The concept of vaccine adjuvants arose over a century ago from the astute observation that the ‘antigenic value of diphtheria toxoid was improved by addition of alum'.1 While their discovery was serendipitous, their potential for enhancing immune response against co-inoculated antigens has made them essential to modern day prophylactic inactivated vaccine formulations. Indeed, from being ‘immunology's dirty, little secret' adjuvants have now become a key component of a vaccinologist's armamentarium.
Adjuvants constitute a wide and diverse range of compounds that enhance or influence the immune response to vaccines in one of many ways.2 Bacterial products such as lipopolysaccharide trigger pattern recognition receptors on the surface of innate cells; nucleoside analogs such as R-848 engage endosomal receptors; aluminum salts and oil-based compounds such as MF59, both of which are licensed for clinical use, increase antigen uptake by dendritic cells and enhance immune cell recruitment to the vaccination site. The net result of adjuvant action is production of cytokines, chemokines and expression of costimulatory signals, which potentiate antigen-specific responses. However, as one would predict, adjuvants can have unwanted and potentially harmful side effects due to multiple and unknown cell targets. This necessitates utmost care in the design and selection of vaccine adjuvants.
There is emerging interest in the use of molecular adjuvants with specific and well-defined cellular targets that lend themselves remarkably well to vector-based vaccines. Costimulatory molecules make for excellent molecular adjuvants due to their ability to potentiate the immune response by directly targeting either T cells or B cells. An inducible T-cell costimulatory molecule of the tumor necrosis factor receptor family, 4-1BB (also called CD137, ILA, TNFSFR9), has received a great deal of interest as a T-cell adjuvant.3 4-1BB is expressed by activated T cells, and is unique among the tumor necrosis factor receptor family in its expression by myeloid cells. Ligation of 4-1BB by its ligand 4-1BBL, expressed by antigen-presenting cells (APC), induces activation of p38 MAPK, JNK and NF-κB pathways in T cells resulting in proliferation, cytokine production, granzyme B and perforin expression, and increased resistance to apoptosis. Importantly, 4-1BB costimulation is potent at eliciting recall CD8 T-cell responses even at a limiting antigen dose4 and there appear to be no additive or synergistic effects with CD28 costimulation. In fact, a number of studies have shown that 4-1BB is superior to CD28 in eliciting recall proliferation and cytolytic function of memory CD8 T cells in vitro.5
A significant question from a therapeutic standpoint is whether 4-1BB co-stimulation can revive exhausted CD8 T cells. There are encouraging data available showing that low-dose agonistic 4-1BB antibody synergizes with programmed death-L1 blockade to rejuvenate exhausted antigen-specific CD8 T cells in a mouse model of chronic viral infection.6 However, a repeat high dose of 4-1BB caused a transient increase followed by rapid decline in antigen-specific CD8 T cells. As with other immune activating agents, dose and timing of 4-1BB are critical to achieve the desired immune potentiating effects. Too much may not be too good.
The role of 4-1BB in eliciting proliferation and cytolytic function of memory CD8 T cells makes it an especially attractive therapeutic target for cancer therapy, where the T-cell response is impaired by persistent antigen and the dearth of costimulatory signals from cancer cells. In the current issue of CMI, Wang and colleagues demonstrate potent anti-tumor effects of a vaccine vector engineered to express 4-1BBL.7 By employing a tumor model in mice, the authors investigated the functional quality of effector and memory CD8 T cells generated after immunization with 4-1BBL-expressing OVA vaccine. Immunization with OVA4-1BBL by vaccine increased the magnitude of tetramer+ CD8 T cells 1.6-fold at the effector phase compared with OVAnull vaccine. Consistent with the biology of 4-1BBL activated cells, CD8 T-cell effectors were highly functional producing interferon-γ and expressing granzyme B. To directly assess cytolytic activity of CD8 T cells in vivo, the authors immunized 6-day tumor-bearing mice with either OVA4-1BBL or with an OVAnull vaccine. Neither unimmunized mice nor OVAnull immunized mice cleared lung tumors completely; however, the number of metastatic colonies was significantly reduced in OVAnull immunized mice. Remarkably, despite only a modest increase in tetramer+ CD8 T cells over OVAnull immunization, all mice vaccinated with OVA4-1BBL cleared tumors from the lung. The authors further demonstrate that mice were completely protected when re-challenged with tumor cells 30 days post-immunization. These data raise the question: in addition to inducing cytolytic effectors, did 4-1BBL also induce highly functional memory CD8 T cells? The authors examined the phenotype of OVA-specific CD8 effectors at day 10 and found evidence of a classic memory CD8 T-cell signature—a CD62L, CD127hi phenotype with high expression of anti-apoptotic genes. Although the frequency of tetramer+ cells at memory was modest in both OVAnull (0.15% of CD8) and OVA4-1BBL (0.73%) vaccinated mice, recall responses were ten-fold higher in response to OVA peptide in OVA4-1BBL immunized mice.
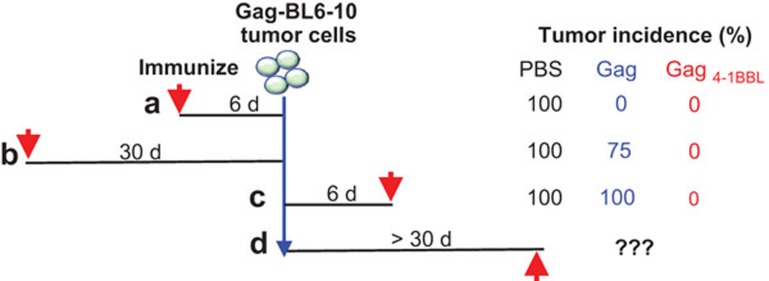
Finally, the authors directly compared 4-1BBL as an adjuvant to a prophylactic and therapeutic vaccine against Gag-expressing tumor cells (Figure 1). Immunization with Gag4-1BBL vaccine was effective at: (i) inducing CD8 T-cell effectors that cleared tumors; (ii) engendering memory cells, which mounted an efficient recall response resulting in tumor clearance; and (iii) generating effector cells in the presence of tumor antigen resulting in clearance of tumor. Whether therapeutic immunization would be effective against well-established tumors is a clinically relevant question that the authors did not address. Additionally, enumeration of the magnitude, phenotype and functional quality of CD8 T-cell responses in this context would be very informative and should be the focus of future studies. A previous study using a mouse model of melanoma found that treatment with 4-1BB agonistic antibody resulted in increased infiltration of a unique KLRG1+Eomes+ CD8 T-cell subset with heightened cytolytic potential.8 The phenotype of 4-1BBL-stimulated CD8 T cells is distinct and relatively little has been done to characterize it. Microarray or RNA sequencing analysis of these cells at effector and memory times points would be a good starting point and could prove to be very informative.
Figure 1.
Induction of anti-tumor immunity by a 4-1BBL-adjuvanted vaccine. Immunization with 4-1BBL adjuvanted Gag vaccine resulted in effective clearance of tumor cells with differentially spaced immunization regimens.
4-1BB agonistic antibodies are in Phase I/Phase II trials against melanoma. While promising, agonistic 4-1BB antibodies are associated with side effects including neutropenia and liver toxicity. There is also evidence for suppression of vaccine-specific antibody responses with agonistic 4-1BB antibodies, but not with membrane-bound 4-1BBL.9 Targeted expression of 4-1BBL by engineering vaccine vectors to express membrane bound forms of 4-1BBL is therefore an attractive strategy to harness the costimulatory potential of 4-1BB while circumventing its side effects. With this strategy, it will be important to determine whether multiple immunizations would be more potent at inducing high magnitude memory CD8 T cells, and whether 4-1BB costimulation is more effective during the prime and/or during the boost. An emerging platform for targeted costimulation utilizing the aptamer-targeted 4-1BB costimulation technology has shown success in models of cancer.10 A better understanding of the biology this molecule and strategies to harness it will undoubtedly aid the design of more effective vaccines against pathogens and cancer.
References
- Glenny AT, Pope C, Waddington H, Wallace U. The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926;29:38–45. [Google Scholar]
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc Natl Acad Sci USA. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V, Penaloza-MacMaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, et al. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol. 2011;187:1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Freywald A, Chen Y, Xu J, Tan X, Xiang J. Transgene 4-1BBL-engineered vaccine stimulates potent Gag-specific therapeutic and long-term immunity via priming increased CD44+CD62LhighIL-7R+ CTLs with up- and down-regulation of anti- and pro-apoptosis genes. Cell Mol Immunol. 2014;12:456–465. doi: 10.1038/cmi.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, et al. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med. 2013;210:743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Liu J, Pillai VB, Mittler RS, Amara RR. Adjuvantive effects of anti-4-1BB agonist Ab and 4-1BBL DNA for a HIV-1 Gag DNA vaccine: different effects on cellular and humoral immunity. Vaccine. 2010;28:1300–1309. doi: 10.1016/j.vaccine.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor F, Kolonias D, McNamara JO 2 nd, Gilboa E. Targeting 4-1BB costimulation to disseminated tumor lesions with bi-specific oligonucleotide aptamers. Mol Ther. 2011;19:1878–1886. doi: 10.1038/mt.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]