Abstract
Although T cells are known to be involved in the pathogenesis of coronary artery disease, it is unclear which subpopulation of T cells contributes to pathogenesis in acute myocardial infarction (MI). We studied the immunological characteristics and clinical impact of CD8+CD57+ T cells in acute MI patients. The frequency of CD57+ cells among CD8+ T cells was examined in peripheral blood sampled the morning after acute MI events. Interestingly, the frequency of CD57+ cells in the CD8+ T-cell population correlated with cardiovascular mortality 6 months after acute MI. The immunological characteristics of CD8+CD57+ T cells were elucidated by surface immunophenotyping, intracellular cytokine staining and flow cytometry. Immunophenotyping revealed that the CD8+CD57+ T cells were activated, senescent T cells with pro-inflammatory and tissue homing properties. Because a high frequency of CD8+CD57+ T cells is associated with short-term cardiovascular mortality in acute MI patients, this specific subset of CD8+ T cells might contribute to acute coronary events via their pro-inflammatory and high cytotoxic capacities. Identification of a pathogenic CD8+ T-cell subset expressing CD57 may offer opportunities for the evaluation and management of acute MI.
Keywords: acute myocardial infarction, CD8+CD57+ T cells, immunosenescence
Introduction
It is well known that inflammation plays a key role in the pathogenesis of coronary artery disease.1,2,3 The pathogenic role of T cells in atherosclerosis is well described in animal models.4,5,6,7 Recently, the association of pathogenic T cells with acute coronary syndrome in humans was addressed.8,9,10 However, it remains unclear which specific subpopulation of T cells plays a major role in acute coronary syndrome. CD4+CD28null T cells have been reported to contribute to acute coronary syndrome,11,12 and expansion of CD4+CD28null T cells is strongly associated with the recurrence of acute coronary events.11 Furthermore, in diabetic patients, an increased frequency of CD4+CD28null T cells correlated with poor outcome after an acute coronary event.12 However, a role for CD8+ T cells in human coronary artery disease has been identified less than for CD4+ T cells.13
The accumulation of CD28null T cells is one of the most prominent changes during the immune aging process.14 Similarly, the expression of CD57, a terminally sulfated glycan carbohydrate epitope, on T cells is currently considered to be a surrogate marker of replicative senescence of T cells.15 CD57+ T cells fail to proliferate after antigen-specific stimulation in vitro and are highly vulnerable to activation-induced apoptosis.16,17 Furthermore, it was demonstrated that replicative senescence of T cells is better defined by the expression of CD57 than by a lack of CD28 expression, as determined by measurement of the T-cell receptor excision circle content and of the proliferative ability of T cells.17 Notably, while CD57+ T cells have been shown to be associated with various inflammatory diseases,18,19,20,21,22 the pathogenic roles of CD57+ T cells have yet to be elucidated in coronary artery disease.
In the present study, we assessed the immunological characteristics of CD57+ T cells, particularly CD8+CD57+ T cells, in patients following acute myocardial infarction (MI) and analyzed their impact on clinical outcome.
Materials and methods
Study population
The present study included 58 prospectively and consecutively enrolled patients diagnosed with acute MI at the Severance Hospital from April 2010 to August 2010. Acute MI was defined as follows: (i) typical ischemic chest pain lasting for more than 30 min; ii) significant elevation of the ST segment or depression of two contiguous leads monitored by a standard 12-lead electrocardiogram; and iii) either elevation of the creatine kinase-MB isoform to greater than twice the normal upper limit or a rise in troponin T exceeding 0.1 ng/ml. Patients underwent a physical examination, electrocardiogram and laboratory examination at the time of initial enrollment. Whole blood was placed into an ACD anti-coagulated tube the morning after the patient was admitted to the hospital. Patients with a history of chronic inflammatory disease or who were taking anti-inflammatory medications were excluded from this study. The baseline characteristics of the study population are summarized in Table 1. Informed consent was provided by all subjects, and the study was approved by the Institutional Review Board (Yonsei University College of Medicine).
Table 1. Clinical characteristics and laboratory findings of analyzed subjects.
| Survival (n=48) | Cardiovascular mortality (n=7) | P value | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 65.6±13.1 | 74.4±8.4 | 0.089 |
| Sex (M/F) | 33∶15 | 6∶1 | 0.660 |
| STEMI/NSTEMI | 26∶22 | 2∶5 | 0.252 |
| Extent of CAD (1-VD/2-VD/3-VD) (%) | 22.7/40.9/36.4 | 0/28.6/71.4 | 0.163 |
| Infarct-related arteries (LM/LAD/LCx/RCA) (%) | 0/48.8/14.6/36.6 | 20.0/40.0/0/40.0 | 0.029* |
| Mode of treatment (PCI/CABG/medical) (%) | 87.5/0/12.5 | 57.1/14.3/28.6 | 0.013* |
| Door-to-balloon time (minutes; in STEMI) | 155.0±470.2 | 70.0±22.6 | 0.804 |
| TIMI flow before PCI (0/1/2/3) | 19/2/4/21 | 2/0/1/4 | 0.820 |
| TIMI flow after PCI (0/1/2/3) | 1/0/0/45 | 1/0/1/5 | 0.009* |
| Laboratory findings | |||
| Hemoglobin (g/dl) | 13.5±2.4 | 11.3±1.6 | 0.021* |
| BUN (mg/dl) | 18.5±7.3 | 32.2±10.1 | <0.001* |
| Cr (mg/dl) | 1.1±0.5 | 3.8±2.2 | 0.019* |
| Total cholesterol (mg/dl) | 155.8±41.5 | 124.7±43.4 | 0.071 |
| Triglyceride (mg/dl) | 109.7±62.4 | 88.9±41.7 | 0.398 |
| HDL cholesterol (mg/dl) | 39.7±11.2 | 32.0±9.6 | 0.093 |
| LDL cholesterol (mg/dl) | 93.8±39.5 | 71.0±29.2 | 0.149 |
| hsCRP (mg/l) | 33.2±55.6 | 105.5±87.7 | 0.073 |
| Initial CK-MB (ng/ml) | 27.9±68.1 | 41.9±57.7 | 0.607 |
| Peak CK-MB (ng/ml) | 141.0±162.5 | 69.4±63.9 | 0.257 |
| Initial Troponin-T (ng/ml) | 0.9±2.7 | 2.0±3.6 | 0.345 |
| Peak Troponin-T (ng/ml) | 1.4±3.2 | 4.0±4.4 | 0.173 |
| NT-proBNP (pg/ml) | 1613.8±3506.2 | 18591.7±15332.7 | 0.026* |
| Echocardiographic data | |||
| LVEF (%) | 53.7±13.6 | 41.4±10.8 | 0.027* |
| LVEDD (mm) | 50.0±5.2 | 53.3±7.3 | 0.147 |
| LVESD (mm) | 35.0±5.9 | 41.7±8.3 | 0.011* |
| LA Vol. index (ml/m2) | 28.2±10.9 | 37.4±21.0 | 0.291 |
Abbreviations: BUN, blood urea nitrogen; CABG, coronary artery bypass surgery; CAD, coronary artery disease; CK-MB, creatine kinase MB; Cr, creatinine; HDL, high-density lipoprotein; hsCRP, high sensitivity C-reactive protein; LAD, left anterior descending artery; LA Vol. index, left atrial volume index; LCx, left circumflex artery; LDL, low-density lipoprotein; LM, left main artery; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic dimension; NSTEMI, non-ST-segment elevation myocardial infarction; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; VD, vessel disease.
Values are presented as a %, or mean±s.d.
P<0.05 is considered significant.
Immunophenotyping analysis of peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated from whole blood using Ficoll-Hypaque (GE Healthcare, Uppsala, Sweden) and immediately analyzed for CD57 and CD28 expression. Any remaining PBMCs were cryopreserved and were later thawed and analyzed by flow cytometry. The cells were stained with fluorochrome-conjugated monoclonal antibodies against surface antigens for 20 min at 4 °C. The antibodies used included anti-CD3-Horizon V500, anti-CD4-PE-Cy7, anti-CD8-APC-H7, anti-CD28-APC, anti-CD45RO-PerCP-Cy5.5, anti-CD56-PE, anti-CD62L-APC, anti-CD94-FITC, anti-CCR5-PE, anti-CCR7-PE, anti-PD-1-PE, anti-NKG2D-APC, anti-HLA-DR-FITC (all from BD Biosciences, San Jose, CA, USA), anti-CD57-eFluor 450, anti-FasL-PE (both from eBioscience, San Diego, CA, USA), anti-CX3CR1-FITC (MBL International, Woburn, MA, USA) and anti-CD127-APC (R&D Systems, Minneapolis, MN, USA). To access intracellular cytotoxic molecules, PBMCs were fixed and permeabilized using a Fixation/Permeabilization Buffer Kit (eBioscience) and further stained for intracellular cytotoxic molecules with anti-perforin-PE, anti-granzyme A-FITC and anti-granzyme B-APC (all from BD Biosciences). Multicolor flow cytometry was performed using an LSR II instrument (BD Biosciences), and FlowJo software (Treestar, San Carlos, CA, USA) was used to analyze the data.
In vitro stimulation of T cells and intracellular cytokine staining
PBMCs were stimulated with anti-CD3 antibody (100 ng/ml) for 6 hours. After 1 h of incubation, brefeldin A (GolgiPlug; BD Biosciences) and monensin (GolgiStop; BD Biosciences) were added to stimulate intracellular cytokine protein accumulation. Following surface staining with anti-CD3-horizon V500, anti-CD4-PE-Cy7, anti-CD8-APC-H7, anti-CD45RO-PerCP-Cy5.5 and anti-CD57-eFluor 450, the cells were fixed and permeabilized using the Fixation/Permeabilization Buffer Kit and further stained for intracellular cytokines with anti-TNF-α-FITC, anti-IL-17A-PE and anti-IFN-γ-APC (all from BD Biosciences).
To analyze the IL-12/IL-18-responsiveness of the T cells, PBMCs were cultured in the presence of IL-12 (20 ng/ml; Peprotech, USA) and IL-18 (100 ng/ml; Peprotech) for 48 h and then stained for the same surface markers and intracellular cytokines, with the exception of IL-17A. FACS analysis was performed using a LSR II flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (Treestar, San Carlos, CA, USA).
Statistical analysis
Continuous variables were reported as the means±s.d. Categorical variables were summarized by the percentage of the group total. Independent t-tests were used for continuous variables, and discrete variables were compared using the Chi-squared method. Intra-group comparisons were summarized using the paired t-test, and the Wilcoxon signed-rank test was used to verify the results. Multivariate logistic regression analysis was performed to identify independent predictors of cardiovascular mortality. The cumulative incidence of mortality was assessed with the Kaplan–Meier method. The statistical significance of the curves was calculated using the log-rank test. Statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
The relationship between the frequency of CD57+ T cells and short-term cardiovascular mortality in acute MI patients
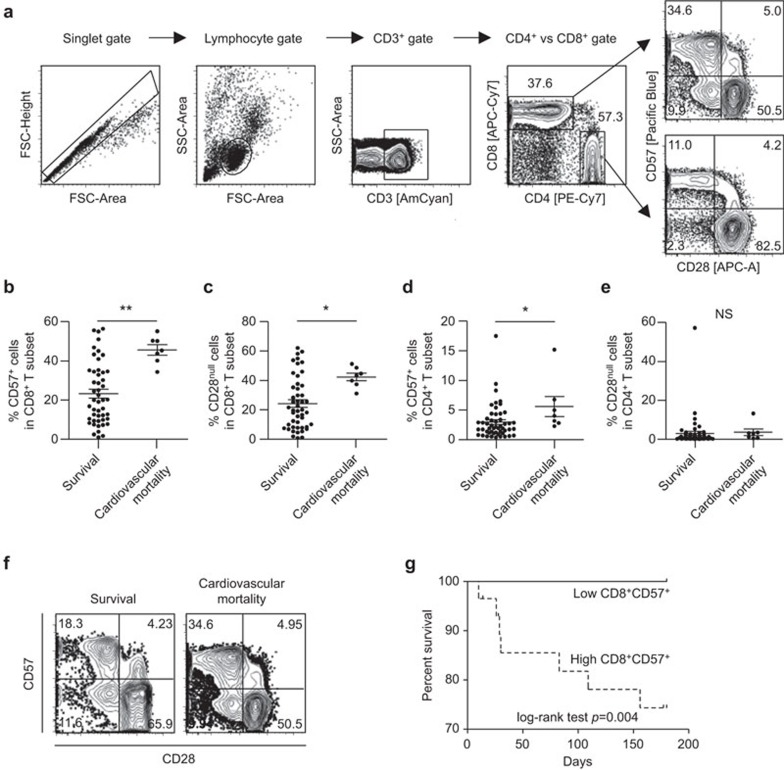
During a 6-month follow-up period, seven (12%) events of cardiovascular mortality and three (5%) events of non-cardiovascular mortality occurred among the 58 subjects. First, we analyzed the frequency of CD57+ or CD28null T cells among the CD8+ or CD4+ T-cell subset present in the peripheral blood of the 48 patients who survived MI on the morning after admission to the hospital and compared the results to the seven cases of cardiovascular death. CD8+ and CD4+ T cells were identified in PBMCs by multicolor flow cytometry by gating single cells (forward scatter-area vs. forward scatter-height), lymphocytes (forward vs. side scatter) and staining for CD3, followed by staining for CD4 and CD8 (Figure 1a). The frequency of CD57+ cells (Figure 1b; 45.6%±7.0% vs. 23.3%±15.7%, P=0.001) or CD28null cells (Figure 1c; 42.3%±6.9% vs. 24.3%±17.7%, P=0.011) in the CD8+ T-cell subset was significantly higher in the group that suffered cardiovascular mortality. We also analyzed the CD4+ T-cell subset. The frequency of CD57+ cells in the CD4+ T-cell subset was significantly higher in the cardiovascular mortality group (Figure 1d; 5.6%±4.5% vs. 3.0%±3.0%, P=0.047), while the frequency of CD28null cells in the CD4+ T-cell subset was not different between the two groups (Figure 1e; 3.6%±4.5% vs. 3.1%±8.5%, P=0.876). In multivariate analysis considering the age, gender and left ventricular ejection fraction (LVEF) of the patients, cardiovascular mortality was only associated with the frequency of CD57+ cells in the CD8+ T-cell subset (Table 2); thus, CD8+CD57+ T cells were a focus of further analyses. In Figure 1f, representative flow cytometry plots are presented for CD57 and CD28 expression in the CD8+ T-cell population. Although the CD57+ cell population overlapped considerably with the CD28null cell population (Figure 1f), only the frequency of CD8+CD57+ cells was independently associated with short-term cardiovascular mortality. Next, we divided the patients (48 cases of survival and seven cases of cardiovascular mortality) into two groups based upon the median percentage of CD8+CD57+ T cells in the peripheral blood, and compared the survival between the two groups. The group with the higher frequency of CD8+CD57+ cells (≥25%) suffered a significantly greater rate of cardiovascular mortality than the group with the lower frequency of CD8+CD57+ cells (P=0.004) (Figure 1g).
Figure 1.
The frequency of CD8+CD57+ T cells and short-term cardiovascular mortality in acute MI patients. The study population was divided into 48 cases of survival and seven cases of cardiovascular mortality, and the frequency of a specific T-cell population was compared between two patient groups. (a) Gating strategy. (b, c) The frequency of CD57+ cells (b) or CD28null cells (c) among CD8+ T cells was compared between the two groups. (d, e) The frequency of CD57+ cells (d) or CD28null cells (e) among CD4+ T cells was compared between the two groups. Data are expressed as the means±s.d. (f) Representative flow cytometry plots are presented for CD57 and CD28 expression in CD8+ T cells in patients who survived (left) and patients who suffered from cardiovascular mortality (right). (g) The patients (48 cases of survival and seven cases of cardiovascular mortality) were divided into two groups by median level (25%) of CD57+ T cell fraction in the CD8+ T-cell population. Kaplan-Meier plot shows that the 6-month survival rate was significantly lower in patients with CD8+CD57+ T cells ≥25% (dotted line) than in patients with CD8+CD57+ T cells <25% (solid line). *P<0.05. MI, myocardial infarction.
Table 2. Multivariate logistic regression analysis for cardiovascular mortality controlled for age, gender and left ventricular ejection fraction.
| Odds ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| CD8+CD57+ | ||||
| Age | 1.050 | 0.936 | 1.178 | 0.406 |
| Male sex | 0.848 | 0.055 | 12.985 | 0.906 |
| LVEF | 0.927 | 0.844 | 1.018 | 0.114 |
| CD8+CD57+ T cells | 1.100 | 1.013 | 1.194 | 0.024* |
| CD8+CD28null | ||||
| Age | 1.061 | 0.950 | 1.184 | 0.295 |
| Male sex | 0.530 | 0.045 | 6.252 | 0.614 |
| LVEF | 0.915 | 0.837 | 1.000 | 0.051 |
| CD8+CD28null T cells | 1.069 | 1.000 | 1.144 | 0.052 |
| CD4+CD57+ | ||||
| Age | 1.064 | 0.957 | 1.182 | 0.251 |
| Male sex | 0.437 | 0.039 | 4.938 | 0.503 |
| LVEF | 0.910 | 0.829 | 1.000 | 0.049* |
| CD4+CD57+ T cells | 1.204 | 0.946 | 1.533 | 0.131 |
| CD4+CD28null | ||||
| Age | 1.087 | 0.984 | 1.202 | 0.102 |
| Male sex | 0.313 | 0.025 | 3.940 | 0.369 |
| LVEF | 0.925 | 0.850 | 1.006 | 0.069 |
| CD4+CD28null T cells | 0.994 | 0.905 | 1.091 | 0.892 |
Abbreviations: CI, Confidence interval; LVEF, left ventricular ejection fraction.
P<0.05 is considered significant.
Immunophenotyping analysis of CD8+CD57+ T cells from acute MI patients
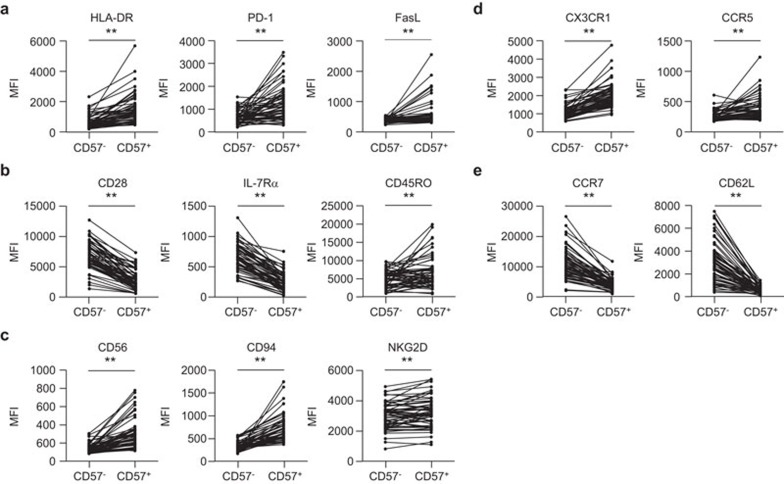
Because CD8+CD57+ T cells were associated with poor prognosis following acute MI, we examined the immunophenotype of the CD8+CD57+ T cells from acute MI patients. Compared with the CD8+CD57− T cells, the CD8+CD57+ T cells expressed significantly higher levels of HLA-DR, PD-1 and FasL, which are all markers associated with the recent activation of T cells (Figure 2a). CD8+CD57+ T cells also showed a senescent and terminally differentiated phenotype, as determined by lower levels of CD28 and IL-7Rα expression and higher levels of CD45RO expression (Figure 2b). Markers associated with natural killer cells, such as CD56, CD94 and NKG2D, were overexpressed on CD8+CD57+ T cells compared with CD8+CD57− T cells (Figure 2c). With respect to chemokine receptors, CD8+CD57+ T cells expressed higher levels of tissue-homing markers (CX3CR1 and CCR5; Figure 2d) and lower levels of central-homing markers (CCR7 and CD62L; Figure 2e) than CD8+CD57− T cells, suggesting that CD8+CD57+ T cells are poised to migrate toward inflamed peripheral tissues. In summary, surface phenotyping analysis revealed that CD8+CD57+ T cells are activated, senescent T cells with cytotoxic and tissue-homing properties.
Figure 2.
Immunophenotyping analysis of CD8+CD57+ T cells from acute MI patients. The MFI was compared for surface markers in both CD8+CD57+ and their paired CD8+CD57− T cell populations (n=58). Cells were stained with monoclonal antibodies against markers indicating the recent activation of T cells, such as HLA-DR, PD-1 and FasL (a), markers for the senescence and memory status of T cells, such as CD28, IL-7Rα and CD45RO (b), natural killer cell-related proteins such as CD56, CD94 and NKG2D (c), tissue-homing markers such as CX3CR1 and CCR5 (d) and central-homing markers such as CCR7 and CD62L (e). The P value for each surface marker was calculated using the paired t-test. **P<0.01. MFI, mean fluorescent intensity; MI, myocardial infarction.
Functional reactivity of CD8+CD57+ T cells from acute MI patients
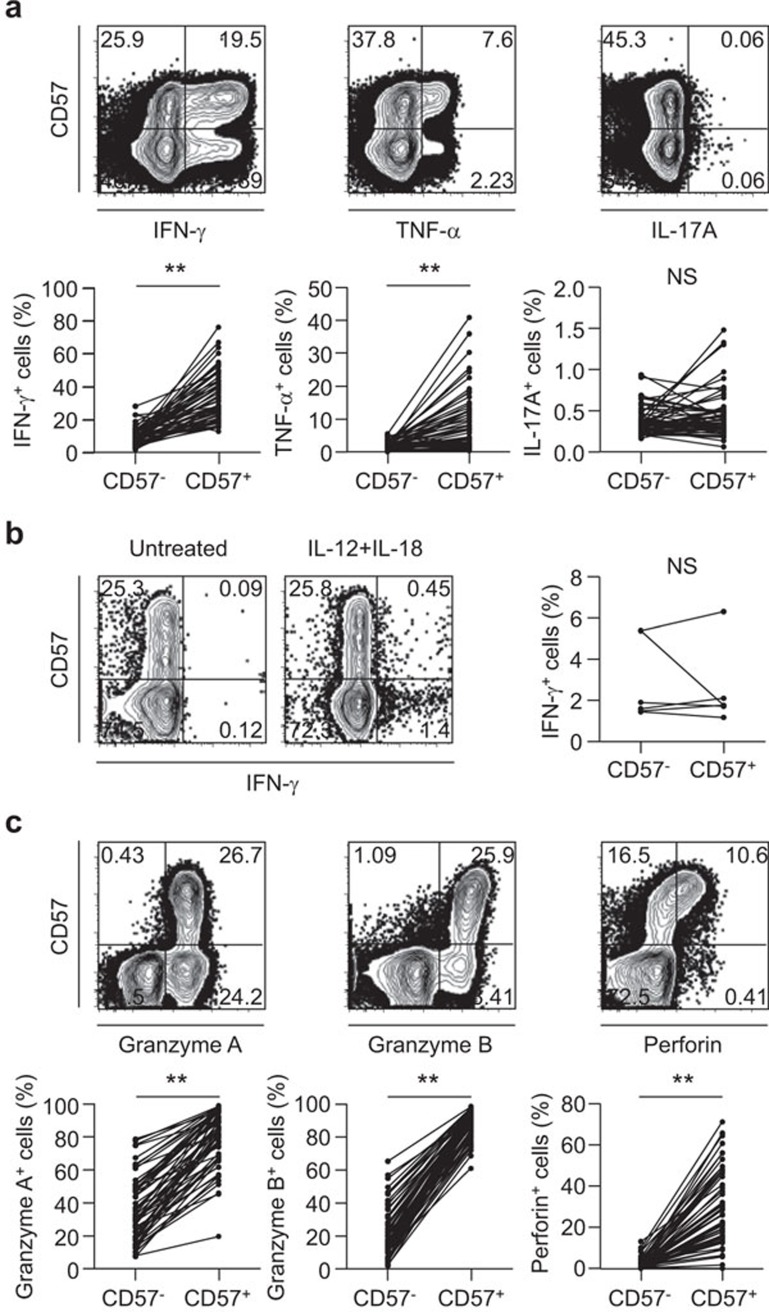
We analyzed the functional characteristics of CD8+CD57+ T cells from acute MI patients. First, we performed intracellular cytokine staining for IFN-γ, TNF-α and IL-17A following anti-CD3 antibody treatment to mimic T-cell receptor (TCR) stimulation. In this regard, we found that while the frequency of IFN-γ- or TNF-α-secreting cells in the CD8+CD57+ T-cell population was significantly greater than that in the CD8+CD57− T-cell population, there was no difference in the frequency of IL-17A-secreting cells between the two populations (Figure 3a). In addition, we examined IFN-γ secretion by CD8+CD57+ T cells in response to IL-12/IL-18 stimulation without TCR stimulation and found that CD8+CD57+ T cells did not show greater IFN-γ secretion than CD8+CD57− T cells (Figure 3b). These data suggest that the functional activation of CD8+CD57+ T cells depends on TCR stimulation rather than IL-12/IL-18 stimulation. We also evaluated the cytotoxic capacity of CD8+CD57+ T cells vs. CD8+CD57− T cells by intracellular staining of cytotoxic granule proteins such as granzyme A, granzyme B and perforin, and we found that these cytotoxic granule proteins were highly expressed by the CD8+CD57+ T-cell population compared with the CD8+CD57− T cell population (Figure 3c). Taken together, these data suggest that CD8+CD57+ T cells might secrete IFN-γ and TNF-α and exert higher cytotoxic function upon TCR engagement in vivo, and thus, they might participate in the pathophysiology of an acute coronary event.
Figure 3.
Functional reactivity of CD8+CD57+ T cells from acute MI patients. (a) PBMCs were stimulated with anti-CD3 antibody for 6 h, and intracellular cytokine staining for IFN-γ, TNF-α and IL-17A was performed (n=58). The frequency of IFN-γ-, TNF-α- or IL-17A-secreting cells in the CD8+CD57+ T-cell population was compared with those in the paired CD8+CD57− T-cell population. (b) IFN-γ secretion by CD8+CD57+ T cells in response to IL-12 and IL-18 stimulation without anti-CD3 antibody for 48 h was examined (n=6). (c) Intracellular staining for cytotoxic granule proteins was performed (n=58). The frequency of granzyme A+, granzyme B+ or perforin+ cells in either the CD8+CD57+ or CD8+CD57− T-cell populations was assessed by flow cytometry. The P value for each protein was calculated using the paired t-test. **P<0.01. MI, myocardial infarction; PBMC, peripheral blood mononuclear cell.
Discussion
In this study, we demonstrated that the frequency of CD57+ cells in the CD8+ T-cell population is associated with short-term cardiovascular mortality in patients with acute MI. This is, to our knowledge, the first demonstration of a relationship between CD8+CD57+ T cells and the clinical outcome of acute coronary syndrome. Immunophenotyping analysis revealed that CD8+CD57+ T cells are activated, senescent T cells that have pro-inflammatory and high cytotoxic capacities and tissue-homing properties.
A role for senescent T cells in atherosclerotic diseases has been reported in several studies.8,9 Previous studies examining CD4+CD28null T cells as a senescent T-cell population suggested a role in promoting vascular inflammation in atherosclerotic diseases, potentially contributing to plaque instability.8 In fact, CD4+CD28null T cells were demonstrated to accumulate in unstable plaques.9 In this regard, CD4+CD28null T cells secrete high amounts of IFN-γ to activate macrophages, which produce metalloproteinases that degrade the extracellular matrix.23,24 Furthermore, CD4+CD28null T cells release perforin and granzymes that lyse endothelial cells and vascular smooth muscle cells.25 In our study, we focused on CD8+CD57+ T cells because cardiovascular mortality was independently associated only with the frequency of CD8+CD57+ T cells and found that CD8+CD57+ T cells also demonstrated senescent, pro-inflammatory and highly cytotoxic properties. Thus, secretion of pro-inflammatory cytokines and the cytotoxic function of CD8+CD57+ T cells may affect atherosclerotic plaque instability, as previously reported for CD4+CD28null T cells. Of note, CD8+CD57+ T cells hardly produced IL-17A upon anti-CD3 stimulation (Figure 3a). Considering a recent report showing that IL-17 stabilizes atherosclerotic plaques through collagen production,26 the inability of CD8+CD57+ T cells to produce IL-17A might also associate with plaque instability in acute coronary syndrome.
Loss of CD28 expression is a well-known, senescent event of T cells, and CD28null T cells accumulate with age.27,28 In addition, loss of CD28 expression is known to be associated with the expression of CD57, a terminally sulfated glycoprotein.29,30 Therefore, both the CD28null and CD57+ phenotypes of T cells have been considered markers of immunosenescence.15 In fact, CD8+CD28null T cells overlapped significantly with the CD8+CD57+ T-cell population in our study (Figure 1f); however, the short-term cardiovascular mortality of acute MI patients was independently associated with only the frequency of CD8+CD57+ cells (Table 2). Current data suggests that the CD8+CD57+ T-cell population is distinct from CD8+CD28null T cells. Brenchley et al.17 found that CD57+CD28+ and CD57−CD28null T cells both exist in the peripheral blood of HIV-seropositive patients. Moreover, it was shown that CD57+CD28+ and CD57+CD28null T cells undergo the same number of cell divisions and have shorter telomeres than CD57− T cells, which undergo fewer cell divisions than CD57+ T cells, regardless of the CD28 expression. Together, these data indicate that CD57 expression better represents T-cell senescence than the loss of CD28 expression. In addition, the association between the frequency of CD8+CD57+ T cells and cardiovascular mortality suggests that CD8+CD57+ T cells might be functionally more relevant to the pathogenesis of acute coronary syndrome than CD8+CD28null T cells. For these reasons, we focused on elucidating the functional capacity and antigen reactivity of CD8+CD57+ T cells in acute MI patients.
We also attempted to identify the stimuli that activate these senescent T cells and cause them to exert effector functions. Several studies have reported the relationship between the IL-12- and IL-18-mediated inflammatory responses and the pathophysiology and prognosis of atherosclerotic diseases.31,32,33 However, in the current study, the IL-12- and/or IL-18-induced IFN-γ secretion by CD8+CD57+ T cells was not greater than that by CD8+CD57− T cells (Figure 3b), while anti-CD3 antibody-induced IFN-γ secretion was significantly higher from the CD8+CD57+ T cell population (Figure 3a).
Recently, Hoffmann et al.22 reported the immunophenotypic profiles of T-cell subsets in patients with acute MI following primary percutaneous coronary intervention. In that study, multiparameter flow cytometric analysis revealed that the frequency of CD4+CD57+ T cells lacking KLRG1 expression were increased in patients with acute MI. Because CD8+CD57+ and CD4+CD57+ T cells are heterogeneous populations, we must further investigate which subpopulations within CD8+CD57+ or CD4+CD57+ T cells are associated with the development of acute MI and its outcome.
Although we demonstrated a relationship between the frequency of CD57+ cells and acute MI patients, the current data must be cautiously interpreted. First, we could not examine the frequency of CD8+CD57+ T cells prior to acute MI events. Considering that CD57 is a marker of the replicative senescence of T cells,15 the frequency of CD8+CD57+ T cells is not expected to increase abruptly after acute MI events. However, the frequencies of CD8+CD57+ T cells before and after acute MI events must be analyzed in relation to cardiovascular mortality in the future. Second, the study population did not include healthy control subjects and thus, immunological characterization of T cells was conducted only in acute MI patients. A direct comparison of T-cell phenotype and function with age- and sex-matched healthy subjects would provide a more valuable immunological perspective for cardiovascular diseases. Third, the small size of the study population and the limited number of primary endpoints restricted a close analysis of the frequency of CD8+CD57+ T cells as a prognostic factor in acute MI in the current study. In particular, we could not determine whether these CD8+CD57+ T cells had an independent incremental value over other traditional factors of cardiovascular mortality in acute MI patients.
In summary, we identified a link between the frequency of CD8+CD57+ T cells and short-term cardiovascular mortality in patients following acute MI. CD8+CD57+ T cells have senescent, pro-inflammatory and high cytotoxic properties. This specific subset of T cells might contribute to plaque instability via high cytotoxic functions and pro-inflammatory cytokines, including IFN-γ and TNF-α. Identification of a pathogenic CD8+ T-cell subset expressing CD57 may offer new opportunities for the prevention and treatment of acute MI.
Acknowledgments
This work was supported by the KAIST Future Systems Healthcare Project from the Ministry of Science, ICT & Future Planning of Korea and by the project of Global PhD Fellowship begun by the National Research Foundation of Korea in 2011. This study was also supported by the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
None to declare.
References
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse. Arterioscler Thromb Vasc Biol. 2006;26:2421–2432. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Kopecky SL, Frye RL, O'Fallon WM, Maseri A, Goronzy JJ, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- Caligiuri G, Paulsson G, Nicoletti A, Maseri A, Hansson GK. Evidence for antigen-driven T-cell response in unstable angina. Circulation. 2000;102:1114–1119. doi: 10.1161/01.cir.102.10.1114. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol. 2007;50:1450–1458. doi: 10.1016/j.jacc.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Giubilato S, Liuzzo G, Brugaletta S, Pitocco D, Graziani F, Smaldone C, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J. 2011;32:1214–1226. doi: 10.1093/eurheartj/ehq499. [DOI] [PubMed] [Google Scholar]
- Kolbus D, Ljungcrantz I, Andersson L, Hedblad B, Fredrikson GN, Björkbacka H, et al. Association between CD8+ T-cell subsets and cardiovascular disease. J Intern Med. 2013;274:41–51. doi: 10.1111/joim.12038. [DOI] [PubMed] [Google Scholar]
- Weng NP, Akbar AN, Goronzy JJ. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- Vogel M, Kowalewski HJ, Zimmermann H, Janetzko A, Margolis RU, Wollny HE. Association of the HNK-1 epitope with 5′-nucleotidase from Torpedo marmorata (electric ray) electric organ. Biochem J. 1991;278:199–202. doi: 10.1042/bj2780199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Maeda T, Yamada H, Nagamine R, Shuto T, Nakashima Y, Hirata G, et al. Involvement of CD4+,CD57+ T cells in the disease activity of rheumatoid arthritis. Arthritis Rheum. 2002;46:379–384. doi: 10.1002/art.10133. [DOI] [PubMed] [Google Scholar]
- Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–8423. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- Le Priol Y, Puthier D, Lecureuil C, Combadiere C, Debre P, Nguyen C, et al. High cytotoxic and specific migratory potencies of senescent CD8+CD57+ cells in HIV-infected and uninfected individuals. J Immunol. 2006;177:5145–5154. doi: 10.4049/jimmunol.177.8.5145. [DOI] [PubMed] [Google Scholar]
- Palmer BE, Mack DG, Martin AK, Maier LA, Fontenot AP. CD57 expression correlates with alveolitis severity in subjects with beryllium-induced disease. J Allergy Clin Immunol. 2007;120:184–191. doi: 10.1016/j.jaci.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Fiser K, Weaver J, Dimmick I, Loeher M, Pircher H, et al. High-throughput 13-parameter immunophenotyping identifies shifts in the circulating T-cell compartment following reperfusion in patients with acute myocardial infarction. PLoS One. 2012;7:e47155. doi: 10.1371/journal.pone.0047155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon MA, Zuckerman S. Gamma interferon: a central mediator in atherosclerosis. Inflamm Res. 2005;54:395–411. doi: 10.1007/s00011-005-1377-2. [DOI] [PubMed] [Google Scholar]
- Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007;5:265–282. doi: 10.1586/14779072.5.2.265. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, et al. T-cell–mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- Gisterå A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, et al. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J Biol Chem. 1998;273:8119–8129. doi: 10.1074/jbc.273.14.8119. [DOI] [PubMed] [Google Scholar]
- Merino J, Martinez-Gonzalez M, Rubio M, Inoges S, Sanchez-Ibarrola A, Subira M. Progressive decrease of CD8 high+ CD28+ CD57− cells with ageing. Clin Exp Immunol. 1998;112:48–51. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M, Phillips JH, Lanier LL. CD28-T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Henry P, Fressonnet R, Alouani S, Scoazec A, Beaufils P, et al. Increased plasma concentrations of interleukin-18 in acute coronary syndromes. Heart. 2002;88:467–469. doi: 10.1136/heart.88.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, et al. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;98:524–531. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]