Lin P, Sun L, Sun Y, et al. Mesenchymal stem cells treated with rat pancreatic extract secrete cytokines that improve the glycometabolism of diabetic rats. Transplant Proc. 2009;41:1878–1884. doi: 10.1016/j.transproceed.2009.01.087. [DOI] [PubMed] [Google Scholar]
- Ferrand J, Noel D, Lehours P, Prochazkova-Carlotti M, Chambonnier L, Ménard A, et al. Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PLoS One. 2011;6:e19569. doi: 10.1371/journal.pone.0019569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- Chang C, Niu D, Zhou H, Zhang Y, Li F, Gong F. Mesenchymal stroma cells improve hyperglycemia and insulin deficiency in the diabetic porcine pancreatic microenvironment. Cytotherapy. 2008;10:796–805. doi: 10.1080/14653240802461924. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Ogihara T, Yamada T, Ishigaki Y, Imai J, Uno K, et al. Bone marrow (BM) transplantation promotes β-cell regeneration after acute injury through BM cell mobilization. Endocrinology. 2007;148:2006–2015. doi: 10.1210/en.2006-1351. [DOI] [PubMed] [Google Scholar]
- Urban VS, Kiss J, Kovacs J, Gócza E, Vas V, Monostori E, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.