Abstract
IL-37 is an anti-inflammatory cytokine that was only recently identified, and it is highly expressed in tissues from patients with inflammatory and autoimmune diseases. Inflammatory cytokines and inflammatory stimuli can induce the upregulation of IL-37. However, it has not been reported whether anti-inflammatory medications induce the expression of IL-37. In this work, we uncovered, for the first time, that two main bioactive components, triptolide and triptonide, from the herb Tripterygium wilfordii Hook f. (TwHF), which possess anti-inflammatory activity, upregulate the expression of IL-37, and this expression was suppressed by ERK1/2 and p38 MAPK inhibitors. Overall, our research demonstrated, for the first time, that anti-inflammatory active components (triptolide and triptonide) upregulated the expression of IL-37 most likely via activation of the ERK1/2 and p38 MAPK pathways.
Keywords: IL-37, THP-1 cells, Tripterygium wilfordii Hook F., triptolide, triptonide
IL-37 is a newly defined member of the IL-1 cytokine family, which is a fundamental inhibitor of innate immunity.1 IL-37 mRNA and protein have been detected in inflammatory and autoimmune diseases such as rheumatoid arthritis,1 atopic dermatitis,2 inflammatory bowel disease3 and systemic lupus erythematosus.4 IL-37 is endogenously kept at low levels in human cells, and can be upregulated by pro-inflammatory cytokines and inflammation stimuli.1 However, it is still unclear whether anti-inflammatory medications induce the expression of IL-37. We document here, for the first time, that IL-37 was upregulated in THP-1 cells induced by two active components, triptolide and triptonide, extracted from the herb Tripterygium wilfordii Hook F (TwHF).
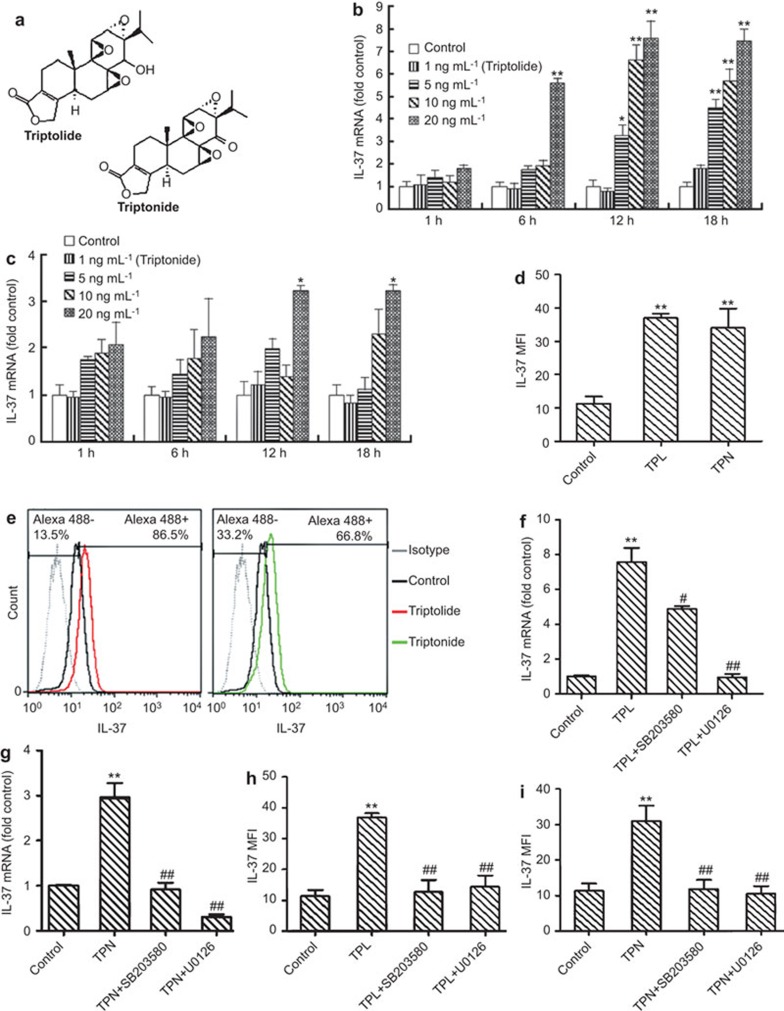
In this study, THP-1-derived macrophages were used as a model system.5 THP-1 cells were treated with five active monomer components (triptolide, triptonide, triptophenolide, celastrol and wilforlide A (Beijing Medicass Biotechnol, Beijing, China)) with purity of more than 98% at different concentrations (1, 5, 10 and 20 ng/ml) for various incubation periods (1, 6, 12 and 18 h). The expression of IL-37 mRNA was analyzed by real-time quantitative PCR using SYBR Premix Ex Taq kit (TaKaRa, Dalian, China) on the ABI prism 7700 Sequence Detection System (Perkin Elmer, Foster City, CA, USA). The sequences of the primers were as follows: IL-37-F (TTAGAAGACCCGGCTGGAAGCC) and IL-37-R (AGATCTCTGGGCGTATGTAGT); GAPDH-F (ACCCAGAAGACTGTGGATGG) and GAPDH-R (TTCTAGACGGCAGGTCAGGT). The expression of the target gene is presented as a ratio, which was normalized to the endogenous reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the comparative CT method was used as reported in the literature.6 The chemical structure of triptolide and triptonide are shown in Figure 1a. The results showed that triptolide and triptonide upregulated the expression of IL-37 mRNA (Figure 1b and c). As shown in Figure 1b, triptolide at concentrations of 5, 10 and 20 ng/ml was found to upregulate IL-37 mRNA, and the maximum upregulation of IL-37 (an approximate 7.4-fold enhancement) was observed in the presence of 20 ng/ml of triptolide at the 12-h incubation period. Similarly, triptonide also induced the upregulation of IL-37 mRNA expression in the presence of 20 ng/ml of triptonide, and the highest upregulated expression of IL-37 mRNA (approximate 3.2-fold enhancement) in the treated THP-1 cells was observed for the 12-h incubation period (Figure 1c). These results suggest that triptolide might have higher ability to induce IL-37 expression than that of triptonide. Three other active components, including triptophenolide, celastrol and wilforlide A, did not upregulate IL-37 mRNA in THP-1 cells.
Figure 1.
Analysis of the effects of triptolide and triptonide on the expression of IL-37 in THP-1 cells. (a) The chemical structure of triptolide and triptonide. (b) Real-time PCR analysis of IL-37 mRNA expression induced by triptolide at different concentrations for various incubation periods. (c) Real-time PCR analysis of IL-37 mRNA expression induced by triptonide at different concentrations for various periods. (d) Flow cytometry analysis of the IL-37 MFI in THP-1 cells treated with triptolide and triptonide. (e) Flow cytometry analysis of intracellular IL-37 protein expression induced by 10 ng/ml of triptolide (left) or 20 ng/ml of triptonide (right) after a 16-h incubation. (f) Real-time PCR analysis of the effect of the p38 MAPK inhibitor SB203580 and the ERK 1/2 inhibitor U0126 on the expression of IL-37 mRNA in THP-1 cells induced by triptolide. (g) Real-time PCR analysis of the effect of the p38 MAPK inhibitor SB203580 and the ERK 1/2 inhibitor U0126 on the expression of IL-37 mRNA in THP-1 cells induced by triptonide. (h) Flow cytometry analysis of the effect of the p38 MAPK inhibitor SB203580 and the ERK 1/2 inhibitor U0126 on the expression of IL-37 in THP-1 cells induced by triptolide. (i) Flow cytometry analysis of the effect of the p38 MAPK inhibitor SB203580 and the ERK 1/2 inhibitor U0126 on the expression of IL-37 in THP-1 cells induced by triptonide. Data are expressed as the mean±s.d. * P<0.05, ** P<0.01 in comparison with the medium alone control. # P<0.05, ## P<0.01 compared with the response to the corresponding uninhibited control, respectively. MFI, mean fluorescence intensity; TPL, triptolide; TPN, triptonide.
Flow cytometry analysis was performed to further investigate the influence of triptolide and triptonide on the expression of intracellular IL-37 protein. The macrophage-like THP-1 cells were treated with triptolide or triptonide for 16 h. The prepared cells were fixed with 100 µl of Fixation/Permeabilization working buffers (eBioscience, San Diego, CA, USA) for 20 min. Then, 2 ml of 1× permeabilization working buffer was added to each tube. After washing twice, the cell pellets were resuspended with 100 µl of 1× permeabilization buffer containing 10% normal goat serum to block non-specific protein-protein interactions and incubated for 60 min. The cells were then incubated with the anti-IL-37 monoclonal antibody (1 µg/1×106 cells; Abcam, Cambridge, UK) for 30 min. The secondary antibody used was Alexa Fluor 488 goat anti-mouse IgG (H+L) (Life Technologies, Eugene, OR, USA) at 1∶500 dilution for 30 min. The isotype control antibody was mouse IgG1 (1 µg/1×106 cells) and was used under the same conditions. The stained cells were resuspended in an appropriate volume of flow cytometry staining buffer and analyzed on a FACSCalibur flow cytometer with FACSDiva software (BD Biosciences). Compared to the control cells without treatment, there was an approximate 3.7-fold increase in IL-37 MFI in the THP-1 cells treated with 10 ng/ml of triptolide for 16 h (Figure 1d), and the positive cells with IL-37 expression increased approximately 6.3-fold (Figure 1e, left). Similarly, compared to the control cells, the positive cells with IL-37 expression increased approximately 5.3-fold (Figure 1e, right), and an approximate 3.4-fold increase in IL-37 MFI was observed in THP-1 cells treated with 20 ng/ml of triptonide for 16 h (Figure 1d).
To further understand the mechanism by which triptolide/triptonide induces the expression of IL-37, the p38 MAPK and ERK1/2 signaling pathways were investigated. Macrophage-like THP-1 cells were pre-treated with the p38 MAKP inhibitor (SB203580) and the ERK 1/2 inhibitor (U0126) (Cell Signaling Technology, Beverly, MA, USA) for 30 min. Then, the treated cells were incubated with triptolide and triptonide for 12 h or 16 h, respectively. The results of the real-time PCR showed that U0126 at 5 µmol/l almost completely abolished the expression of IL-37 mRNA induced by triptolide or triptonide in THP-1 cells (Figure 1f and g). The other inhibitor (SB203580), used at 10 µmol/l, almost completely abolished the expression of triptonide-induced IL-37 mRNA in THP-1 cells after 12 h of incubation (Figure 1g). However, SB203580 inhibited approximately 41.5% of IL-37 mRNA expression induced by triptolide at 12 h of incubation (Figure 1f). As for the cytometry analysis, U0126 and SB203580 almost completely abolished the expression of IL-37 protein in THP-1 cells induced by triptonide after 16 h of incubation (Figure 1i). However, when THP-1 cells were stimulated with triptolide for 16 h, SB203580 inhibited 90.9% of IL-37 expression and U0126 inhibited 85.1% of IL-37 expression (Figure 1h). These findings indicate that SB203580 or U0126 suppressed the expression of triptolide or triptonide-induced IL-37 significantly in THP-1 cells and that SB203580 has lower inhibitory effects on triptolide-induced IL-37 expression in THP-1 cells, which suggests that the p38 MAPK and ERK1/2 pathways may be involved in the upregulation of triptolide and triptonide-induced IL-37.
TwHF has been used in traditional Chinese medicine for the treatment of inflammatory and autoimmune diseases.7 Ninety-five percent of the active components of TwHF are diterpenes, triterpenes and alkaloids. Many diterpenes and triterpenes compounds isolated from TwHF exhibit anti-inflammatory and immunosuppressive activities. Triptolide, a diterpenoid triepoxide purified from TwHF, has been identified as the major component of TwHF.8 In this study, only triptolide and triptonide were observed to induce the upregulation of IL-37. Although the other active components (triptophenolide, celastrol and wilforlide A) showed no effect on IL-37 expression in this study, it cannot be declared that IL-37 expression cannot be induced by other diterpenes and triterpenes components of TwHF. To date, over 300 secondary metabolites are reported from TwFH. Obviously, more of the diterpenes and triterpenes components should be examined in the future to further discover more active components of TwFH that can induce IL-37 expression.
The MAPK signaling pathway is one of the major signaling pathways in inflammation. Triptolide not only inhibited NF-κB activation but also activated p38 MAPK and MEK/ERK phosphorylation in THP-1 cells.9 Triptolide was also reported to activate ERK 1/2 and p38 MAPK.10 Our experimental results showed that the p38 MAPK inhibitor (SB203580) and the ERK1/2 inhibitor (U0126) suppressed the upregulation of IL-37 induced by triptolide and triptonide, respectively. However, this study is only a preliminary exploration, and further investigation is required to address the mechanism of IL-37 upregulation induced by triptolide and triptonide. IL-37 could be upregulated by pro-inflammatory cytokines and inflammatory stimuli.1 In contrast, this study, for the first time, uncovered that the active components triptolide and triptonide possess anti-inflammatory activity and upregulate IL-37 expression. Whether it is a new mechanism that triptolide and triptonide act as anti-inflammatory agents via the upregulation of IL-37 is still unclear and needs to be investigated further.
In conclusion, this study demonstrated that triptolide and triptonide extracted from TwHF have anti-inflammatory activities and can induce the upregulation of IL-37 in THP-1 cells, an effect that is suppressed by inhibitors of ERK1/2 and p38 MAPK. These findings have revealed previously unrecognized effects of the anti-inflammatory components triptolide and triptonide on the upregulation of IL-37.
Acknowledgments
This work was supported by the grants from the Science & Technology Innovation Fund of Guangdong Medical College (No. STIF201107) and the College Students' Innovative Training Program of Guangdong Province, China (No. 1057113038).
The authors declare no financial or commercial conflict of interest.
References
- Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Inoue Y, Seto K, Komitsu N, Aihara M. Interleukin-37 is elevated in subjects with atopic dermatitis. J Dermatol Sci. 2013;69:173–175. doi: 10.1016/j.jdermsci.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Ban H, Bamba S, et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172:410–416. doi: 10.1111/cei.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: its correlation with disease activity. J Transl Med. 2014;12:69. doi: 10.1186/1479-5876-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol. 2012;74:424–436. doi: 10.1111/j.1365-2125.2012.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta R, Wang X, Ge H, Ray W, Nelin LD, Liu Y. Triptolide induces anti-inflammatory cellular responses. Am J Transl Res. 2009;1:267–282. [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kim YI. Triptolide induces apoptosis of PMA-treated THP-1 cells through activation of caspases, inhibition of NF-kappaB and activation of MAPKs. Int J Oncol. 2013;43:1169–1175. doi: 10.3892/ijo.2013.2033. [DOI] [PubMed] [Google Scholar]
- Zhu B, Wang YJ, Zhu CF, Lin Y, Zhu XL, Wei S, et al. Triptolide inhibits extracellular matrix protein synthesis by suppressing the Smad2 but not the MAPK pathway in TGF-beta1-stimulated NRK-49F cells. Nephrol Dial Transplant. 2010;25:3180–3191. doi: 10.1093/ndt/gfq239. [DOI] [PubMed] [Google Scholar]