Abstract
Objective
Direct visualization of the velopharynx and, in particular, the levator muscle is particularly important in the assessment of velopharyngeal function and normal speech production. The purpose of this study is to demonstrate the development of a static and dynamic magnetic resonance imaging protocol for evaluation of velopharyngeal structures and function.
Methods
A high-resolution, T2-weighted turbo-spin-echo three-dimensional anatomical scan (sampling perfection with application optimized contrasts using different flip angle evolution) was used to acquire a large field of view covering the velopharyngeal anatomy. Dynamic speech assessment was obtained using a fast-gradient echo, fast low-angle shot, multi-shot spiral technique to acquire 15.8 frames per second (fps) of the sagittal and oblique coronal image planes.
Results
Using a three-dimensional data set, as opposed to two-dimensional data, the full contour of the levator muscle can be appreciated. Dynamic images were obtained at 15.8 fps in the sagittal and oblique coronal planes, enabling visualization of the movements of the velum, posterior pharyngeal wall, lateral pharyngeal walls, and levator muscle during speech.
Conclusions
A three-dimensional magnetic resonance imaging sequence, such as that used in the present study, may provide better analyses and more precise measurements. A dynamic fast low-angle shot sequence allows for visualization of the levator muscle and the velum during speech at a high image rate. This protocol could have a significant impact in improving the process of visualizing pathology and promoting clinical treatment plans for individuals born with cleft lip and palate.
Keywords: cleft palate, dynamic MRI during speech, MRI
The complex and dynamic nature of the velopharynx makes it a difficult area to assess, particularly during speech. The velopharynx is deep within the head and surrounded by air spaces, muscles, and bone. The main muscle for velar elevation is the levator veli palatini (levator) muscle, which courses from the base of the skull and converges into the midline of the velum. In normal anatomy, the levator muscle functions to elevate and retract the velum to provide a seal against the posterior pharyngeal wall.
Direct visualization of the velopharynx and, in particular, the levator muscle is important in the assessment of speech. Despite advances in surgical management, many children born with cleft lip and palate continue to demonstrate velopharyngeal dysfunction (VPD) following primary palatoplasty (McWilliams, 1990). The most commonly used clinical methods for visualizing the velopharynx are nasendoscopy and multi-view videofluoroscopy (Skolnick and Cohn, 1989; Witzel and Stringer, 1990; Sader et al., 1994; Hess et al., 1996). Nasendoscopy is invasive and can provide distorted depth cues (Pigott, 2002). Videofluoroscopy uses ionizing radiation, thus limiting the safety of prolonged and repeated use for assessment and follow-up, and can present with errors, particularly due to misalignment of the patient and measurement error (Birch et al., 1999). Neither method allows for visualization of underlying muscles, including the levator muscle. In contrast, magnetic resonance imaging (MRI) offers an imaging method that is noninvasive, easily repeatable, allows for views of underlying musculature, and can be reproduced easily (Beer et al., 2004; Kuehn et al., 2004).
Imaging protocols have been developed to improve visualization of the levator muscle. By using an oblique coronal MRI image plane, the levator muscle can be sampled along the long axis of the muscle (Ettema et al., 2002; Kuehn et al., 2004). Such views have enabled assessment of morphologic variations between individuals with cleft palate and those with normal anatomy. Dynamic MRI in the oblique coronal image plane is the only imaging modality that enables visualization of the levator muscle during speech production using a safe, noninvasive method.
Beer et al. (2004) used a turbo-spin-echo (TSE) “zoom” sequence using a 1.5 Tesla Gyroscan NT scanner (Philips, Best, The Netherlands) to assess velopharyngeal function among seven patients with perceived nasality. Comparisons to videofluoroscopy suggested that with continued development, MRI with near real-time temporal resolution could be used for accurately identifying closure patterns, with the benefit of no ionizing radiation. Noted as a limitation of the Beer et al. (2004) study is that only six images per second were obtained compared with the real-time nature of traditional methods (nasendoscopy and videofluoroscopy) that produce a video display. Fast gradient-echo sequence, echo-planar imaging has been used to produce images in near real-time (Wein et al., 1991; Suto et al., 1993; Gilber et al., 1998; Anagnostara et al., 2001). These sequences suffer from low signal-to-noise ratios and are susceptible to artifacts. Frame rates under 10 frames per second (fps) are particularly disadvantageous in the region of the velopharynx where elevation rates may occur under 100 ms (Kuehn, 1976).
Gated acquisition procedures have been used to increase imaging speeds (Kane et al., 2002; Shinagawa et al., 2005). These methods are sufficient for cyclic processes that have a high degree of repetition across cycles (e.g., cardiac cycles); however, they are inadequate for analyzing the temporal variations in the elevation of the velum across multiple speech samples. Data suggest that even simple, complete words show significant variations in movements of speech structures and muscles between repetitions (NessAiver et al., 2006). True fast imaging with steady-state precession (True FISP) imaging has been used to assess dynamic images of the velopharyngeal mechanism (Atik et al., 2008; Drissi et al., 2011). True FISP is a dynamic imaging process that preserves a steady-state signal and requires a relatively homogeneous magnetic field. Imaging typically has a relatively high signal-to-noise ratio and poor rate of image acquisition.
Research continues to emphasize the need for a clinical dynamic tool for assessing velopharyngeal function (Shinagawa et al., 2005; Atik et al., 2008; Kao et al., 2008; Bae et al., 2011a; Silver et al., 2011). An ideal imaging protocol for children would provide three-dimensional (3D) analysis of the anatomy as well as a functional assessment that can be obtained relatively quickly (e.g., under a minute). Dynamic image sequences should allow for speech samples (e.g., word- or sentence-level productions) as opposed to prolonged speech sounds, which most clinical studies of the velopharynx to date have used (McGowen et al., 1992; Özgür et al., 2000; Vadodaria et al., 2000; Ha et al., 2007; Atik et al., 2008; Kao et al., 2008; Drissi et al., 2010; Tian et al., 2010). The sampling planes of the functional imaging should be along the plane of velopharyngeal closure, which in normal anatomy is in the direction of the oblique coronal image plane. Studies using MRI for functional analysis at word- or sentence-level production have focused exclusively on the midsagittal and axial image planes (Kane et al., 2002; Bae et al., 2011a; Drissi et al., 2011). Neither of these planes can display the nature of the primary muscle function (i.e., the levator muscle).
The purpose of this study is to demonstrate the development and implementation of a static and dynamic MRI protocol for assessing velopharyngeal structures and functions. Ten adults with normal anatomy were examined to determine a method for volumetric assessment of the levator muscle and velopharyngeal function. This study demonstrates a potential diagnostic imaging method to assist in identifying the cause of velopharyngeal dysfunction, determining whether surgery is warranted, and conducting preoperative planning that is specific to the individual’s anatomy and function.
Method
Subjects
In accordance with the local institutional review boards, 10 healthy male subjects with normal anatomy between 19 and 24 years of age (mean, 21 ± 1.5 years) participated in the study. All subjects were white and were native English speakers. Subjects indicated no history of craniofacial anomalies, swallowing disorders, sleep apnea, or neurologic disorders that might affect measures of structures and their movements. All subjects were judged informally by a speech-language pathologist to have normal oral-to-nasal resonance balance. All subjects had a body mass index under 27 (mean, 23 ± 2.4) to control for possible variations in the pharyngeal airway as a result of obesity.
Magnetic Resonance Imaging
Subjects were scanned in the supine position using a Siemens 3 Tesla Trio (Erlangen, Germany) MRI scanner and a 12-channel Siemens Trio head coil. A Velcro-fastened elastic strap was positioned above the level of the nasion and fastened to the head coil to minimize motion during the scanning session. Simultaneous speech recordings were obtained following previously described methods (Sutton et al., 2010; Bae et al., 2011a). Subjects wore an MR-compatible headset with an attached optical microphone (Dual Channel-FOMRI; Optoacoustics Ltd., Or Yehuda, Israel) to actively cancel the loud MR gradient noise while preserving the speech samples.
The imaging protocol (Table 1) was developed for research analyses using a non-Cartesian spiral sequence that is supported by the Siemens 3 Tesla Trio scanner. This is not a standard protocol currently available on clinical MRI scanners and required custom pulse-sequence programming for the dynamic imaging sequence. A high-resolution, T2-weighted TSE, variable flip angle, 3D anatomical scan called SPACE (short for “sampling perfection with application optimized contrasts using different flip angle evolution”) was used to acquire a large field of view covering the oropharyngeal anatomy (256 × 192 × 153.6 mm) with 0.8-mm isotropic acquired resolution with an acquisition time of slightly less than 5 minutes (4 minutes 52 seconds). The field of view and matrix sizes were identical between subjects. Parameters include a repetition time of 2500 milliseconds, echo time of 268 milliseconds and an echo-train length of 171, and parallel imaging acceleration with a factor of two in both phase encode and slice direction. Amira 3.1.1 software (Visage Imaging GmbH, Berlin, Germany) was used to resample the data to visualize the levator muscle from the origin to the insertion using previously reported methods (Perry et al., 2011).
TABLE 1.
MRI Protocol, 3 Tesla
| Static 3D MRI Parameters* | Dynamic MRI Parameters | |
|---|---|---|
| Pulse sequence | SPACE: T2 turbo-spin-echo. Variable flip angle | FLASH: GRE six-shot spiral |
| Field of view | 256 × 192 × 153.6 mm3 | 240 × 240 × 8 mm3 |
| Repetition time | 2500 ms | 9 ms |
| Echo time | 268 ms echo train length: 171 | Alternating between 1.3 and 1.8 ms |
| Resolution | 0.8 mm isotropic | 1.875 × 1.875 × 8 mm3 |
| Length of scan | 4 min 52 s for 1 static volume | 50.5 s for 799 native frame rate images or 1515 sliding window images at 30 fps |
3D = three-dimensional.
Dynamic speech assessment was obtained using a fast-gradient echo, fast low-angle shot (FLASH), multi-shot spiral technique to acquire 15.8 fps as previously described (Sutton et al., 2010). The sequence uses a time-efficient acquisition of a six-shot spiral pulse sequence with an alternating TE between 1.3 and 1.8 milliseconds to allow for dynamic estimation and correction of the magnetic field map. Multiple saturation bands were used to suppress the signal from regions outside of the area of interest and also to decrease the signal created from regions with higher fat concentrations such as the cheeks. These methods provide a higher image quality with an improved signal-to-noise ratio and reduce the overall rate of image acquisition. Fast frame rates are achieved through the use of an optimized acquisition strategy coupled with an image reconstruction method that corrects for effects caused by imperfections in the magnetic field in the oropharyngeal region (Sutton et al., 2010). Subjects were instructed to repeat the utterance “ansa” while images were obtained in the oblique coronal and midsagittal image planes. The oblique coronal plane was sampled along the length of the levator muscle. The speech sample was carefully selected to represent movements of the velum between fully lowered (i.e., nasal), elevated (i.e., consonants), and transitions between the fully lowered and elevated positions. A metronome was played over the headphones at a rate of 2 Hz to instruct the subject on the pace of one syllable per beat. This imaging speed allows for at least one full image during each lowered and each elevated production to analyze the data for nasal and oral sounds.
Images were reconstructed with an output time-driven, sliding-window process. For this process, the desired frame rate for the output images is chosen (30 fps) and the necessary data for reconstructing a single image are gathered from the data closest to the desired time point. This results in a minimal amount of interpolation across time. Alternatively, the images in the native frame rate (15.8 fps) could be interpolated to the desired output rate, but this would result in significant blurring of information across time. The sliding-window reconstruction process minimizes redundant information in adjacent time points and minimizes temporal blurring (Sutton et al., 2009).
Acquisition simulation software provided by the vendor of the MRI scanner provides timing data that were used to align the audio speech recordings with the dynamic images. This software allows for accurate simulations of sequence timing using the exact acquisition protocol, giving information about the actual time location of data acquisition events with 10-microsecond accuracy.
Image Analyses
Image analyses were performed to evaluate the effectiveness of using the created MRI scanning protocol for 3D and dynamic image analysis of velopharyngeal structure and function. Three-dimensional computer reconstructions were performed following previously reported methods (Perry and Kuehn, 2007). Sequential MR images in the oblique coronal image plane displaying the levator muscle sling were segmented manually, and rendered data were exported into a polygonal mesh. Measures were selected along three planes to demonstrate the variety of anatomical features that can be observed using 3D image analysis. From the midsagittal plane, the velar length, palate length, and velopharyngeal depth were obtained. The coronal plane was used to measure palatal width and height, which may be clinically relevant in patients with syndromes or in evaluating the effect of surgical procedures on the palatal vault (Kharbanda et al., 2002; Bakri et al., 2012). Last, the oblique coronal plane was used to measure the levator muscle. Descriptions of these measures are provided in Table 2 and displayed in Figure 1. A qualitative analysis was performed to evaluate the movement of the velum during phonation in the midsagittal and oblique coronal image planes. Angle of origin and levator muscle length changes were analyzed across subjects during the production of “ansa” using similar methods to Ettema et al. (2002).
TABLE 2.
Description of Measures Obtained
| Midsagittal Measures | |
| Hard palate length | Linear distance between anterior nasal spine and posterior nasal spine |
| Velar length | Distance between posterior nasal spine and the tip of the velum (uvula proper) as measured through the midline of the velar structure |
| Velopharyngeal depth | Linear distance between the posterior nasal spine and the posterior pharyngeal wall along an extended line from the hard palate plane |
| Coronal Measures | |
| Palate width | Linear distance between the free gingival lingual margin of the posterior cusp of the second molar of one side to the same region on the other side |
| Palate height | Linear distance as measured with a line perpendicular to the palatal width line extending (at the region of the palatal vault) to the maximal height of the palate |
FIGURE 1.
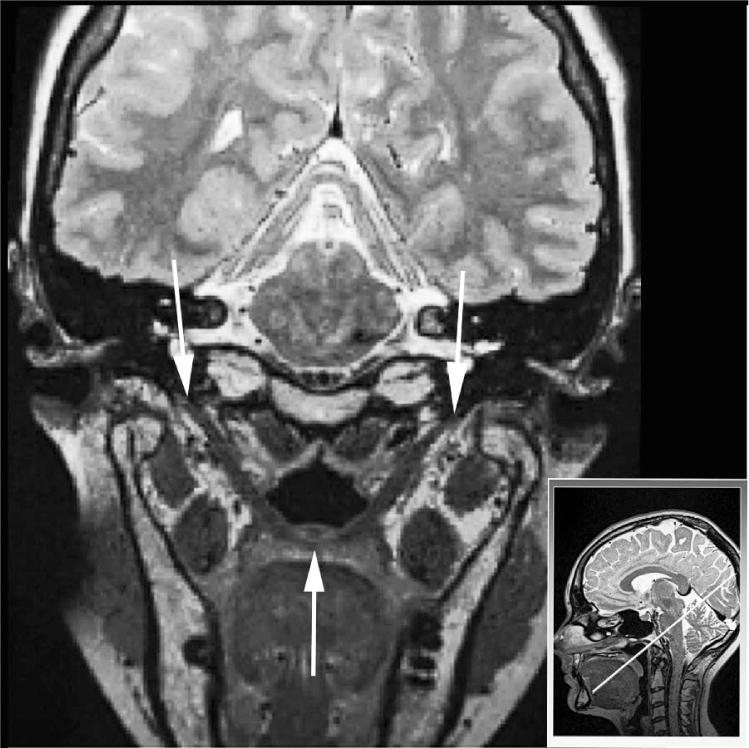
Oblique coronal image showing the cohesive levator sling from origin (white arrows at the top) at the base of the skull to insertion in the velum (single white arrow at the bottom). The image in the lower right displays the sampling plane used to obtain the oblique coronal image. Note the dark circle just above the lowest portion of the levator sling, which is the musculus uvulae cut in cross-section.
Results
The imaging protocol developed in the present study allows structural and functional assessments of velopharyngeal structures. Figure 2 demonstrates the oblique coronal image plane of the velopharynx with specific focus on the cohesive sling created by the two levator muscle bundles. The muscle can be observed from the origin to the insertion points with 0.8-mm isotropic resolution. Using the 3D data, the levator muscle was segmented and extracted from surrounding muscles and structures using variable image planes along the length of the muscle and was combined with 3D computer reconstructions, as previously described (Perry et al., 2012a). Using this method on a 3D data set, as opposed to a two-dimensional (2D) data set, the contour and morphology of the levator muscle can be appreciated as it descends from the base of the skull into the body of the velum. Before meeting the opposing muscle bundle, the levator bundles curve anteriorly and broaden. This broad fanning of the intravelar muscle segment was observed in 4 of the 10 subjects. A similar observation was reported by Perry et al. (2012a).
FIGURE 2.
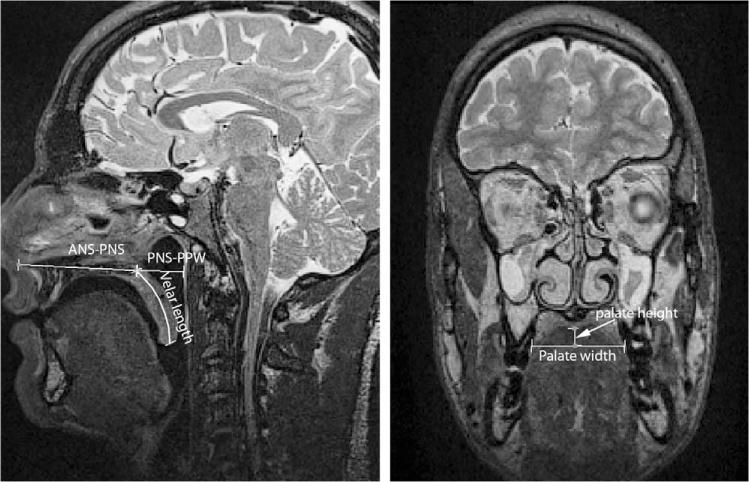
Demonstration of the measures in the midsagittal image plane and coronal image plane. ANS = anterior nasal spine; PNS = posterior nasal spine.
Quantitative measures of the velopharyngeal structures at rest are presented in Table 3. Because image data were obtained using a 3D SPACE sequence, the images were resampled from multiple views (Tables 2 and 3). The mean hard palate length was 60.6 ± 5.7 mm, mean velar length was 37.6 ± 3.2 mm, and velopharyngeal depth was 20.7 ± 2.0 mm. Mean palatal width was 38.9 ± 3.0 mm and mean palatal height was 9.3 ± 3.1 mm.
TABLE 3.
Values Obtained for Each Measure (mm) Across 10 Male Subjects
| Subject | Hard Palate | Velar Length | Velopharyngeal Depth | Palate Width | Palate Height |
|---|---|---|---|---|---|
| 1 | 63.8 | 41.4 | 24.3 | 37.1 | 10.8 |
| 2 | 72.1 | 37.8 | 21.5 | 38.7 | 14.6 |
| 3 | 57.9 | 39.0 | 17.5 | 40.1 | 5.4 |
| 4 | 66.0 | 33.8 | 20.5 | 35.0 | 12.6 |
| 5 | 57.5 | 41.8 | 19.1 | 44.6 | 12.1 |
| 6 | 61.1 | 35.2 | 19.6 | 37.3 | 9.5 |
| 7 | 51.0 | 35.8 | 21.5 | 35.1 | 7.3 |
| 8 | 61.1 | 35.2 | 19.3 | 41.2 | 7.0 |
| 9 | 57.1 | 34.3 | 20.1 | 41.4 | 5.6 |
| 10 | 58.7 | 41.6 | 23.2 | 38.4 | 7.7 |
| Mean ± SD | 60.6 ± 5.7 | 37.6 ± 3.2 | 20.7 ± 2.0 | 38.9 ± 3.0 | 9.3 ± 3.1 |
Dynamic images were obtained at 15.8 fps in the sagittal and oblique coronal planes, enabling visualization of the movements of the velum, posterior pharyngeal wall, lateral pharyngeal walls, and levator muscle. Figures 3 and 4 demonstrate the location of image acquisition and velopharyngeal movements during “ansa” production. Dynamic imaging in the oblique coronal image plane allow for velopharyngeal port closure to be appreciated in the plane at which closure occurs. The superior and posterior movement of the velum and medial and anterior movement of the pharyngeal walls can be visualized to determine whether closure was achieved. In all subjects, complete closure of the velopharyngeal port was visualized. As seen in Figure 4, the initial /a/ in the utterance “ansa” demonstrated anticipatory nasal positioning. The velum was lowered during the nasal consonant production and elevated with complete closure during the following phonemes /s/ and /a/.
FIGURE 3.

Demonstration of the process of acquiring an oblique coronal image. A: The oblique line in the midsagittal image is used to acquire the view of the levator muscle. B: The levator muscle can be viewed as the U-shaped muscle sling inside the white box. The white box represents the selected region used to acquire the dynamic images. C: The levator sling can be visualized as the central U-shaped muscle sling in the dynamic images.
FIGURE 4.

Series of MR images to represent the movement of the velopharyngeal structures in the oblique coronal (bottom row) image planes during “ansa” production. NC = nasal cavity; OC = oral cavity; Levator = levator veli palatini muscle.
The levator muscle length and angle of origins were measured during “ansa” production. Data provided in Table 4 display the length and angle of origin changes between the production of the vowel, nasal, consonant, and vowel productions. The values from the left and right levator lengths and angles were averaged and distances between points of origin were the same as those obtained from static images. The levator length mean values showed a longer levator muscle length for nasals compared with the /a/ and /s/ productions, as consistent with the images in Figure 4. The levator length was shortest during the final /a/ production. Based on these data, the percentage of contraction (amount of muscle shortening relative to rest position) was 14% for the prenasal vowel, 5% for the nasal, 13% for the postnasal consonant (/s/), and 16% for the final /a/ production. The mean angles of origin demonstrated a larger angle for the nasal production and smaller angles for the vowels and /s/ productions.
TABLE 4.
Mean Values for Dynamic Speech Measure Across 10 Male Subjects With Length Measures (mm) and Angle Measures (°); The Mean Percentage of Muscle Contraction Relative to the Rest Position Is Displayed for the Levator Muscle Length
| Rest | /a/ | /n/ | /s/ | /a/ | |
|---|---|---|---|---|---|
| Levator length | 51.12 | 43.76 (14%) | 48.25 (5%) | 45.17 (13%) | 42.71 (16%) |
| Angle of origin | 60 | 56 | 58 | 55 | 53 |
Discussion
Anatomical measurements were used to demonstrate the types of measures that can be obtained and to serve as preliminary data to compare with previously reported studies (Table 5). Velar length values in the present study are larger than those obtained in similar studies (Tian and Redett, 2009; Tian et al., 2010; Bae et al., 2011b). Other reported studies in Table 5 measured the velar length as a straight line from the posterior nasal spine to the tip of the uvula. Kuehn and Kahane (1990) explained a method for measuring the curvilinear form of the velum from the anterior to the most posterior segment of the velum along the midline region between the oral and nasal velar surfaces. A curvilinear line, as used by Kuehn and Kahane (1990) and the present study, passing through the middle of the velar structure represents a more accurate measure of velar length. Values in the present study for velar length (mean, 37.6 ± 3.2 mm) are similar to corresponding male velar length values (mean, 40.5 mm) observed by Kuehn and Kahane (1990) obtained from cadaver dissection of four male subjects.
TABLE 5.
Summary of Related Measurements From Previous Studies (mm)
| Current Study: 10 Men (White) | Bae et al., 2011: 5 Men (White), 5 Women (White) | Tian et al., 2010: 5 Men (Chinese), 7 Women (Chinese) | Tian & Redett, 2009: 5 Men (3 Chinese; 2 White), 12 Women (3 Chinese; 9 White) | Patel, 2012: 223 Men (Indian) | Yumusova et al., 2012: 11 Men | |
|---|---|---|---|---|---|---|
| Hard palate length | 60.6 ± 5.7 | 52.4 ± 4.8 | 48.4 ± 4.4 | 52.9 ± 5.3 | – | – |
| Velar length* | 37.6 ± 3.2 | 33.6 ± 3.1 | 33.7 ± 2.6 | 31.8 ± 2.8 | – | – |
| Velopharyngeal depth | 20.7 ± 2.0 | 23.9 ± 4.2 | 25.99 ± 4.39 | 23.8 ± 4.2 | – | – |
| Palate width | 38.9 ± 3.0 | – | – | – | 37.17 ± 2.9 | – |
| Palate height | 9.3 ± 3.1 | – | – | – | – | 13.52 ± 2.5 |
Velar length measured differently between studies.
Values obtained for palate width and height are similar to previous reports (Ferrario et al., 2001; Ferrario et al., 2002; Patel, 2012; Yunusova et al., 2012). The minimal variability noted in Table 5 may be due differences in the number of subjects, sex, and racial groups used in other reported studies. Bae et al. (2011b) reported measures on 10 white adult subjects (five men and five women) between 20 and 31 years of age. Tian et al. (2010) reported data from 12 Chinese adult subjects (five men and seven women) from 19 to 40 years of age and do not represent a 3D image sequence acquisition. Tian and Redett (2009) reported mean values from 2D data from 17 adult subjects (five men and 12 women) of different races (11 white and six Chinese) and age (19 to 43 years of age). Due to the known craniofacial and vocal tract variations related to sex, race, and age (Akgüner, 1999; Ettema et al., 2002; Bae et al., 2011b) caution should be taken when making direct comparisons between studies. Data in the present study represent a more homogeneous population compared with data from other similar studies. The individual’s height and weight may also be factors that affect the velopharyngeal structures. Perry et al. (2011) suggested that head circumference in infants may be predictors of the levator muscle length. Further research should investigate the interaction of these variables on velopharyngeal structures.
A test for equal variance using F-test (Minitab 15; Minitab, State College, PA) was used to compare the amount of variance among reported studies in Table 5. The present study demonstrates a smaller amount of variance particularly in regard to velopharyngeal depth. There was no significant difference between the variances in the hard palate length reported in the present study and those of Bae et al. (2011; P=.597), Tian et al. (2010; P=.383), and Tian and Redett (2009; P=.721). There was no significant difference between the variances in the velar length reported in the present study and those of Bae et al. (2011; P=.798), Tian et al. (2010; P = .609), or Tian and Redett (2009; P=.931). There was a significant difference between the variances in the velopharyngeal depth measurements of the present study and those of the other three reported studies (P=.03, .02, and .02, respectively), demonstrating a significantly smaller standard deviation.
A major drawback of MRI over other clinical imaging methods (e.g., videofluoroscopy, nasendoscopy, lateral view x-ray) is the use of sedation, which is generally needed in young children (under 4 years of age) to reduce motion artifacts. A clinical 2D MRI protocol for imaging the levator muscle can be time consuming because it requires on-site multiple adjustments to the sampling field in order to obtain the ideal image plane, thus extending the total scan time (Perry et al., 2012b). Three-dimensional image sequences, such as SPACE, create a dataset in under 3 minutes that can be postprocessed to identify the levator muscle. A 3D MRI sequence using a 3 Tesla scanner is capable of producing greater contrast and through-plane image resolution for imaging muscle structures such as the levator muscle (Bae et al., 2011b). This may provide more precise anatomical measurements of the levator muscle compared with image analyses using a magnet with lower strength. More research is needed to determine the efficiency of using this protocol on a younger population.
Drissi et al. (2011) emphasized the importance of three imaging planes during dynamic assessments including the sagittal view (for velar elevation assessments), frontal view (for medial movement of the lateral pharyngeal walls), and axial view (for viewing the velopharyngeal port). The present study demonstrates the importance of an additional image plane for static and dynamic assessments, that is, the oblique coronal image plane. Bae et al. (2011) and Sutton et al. (2009) demonstrated a similar high temporal, serial acquisition during speech production; however, data were obtained only in the midsagittal image plane. Although the imaging rate was high (21 fps), both studies used a head-only MRI system that is typically designed for brain imaging. Due to the limited imaging region from the head-only scanner, the intensity of the signal fell off near the region of the oropharynx, producing poorer image quality. The present study demonstrates the first use of dynamic imaging using an optimized FLASH sequence along the axis of velopharyngeal closure at word-level productions. This axis of closure is not only valuable for further research analyses, but it may be a valuable clinical component for assessing velopharyngeal function in individuals with cleft palate. The advantage of dynamic MRI over traditional-view nasendoscopy is the ability to specify the exact plane of interest for imaging, which eliminates the depth-perception distortions found in nasendoscopy. Information such as the orifice size and velopharyngeal gap may be clinically useful data that can be measured using dynamic MRI. Further research is needed to decrease the slice thickness and increase spatial and temporal resolution. Future research should also aim to examine the direct benefits of imaging in the patient-customized plane of closure. It is expected that small or discrete openings might be more obvious on an oblique coronal image plane compared with a view along the axial plane.
Dynamic imaging findings from the present study can in part be compared with those observed by Ettema et al. (2002), who used the same speech sample (“ansa”). The authors noted a progressive decrease in the levator length from rest to nasal productions to low vowels to high vowels to fricatives. The levator muscle showed an average 19% reduction in length from rest position to the fricative production. The present study demonstrated a 14% reduction in the prenasal vowel and a 13% reduction in the levator length for the postnasal sibilant. These differences between the present study and those of Ettema et al. (2002) can likely be explained by the coarticulatory effect, which can only be appreciated during a real-time speech sample, as in the present study. Participants in the comparative study (Ettema et al., 2002) produced the same speech sample (“ansa”); however, each phoneme was sustained for 4 seconds. By sustaining the productions, the dynamic interaction of adjacent phonemes (such as the effect of the nasal sound) may, in part, remove the coarticulatory effect.
True FISP studies typically obtain images at a rate of two images per second, which is inadequate for proper speech assessments. The total duration of our scan was 50.5 seconds, resulting in 799 images in the native frame rate of 15.8 fps and 1515 images when reconstructed on a grid of 30 fps. The present study demonstrates an acquisition that does not rely on either repeated acquisitions or uniform magnetic fields for steady-state sequences. Previous dynamic assessments have used prolonged speech sound productions (McGowen et al., 1992; Akgüner et al., 1998; Özgür et al., 2000; Vadodaria et al., 2000; Ettema et al., 2002; Ha et al., 2007; Atik et al., 2008; Kao et al., 2008; Drissi et al., 2010; Tian et al., 2010). A few studies have demonstrated the use of sentence-level productions (Beer et al., 2004; Silver et al., 2011). However, these studies have used only traditional image planes including axial and midsagittal and at a much slower imaging speech compared with that of the current study.
Limitations of the present study are the use of a 3 Tesla scanner and nonstandard clinical protocol. The protocol used involves a non-Cartesian spiral sequence that is not available on clinical scanners and required custom programming of the pulse sequence. It is expected that further development, particularly on clinically relevant populations, will result in translation of this sequence and corresponding protocol to clinical sites. Future research should explore the potential benefit of this protocol in cleft palate speech. Clinical decisions that might be drawn from such data include type or modifications of secondary surgical procedure, existence of abnormal muscle arrangement in submucous cleft palate, residual function of the levator muscle following different surgical procedures, and the need for a palate re-repair (Sommerlad et al., 2002) or Furlow palatoplasty (Furlow, 1986).
Conclusion
This study demonstrated the development and implementation of a static and dynamic MRI protocol. Dynamic images were obtained at 15.8 fps in the sagittal and oblique coronal planes, enabling visualization of the movements of the velum, posterior pharyngeal wall, lateral pharyngeal walls, and levator muscle. Data from the present study demonstrate the variety of image planes that can be acquired for anatomical analyses and the information that can be gathered from dynamic oblique coronal image acquisition. Although this protocol is not currently available for clinical MRI scanners, this paper supports the need for future research to translate these research developments into the clinical practice.
Acknowledgments
This publication was made possible by grant number 1R03DC009676-01A1 from the National Institute of Deafness and Other Communicative Disorders. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Jamie L. Perry, Assistant Professor, Department of Communication Sciences and Disorders, East Carolina University, Greenville, North Carolina.
Bradley P. Sutton, Associate Professor, Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, Illinois.
David P. Kuehn, Professor Emeritus, Department of Speech and Hearing Science, University of Illinois at Urbana-Champaign, Champaign, Illinois.
Jinadasa K. Gamage, Professor, Department of Mathematics, Illinois State University
References
- Akgüner M. Velopharyngeal anthropometric analysis with MRI in normal subjects. Ann Plast Surg. 1999;43:142–147. [PubMed] [Google Scholar]
- Akgüner M, Karaca C, Barutçu¸ A, Özaksoy D, Yurt A, Vayvada H. Evaluation of velopharyngeal pathophysiology and velopharyngeal insufficiency with magnetic resonance imaging. Eur J Plast Surg. 1998;21:118–128. [Google Scholar]
- Anagnostara A, Stoeckli S, Weber OM, Kollias MD. Evaluation of the anatomical and functional properties of deglutition with various kinetic high-speed MRI sequences. J Magn Reson Imaging. 2001;14:194–199. doi: 10.1002/jmri.1172. [DOI] [PubMed] [Google Scholar]
- Atik B, Bekerecioglu M, Tan O, Etik O, Davran R, Arslan H. Evaluation of dynamic magnetic resonance imaging in assessing velopharyngeal insufficiency during phonation. J Craniofac Surg. 2008;19:566–572. doi: 10.1097/SCS.0b013e31816ae746. [DOI] [PubMed] [Google Scholar]
- Bae Y, Kuehn DK, Conway CA, Sutton BP. Real-time magnetic resonance imaging of velopharyngeal activity with simultaneous speech recordings. Cleft Palate Craniofac J. 2011a;48:695–707. doi: 10.1597/09-158. [DOI] [PubMed] [Google Scholar]
- Bae Y, Kuehn DP, Sutton BP, Conway CA, Perry JL. Three-dimensional magnetic resonance imaging of velopharyngeal structures. J Speech Hear Res. 2011b;54:1538–1545. doi: 10.1044/1092-4388(2011/10-0021). [DOI] [PubMed] [Google Scholar]
- Bakri S, Rizell S, Saied S, Lilja J, Mark H. Height of the palatal vault after two different surgical procedures: study of the difference in patients with complete unilateral cleft lip and palate. J Plast Surg Hand Surg. 2012;46:155–158. doi: 10.3109/2000656X.2012.683796. [DOI] [PubMed] [Google Scholar]
- Beer JA, Hellerhoff P, Zimmermann A, Mady K, Sader R, Rummeny EJ, Hannig C. Dynamic near-real-time magnetic resonance imaging for analyzing the velopharyngeal closure in comparison with videofluoroscopy. J Magn Reson Imaging. 2004;20:791–797. doi: 10.1002/jmri.20197. [DOI] [PubMed] [Google Scholar]
- Birch MJ, Sommerlad BC, Fenn C, Butterworth M. A study of the measurement errors associated with the analysis of velar movements assessed from lateral videofluoroscopic investigations. Cleft Palate Craniofac J. 1999;36:499–507. doi: 10.1597/1545-1569_1999_036_0499_asotme_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Drissi C, Mitrofanoff M, Talandier C, Falip C, Le Couls V, Adamsbaum C. Feasibility of dynamic MRI for evaluating velopharyngeal insufficiency in children. Eur Radiol. 2011;21:1462–1469. doi: 10.1007/s00330-011-2069-7. [DOI] [PubMed] [Google Scholar]
- Ettema SL, Kuehn DK, Perlman AL, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Craniofac J. 2002;39:130–144. doi: 10.1597/1545-1569_2002_039_0130_mriotl_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Colombo A, Dellavia C, Colombo A, Ferrari RP. Three-dimensional hard tissue palatal size and shape: a 10-year longitudinal evaluation in healthy adults. Int J Adult Orthod. 2002;17:51–58. [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Colombo A, Dellavia C, Dimaggio FR. Three-dimensional hard tissue palatal size and shape in human adolescents and adults. Clin Orthod Res. 2001;4:141–147. doi: 10.1034/j.1600-0544.2001.040304.x. [DOI] [PubMed] [Google Scholar]
- Furlow L. Cleft palate repair by double-opposing Z-plasty. Plast Reconstr Surg. 1986;78:724–735. doi: 10.1097/00006534-198678060-00002. [DOI] [PubMed] [Google Scholar]
- Gilber RJ, Daftary S, Campbell TA, Weisskoff RM. Patterns of lingual tissue deformation associated with bolus containment and propulsion during deglutition as determined by echo-planar MRI. J Magn Reson Imaging. 1998;8:554–560. doi: 10.1002/jmri.1880080307. [DOI] [PubMed] [Google Scholar]
- Ha S, Kuehn DP, Cohen M, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle in speakers with repaired cleft palate. Cleft Palate Craniofac J. 2007;44:495–505. doi: 10.1597/06-220.1. [DOI] [PubMed] [Google Scholar]
- Hess U, Hanning C, Sader R, Cavallaro A, Wuttge-Hannig A, Zeilhofer H. Evaluation of velopharyngeal closure in preoperative planning of maxillary advancement. Rontgenpraxis. 1996;49:25–26. [PubMed] [Google Scholar]
- Kane AA, Butman JA, Mullick R, Skopec M, Choyke P. A new method for the study of velopharyngeal function using gated magnetic resonance imaging. Plast Reconstr Surg. 2002;109:472–481. doi: 10.1097/00006534-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Kao DS, Soltysik DA, Hyde JS, Gosain AK. Magnetic resonance imaging as an aid in dynamic assessment of the velopharyngeal mechanism in children. Plast Reconstr Surg. 2008;122:572–577. doi: 10.1097/PRS.0b013e31817d54d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda P, Shaw C, Worthington H. Palatal height: another indicator of surgical outcome in unilateral cleft lip and palate. Cleft Palate Craniofac J. 2002;39:308–311. doi: 10.1597/1545-1569_2002_039_0308_phaios_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Kuehn DP. A cineradiographic investigation of velar movement variables in two normals. Cleft Palate Craniofac J. 1976;13:88–103. [PubMed] [Google Scholar]
- Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC. Magnetic resonance imaging of the levator veli palatini muscle before and after primary palatoplasty. Cleft Palate Craniofac J. 2004;41:584–592. doi: 10.1597/03-060.1. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Kahane JC. Histologic study of the normal human adult soft palate. Cleft Palate Craniofac J. 1990;27:26–35. doi: 10.1597/1545-1569(1990)027<0026:hsotnh>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- McGowen J, Hatabu H, Yousem D, Randall P, Kressel H. Evaluation of soft palate function with MRI: application to the cleft palate patient. J Comput Assist Tomogr. 1992;16:877–882. doi: 10.1097/00004728-199211000-00009. [DOI] [PubMed] [Google Scholar]
- McWilliams BJ. The long term speech results of primary and secondary surgical correction of palatal clefts. In: Bardach J, Morris HL, editors. Multidisciplinary Management of Cleft Lip and Palate. Philadelphia: WB Saunders; 1990. [Google Scholar]
- NessAiver MS, Stone M, Parthasarathy V, Kahana Y, Paritsky A. Recording high quality speech during tagged cine-MRI studies using a fiber optic microphone. J Magn Reson Imaging. 2006;23:92–97. doi: 10.1002/jmri.20463. [DOI] [PubMed] [Google Scholar]
- Özgür F, Tunçbilek G, Cila A. Evaluation of velopharyngeal insufficiency with magnetic resonance imaging and nasoendoscopy. Ann Plast Surg. 2000;44:8–13. doi: 10.1097/00000637-200044010-00002. [DOI] [PubMed] [Google Scholar]
- Patel M. A study of the hard palate in the skulls of central Indian population. Int J Pharm Biol Sci. 2012;3:B527–B533. [Google Scholar]
- Perry JL, Kuehn DP. Three-dimensional computer reconstruction of the levator veli palatini muscle in situ using magnetic resonance imaging. Cleft Palate Craniofac J. 2007;69:214–216. doi: 10.1597/06-137.1. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP. Morphology of the levator veli palatini muscle using magnetic resonance imaging. Cleft Palate Craniofac J. 2012a doi: 10.1597/11-125. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Goldwasser M. Craniometric and velopharyngeal assessment of infants with and without cleft palate. J Craniofac Surg. 2011;22:499–503. doi: 10.1097/SCS.0b013e3182087378. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Wachtel JM, Bailey JS, Luginbuhl LL. Using magnetic resonance imaging for early assessment of submucous cleft palate: a case report. Cleft Palate Craniofac J. 2012b;49:e35–e41. doi: 10.1597/10-189. [DOI] [PubMed] [Google Scholar]
- Pigott RW. An analysis of the strengths and weaknesses of endoscopic and radiological investigations of the velopharyngeal incompetence based on 20-year experience of simultaneous recording. Br J Plast Surg. 2002;55:32–35. doi: 10.1054/bjps.2001.3732. [DOI] [PubMed] [Google Scholar]
- Sader R, Horch HH, Herzog M, Zeihofer HF, Hannig C, Hess U, Bünte E, Böhme G. High-frequency videocinematography for the objective imaging of the velopharyngeal closure mechanism in cleft palate patients. Fortschr Keiferothop. 1994;55:169–175. doi: 10.1007/BF02285407. [DOI] [PubMed] [Google Scholar]
- Shinagawa HT, Ono E, Honda S, Masaki S, Shimada Y, Iriki A, Ohyama K. Dynamic analysis of articulatory movement using magnetic resonance imaging movies: methods and implications in cleft lip and palate. Cleft Palate Craniofac J. 2005;42:225–230. doi: 10.1597/03-007.1. [DOI] [PubMed] [Google Scholar]
- Silver AL, Nimkin K, Ashland JE, Ghosh SS, van der Kouwe AJ, Brigger MT, Hartnick C. Cine magnetic resonance imaging with simultaneous audio to evaluation pediatric velopharyngeal insufficiency. Arch Otolaryngol Head Neck Surg. 2011;137:258–263. doi: 10.1001/archoto.2011.11. [DOI] [PubMed] [Google Scholar]
- Skolnick ML, Cohn ER. Videofluoroscopic Studies of Speech in Patients with Cleft Palate. New York: Springer-Verlag; 1989. pp. 1–48. [Google Scholar]
- Sommerlad BC, Mehendale FV, Birch MJ, Sell D, Hattee C, Harland K. Palate re-repair revisited. Cleft Palate Craniofac J. 2002;39:295–307. doi: 10.1597/1545-1569_2002_039_0295_prrr_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Suto Y, Matsuo T, Kato T, Inoue Y, Ogawa S, Suzuki T, Yamada M, Ohta Y. Evaluation of the pharyngeal airway in patients with sleep apnea. Am J Roentgenol. 1993;160:311–314. doi: 10.2214/ajr.160.2.8424340. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Conway C, Bae Y, Brinegar C, Liang Z, Kuehn DP. Dynamic imaging of speech and swallowing with MRI. Conf Proc IEEE Eng Med Biol Soc. 2009:6651–6654. doi: 10.1109/IEMBS.2009.5332869. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Conway CA, Bae Y, Seethamraju R, Kuehn DK. Faster dynamic imaging of speech with field inhomogeneity correlated spiral fast low angle shot (FLASH) at 3T. J Magn Reson Imaging. 2010;32:1228–1237. doi: 10.1002/jmri.22369. [DOI] [PubMed] [Google Scholar]
- Tian W, Regett RJ. New velopharyngeal measurements at rest and during speech: implication and applications. J Craniofac Surg. 2009;20:532–539. doi: 10.1097/SCS.0b013e31819b9fbe. [DOI] [PubMed] [Google Scholar]
- Tian W, Yin H, Redett R, Shi B, Shi J, Zhang R, Zheng Q. Magnetic resonance imaging assessment of the velopharyngeal mechanism at rest and during speech in Chinese adults and children. J Speech Lang Hear Res. 2010;53:1595–1615. doi: 10.1044/1092-4388(2010/09-0105). [DOI] [PubMed] [Google Scholar]
- Vadodaria S, Goodacre T, Anslow P. Does MRI contribute to the investigation of palatal function? Br J Plast Surg. 2000;53:191–199. doi: 10.1054/bjps.1999.3308. [DOI] [PubMed] [Google Scholar]
- Wein BB, Drobnitzky M, Klajman S, Angerstein W. Evaluation of functional positions of tongue and soft palate with MR imaging: initial clinical results. J Magn Reson Imaging. 1991;1:381–383. doi: 10.1002/jmri.1880010317. [DOI] [PubMed] [Google Scholar]
- Witzel MA, Stringer DA. Methods of assessing velopharyngeal function. In: Bardach J, Morris HL, editors. Secondary Surgical Treatment of Cleft Palate. Philadelphia: WB Saunders; 1990. pp. 763–773. [Google Scholar]
- Yunusova Y, Baljiko M, Pintilie G, Rudy K, Faloutsos P, Daskalogiannakis J. Acquisition of the 3D surface of the palate by in-vivo digitization with Wave. Speech Commun. 2012;54:923–931. [Google Scholar]


