Progressive deterioration of innate and adaptive immune functions, a status termed ‘immunosenescence', is associated with the higher frequency and severity of diseases in the elderly, such as chronic infections, cancer and autoimmune disorders.1 Age-related immunosenescence can be characterized by a decrease in adaptive immunity and increase in low-grade chronic inflammation, also referred to as ‘inflamm-aging'.2 This dysfunctional immunity is characterized by perturbations of the T-lymphocyte compartment, largely attributed to thymus involution, shrinkage of the naive T-cell repertoire and oligoclonal expansions of memory T cells, particularly of CD8+ effector memory subsets.3 Age and latent viral infections, especially human cytomegalovirus (CMV), are both considered a driving force of CD8+ T-cell expansion and senescence, which is characterized by shortened telomeres, progressive loss of costimulatory receptors, CD28 and CD27, and expression of senescence-associated surface markers, such as KLRG1 and the glycoepitope CD57, which also reflects the proliferative history of cells.4
In this issue of the Journal, Yu and colleagues report on the association between the frequencies of circulating CD8+ T cells that express CD57 and the short-term cardiovascular mortality in patients following acute myocardial infarction. Using flow immunophenotyping and functional assays the authors characterized the CD8+CD57+ subset as differentiated, senescent-like, mostly CMV-reactive cytotoxic T cells. There are several limitations of this study. The primary endpoint (cardiovascular death) was only reached in seven patients, which makes the study underpowered and too small to draw definite conclusions. In part, this seems due to some selection process in choosing patients with relatively small infarcts, but mainly the overall small sample size of 58 patients. Another limitation is clearly the lack of distinction between CMV-seropositive and -seronegative patients. This would be important, since CMV-specific cells express high levels of CD57, and as is shown, CD8+CD57+ cells react to CMV-specific antigen stimulation by secretion of the pro-inflammatory cytokines TNF-α and IFN-γ. The authors assume, which is most plausible, that expansion of CD8+CD57+ T cells would most likely had occurred already long before the onset of myocardial infarction, indicating long-term immune activation or chronic low-grade inflammation in these patients. If this would be the case, then what is the likely mechanism behind the upregulation of CD57 on CD8+ T cells in these patients? It is conceivable that general chronic inflammation can drive T-cell proliferation and thereby CD57 expression simply as a marker of proliferation history of a cell, also reflected by short telomere length.4 In addition, CD57 also identifies a population of CMV-specific cytotoxic T cells, which can induce chemokine-mediated endothelial damage, potentially accelerating the progression of atherosclerosis.5
Even though other antigenic epitopes might potentiate persistent T-cell expansion, the most common cause of increased proliferation and terminal differentiation of CD28−CD8+ T cells is chronic CMV infection, resulting in gradual enrichment for terminally differentiated cells that express CD57.6,7 Yu and colleagues proved reactivity (at least in part) of CD57+ cells towards CMV epitopes. Are therefore CMV-seropositive myocardial infarction patients the actual risk group? There is increasing evidence that CMV seropositivity may account for progression of coronary heart disease and increased cardiovascular mortality. The largest study to date has demonstrated a significant association of CMV and cardiovascular disease in 7700 participants.8 A recent study by Savva and colleagues9 has found a surprising increase in CHD-related mortality in CMV-seropositive, but otherwise healthy individuals over the course of 17 years. This translated into a decrease in lifespan of almost 4 years. We found that CMV-seropositive patients with chronic myocardial infarction have increased telomere shortening in their CD8+ T cells when compared to CMV-seropositive healthy controls, suggesting a bidirectional relation between CMV-driven immunosenescence and chronic vascular inflammation, the underlying cause of coronary artery disease.10 However, chronic inflammation during healthy aging can also lead to immunosenescence without prior infection with CMV or other herpes viruses.
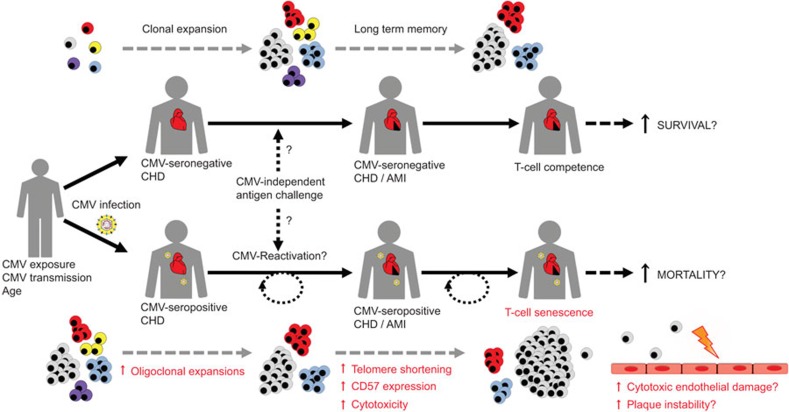
In conclusion, this new study further supports a link between T-cell immunosenescence and progression of coronary heart disease (Figure 1), even though several questions remain unanswered. Should we treat patients with coronary heart disease who express features of (premature) CD8+ T-cell senescence more aggressively? Can we use senescence markers to distinguish high-risk patients from otherwise ‘healthy aged' individuals? Although an inverse relationship between telomere length within leukocytes and risk of coronary heart disease has recently been settled in a large meta-analysis,11 telomere length of total leukocytes is not an ideal biomarker for cardiovascular outcome due to the significant variation in telomere length among healthy individuals, both at birth and over the life course.
Figure 1.
T-cell immunosenescence as a risk factor for patients with CHD. Chronic low-grade inflammation is a hallmark in patients with CHD, which may cause antigen-dependent or homeostatic T-cell proliferation (upper panel). In addition, CMV-seropositive patients with CHD and MI may have experienced episodes of virus reactivation and repeated CMV-antigenic challenge, leading to activation and oligoclonal expansion of virus specific memory T cells. This in turn could finally accelerate the process of gradual clonal loss (‘memory inflation') and progression of T-cell immunosenescence in CMV-seropositive CHD patients (lower panel), characterized by the occurrence of highly differentiated CD57-positive cytotoxic T cells with shortened telomeres. T-cell senescence could therefore potentiate systemic inflammation, but also induce local endothelial damage and plaque instability (by secretion of TNF-α and IFN-γ), which may impact the prognosis of patients following MI. CHD, coronary heart disease; CMV, cytomegalovirus; MI, myocardial infarction.
Further prospective studies are needed to validate T-cell senescence as a biomarker and possible risk marker for vascular disease progression. For this, large-scale studies that adjust for gender, age, CMV status and left ventricular function following myocardial infarction are needed. Studies in healthy volunteers or patients with lower disease burden would require far longer follow-up periods due to the much smaller number of expected clinical events. These long-needed studies could provide additional mechanistic insight and further guidance to develop individual risk-assessment strategies. Eventually, cytotoxic T cells could prove to be horsemen of the vascular apocalypse.
References
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, de Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Bolovan-Fritts CA, Trout RN, Spector SA. High T-cell response to human cytomegalovirus induces chemokine-mediated endothelial cell damage. Blood. 2007;110:1857–1863. doi: 10.1182/blood-2007-03-078881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EC, Moss PA, Frodsham P, Lehner PJ, Bell JI, Borysiewicz LK. CD8highCD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J Immunol. 1995;155:5046–5056. [PubMed] [Google Scholar]
- Lee SA, Sinclair E, Hatano H, Hsue PY, Epling L, Hecht FM, et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One. 2014;9:e89444. doi: 10.1371/journal.pone.0089444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Aiello AE. Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. Int J Epidemiol. 2009;38:775–787. doi: 10.1093/ije/dyn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva GM, Pachnio A, Kaul B, Morgan K, Huppert FA, Brayne C, et al. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12:381–387. doi: 10.1111/acel.12059. [DOI] [PubMed] [Google Scholar]
- Spyridopoulos I, Hoffmann J, Aicher A, Brummendorf TH, Doerr HW, Zeiher AM, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–1372. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]