Abstract
Polyreactive innate-type B cells account for many B cells expressing self-reactivity in the periphery. Improper regulation of these B cells may be an important factor that underlies autoimmune disease. Here we have explored the influence of self-reactive innate B cells in the development of collagen-induced arthritis (CIA), a mouse model of rheumatoid arthritis. We show that splenic marginal zone (MZ), but not B-1 B cells exhibit spontaneous IgM reactivity to autologous collagen II in naïve mice. Upon immunization with heterologous collagen II in complete Freund's adjuvant the collagen-reactive MZ B cells expanded rapidly, while the B-1 B cells showed a modest anti-collagen response. The MZ B cells were easily activated by toll-like receptor (TLR) 4 and 9-ligands in vitro, inducing proliferation and cytokine secretion, implying that dual engagement of the B-cell receptor and TLRs may promote the immune response to self-antigen. Furthermore, collagen-primed MZ B cells showed significant antigen-presenting capacity as reflected by cognate T-cell proliferation in vitro and induction of IgG anti-collagen antibodies in vivo. MZ B cells that were deficient in complement receptors 1 and 2 demonstrated increased proliferation and cytokine production, while Fcγ receptor IIb deficiency of the cells lead to increased cytokine production and antigen presentation. In conclusion, our data highlight self-reactive MZ B cells as initiators of the autoimmune response in CIA, where complement and Fc receptors are relevant in controlling the self-reactivity in the cells.
Keywords: arthritis; complement receptors; Fc receptors; marginal zone B cells, mice
Introduction
The presence of autoantibodies is a hallmark of many autoimmune diseases and B-cell depletion therapy has proved to ameliorate several autoimmune conditions,1,2 strengthening the view of a direct role for B cells in autoimmunity. The specific autoantibodies can be detected in the blood several years before the onset of clinical disease, indicating that the autoimmune disease process takes place several years before overt disease.3 This raises the question of what takes place in the interval between the etiological steps and the manifest autoimmune disease. Animal models for human autoimmune diseases, in which the induction phase of the disease can be explored, can be generated by immunizing susceptible species with self-antigens in adjuvant. A widely accepted model for rheumatoid arthritis (RA) is collagen-induced arthritis (CIA), which is elicited by immunizing susceptible mouse strains with native type II collagen (CII).4 CII is the major structural component of articular cartilage and CII genes are highly conserved in the genomic organization among mammals.5,6,7 Heterologous CII is superior to autologous CII in inducing the disease in mice, and bovine CII is often administered as the immunogen. By definition, the CII is a type II thymus-independent antigen as it contains, in resemblance with many infectious agents, repetitive epitopes that can cross-link B-cell receptors (BCR) inducing a T-cell independent antibody response.5,8 Moreover, B-cell deficient mice are resistant to CIA9 and mice lacking B-cell regulatory receptors such as Fc gamma receptor IIb (FcγRIIb) or complement receptors 1 and 2 (CR1/2) develop enhanced anti-CII antibody production and susceptibility to CIA,10,11 demonstrating the importance of B cells in CIA pathogenesis. Importantly, not only antibody production, but also antigen presentation and cytokine secretion with subsequent T-cell activation and polarization may well contribute to the B-cell pathogenicity.
A high proportion of innate type of B cells in the periphery, such as marginal zone (MZ) B cells and B-1 B cells, carry BCRs specific for self-antigens. These cells are not deleted by self-tolerance mechanisms and may exert biological effects in maintaining homeostasis. A series of autoantigens have been found to be the targets of the innate self-reactive B cells,12 but whether these cells can trigger an autoimmune response and present autoantigen to T cells is not fully known. We have previously demonstrated that MZ B cells are naturally reactive to heterologous CII and expand rapidly following immunization with this antigen for induction of CIA.13 The MZ B cells are distinguishable from the more common follicular (FO) B cells by their anatomical distribution in the splenic MZ of follicles and their high expression of IgM, CR1/2, FcγRIIb, and CD1d.13,14,15 They quickly mount immune responses to type I and type II thymus-independent antigens, inducing extensive BCR cross-linking and co-engagement of multiple pattern recognition receptors including toll-like receptors (TLRs), which enables them to quickly respond with IgM production in a first line of defense.16,17,18,19,20 Furthermore, owing to their pre-activated state and high surface expression class II MHC (MHCII), CD80 and CD86, the MZ B cells represent powerful activators of CD4+ T cells.19,21,22
Here we addressed whether B cells in CIA are true self-reactive, i.e. responsive to autologous CII (in a similar manner as to heterologous CII), and if so, how self-reactive MZ B cells contribute to the CII autoimmunity in comparison to FO B cells in terms of antibody production, proliferation, cytokine secretion, and antigen presentation to T cells. Additionally, we raised the question whether FcγRIIb and CR1/2 regulate the self-reactivity in MZ B cells, and if B-1 B cells also contribute to the CII autoreactivity in CIA. Our results reveal MZ B cells as major players in the initiation phase of CIA, not only by driving the B-cell response to autologous CII but also by the activation of CII-specific T-cells, activities that are regulated by CR1/2 and FcγRIIb.
Materials and Methods
Ethics statement
All animal experiments were approved by a local animal research ethics committee (permit numbers C71/11, C72/11, and N18/14). All animals were bred and maintained at the animal facilities at the Biomedical Centre, Uppsala University, Uppsala, Sweden. The mice were fed rodent chow and water ad libitum, and were negative for routine-screened pathogens.
Mice
Arthritis-susceptible DBA/1 mice were originally obtained from Bommice, Bomholt Gaard Ltd (Ry, Denmark). Mice deficient in CR1/223,24 and FcγRIIb25 had been back-crossed onto the DBA/1 background (>15 generations) and expanded by interbreeding as previously described.10,11 A transgenic mouse expressing a TCR specific for an immunodominant epitope on CII had previously been developed on the DBA/1 background (qCII24 mice).26 Male qCII24 mice were bred onto wild-type (WT) DBA/1 females and offspring heterozygous for the CII-reactive TCR were used for antigen-presenting experiments. The mice used in the experiments were of both sexes and 7–17 weeks old, with the exception of the qCII24 mice that were up to 37 weeks old.
Collagen type II
Bovine CII was prepared from bovine nasal cartilage by pepsin digestion followed by purification as described previously.27 Murine CII was purchased from Chondrex (Redmond, WA, USA) and the CII(245-270) peptide (GPLGPKGQTGEPGIAGFKGEQGPK) from Genscript (Piscataway, NJ, USA).
Immunization
Native BCII was dissolved in 0.01 M acetic acid with constant mixing overnight at 4 °C and was subsequently emulsified 1:1 in complete Freund's adjuvant (CFA; Difco, Detroit, MI, USA) to a final concentration of 1 mg/ml. The mice were immunized intradermally at the base of the tail with 50 μl emulsion, constituting a dose of 50 μg BCII. Control mice were immunized similarly but with 50 µg of ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO, USA) in CFA, or CFA only. For investigating the B-cell response to CII in CR1/2- and FcγRIIb-deficient mice, a low-dose immunization protocol was used (20 µg BCII).
ELISA
Naïve mice and mice immunized with BCII were bled from the tail vein at 5, 12, 21, and 28 days post-immunization and the serum was isolated. The measurement of anti-CII antibodies was done using ELISA as previously described.28 Briefly, 96-well MaxiSorp plates (NuncBrand Thermo Fischer Scientific, Roskilde, Denmark) were coated over night at 4 °C with BCII, MCII, or bovine serum albumin (BSA) fraction V (Merck KGaA, Darmstadt, Germany) and subsequently blocked with BSA followed by addition of the isolated serum, diluted ×25 or ×100 for CII-specific IgM or IgG detection, respectively. For detection of antigen-specific IgM and IgG, we used alkaline phosphatase-conjugated sheep anti-mouse IgM or IgG (Sigma-Aldrich). After each step, the plates were washed in phosphate-buffered saline (PBS) with 0.05% Tween (Sigma-Aldrich) and finally developed using ρ-nitrophenyl phosphate substrate (Sigma-Aldrich) diluted in diethanolamine buffer (1 mg/ml). The absorbance was measured at 405 nm using a spectrophotometer (VersaMax, Molecular devices, Sunnyvale, CA, USA). Cell culture supernatants were analyzed in the same way but were not diluted for analysis.
Preparation of cells
The spleen and pooled popliteal, inguinal, axillary, and brachial lymph nodes were collected post mortem from naïve and BCII-immunized mice at the indicated time points after immunization. Single-cell suspensions were prepared from individual mice by gently mashing the spleen and lymph nodes through a stainless steel mesh. To lyse splenic erythrocytes, ACK buffer (0.15 M NH4Cl (Merck KGaA), 0.1 mM EDTA (Merck KGaA), and 1.0 M KHCO3 (Sigma-Aldrich)) was added followed by a wash in PBS. Finally, the single-cell suspensions from both spleen and lymph nodes were diluted in DMEM (National Veterinary Institute, Uppsala, Sweden) supplemented with 100 U/ml penicillin (Sigma), 100 µg/ml streptomycin (Sigma), 2 mM glutamine (Sigma), 50 µM β-mercaptoethanol, and 10% fetal calf serum (Sigma); referred to as complete DMEM 10% FCS. The cells were kept on ice throughout the preparation. Cell counts and determination of viability were made using trypan blue (Gibco, Grand Island, NY, USA).
B-cell staining and sorting
For sorting of FO and MZ B cells, the splenocytes were first enriched for B cells using MACS magnetic cell separation according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). In brief, the splenocytes were labeled with anti-mouse CD43 MicroBeads (Miltenyi Biotec) at 4 °C for 30 minutes. Labeling was followed by washing and resuspension in MACS buffer (0.5% BSA, 2 mM EDTA (Merck KGaA) in PBS). The cells were subsequently loaded onto an LS column (Miltenyi Biotec) in a magnetic field and the effluent was collected. The CD43− B cells were labeled with fluorochrome-conjugated anti-mouse CD1d (clone 1B1; Biolegend, San Diego, CA, USA) and anti-mouse CD23 (clone B3B4; Biolegend) at 4 °C for 30 minutes. After washing, the cells were sorted into FO and MZ B cells using either a FACSVantage and FACSDiva software v. 5.1 (BD Biosciences, San Jose, CA, USA) or a BD FACSAriaIII and FACSDiva software v. 6.1.3 (BD Biosciences). FO B cells were defined as CD23highCD1dlow, whereas MZ B cells were defined as CD23lowCD1dhigh (for sorting strategy and purity, see Supplementary Figure S1). Any possible contamination of transitional B cells or CD5+ cells was considered negligible using this sorting protocol.13
For sorting of B-1 and MZ B cells, the splenocytes were labeled with fluorochrome-conjugated anti-mouse CD1d (clone 1B1; Biolegend) and anti-mouse B220 (clone RA3-6B2; Biolegend) together with biotinylated anti-mouse CD43 (clone eBioR2/60; eBioscience, San Diego, CA, USA) followed by fluorochrome-conjugated streptavidin (Biolegend), both steps at 4 °C for 30 minutes followed by washing. The cells were subsequently sorted into B-1 and MZ B cells using a BD FACSAriaIII and FACSDiva software v. 6.1.3 (BD Biosciences). B-1 B cells were defined as B220+CD43+, whereas MZ B cells were defined as B220+CD43−CD1dhigh (for sorting strategy and purity, see Supplementary Figure S2).
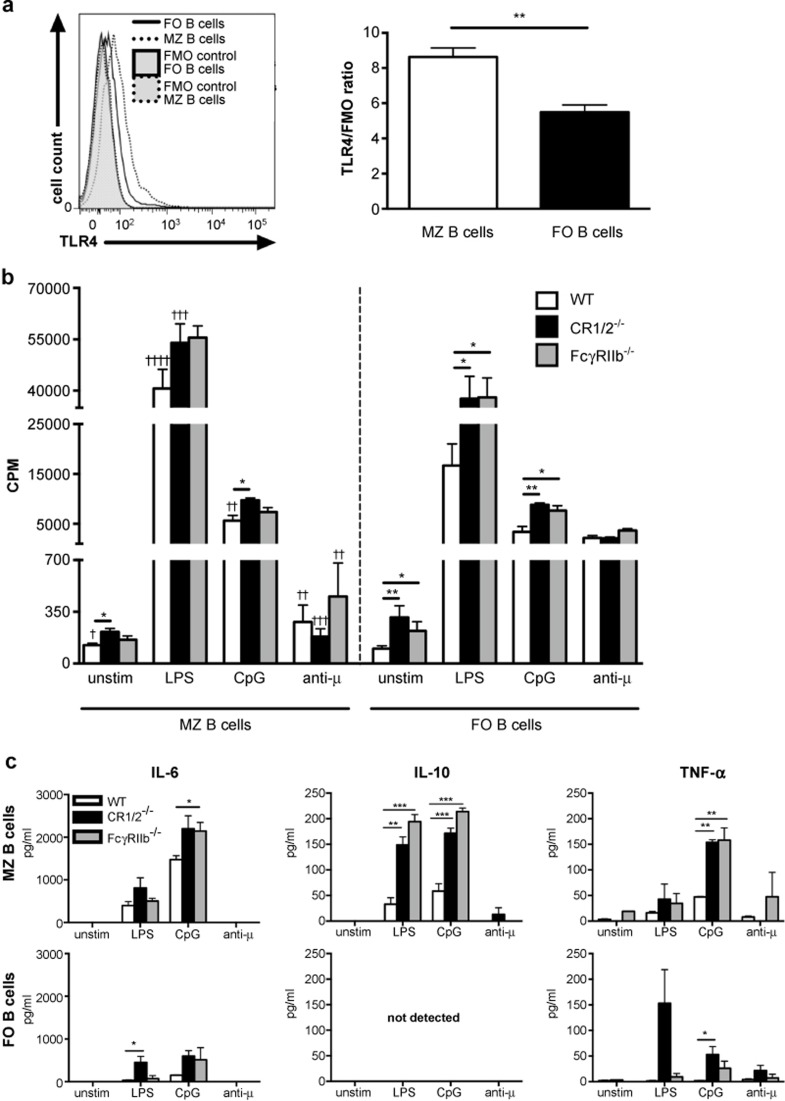
B cells were also stained for their surface expression of TLR4 (clone MTS510; BD Pharmingen, BD Biosciences), MHCII (clone KH116; BD Pharmingen, BD Biosciences), CD80 (clone 16-10A1; Biolegend), or CD86 (clone GL1; Biolegend) in combination with the antibodies to CD1d, CD23, and B220 described above. The cells were stained for 30 minutes at 4 °C and subsequently washed twice. Flow cytometry was performed on an LSRII flow cytometer (BD Biosciences), and B cells were gated as B220+ and further as MZ and FO B cells as described above. Flow cytometric data were analyzed using FlowJo software v. 7.6.4 or 9.6.1 (Treestar, Ashland, OR, USA). When analyzing the TLR4 expression, difference in background fluorescence between the two populations was corrected for by calculating a ratio between median fluorescence intensity of the population in the sample to the median fluorescence intensity of the corresponding population in the fluorescence-minus-one (FMO) control. The FMO control contains all fluorochromes in the panel except the one of interest, thus revealing any spillover or auto-fluorescence in that channel.
ELISpot assay
Ninety-six-well MaxiSorp plates were coated over night at 4 °C with BCII, MCII, or BSA at 10 μg/well. Splenocytes from naïve or BCII-immunized WT-, CR1/2-, and FcγRIIb-deficient mice and lymph node cells from WT mice were diluted in complete DMEM 10% FCS and added at 1 × 106 cells/well in replicate (4 wells per antigen for splenocytes; 1–4 wells per antigen for lymph node cells). The cells were incubated for 3 hours at 37 °C 5% CO2 followed by washing with PBS 0.05% Tween and subsequent addition of alkaline phosphatase-conjugated sheep anti-mouse IgM or IgG (Sigma-Aldrich) for 2 hours at room temperature. After additional washing, BCIP/NBT liquid substrate system (Sigma-Aldrich) was added at 50 μl/well. The reaction was stopped with deionized water after 1 hour and the spots were manually calculated under an inverted light microscope (Leitz Diavert, Wetzlar, Germany). A mean value was determined for each replicate and was expressed as the number of antibody-forming cells (AFC) per 106 cells.
The response to CII in FO, MZ, and B-1 B-cell subsets was analyzed by ELISpot as described above, with the exception that splenocytes from two or three mice (naïve or treated in the same way) were pooled to recover sufficient number of MZ or B-1 B cells after sorting (considered one observation). After sorting, the B cells were diluted in complete DMEM medium 10% FCS and plated at 0.5–1 × 106 cells/well in 1–4 wells. The cells were then cultured for 18–20 hours at 37 °C 5% CO2. After washing, alkaline phosphatase-conjugated sheep anti-mouse IgM or IgG was added and plates were incubated for 4 hours at room temperature before washing, addition of the substrate and counting of AFC as described above.
B-cell stimulation
MZ, FO, or B-1 B cells from naïve or immunized WT-, CR1/2-, and FcγRIIb-deficient mice were plated at 1 × 105 cells per well (2–4 wells per subset) in round-bottomed 96-well cell culture plates (BD Biosciences). The cells were cultured at 37 °C and 5% CO2 in complete DMEM 10% FCS alone or in the presence of lipopolysaccharide (LPS; Sigma-Aldrich) at 5 µg/ml, CpG-B DNA oligodeoxyribonucleotide 2006 (CpG; Hycult Biotech, Uden, the Netherlands) at 3 µg/ml or anti-µ F(ab')2 fragment-specific antibody (anti-µ; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at 5 µg/ml. After 2 days in culture, 100 µl culture supernatant was collected from each well, replicates pooled and stored at –20 °C until analysis for cytokines or antibodies. The wells were subsequently filled up with 100 µl complete DMEM 10% FCS and pulsed with 3H-thymidine (Perkin Elmer, Waltham, MA, USA) at 1 µCi per well for the last 18–22 hours of culture. Proliferation was measured using a liquid scintillation β-counter (Wallac 1450 MicroBeta Trilux, Perkin Elmer).
Cytokine production in the culture supernatants was analyzed using the Mouse Th1/Th2 10plex FlowCytomix Multiplex kit (eBioscience) according to the manufacturer's protocol. The assay was performed on an LSRII flow cytometer and the data analyzed using the software FlowCytomix Pro 3.0 (eBioscience).
Antigen presentation
CII-specific Vβ8.3 TCR+ T cells from the qCII24 mice were isolated from spleens using positive selection in MACS according to the manufacturer's protocol (Miltenyi Biotec). Briefly, the splenocytes were stained for 30 minutes at 4 °C in the dark using an anti-Vβ8.3 TCR antibody conjugated to PE (clone 1B3.3; BD Pharmingen). The cells were subsequently washed and further labeled with anti-PE MicroBeads (Miltenyi Biotec) for 30 minutes at 4 °C. After the secondary labeling, the cells were washed again and run over an LS separation column and the positive fraction was collected. After isolation, the Vβ8.3 TCR+ T cells were labeled with CFSE using the Vybrant® CFDA SE Cell Tracer kit (Molecular Probes, Leiden, the Netherlands) according to the manufacturer's protocol. In brief, 10 × 106 cells were labeled in 5 µM of the dye for 7 minutes followed by washing four times in complete DMEM 10% FCS. The cells were suspended in F-DMEM (National Veterinary Institute) supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µM β-mercaptoethanol, 2 mM L-glutamine and 5% FCS. The CFSE-labeled CII-specific T cells (5 × 104 cells per well) were plated to a round-bottomed 96-well cell culture plate already containing MZ or FO B cells (1 × 105 cells per well) from naïve or CII-immunized (5 days post-immunization) WT-, CR1/2-, or FcγRIIb-deficient mice. Control wells were set up with CII-specific T cells alone. Half of the wells were further stimulated with 1 µg CII(245-270) peptide per well. The volume of growth medium was 200 µl, and 2–4 wells were set up for each combination. The cells were incubated for 3 days at 37 °C and 5% CO2 before being analyzed using flow cytometry. To gate out viable CII-specific T cells, the culture replicates were pooled and stained for 30 minutes at 4 °C with anti-Vβ8.3 TCR-PE. After washing, 5 µl of the viability dye 7-AAD (Biolegend) was added to the samples 5–15 minutes before flow cytometry on an LSRII flow cytometer. For analysis the proliferation platform in FlowJo was used, where percent divided equals the percentage of the original population that went into cell division, while proliferation index describes the average number of divisions for a responding cell.
For antigen presentation in vivo, splenocytes from BCII-immunized WT mice (5 days post-immunization) were sorted into MZ and FO B cells and subsequently stimulated with 3 µg/ml CpG (Hycult Biotech) in complete DMEM 10% FCS over night at 37 °C 5% CO2. The cells were thereafter washed twice in PBS and 1 × 106 cells were adoptively transferred by intravenous or intraperitoneal route to WT or qCII24 mice. qCII24 mice receiving PBS only served as controls. The recipient mice were bled at 5, 12, and 21 days post-transfer, and the serum was analyzed for IgG anti-MCII antibodies by ELISA as described above. A fold change from the background antibody level in control serum was determined.
Statistical analysis
Statistical analyses were performed using Prism 4.03 or 6.0d from GraphPad Software, Inc. (La Jolla, CA, USA). Statistical differences between groups were determined using a two-tailed Student's t-test. When comparing MZ and FO B cells or B-1 and MZ B cells, a paired Student's t-test was used, for all other comparisons we used an unpaired test. All results are presented as mean + SEM. P-values < 0.05 were considered significant.
Results
Autoreactivity in CIA is initiated in the spleen and precedes a strong IgG response in lymph nodes and blood
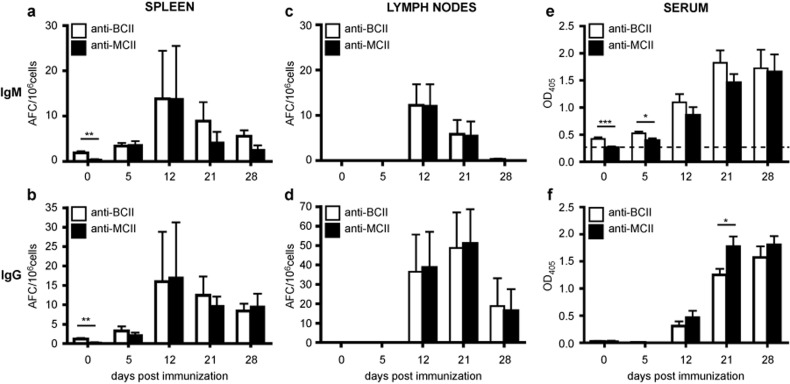
The early autoreactive B-cell response in CIA was investigated by analyzing the response to heterologous and autologous CII in the spleen, lymph nodes, and sera of naïve and BCII-immunized DBA/1 mice. In naïve mice (day 0), we could demonstrate naturally CII-reactive B cells in the spleen but not in the lymph nodes (Figure 1a–d). Both IgM- and IgG-positive B cells reactive to BCII and MCII were observed, but the number of BCII-reactive B cells was significantly higher than the number of B cells reactive to MCII (Figure 1a–b). After BCII immunization the splenic B-cell response to BCII and MCII developed in a similar manner and to a similar magnitude. Within the first week after immunization, IgM- and IgG-positive B cells reactive to both BCII and MCII were observed in the spleen and within the second week also in the lymph nodes (Figure 1a–d). The autoimmune response to CII progressed and showed the highest number of IgG+ B cells in the lymph nodes 3 weeks after immunization (Figure 1d). These results imply that BCII and MCII can be used interchangeable to detect the autoimmune B-cell response in lymphoid tissue of CIA mice. No B-cell response to the control protein BSA was detected in either naïve mice or after BCII immunization at any time point.
Figure 1.
The autoimmune response towards MCII parallels that to BCII and is initiated in the spleen. (a–d) The number of BCII- and MCII-specific IgM- and IgG-positive B cells was investigated by ELISpot in the spleen and lymph nodes of naïve and mice immunized with BCII for CIA (n = 6–8). (e–f) The serum levels of BCII- and MCII-specific IgM and IgG was measured by ELISA in naïve and BCII-immunized mice (n = 5–27). Data were presented as mean ± SEM and represent two to three independent experiments. The dotted line in e represents the mean antibody response against a control antigen (bovine serum albumin) over time. AFC = antibody-forming cells; BCII = bovine collagen type II; MCII = murine collagen type II; OD = optical density.
In sera of naïve mice IgM antibodies to BCII, but not to MCII, were detected (Figure 1e). At 5 days after BCII-immunized IgM antibodies to both BCII and MCII were found, but the levels of BCII-specific IgM were slightly higher. However, the IgM antibodies to MCII advanced and peaked at similar level as IgM anti-BCII 4 weeks after immunization. Serum IgG against BCII and MCII could not be detected in naïve mice but developed at similar levels in mice within 2 weeks after BCII immunization (Figure 1f). Notably, at 3 weeks (a time point when clinical arthritis starts to develop in the mice) the amount of IgG anti-MCII was significantly higher than to BCII (Figure 1f).
Self-reactive IgM+ B cells are regulated by CR1/2 but not by FcγRIIb
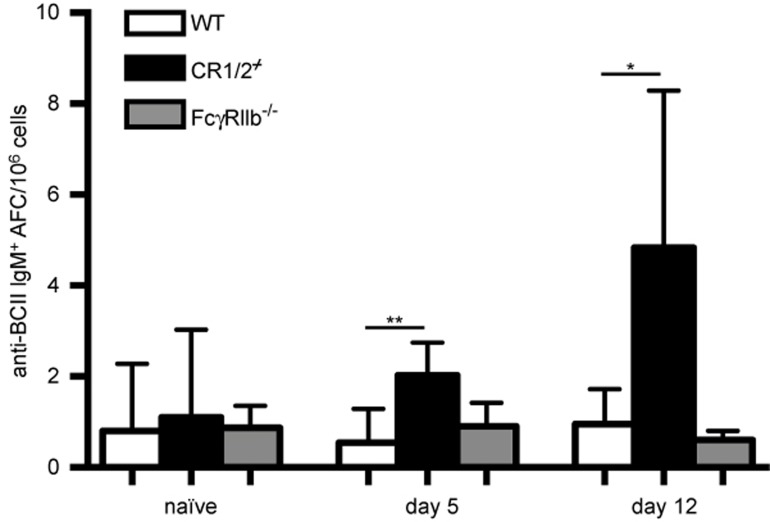
The next question we raised was to what extent the spontaneous and early splenic IgM+ B-cell response to CII was regulated by CR1/2 and FcγRIIb, inhibitory receptors shown to restrict the anti-CII antibody response and arthritis development in CIA.10,11 Indeed, we recognized that CR1/2 control the development of self-reactive B cells as the number of CII-specific IgM+ B cells was higher in the CR1/2-deficient mice than in the WT mice following immunization with 20 µg of BCII (Figure 2). In contrast, the frequency of IgM+ CII-reactive B cells in the FcγRIIb-deficient mice was of the same magnitude as that in the WT mice after immunization.
Figure 2.
CR1/2-deficient B cells show increased CII reactivity. The number of BCII-specific IgM-positive B cells was investigated by ELISpot in the spleen of naïve and low-dose BCII-immunized WT-, CR1/2-, and FcγRIIb-deficient mice (n = 5–6). Data were presented as mean + SEM and represent seven independent experiments. AFC = antibody-forming cells.
MZ B cells are prone to the autoreactive CII-response in the spleen
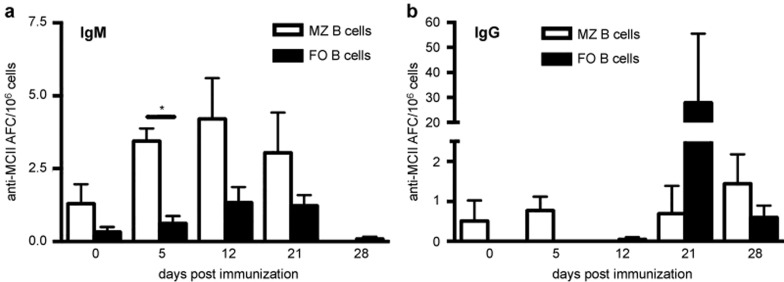
We then asked whether the MZ or the FO B-cell subset was responsible for the autoreactive response to CII in the spleen of naïve mice and following BCII immunization. Splenocytes were separated into FO and MZ B cells based on their expression of CD1d and CD23 and were subsequently analyzed for MCII-reactive clones using ELISpot. Albeit low in numbers, the MZ B cells demonstrated natural IgM+ CII reactivity in naïve mice, whereas the FO B cells tended not to display any CII reactivity (Figure 3a). Upon BCII immunization the IgM+ MZ B cells expanded rapidly, reaching elevated numbers on day 5, peaked on day 12 and declined thereafter. In contrast, low frequencies of IgM anti-CII FO B-cell clones were observed after immunization. Instead, high numbers of IgG+ CII-reactive FO clones could be detected 21 days after immunization (Figure 3b). The FO B cells reactive to CII switched to IgG production at a greater extent than CII-reactive MZ B cells as very few IgG+ MZ B cells were observed at all investigated time points. No B-cell response to the control protein BSA was detected in either naïve mice or after BCII immunization at any time point.
Figure 3.
The early autoreactive response in the spleen is driven by MZ B cells. Splenic B cells from naïve and BCII-immunized mice were separated into MZ and FO B cells by FACS and the number of MCII-reactive clones in either subset was investigated by ELISpot (n = 3–8). The number of IgM+ (a) and IgG+ (b) MCII-reactive MZ and FO B cells. Data were presented as mean + SEM and represent three independent experiments. AFC = antibody-forming cells.
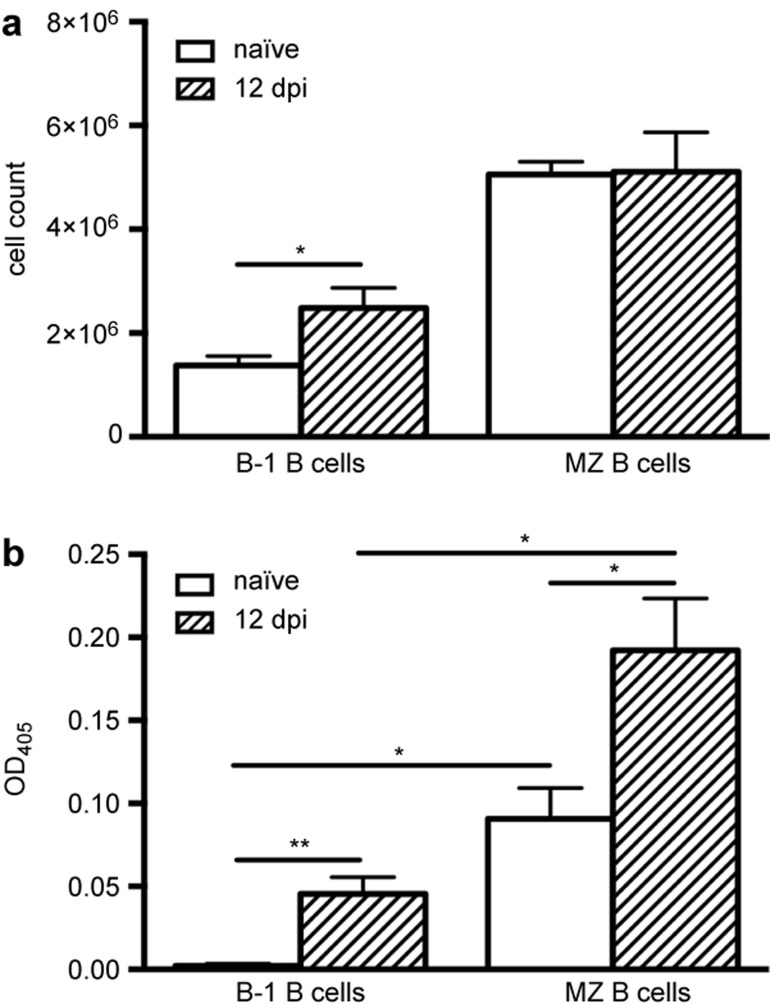
To elucidate whether the early CII reactivity in the spleen also included other innate-type B-cell subsets we investigated splenic B-1 B cells in naïve and BCII-immunized mice. We first observed that the total number of B-1 B cells in the spleen increased after BCII immunization, an effect not seen in the MZ B-cell population (Figure 4a). Nevertheless, the B-1 B cells from naïve or BCII-immunized mice did not show any CII reactivity by ELISpot (data not shown), implying that the increase in B-1 B cell numbers was not likely an antigen-specific effect. However, to see if the B-1 B cells could be stimulated to produce antibodies to CII we cultured the B-1 B cells with CpG in vitro and then analyzed the cell culture supernatants for IgM anti-CII antibodies using ELISA. Consistent with our previous data, MZ B cells from naïve mice secreted IgM anti-CII antibodies, but B-1 B cells from the same mice did not display any CII reactivity following CpG stimulation (Figure 4b). However, CpG-stimulated B-1 B cells from BCII-immunized mice secreted IgM anti-CII antibodies, although the response was much lower than from the MZ B cells (Figure 4b). Thus, of the two innate-type B-cell subsets in the spleen, the B-1 B cells contributed very little to the early CII-response and we therefore chose to focus our further investigations on the MZ B cells.
Figure 4.
Spontaneous CII reactivity in MZ B cells but not in B-1 B cells. Splenic B cells from naïve and BCII-immunized mice (n = 4–6) were separated into B-1 and MZ B cells by FACS. (a) The total cell count of splenic B-1 B cells and MZ B cells in naïve and BCII-immunized mice. (b) IgM anti-BCII antibodies in culture supernatants of B-1 B cells and MZ B cells stimulated with CpG. Data were presented as mean + SEM and represent three independent experiments. OD = optical density; dpi = days post-immunization.
MZ B cells proliferate and secrete cytokines upon TLR stimulation and are regulated by CR1/2 and FcγRIIb
Having revealed the initiating autoreactive response in MZ B cells, we wanted to understand the functional properties of MZ B cells, in comparison to FO B cells, in DBA/1 mice, in terms of TLR and BCR activation (to simulate CII and CFA exposure). We also addressed whether CR1/2 and FcγRIIb could control the response in the MZ B cells. Prior to the functional studies we determined the surface expression of TLR4, which was almost 60% higher on MZ B cells compared to FO B cells (Figure 5a). The TLR4 expression on FcγRIIb- or CR1/2-deficient MZ B cells did not differ from that of WT MZ B cells (data not shown). During cultivation we noted that WT MZ B cells were more prone to spontaneous proliferation in comparison to WT FO B cells (Figure 5b). Similarly, upon TLR4 or TLR9 stimulation with LPS and CpG respectively, the WT MZ B cells proliferated significantly better than the FO B cells, while BCR stimulation with anti-μ induced only modest proliferation in the MZ B cells compared to the FO B cells (Figure 5b). Furthermore, deficiency of CR1/2 in the MZ B cells led to increased proliferation compared to WT MZ B cells, both spontaneous and upon TLR9 stimulation. There was also a strong trend for increased proliferation in the CR1/2-deficient MZ B cells after stimulation through TLR4. MZ B cells lacking FcγRIIb showed a tendency of greater proliferation after TLR stimulation. Likewise, FO B cells deficient in CR1/2 or FcγRIIb had more spontaneous proliferation and were also more sensitive to TLR-stimulated proliferation compared to WT FO B cells. Notably, BCR-stimulated proliferation of MZ B cells, as well as of FO B cells, was not affected by CR1/2- or FcγRIIb- deficiency (Figure 5b). Concerning cytokine secretion, TLR4 and TLR9 activation of WT MZ B cells induced IL-6, IL-10, and TNF-α, while little or no cytokine secretion was detected in WT FO B cells upon similar stimulation (Figure 5c). Small amounts of interferon-γ were also detected in the MZ B-cell cultures stimulated through TLR9 (data not shown). The cytokine production induced by TLR4 and especially TLR9 activation was greatly enhanced in MZ B cells lacking CR1/2 or FcγRIIb, particularly IL-10 and TNF-α (Figure 5c). BCR stimulation of WT MZ B cells induced very little cytokine production, while likewise stimulated CR1/2- or FcγRIIb-deficient MZ B cells produced some IL-10 or TNF-α respectively (Figure 5c). IL-6 and/or TNF-α secretion was induced in CR1/2-deficient FO B cells upon TLR or BCR stimulation.
Figure 5.
TLR-activation is enhanced in MZ B cells and is controlled by CR1/2 and FcγRIIb. (a) Representative histogram and mean + SEM cell surface expression of TLR4 in MZ and FO B cells. Data represent two independent experiments (n = 5). (b) MZ and FO B cells from WT (n = 9), CR1/2- (n = 6) and FcγRIIb-deficient (n = 5) mice were stimulated through TLR4 (LPS), TLR9 (CpG), or BCR (anti-µ) for 3 days. The cells were pulsed with 3H-thymidine for the last 18–22 hours of culture and proliferation was measured as cpm. Data were presented as mean + SEM and represent six independent experiments. (c) Cell supernatants obtained from unstimulated and stimulated WT, CR1/2-, and FcγRIIb-deficient MZ and FO B-cell cultures in b were analyzed for secreted cytokines using a multiplex cytometric bead array (n = 3). Data were presented as mean + SEM and represent three independent experiments. *annotates statistically significant difference compared to the corresponding WT B-cell subset; †annotates statistically significant difference compared to FO B cells. FMO = fluorescence-minus-one control; cpm = counts per minute.
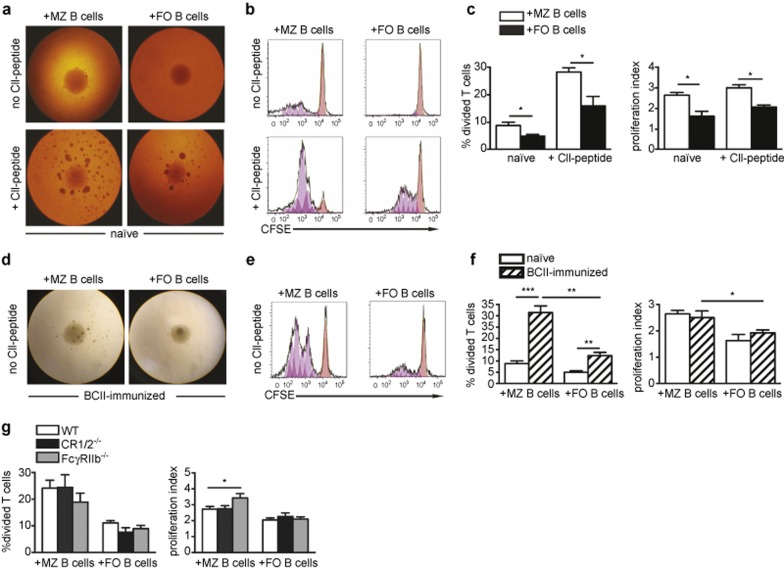
MZ B cells are potent presenters of CII to T cells
We then investigated if the MZ B cells had the capacity to present CII to T cells in vitro. Thus, CFSE-labeled CII-specific T cells from the transgenic qCII24 mice were cultured with MZ or FO B cells from naïve or BCII-immunized WT mice. Antigen presentation was assessed as T-cell proliferation measured with flow cytometry. The results showed that MZ B cells from naïve mice could induce proliferation of the CII-specific T cells, which was improved when a CII(245-270) peptide was added to the cultures (Figure 6a–c). Naïve FO B cells could also present the CII, but the MZ B cells were superior in driving the antigen-specific T-cell proliferation. This effect was seen both for percent divided and proliferation index. When investigating B cells that had been primed with CII in vivo (5 days after BCII immunization), we found that the MZ B cells were very potent antigen-presenting cells, inducing T-cell proliferation three times more efficiently than the naïve MZ B cells as well as their CII-primed FO B cell counterpart (Figure 6d–f). The proliferation index for T cells co-cultured with CII-primed MZ B cells was also higher than when co-cultured with FO B cells (Figure 6f, right).
Figure 6.
MZ B cells are superior to FO B cells in presenting autoantigen to T cells and show enhanced capacity when deficient in FcγRIIb. CFSE-labeled Vβ8.3+ CII-specific T cells were cultured with splenic MZ or FO B cells from naïve or BCII-immunized mice, with or without addition of CII(245-270)-peptide. (a) Cultures of Vβ8.3+ CII-T cells and B-cell subsets from naïve mice at 3 days with or without CII-peptide (×4 magnification). (b) Cultures in a shown as histograms of CFSE dilution. The orange peaks show undivided cells, the pink peaks show generations of cells in division. (c) Percentage of CII-specific T cells that went into division (left) and the proliferation index of the dividing T cells (right) after culture as described in a (n = 4). Data were presented as mean + SEM and represent three independent experiments. (d) Cultures of Vβ8.3+ CII-specific T cells and B-cell subsets from BCII-immunized mice (×4 magnification). (e) Cultures in d shown as histograms of CFSE dilution. The orange peaks show undivided cells, the pink peaks show generations of cells in division. (f) Percentage of CII-specific T cells that went into division (left) and the proliferation index of the dividing T cells (right) after culture as described in a and d (n = 4). Data were presented as mean + SEM and represent five independent experiments. (g) Percentage of CII-specific T cells that went into division (left) and the proliferation index of the dividing T cells (right) after culture with MZ or FO B cells from the spleen of BCII-immunized WT-, CR1/2-, or FcγRIIb-deficient mice (n = 5–9). Data were presented as mean + SEM and represent seven independent experiments.
We also explored whether CR1/2 or FcγRIIb affected the antigen presentation by the MZ B cells. When CR1/2- and FcγRIIb-deficient MZ or FO B cells were primed with CII in vivo, the percentage of T cells that proliferated was the same as in cultures with WT counterparts (Figure 6g; left). However, MZ B cells lacking FcγRIIb showed more efficient antigen presentation by stimulating the T cells to go through more divisions than WT MZ B cells (Figure 6g; right).
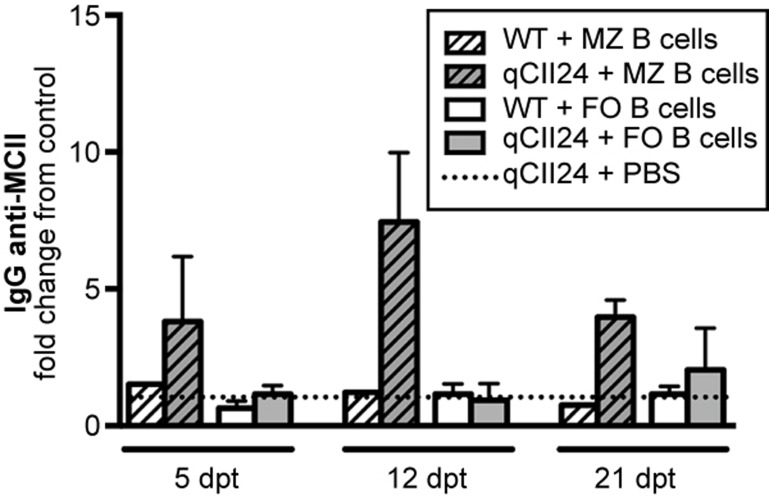
To verify the antigen-presenting capacity of the MZ B cells in vivo, we adoptively transferred CpG-stimulated MZ B cells, or FO B cells as a comparison, from BCII-immunized mice to qCII24 mice or WT recipients. qCII24 mice injected with PBS served as controls. At 5, 12, and 21 days after transfer the recipient mice were screened for IgG anti-MCII antibodies in the blood. Interestingly, qCII24 mice receiving MZ B cells displayed MCII-specific IgG levels above background levels measured in the qCII24 controls given PBS (figure 7). The IgG anti-MCII antibodies peaked at 12 days and decreased at 21 days after transfer. Similar increase in IgG anti-MCII antibodies was not seen in WT recipients, nor in qCII24 and WT mice receiving FO B cells. These data suggest that self-reactive MZ B cells can present CII and activate CII-specific T cells in vivo.
Figure 7.
MZ B cells can efficiently present autoantigen in vivo. CpG-stimulated MZ or FO B cells from BCII-immunized WT mice were adoptively transferred to WT or qCII24 mice. Sera from the recipient mice were analyzed for IgG anti-MCII antibodies by ELISA and a fold change from the background antibody level in serum from a qCII24 mouse receiving PBS only was determined. Data from one representative experiment out of two were presented as mean + SEM (n = 1–3). dpt = days post-transfer.
Discussion
Previous work by us demonstrated that MZ B cells are spontaneously reactive to heterologous CII and are initiators of the immune response in CIA.13 In this article, we report the analysis of the autoimmune response to autologous CII in MZ B cells and by what mechanisms these cells may contribute to autoimmune arthritis and how they are regulated.
We show that the naturally BCII reactivity in MZ B cells from naïve mice13 also includes a spontaneous reaction to autologous MCII. The parallel response to both heterologous and autologous CII is most likely due to the fact that there are only minor differences in the amino acid sequence between the two proteins, with the immunodominant epitope of CII(257-270) differing in one single amino acid residue between BCII and MCII.6,7 The kinetics of the B-cell response to MCII in comparison with BCII is basically similar in BCII-immunized mice, although the response to BCII is initially stronger. This demonstrates that BCII immunization trigger MCII autoimmunity to an equal degree as to the immunogen.
CII is classified as a type II thymus-independent antigen and would therefore potently and preferentially activate innate B cells.18,29 Indeed, the BCII immunization amplified the naturally occurring CII-reactive IgM+ MZ B-cell population, whereas the few CII-reactive IgG+ MZ B cells present in naïve mice did not expand. This implies that the self-reactive IgM+ MZ B cells do not preferentially class switch but rather develop quickly into short-lived IgM-producing plasma cells.19 In comparison, naive splenic B-1 B cells did not show any CII reactivity and even though B-1 B cells in immunized mice expanded in numbers they showed only IgM anti-CII upon CpG activation and at levels significantly lower than from MZ B cells from the same mice. These findings underline the MZ B cells as initiators of the splenic autoimmune response to CII and the major producers of early IgM anti-CII antibodies.
The spontaneous BCII reactivity, as well as the one induced by the BCII immunization, was verified by the IgM anti-CII antibodies in the sera of naïve and BCII-immunized mice. The IgM antibodies may not be pathogenic per se, but they are certainly of importance given that mice deficient in IgM are resistant to CIA.30 By binding to the recently identified FcµR on B cells, secreted IgM can enhance B-cell survival and the following humoral immune response.31 IgM also activates complement, and complement-opsonized immune complexes containing CII can be deposited on FO dendritic cells, thereby facilitating a germinal center reaction with affinity maturation and isotype switching of CII-reactive FO B cells. Indeed, this initial IgM-dominated autoimmunity observed in the spleen preceded the development of numerous IgG+ anti-CII FO B cells, mainly residing in the lymph nodes. This number peaked at 3 weeks after immunization, indicating that class switching and affinity maturation occurs mainly after breakage of T-cell tolerance to CII. Accordingly, IgG anti-CII antibodies was first detected in serum 12 days after immunization and peaked at 3 weeks, timing the expectance of clinical disease development. In addition, the serum levels of IgG anti-MCII were even higher than the IgG anti-BCII antibodies, further indicating a complete breakage of tolerance to MCII by this time point.
Regulation by CR1/2 and FcγRIIb is substantial to avoid autoimmunity,32,33,34,35,36,37 and we show here that CR1/2-deficiency allows the expansion of the early splenic CII-reactive IgM+ B cells in CIA, an effect likely contributing to the enhanced IgM anti-CII antibodies and arthritis development reported in CR1/2-deficient DBA/1 mice.11 However, the development of the early CII-reactive IgM+ B cells was not affected in the BCII-immunized FcγRIIb-deficient mice, suggesting that FcγRIIb is not involved in the early phase of CIA when expansion of CII-specific B cells take place.
The natural ability of MZ B cells to recognize and react to CII together with their rapid response to innate signals gives them an advantage in CIA when responding to CII and the various TLR ligands in the mycobacterial components of CFA. Our present data suggest that BCR signaling alone is insufficient for full MZ B cell activation, but rather needs to synergize with strong signals from other receptor families, such as the TLRs.15,17,19,38,39,40,41 High levels of TLRs expressed on cells can impact the magnitude of the response to ligands, and the greater levels of the TLR4 protein on MZ B cells compared to FO B cells shown by us in the DBA/1 mice likely explain the greater proliferative response in the MZ B cells upon LPS stimulation. Notably, the TLR activation, especially through TLR9, contributed to secretion of cytokines in the MZ B cells but not in the FO B cells. Particularly pro-inflammatory (TNF and IL-6) and immune regulatory (IL-10) cytokines were produced. These are critical regulators of inflammation due to their respective ability to promote Th17 and inhibit T regulatory cells, demonstrating that MZ B cells can contribute to an inflammatory milieu.
As MZ B cells have a low threshold for activation and are long-lived, they are potentially more harmful than cells with a shorter life span and therefore need tight regulation. Indeed, CR1/2 and FcγRIIb regulated the proliferative and cytokine reaction triggered by TLR in the MZ B cells. In contrast, BCR-stimulated responses in the MZ B cells were not affected by CR1/2- or FcγRIIb-deficiency. FO B cells were also controlled by the inhibitory receptors, especially TLR-stimulated proliferation.
Antigen presentation is a critical step in activating T cells and, in the case of autoimmunity, breaking of self-tolerance. Here, we demonstrate that CII-primed MZ B cells can efficiently present CII and induce proliferation of CII-specific T cells. FO B cells also have this ability, but are far inferior to the MZ B cells, which have the advantage of the higher levels of MHCII, CD80, and CD86 as demonstrated by us (Supplementary Figure S3) and others.19,21 The co-stimulatory molecules regulate the CD4+ T-cell expansion (reviewed by Lenschow and colleagues42). Consistent with reports of MZ B cells as important antigen-presenting cells in murine models for type 1 diabetes and systemic lupus erythematosus,38,43,44 we also show that CII-primed MZ B cells can break tolerance in naïve cognate T cells in vivo giving rise to IgG anti-CII antibodies. However, this response was not sufficient to induce clinical arthritis in the recipient mice. Furthermore, it was also interesting to note that the antigen-presenting capacity of MZ B cells is regulated by FcγRIIb, while CR1/2 do not have such effect. Thus, the CII-primed FcγRIIb-deficient MZ B cells produced more cell divisions in the self-reactive T cells compared with the WT MZ B cells. The reason for this we believe is due to the fact that FcγRIIb can act as a dominant negative inhibitor of BCR endocytosis, preventing the ability of B cells to generate MHCII-bound TCR ligands.45 Consequently, FcγRIIb-deficient MZ B cells may be able to endocytose more CII than WT MZ B cells resulting in further MHCII-bound CII peptides to T cells. Interestingly, this effect was only demonstrated in the MZ B cells, as the antigen-presenting properties of FO B cells were not affected by FcγRIIb-deficiency, possibly due to less expression of CII-specific BCR. In view of the fact that FcγRIIb expression is downregulated on B cells in RA patients46 our finding suggests an enhanced autoantigen-presenting capacity by B cells in RA.
Understanding the important role of MZ B cells in autoimmunity, the key question is how central they are for the outcome of clinical disease in CIA. A deletion of MZ B cells in vivo would answer this question. However, this is experimentally challenging, as there is no single marker that selectively target MZ B cells. One may also consider if the MZ B cells, rather than merely being initiators of the autoimmune response, are actually exerting a B-cell regulatory function by their early anti-CII IgM and IL-10 secretion,47,48 although further mechanistic experiments are needed to distinguish this.
In conclusion, the superior role of MZ B cells in antigen-recognition, IgM production, cytokine secretion, and antigen-presenting function in CIA marks MZ B cells as important players in the series of events leading toward autoimmune arthritis. Furthermore, CR1/2 and FcγRIIb are potent regulators of self-reactive MZ B cells.
Acknowledgments
This work was supported by grants from the Swedish Research Council, the King Gustaf V:s 80-years Foundation, the Swedish Rheumatism Association and the O. & E. Ericsson's Foundation. We thank Cecilia Carnrot for technical assistance. Flow cytometric experiments and sorting were performed with equipment maintained by the Science for Life Lab BioVis Platform, Uppsala.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Figures 1 and 2 show the sorting strategy and post-sort purity checks for marginal zone, follicular, and B-1 B cells.
Supplementary Figure 3 shows the surface expression of CD80, CD86, and class II MHC on marginal zone and follicular B cells, as determined by flow cytometry.
Supplementary Information
References
- Gurcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9:10–25. doi: 10.1016/j.intimp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Mariño E, Silveira PA, Stolp J, Grey ST. B cell-directed therapies in type 1 diabetes. Trends Immunol. 2011;32:287–294. doi: 10.1016/j.it.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Rantapää-Dahlqvist S, de Jong BAW, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsaranta M, Toman D, de Crombrugghe B, Vuorio E. Mouse type II collagen gene. Complete nucleotide sequence, exon structure, and alternative splicing. J Biol Chem. 1991;266:16862–16869. [PubMed] [Google Scholar]
- Rosloniec EF, Whittington KB, Brand DD, Myers LK, Stuart JM. Identification of MHC class II and TCR binding residues in the type II collagen immunodominant determinant mediating collagen-induced arthritis. Cell Immunol. 1996;172:21–28. doi: 10.1006/cimm.1996.0210. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Brand DD, Tang B, Rosloniec EF, Stuart JM, Kang AH, et al. Analog peptides of type II collagen can suppress arthritis in HLA-DR4 (DRB1*0401) transgenic mice. Arthr Res Ther. 2006;8:R150. doi: 10.1186/ar2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA). Clin Exp Immunol. 1998;111:521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinau S, Martinsson P, Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct Fc gamma receptors. J Exp Med. 2000;191:1611–1616. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson KE, Andrén M, Diaz de Ståhl T, Kleinau S. Enhanced susceptibility to low-dose collagen-induced arthritis in CR1/2-deficient female mice – possible role of estrogen on CR1 expression. FASEB J. 2009;23:2450–2458. doi: 10.1096/fj.08-125849. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Carnrot C, Prokopec KE, Rasbo K, Karlsson MCI, Kleinau S. Marginal zone B cells are naturally reactive to collagen type II and are involved in the initiation of the immune response in collagen-induced arthritis. Cell Mol Immunol. 2011;8:296–304. doi: 10.1038/cmi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- Bankoti R, Gupta K, Levchenko A, Stager S. Marginal zone B cells regulate antigen-specific T cell responses during infection. J Immunol. 2012;188:3961–3971. doi: 10.4049/jimmunol.1102880. [DOI] [PubMed] [Google Scholar]
- Diaz de Stahl T, Dahlstrom J, Carroll MC, Heyman B. A role for complement in feedback enhancement of antibody responses by IgG3. J Exp Med. 2003;197:1183–1190. doi: 10.1084/jem.20022232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- Brand DD, Myers LK, Whittington KB, Latham KA, Stuart JM, Kang AH, et al. Detection of early changes in autoimmune T cell phenotype and function following intravenous administration of type II collagen in a TCR-transgenic model. J Immunol. 2002;168:490–498. doi: 10.4049/jimmunol.168.1.490. [DOI] [PubMed] [Google Scholar]
- Miller EJ. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972;11:4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Magnusson SE, Andren M, Nilsson KE, Sondermann P, Jacob U, Kleinau S. Amelioration of collagen-induced arthritis by human recombinant soluble FcgammaRIIb. Clin Immunol. 2008;127:225–233. doi: 10.1016/j.clim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- Zheng B, Zhang X, Guo L, Han S. IgM plays an important role in induction of collagen-induced arthritis. Clin Exp Immunol. 2007;149:579–585. doi: 10.1111/j.1365-2249.2007.03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci USA. 2012;109:E2699–E2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, et al. Deletion of Fcγ receptor IIB renders H-2b mice susceptible to collagen-induced arthritis. J Exp Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland S, Ravetch JV. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- Prodeus AP, Goerg S, Shen L-M, Pozdnyakova OO, Chu L, Alicot EM, et al. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- Dreja H, Annenkov A, Chernajovsky Y. Soluble complement receptor 1 (CD35) delivered by retrovirally infected syngeneic cells or by naked DNA injection prevents the progression of collagen-induced arthritis. Arthritis Rheum. 2000;43:1698–1709. doi: 10.1002/1529-0131(200008)43:8<1698::AID-ANR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Goodfellow RM, Williams AS, Levin JL, Williams BD, Morgan BP. Soluble complement receptor one (sCR1) inhibits the development and progression of rat collagen-induced arthritis. Clin Exp Immunol. 2000;119:210–216. doi: 10.1046/j.1365-2249.2000.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiniene E, Bremell T, Tarkowski A. Complement depletion aggravates Staphylococcus aureus septicaemia and septic arthritis. Clin Exp Immunol. 1999;115:95–102. doi: 10.1046/j.1365-2249.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino E, Batten M, Groom J, Walters S, Liuwantara D, Mackay F, et al. Marginal-zone B-cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-cells. Diabetes. 2008;57:395–404. doi: 10.2337/db07-0589. [DOI] [PubMed] [Google Scholar]
- Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov AV, Swanson CL, Troy S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008;180:3882–3888. doi: 10.4049/jimmunol.180.6.3882. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Yamada H, Smoot D, Sneed R, Lees A, Mond JJ. Comparative in vitro analysis of proliferation, Ig secretion, and Ig class switching by murine marginal zone and follicular B cells. J Immunol. 1993;150:2737–2745. [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Silveira PA, Grey ST. B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab. 2006;17:128–135. doi: 10.1016/j.tem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Niu H, Zheng YY, Morel L. Autoreactive marginal zone B cells enter the follicles and interact with CD4+ T cells in lupus-prone mice. BMC Immunol. 2011;12:7. doi: 10.1186/1471-2172-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minskoff SA, Matter K, Mellman I. Cutting edge: FcγRII-B1 regulates the presentation of B cell receptor-bound antigens. J Immunol. 1998;161:2079–2083. [PubMed] [Google Scholar]
- Prokopec KE, Rhodiner M, Matt P, Lindqvist U, Kleinau S. Downregulation of Fc and complement receptors on B cells in rheumatoid arthritis. Clin Immunol. 2010;137:322–329. doi: 10.1016/j.clim.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthr Res Ther. 2012;14:R32. doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheum. 2010;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.