Abstract
Background
No studies have reported the circumference and diameter of the levator veli palatini muscle at multiple points along its length and from both views (frontal and lateral). The purpose of this study was to provide quantitative data regarding the levator muscle morphology along the length of the muscle using magnetic resonance imaging and advanced three-dimensional computer technology.
Methods
Ten Caucasian male subjects participated in the study. Subjects were scanned using a Siemens 3 T Trio. Levator muscle measures were obtained using a two-dimensional image plane. A three-dimensional model was used to measure the circumference and muscle diameter (in two directions) at six points along the length of the levator muscle.
Results
Levator muscle length ranged from 41.67 mm to 52.85 mm across all subjects. Mean extravelar muscle length was 30.55 mm (SD, 2.8 mm) and 30.01 mm (SD, 2.9 mm) for right and left muscles. The mean circumference at the origin was 18.90 mm (SD, 2.6 mm). At the second point, the muscle circumference mean increased slightly (mean, 22.40 mm; SD, 4.9 mm). The means for the remainder of the measures (points 3, 4, 5, and 6) were consistent, showing little to no change.
Conclusion
Circumference and diameter values were similar to those reported in previous literature. The muscle did diverge at the point where the muscle bundle entered the velum, as it has been previously described. Instead, the muscle diverges near the midline insertion becoming sparser (smaller superior-to-inferior diameter).
Keywords: levator veli palatini muscle, magnetic resonance imaging, muscle morphology
Introduction
The levator veli palatini (levator) muscle is considered to be the primary muscle responsible for velar elevation. As its name implies, it functions to elevate the velum to create closure between the oral and nasal cavities (Moon and Kuehn, 2004). The levator muscle pulls the velum along a curvilinear (superior and posterior) trajectory to assist in closure of the velopharyngeal port (Kent et al., 1974; Kuehn, 1976). It is suggested that the levator muscle may be responsible, in part, for lateral wall movement during phonation (Yamawaki et al., 1999). Although the levator muscle is considered to be the major muscle for velar elevation, velar positioning is thought to be related to other velar muscles including the palatopharyngeus and palatoglossus (Seaver and Kuehn, 1980; Kuehn et al., 1982; Moon et al., 1994). Electromyographic data during velar elevation further displayed this interaction between the levator, palatoglossus, and palatopharyngeus muscles during normal velopharyngeal closure (Kuehn et al., 1982).
The levator muscle originates from the base of the skull near the apex of the petrous portion of the temporal bone (Moon and Kuehn, 2004). Some authors have observed an attachment to the posterior aspect of the Eustachian (auditory) tube (Huang et al., 1997). The muscle lies directly inferior to the Eustachian tube and runs parallel to the tube coursing from the origin to the insertion in a medial, inferior, and anterior direction (Huang et al., 1998). The levator muscle bundles insert into the midsection of the velum at approximately 40% of the velar length (Boorman and Sommerlad, 1985). This is consistent with histology study results from Ettema and Kuehn (1994), which demonstrated the greatest proportion of muscle tissue in the velum to be at 40% of the velar length. The added muscle bulk is likely due to the presence of the musculus uvulae, which is cradled by the levator muscle sling (Ettema and Kuehn, 1994). Fritzell (1969, 1979) reported electromyographic data to support the notion that the mass of the levator muscle is located and pulls the velum from this point. Levator muscle contraction was associated with superior and posterior movement of the velum from the midportion of the velum. This has been supported by other similar studies (Lubker et al., 1970; Dickson and Dickson, 1972; Bell-Berti, 1976; Kuehn, 1979).
After entering the velum, the levator muscle broadens and crosses the midline of the velum (Kuehn and Moon, 2005). Kuehn and Moon (2005) reported the crossing of the levator muscle fibers at about 25% of the distance of the posterior nasal spine and the uvula. Through the midline, there was no septum separating the two levator muscle bundles.
While the literature is consistent regarding the main function of the levator muscle serving in velopharyngeal closure, there is disagreement about the relationship of the muscle for opening the Eustachian tube. Some authors propose that the muscle assists in dilating the orifice of the Eustachian tube (Seif and Dellon, 1978; Shprintzen and Croft, 1981; Rood and Doyle, 1982; Tomoda et al., 1984; Swarts and Rood, 1990; Spauwen et al., 1991; Sudo et al., 1998), whereas others have been unable to confirm such a relationship (Honjo et al., 1979; Finkelstein et al., 1990). Some authors propose that the levator muscle has attachments only onto the Eustachian tube, thus having a direct function in opening the Eustachian tube (Huang et al., 1997; Arnold et al., 2005). Huang et al. (1997) reported 15 adult fresh cadaveric specimens of Asian descent (nine males and six females) had attachment of the muscle fibers to the posteriomedial aspect of the Eustachian tube at the isthmus (junction between the cartilaginous and bony aspects). This muscle attachment results in an upward, medial, and posterior displacement of the medial tubal cartilage, which functions to open the lumen of the tube. In the case of a cleft palate, if the muscle is attached at the Eustachian tube, the levator muscle can obstruct the opening to the Eustachian tube. This has been speculated as a cause, in part, to the high frequency of otitis media observed in children with cleft palate (Arnold et al., 2005).
Mehendale (2004) described a tendinous insertion of the levator muscle via a small bundle of anteriolateral fibers. Through examination of 10 adult Caucasian human specimens (five males and five females), the small bundle was observed to branch away from the main mass of the levator muscle at the inferior one third of the length of the levator muscle. The anteriolateral fibers terminated in a collection of fine tendons, which inserted into the posterior and lateral edge of the palatal aponeurosis near the hamulus (Mehendale, 2004). These fibers are thought to provide traction on the main part of the levator muscle and to provide posterior and superior extension of the palatal aponeurosis, which may facilitate velar extension during velopharyngeal closure (Mehendale, 2004).
Studies have demonstrated that the levator muscle is very consistent in size, shape, and location (Ettema et al., 2002; Moon and Kuehn, 2004). This has been demonstrated through histologic studies (Moon and Kuehn, 2004), gross dissection (Azzam and Kuehn, 1977; Kuehn and Azzam, 1978; Huang et al., 1997; Moon and Kuehn, 2004), and magnetic resonance imaging (Ettema et al., 2002; Ha et al., 2007; Perry and Kuehn, 2009; Perry, 2011; Perry et al., 2011; Tian et al., 2010). From the sagittal view, the muscle inserts into the velum at a 45° angle relative to a vertical reference line (Moon and Kuehn, 2004). It is said to have a broad insertion as it extends into the velum (Dickson et al., 1974; Boorman and Sommerlad, 1985; Kuehn and Kahane, 1990; Carrasco, 1996; Huang et al., 1998).
The muscle is often described as a flattened cylinder with the largest diameter in the midline where the bundles diverge out to intermingle with the opposing levator bundle (Azzam and Kuehn, 1977; Kuehn and Azzam, 1978). Huang et al. (1997) obtained samples of 15 adult specimens (nine males and six females) of Asian origin and measured the muscle at the origin of the muscle and at the level of the torus tubarius. The authors described the extravelar portion of the muscle to be consistently cylindrical and uniform to the point of entry into the velum with a mean diameter of 7.96 mm (right muscle bundle) and 7.94 mm (left muscle bundle) at the base of the skull. At the level of the torus tubarius, the muscle had a mean diameter of 8.14 mm (right muscle bundle) and 8.11 mm (left muscle bundle). All individuals had nearly (within 0.7 mm) the same values at the origin and at the level of the torus tubarius.
Azzam and Kuehn (1977) studied seven adult human cadavers (four males and three females) to examine the morphology of the musculus uvulae. The authors measured the diameter of the levator muscle as a comparison to those obtained from the musculus uvulae. The muscle was measured using calipers (lateral extent) at the position midway between the medial extent of the cartilage of the Eustachian tube and the point of levator insertion into the body of the velum. The mean diameter of the left and right levator muscle bundle was 9.1 mm and 9.9 mm (range between 7.1 mm and 12.6 mm). Kuehn and Azzam (1978) conducted a study of the palatoglossus muscles from 25 human cadavers. The authors reported muscle diameter of the left and right levator muscle bundles as a comparison to those measures taken on the palatoglossus muscle. The levator muscle diameter was taken at the area deep to the torus tubarius and ranged from 5.5 mm to 11.4 mm. The right and left levator muscle bundles showed a mean of 8.14 mm (SD, 2.10 mm) and 8.34 mm (SD, 1.76 mm). Although Kuehn and Azzam (1978) reported a greater range, overall mean diameter measures were similar (mean scores within 0.23 mm) to those obtained by Huang et al. (1997) at the same point along the muscle.
Studies related to the size and shape of the levator muscle are predominately from cadaver specimens (histology or gross dissection). Studies on the morphology of the levator muscle in vivo have been related to levator muscle length, distance between muscle origins, and point of insertion into the body of the velum (Ettema et al., 2002; Ha et al., 2007; Perry, 2011; Perry et al., 2011; Tian et al., 2010). These studies have used magnetic resonance imaging, which is the only imaging modality that allows for visualization of the levator muscle in a living subject. Ettema et al. (2002) reported levator muscle length at 44.7 mm for five women and 45.8 mm for five males. Average thickness was 5.4 mm for both men and women. The average angle at the origin, between the base of the skull and the course of the muscle bundle during rest, was 64.5° for women and 60.4° for men. Levator muscle thickness has been reported in several studies (Ettema et al., 2002; Ha et al., 2007; Perry et al., 2011; Perry, 2011), although some have questioned the validity of these reports (Ha et al., 2007). This is because studies using magnetic resonance imaging have measured the diameter using a two-dimensional image plane. It is possible that the measure of the muscle diameter in the midpoint (in the velum) or at the location where the muscle bundles insert into the body of the velum likely includes fibers from the musculus uvulae or palatoglossus muscles, thus resulting in values that do not represent the levator muscle alone. Dissection studies have demonstrated consistently that the levator muscle is a flattened cylinder and is quite consistent in diameter until the point of insertion into the velum. Most studies related to magnetic resonance imaging obtained the muscle diameter in a frontal view (medial-to-lateral or superior-to-inferior), whereas most dissection studies report a lateral view (anterior-to-posterior measurement).
From these data combined, it is hard to determine the full nature of the muscle from the origin to the insertion. No studies have reported the circumference and diameter of the muscle at several points along its length and from both views (frontal and lateral) in living subjects. Understanding the muscle morphology can provide insight into muscle function for normal and abnormal velopharyngeal control for speech and swallowing. In agreement with findings from previous studies (Ettema et al., 2002; Perry et al., 2011; Perry, 2011), it was hypothesized that the levator muscle would be consistent in size and shape across subjects (given the same sex and race). It has been proposed that this limited variability across subjects with normal anatomy demonstrates that there may be dimensional features of the levator muscle that are unique to developing normal oral-to-nasal resonance balance (Ettema et al., 2002; Perry et al., 2011). Studies have shown that significant variations in the levator muscle form can result in hypernasal speech (Ha et al., 2007). It is important that analyses of muscle morphology and function be through the use of three-dimensional methods on living subjects. It was hypothesized that three-dimensional analysis would prove to be a viable source for analysis of the muscle shape and provide details that are not available through traditional methods (histology, radiology, magnetic resonance imaging alone, and nasopharyngoscopy). The velopharyngeal mechanism is a complex system, in which levator function accounts for only one part. Creating a method for three-dimensional analysis of the levator muscle and, in the future, surrounding structures will provide a significant role in improving the understanding of the velopharyngeal mechanism in individuals with normal and abnormal anatomy (e.g., cleft palate). The purpose of this study is to demonstrate a method of morphologic analysis and to provide quantitative data regarding levator muscle morphology along the length of the muscle using magnetic resonance imaging and advanced three-dimensional computer technology.
Methods
Participants
In accordance with the Institutional Review Boards at the University of Illinois at Urbana-Champaign and Illinois State University (Normal), 10 Caucasian male subjects were recruited to participate in the study. Subjects were between the ages of 20 and 32 years (mean, 23.8; SD, 4.6). Subjects reported no history of swallowing, neurological, or musculoskeletal disorders. Sex and/or racial differences in craniometric measures, vocal tract dimensions, and levator muscle morphology have been reported (Chung et al., 1985; Chung et al., 1986; Yuen et al., 1989; Liu et al., 2000; Simpson, 2001; Ettema et al., 2002; Johannsdottir et al., 2004; Yeong and Huggare, 2004). These reported hard tissue variations may result in further soft tissue variations, particularly in the area of the velopharyngeal mechanism. For this reason, only male Caucasian subjects were recruited to control for sex and racial influences.
Individuals with history of a tonsillectomy, adenoidectomy, or any oropharyngeal structural or functional abnormalities were excluded from the study. All subjects were native English speakers and were judged informally by a speech language pathologist to have normal oral-to-nasal resonance balance. All subjects had a body mass index between 19 and 26 with a mean of 23 (SD, 2.5) to control for possible variations in the pharyngeal airway as a result of obesity. In addition, a large individual or a person with very broad shoulders may have difficulty fitting into the scanner. Subject demographics are provided in Table 1.
TABLE 1.
Demographics of the 10 Caucasian Male Participants
| Subject | Age, y | Weight, lb | Height | Index, kg/m2 |
|---|---|---|---|---|
| S1 | 32 | 160 | 5′6″ | 25.8 |
| S2 | 21 | 170 | 6′2″ | 21.8 |
| S3 | 24 | 130 | 5′9″ | 19.2 |
| S4 | 20 | 170 | 5′7″ | 26.6 |
| S5 | 20 | 140 | 5′11″ | 19.5 |
| S6 | 22 | 183 | 5′11″ | 25.5 |
| S7 | 25 | 135 | 5′5″ | 22.5 |
| S8 | 22 | 170 | 5′11″ | 23.7 |
| S9 | 20 | 170 | 5′11″ | 23.7 |
| S10 | 32 | 135 | 5′6″ | 21.8 |
| Mean/SD | 23.8 (4.6) | 156 (19) | 5′9″ (3″) | 23 (2.5) |
Magnetic Resonance Imaging
Subjects were scanned using a Siemens 3 T Trio and a 12-channel Siemens Trio head coil. During the 5-minute scan, subjects were instructed to breathe through their nose with their mouth closed. Imaging was obtained while in the supine position. Thus, the velum was in a relaxed and lowered position. To minimize motion during the scanning session, a Velcro-fastened elastic strap was placed around the subject’s head, passing above the nasion, and fastened to the head coil. A high-resolution, T2-weighted turbo-spin-echo three-dimensional anatomical scan called SPACE (Sampling Perfection with Application optimized Contrasts using different flip angle Evolution) was used to acquire a large field of view covering the oropharyngeal anatomy (25.6 × 19.2 × 15.5 cm) with 0.8 mm isotropic resolution with an acquisition time of slightly less than 5 minutes (4:52). Echo time (TE) was 268 milliseconds, and repetition time (TR) was 2.5 seconds.
Image Processing and Levator Muscle Measures
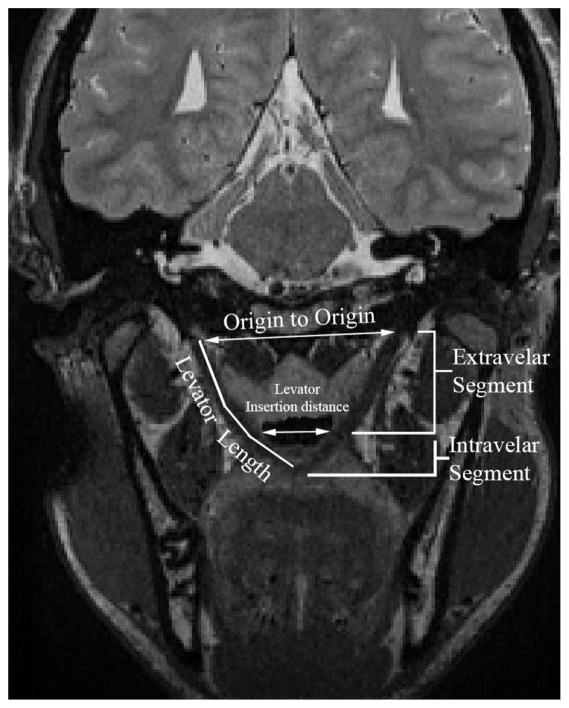
Image-processing methods were consistent with previously reported methods (Perry and Kuehn, 2007; Perry and Kuehn, 2009; Perry et al., 2011). The magnetic resonance images were transferred into Amira 4 Visualization and Volume Modeling software (Mercury Company Systems Inc, Chelmsford, MA), which has a built-in native Digital Imaging and Communications in Medicine (DICOM) support program. The addition of the DICOM support system to the Amira software program ensures that magnetic resonance imaging data can be transferred into Amira while maintaining the anatomical geometry (e.g., aspect ratio, scaling dimension, and image resolution). Using the three-dimensional anatomical scan, the entire data set was resampled to obtain the oblique coronal image that displayed the levator muscle in the most cohesive complete form. Figure 1 displays a subject’s levator muscle in the oblique coronal plane. The measures obtained on the two-dimensional image plane included muscle length (origin to midline velar insertion), extravelar length (origin to insertion point into the velum), intravelar length (entire length of the levator muscle that is contained within the body of the velum), distance between muscle origins, and the distance between the locations where the levator muscle bundles insert into the body of the velum. Figure 2 displays the measures obtained on the levator muscle.
FIGURE 1.
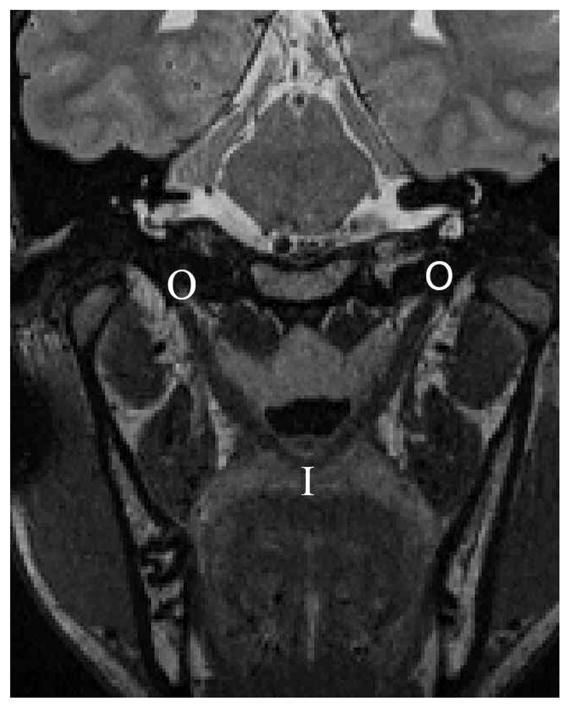
Magnetic resonance imaging in the oblique coronal plane displaying the levator muscle from the origin to the insertion in the velum. “I” represents the insertion of the levator muscle into the velum, and “O” represents the origin at the base of the skull. Note that the muscle forms a sling through the middle of the velum.
FIGURE 2.
Magnetic resonance imaging of the oblique coronal plane displaying the levator muscle and the measures obtained on the muscle. The extravelar segment consists of the portion of the levator muscle bundle that exists outside of the velum, and the intravelar segment consists of the entire levator muscle that exists inside the body of the velum (right and left segments combined). Levator length represents the entire length of the muscle bundle from origin to insertion at the velar midline. The levator insertion distance represents the distance between the two points where the levator muscle bundles insert into the body of the velum.
The successive oblique coronal images that represent the muscle in its entirety were used to define the levator muscle fibers. Voxels of the combined slices were used to create a voxel set. The levator bundle fibers were segmented by hand using a paintbrush tool with one pixel resolution to highlight the boundaries of the levator muscle through successive slices. When the muscle appeared to be curved (as observed in three individuals), the data set was resampled and segmented from multiple image planes. The segmented highlighted voxels were generated into surfaces (Fig. 3) that were imported into the Maya 7.0 (Autodesk, Ontario, Canada) software system without changing the pixel aspect ratio. The surfaces (outlines of voxel sets) created by Amira are rigid and irregular (Fig. 3). Imported surfaces in Maya preserve the original orientation, aspect ratio, and polygonal count of the original data (Perry et al., 2011).
FIGURE 3.
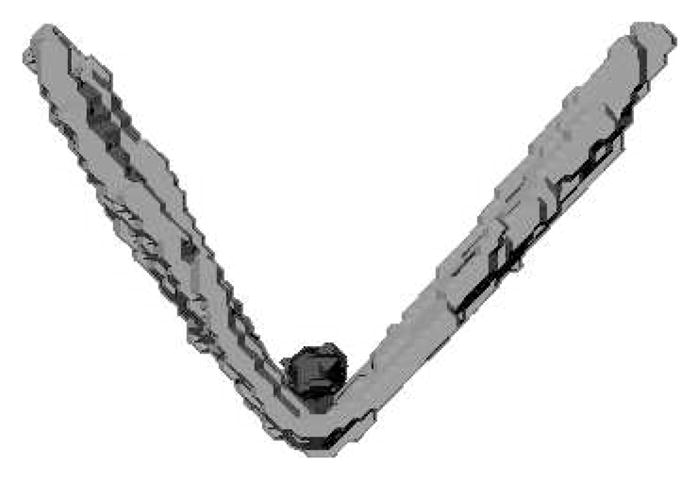
Surface generation of the levator muscle created from Amira and imported into Maya. Note the rigid and irregular shape of the muscle, which is due to voxel segmentation done in Amira. The levator muscle forms a “V” shape, and the musculus uvulae is a cradled by the levator sling (shown as a small sphere inside the “V”).
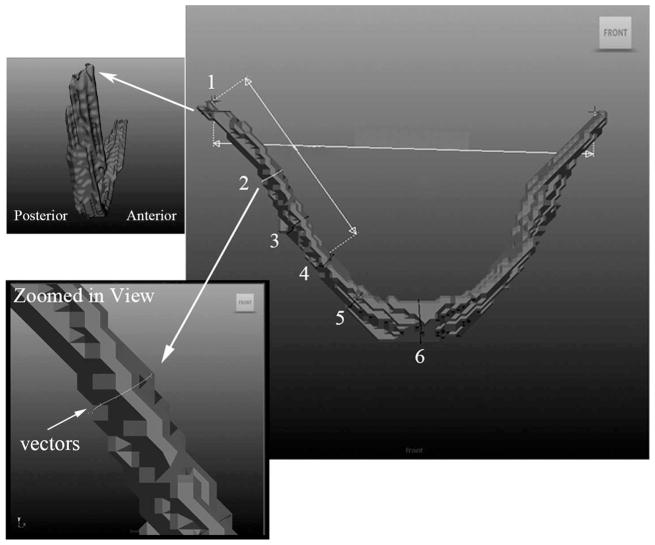
The levator muscle length measures obtained in Amira showed very little difference between the right and left muscle bundle (between 0.01 mm and 2.9 mm) within each subject (Table 2). Therefore, the muscle circumference was measured on only one muscle bundle (subject’s right muscle bundle). Using Maya, a curve-vector arc tool was used to create outlines around the muscle bundle at six points along the length of the muscle from the origin to the insertion (Fig. 4). The measure points include (1) origin of the muscle, (2) halfway between origin and velum, (3) halfway between measure 2 and measure 4, (4) point where the muscle inserts into the velum, (5) half way between measure 4 and midline of the muscle at velum, and (6) midline insertion within the velum. The six points that were measured are displayed in Figure 4. At the origin, the muscle bundle was measured along the plane of attachment to the base of the skull. As evident in Figure 4, the top surface of the levator muscle origin is oblique to the long axis of the muscle. Therefore, the origin of the muscle bundle was measured as a line coursing along the upper surface of the muscle attachment. All other measure points were obtained by creating a circle around the muscle bundle perpendicular to the long axis.
TABLE 2.
Lengths in Millimeters of Levator Muscle, Extravelar Length (Length of Muscle From Origin to the Point at Which the Muscle Inserts Into the Body of the Velum), Distance Between Origins at the Base of the Skull, Linear Distance Between the Points Where the Levator Inserted Into the Body of the Velum (“Velar Insertion”), and the Intravelar Segment (IVS; Distance of the Levator Muscle That is Within the Body of the Velum)
| Subject | Levator Length
|
Extravelar Length
|
Origin-Origin | Velar Insertion | IVS | ||
|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | ||||
| S1 | 48.20 | 48.60 | 29.05 | 28.63 | 56.89 | 25.25 | 39.12 |
| S2 | 50.58 | 52.51 | 32.60 | 30.47 | 58.60 | 27.45 | 40.02 |
| S3 | 41.67 | 42.17 | 26.88 | 26.82 | 51.53 | 22.35 | 30.14 |
| S4 | 47.19 | 48.75 | 33.13 | 34.66 | 58.12 | 27.52 | 28.15 |
| S5 | 48.50 | 46.95 | 34.08 | 32.34 | 57.88 | 23.99 | 29.03 |
| S6 | 52.85 | 52.68 | 34.54 | 34.63 | 60.04 | 26.58 | 36.36 |
| S7 | 45.43 | 44.82 | 28.12 | 27.10 | 52.45 | 26.42 | 35.03 |
| S8 | 46.25 | 45.48 | 30.11 | 28.35 | 58.50 | 26.37 | 33.27 |
| S9 | 48.08 | 48.07 | 29.10 | 29.18 | 52.38 | 27.66 | 37.87 |
| S10 | 43.24 | 46.12 | 27.89 | 27.89 | 53.70 | 28.69 | 33.58 |
| Mean/SD | 47.20 (3.3) | 47.72 (3.3) | 30.55 (2.8) | 30.01 (2.9) | 56.01 (3.1) | 26.23 (1.9) | 34.26 (4.2) |
FIGURE 4.
Image of the levator muscle displayed in the Maya software tool. The six points along the length of the muscle bundle are shown as lines appearing perpendicular to the long axis (transverse). The six points include (1) origin of the muscle, (2) halfway between origin and velum, (3) halfway between measure 2 and measure 4, (4) point where the muscle inserts into the velum, (5) halfway between measure 4 and midline of muscle at velum, and (6) midline insertion within the velum. Figure 4 also demonstrates a magnified image of the oblique origin (top left) where the first measure was taken and a closer view of the curve tool and vectors applied (lower left) to the circle. The vectors appear as small dots around the circle.
Eight to 10 vectors were placed around the muscle outlines to allow for the outlines to be manually positioned against the levator muscle bundle surface and perpendicular to the long axis (Fig. 4). The muscle was not perfectly cylindrical; therefore, it was important to have numerous control vertices to account for the variation in the outlines around the muscle surface. The three-dimensional model of the levator muscle with the six circumference markers was rotated on the computer screen to view the muscle from multiple views. By rotating the three-dimensional model in space, the vectors could be tightly positioned onto the surface of the model.
The transformed circumferences (outlines) were relocated onto a flat surface plane in the three-dimensional software (Maya 7.0). The anterior-to-posterior diameter represents the diameter of the muscle bundle from front to back (Fig. 5, lateral view) and, in general, is the largest diameter of the muscle bundle. Because the levator muscle has a curvilinear form as it bends medially toward the velum, the directional terms used to describe the smaller diameter measure vary depending on the location of the measurement. Thus, as seen in Figure 5 (top-down view), the smaller diameter taken along the extravelar segment of the levator muscle bundle represents the distance from medial to lateral. The diameter in the intravelar segment (Fig. 5, lateral view) represents a distance from superior to inferior. Both directional terminologies (superior-to-inferior or medial-to-lateral) will be used for this study and represent the smaller diameter compared with that of the larger anterior-to-posterior muscle diameter.
FIGURE 5.
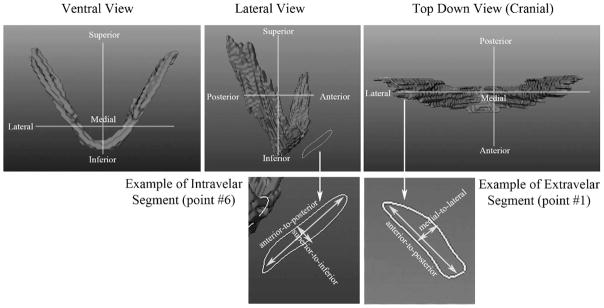
Demonstration of the two diameter measures (anterior to posterior; medial to lateral and superior to inferior) obtained for the intravelar segment and extravelar segment. Note that the diameter of the intravelar segment of the levator muscle represents a diameter in the superior-to-inferior direction. The diameter in the extravelar segment of the levator muscle represents a diameter in the medial-to-lateral direction.
Results
Levator Muscle Measures
The measures obtained on the two-dimensional image plane included muscle length (origin to midline velar insertion), extravelar length (origin to insertion point into the velum), intravelar length (length of the entire muscle segment contained within the velum), distance between muscle origins, and the distance between the points where the muscle bundles insert into the body of the velum (Fig. 2). Levator muscle length ranged from 41.67 mm to 52.85 mm across all subjects (Table 2). Mean levator muscle length was 47.20 mm (SD, 3.3 mm) and 47.72 mm (SD, 3.3 mm) for the right and left levator muscle bundles, respectively. The right and left muscle bundles were quite similar within subjects, showing differences less than 2.9 mm.
Extravelar muscle length was determined as the distance from the origin to the point of insertion into the body of velum. Mean extravelar muscle length was 30.55 mm (SD, 2.8 mm) and 30.01 mm (SD, 2.9 mm) for right and left muscle bundles, respectively (Table 2). The differences between the right and left muscle bundle lengths were minimal (under 1.7 mm) within subjects. The extravelar segment accounts for approximately 64% (SD, 4%) of the entire levator muscle bundle length. The mean intravelar segment length was 34.26 mm (SD, 4.2 mm) and ranged between 28.15 mm to 40.02 mm. The intravelar length appears to have more variability across subjects (ranging from 28.15 mm to 40.02 mm) compared with that of the extravelar length (ranging from 26.82 to 34.63 mm).
The range of distance measurements between muscle origins was 51.53 mm to 60.04 mm across subjects with a mean distance of 56.01 mm (SD, 3.1 mm). The distance between the two points where the levator muscle bundles insert into the body of the velum was measured as a straight line between the inferior ends of the extravelar segments of the levator muscle (Fig. 2). The measurement was taken on the medial edge of the muscle (Fig. 2). As seen in Table 2, the distance between the points where the levator muscle bundles insert into the velum ranged from 22.35 mm to 28.69 mm across subjects, with a mean of 26.23 mm (SD, 1.9 mm). In all subjects, the levator muscle displayed a muscle sling through the midline of the velum with no separation or septum between muscle bundles at the junction in the velum. The middle point in the sling (at the velum) was arbitrarily determined as the inferior end of each muscle bundle.
Circumference of the Levator Muscle
The circumference of the levator muscle bundle was measured at six points along the length of the levator muscle (Fig. 4). Table 3 lists each value obtained at each point, and Figure 6 provides a graphical display of the measure points across each subject. At the origin of the levator muscle (point 1; Table 3), the muscle measured between 16.60 mm and 25.35 mm across subjects. The mean circumference was 18.90 mm (SD, 2.6 mm). As evident in Table 3, the levator muscle demonstrated the greatest amount of change in the overall circumference from point 1 (muscle origin) to point 2 (halfway point between origin and the velum). At the second point, the muscle circumference mean increased slightly (mean, 22.40 mm; SD, 4.9 mm). This increase between circumference measures between points 1 and 2 was observed in all subjects. The means for the remainder of the measures (points 3, 4, 5, and 6) were consistent, showing a minimal change in the muscle circumference as it descends toward the velum. At the third measure point (halfway between measure 2 and the velum), the mean circumference was 22.76 mm (SD, 4.0 mm). Measure point 4 represents the point where the levator muscle inserts into the body of the velum. The mean muscle circumference at this point was 22.02 mm (SD, 3.6 mm) showing little to no increase in total muscle circumference compared with that of measure points 2 and 3. Measure point 5 represents halfway between the velar insertion point (measure point 4) and the midline insertion in the velum (measure point 6). Both measures 5 and 6 also showed little to no increase in muscle circumference, with means at 21.96 mm (SD, 5.3 mm) and 23.71 mm (SD, 6.5 mm), respectively. Overall (Table 3; Fig. 6), the muscle made the largest change in circumference between the origin and the point halfway between the muscle origin and the insertion into the velum.
TABLE 3.
Circumferences in Millimeters Along the Length of the Levator Muscle at the Following Points: (1) Origin of the Muscle, (2) Halfway Between Origin and Velum, (3) Halfway Between Measure 2 and Measure 4, (4) Point Where the Muscle Inserts Into the Velum, (5) Halfway Between Measure 4 and Midline of Muscle at Velum, and (6) Midline Insertion Within the Velum
| Subject | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| S1 | 17.83 | 25.02 | 24.29 | 23.58 | 24.75 | 24.25 |
| S2 | 16.67 | 18.97 | 16.76 | 19.35 | 18.89 | 20.28 |
| S3 | 18.95 | 18.81 | 19.53 | 16.06 | 13.00 | 13.61 |
| S4 | 20.35 | 25.06 | 25.40 | 24.98 | 24.72 | 27.16 |
| S5 | 17.39 | 22.21 | 21.83 | 19.84 | 20.41 | 20.89 |
| S6 | 25.24 | 32.21 | 29.63 | 27.32 | 31.59 | 36.77 |
| S7 | 16.60 | 20.25 | 20.80 | 20.65 | 18.85 | 18.82 |
| S8 | 16.92 | 14.95 | 20.78 | 21.09 | 25.77 | 30.27 |
| S9 | 19.81 | 26.77 | 28.21 | 27.21 | 24.28 | 24.34 |
| S10 | 19.28 | 19.78 | 20.34 | 20.14 | 17.31 | 20.76 |
| Mean/SD | 18.90 (2.6) | 22.40 (4.9) | 22.76 (4.0) | 22.02 (3.6) | 21.96 (5.3) | 23.71 (6.5) |
FIGURE 6.
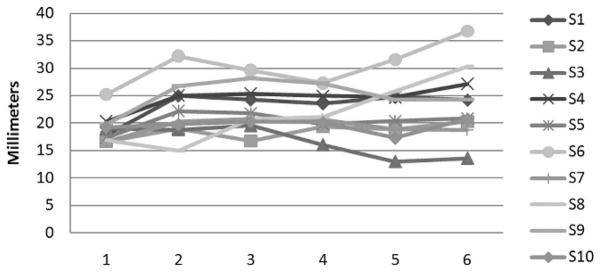
Graphical display of the circumference measurements obtained from each subject (in millimeters) at each point along the levator muscle.
Diameter of the Levator Muscle
The muscle diameter was measured by determining the diameter of the circumference in both directions. The anterior-to-posterior measure is the largest diameter of the muscle, and the smaller diameter is taken perpendicular to this diameter. Diameter data are presented in Table 4. The muscle diameters at the origin near the base of the skull were 7.9 mm (anterior to posterior) and 3.1 mm (medial to lateral). At measure point 2, the muscle diameters in both directions increased slightly (mean, 8.7 mm anterior-to-posterior diameter; mean, 4.4 mm medial-to-lateral diameter). Beginning at the origin, the muscle appears to be a flattened cylinder. This muscle shape continued along the entire length of the muscle bundle and is a consistent feature (greater diameter in the anterior-to-posterior dimension compared with the medial-to-lateral diameter) among all subjects. The muscle remains somewhat consistent in its diameter from points 2 through 5, with a slight increase in anterior-to-posterior diameter at measure point 6. The greatest anterior-to-posterior diameter was observed at the midline of the velum (mean, 9.6 mm), whereas the greatest medial-to-lateral or superior-to-inferior diameter was observed at the insertion point into the velum (measure 4) with a mean of 4.2 mm. The smallest mean diameters in both directions can be observed at the origin (mean, 7.9 mm and 3.1 mm). Nearly all subjects displayed a diverging of the muscle at the insertion into the velum in which the muscle was two to three times greater in the anterior-to-posterior dimension compared with that of the diameter in the superior-to-inferior dimension. Two subjects showed minimal to no difference (subjects 2 and 3) between the two diameters at the midline insertion into the velum.
TABLE 4.
The Larger Diameter, Anterior-to-Posterior (A-P), and the Smaller Diameter, Medial-to-Lateral (M-L) and Superior-to-Inferior (S-L), Shown in Millimeters Along the Length of the Levator Muscle at the Following Points: (1) Origin of the Muscle, (2) Halfway Between Origin and Velum, (3) Halfway Between Measure 2 and Measure 4, (4) Point Where the Muscle Inserts Into the Velum, (5) Halfway Between Measure 4 and Midline of Muscle at Velum, and (6) Midline Insertion Within the Velum
| Subject | 1
|
2
|
3
|
4
|
5
|
6
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-P | M-L | A-P | M-L | A-P | M-L | A-P | S-I | A-P | S-I | A-P | S-I | |
| S1 | 7.5 | 2.8 | 9.1 | 6.0 | 9.3 | 5.5 | 9.2 | 5.6 | 8.9 | 5.2 | 9.6 | 4.1 |
| S2 | 6.6 | 3.5 | 5.7 | 4.9 | 6.1 | 4.7 | 5.8 | 5.3 | 6.2 | 5.8 | 6.1 | 6.1 |
| S3 | 7.9 | 3.2 | 6.7 | 3.5 | 8.1 | 3.5 | 6.2 | 3.5 | 4.5 | 2.7 | 4.6 | 3.6 |
| S4 | 8.3 | 3.9 | 9.1 | 5.2 | 10.1 | 3.9 | 9.7 | 4.7 | 9.9 | 4.2 | 11.3 | 3.2 |
| S5 | 7.1 | 3.5 | 9.7 | 3.1 | 9.1 | 3.3 | 8.4 | 3.1 | 8.7 | 2.5 | 9.0 | 2.2 |
| S6 | 11.1 | 2.4 | 13.3 | 5.5 | 12.1 | 4.9 | 11.1 | 4.1 | 13.6 | 3.6 | 17.1 | 3.4 |
| S7 | 7.1 | 2.3 | 8.7 | 3.3 | 8.7 | 3.2 | 8.0 | 4.6 | 7.6 | 3.2 | 7.6 | 3.7 |
| S8 | 7.3 | 2.3 | 6.1 | 3.1 | 8.6 | 2.6 | 9.1 | 3.4 | 11.5 | 3.6 | 12.6 | 3.0 |
| S9 | 7.9 | 4.2 | 10.4 | 5.9 | 11.1 | 5.4 | 11.2 | 4.2 | 10.1 | 4.3 | 9.9 | 4.1 |
| S10 | 8.1 | 3.1 | 8.3 | 3.9 | 8.4 | 3.7 | 8.5 | 4.0 | 7.4 | 2.5 | 8.2 | 2.4 |
| Mean/SD | 7.9 (1.2) | 3.1 (0.7) | 8.7 (2.2) | 4.4 (1.2) | 9.2 (1.7) | 4.1 (1.0) | 8.7 (1.8) | 4.2 (0.8) | 8.8 (2.6) | 3.8 (1.1) | 9.6 (1.1) | 3.6 (1.1) |
Discussion
The magnetic resonance imaging sequences used in this study provided good spatial resolution (0.8 mm isotropic resolution), which allowed for clear visualization of the boundaries between neighboring muscles, including the musculus uvulae and the palatoglossus (Fig. 7). Clear muscle boundaries ensure that the muscle that is extracted represents the levator muscle and does not include surrounding muscles, which has been a limitation of previous studies (Ettema et al., 2002). Three-dimensional technology coupled with magnetic resonance imaging provides a complete view of the muscle morphology. A short animation is provided demonstrating the muscle rotating around a vertical axis (Fig. 8).
FIGURE 7.
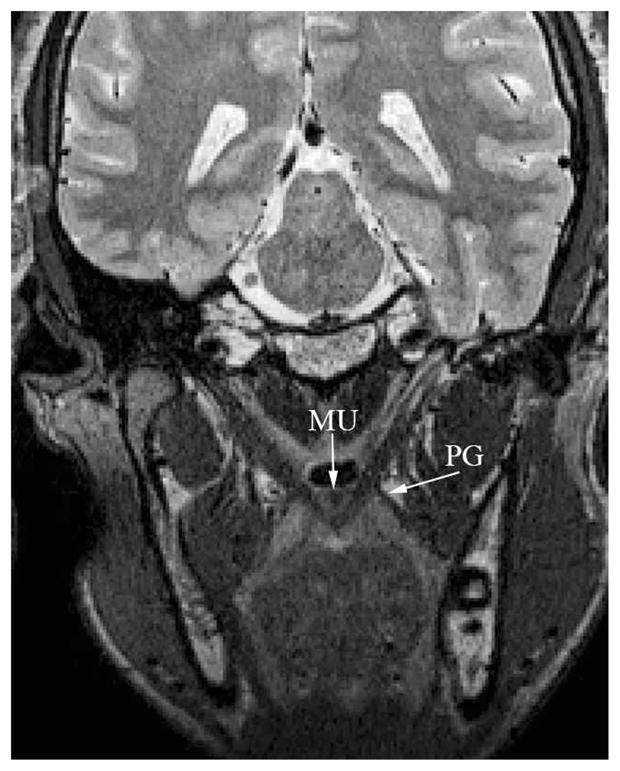
Image of the musculus uvulae (“MU”) on an oblique coronal image plane. Note that the muscle appears near the nasal surface of the velum and is clearly delineated from the levator muscle bundle through the midline of the velum. The palatoglossus (PG) is also evident in the image, and the boundary, as it joins the levator muscle, can be visualized.
Histologic studies are destructive in nature and are done only on cadaveric material. Lateral view x-ray and nasoendoscopy do not provide visualization of muscle tissue. Magnetic resonance imaging is the only imaging method for examining muscle tissue, such as the levator muscle, in living individuals and from multiple views. Variations observed in the present study compared to previous literature (using histology, x-ray, nasoendoscopy, or magnetic resonance imaging alone) may be due to the inherent benefits of using imaging in living subjects (magnetic resonance imaging) combined with three-dimensional technology to examine structures that exist as a complex system, such as that of the velopharynx. Three-dimensional technologies allow for imaging extraction of muscle structures from their surrounding environment and careful examination from multiple viewpoints. Although the levator muscle was the only focus of this article, further use of this method for imaging other velopharyngeal structures is an important area in need of further development. The velopharynx exists as a complex system controlled by many muscles and structures. The biomechanics of the entire velopharyngeal system appear to be controlled by craniometry (Chung and Kau, 1985; Chung et al., 1986; Johannsdottir et al., 2004; Konigsberg et al., 2009). Hence, it would be expected that variations would exist based on gender and sex. Three-dimensional computer technology and magnetic resonance imaging may be a viable method for direct comparisons across groups to carefully examine these variations relative to surrounding structures. In addition, these methods would be beneficial in examining the development of the levator muscle and surrounding structures from infancy to adulthood.
Measures of the levator muscle including length, extra-velar length, intravelar muscle segment length, distance between origins, and distance between the two points where the levator muscle bundles insert into the body of the velum were similar across subjects and also relative to previous studies (Table 5). Levator muscle length has been reported in three other studies. Studies related to normal anatomy (Ettema et al., 2002; Perry, 2011) demonstrated levator muscle values that were within 1.86 mm of each other. Distances between muscle origins were within 6.32 mm. Extravelar segment values were within 3.19 mm of each other (present study compared with Tian et al., 2010). The values reported by Ettema et al. (2002) are the only comparisons that are for Caucasian males (five male subjects). Tian et al. (2010) examined 12 Chinese adults (males and females), Perry (2011) examined four Caucasian females, and Ha et al. (2007) examined four males with a repaired cleft palate. Because participant groups across these reported studies are not autonomous (varying by race, gender, and normal or abnormal anatomy), it would not be expected that values in each area would be related closely to one another.
TABLE 5.
Comparison of Group Means Between Current Study and Similar Studies Using Magnetic Resonance Imaging Analyses of Levator Muscle Morphology*
| Parameter | Present Study, mm (N = 10) | Ettema et al. (2002), mm (N = 5) | Perry (2011), mm (N = 4) | Ha et al. (2007), mm (N = 4) | Tian et al. (2010), mm (N = 12) |
|---|---|---|---|---|---|
| Levator muscle length | 47.46 | 46.4 | 45.6 | 41 | — |
| Origin to origin | 56.01 | 54.6 | — | 53 | 60.92 |
| Extravelar length | — | — | — | ||
| Right | 30.55 | 32.97 | |||
| Left | 30.01 | 33.20 | |||
| Distance between 2 points where muscle bundles insert into velum | 26.23 | — | — | — | 24.99 |
Values reported under Ettema et al. (2002) represent the mean for Caucasian subjects only. Values reported under Tian et al. (2010) represent the mean for 12 adult Chinese subjects (male and female) because values are not reported by gender. Ha et al. (2007) represent the mean for four male subjects with repaired cleft palate. Note that not all studies measured the muscle at each point related to the present study.
The levator muscle has been described as a flattened cylinder that diverges once it enters the body of the velum (Azzam and Kuehn, 1977; Kuehn and Azzam, 1978; Huang et al., 1997). Huang et al. (1997) described the extravelar part of the muscle to be consistently cylindrical and uniform in size from the origin to the point at which the muscle enters the velum. In the present study, the muscle also demonstrated a shape of a flattened cylinder. Huang et al. (1997) reported a mean diameter of 7.96 mm (right muscle) and 7.94 mm (left muscle) at the cranial base (Table 6). The present study demonstrated an anterior-to-posterior mean diameter of 7.9 mm.
TABLE 6.
Comparison of the Measurements Along the Length of the Levator Veli Palatini Muscle in the Present Study (Anterior-to-Posterior Diameter Means) Compared With Those Reported by Previous Literature (Anterior-to-Posterior Diameter Means)*
| Measure Point | Present Study, mm (N = 10) | Azzam and Kuehn (1977), mm | Huang et al. (1997), mm | Kuehn and Azzam (1978), mm |
|---|---|---|---|---|
| 1: Origin | 7.9 | — | 7.95 | — |
| 2: Halfway between origin and velum | 8.7 | — | 8.13 | 8.24 |
| 3: Halfway between measure 2 and velum | 9.2 | 9.5 | — | — |
| 4: Velum insertion | 8.7 | — | — | — |
| 5: Halfway between measure 4 and midline | 8.8 | — | — | — |
| 6: Midline insertion | 9.6 | — | — | — |
Note that not all studies measured the muscle at each point related to the present study.
The second measure point (halfway between origin and the insertion into the velum) was most related to the level of the torus tubarius. The torus tubarius was identified using a sagittal image plane positioned along the right lateral pharyngeal wall and segmented in the same manner as that of the levator muscle. The torus tubarius was often found to be near (within 2 to 3 mm) the second measure point; however, the location of the torus tubarius relative to the muscle origin varied between subjects. Studies taking measures at the level of the torus tubarius may not be assessing the levator muscle at the same position relative to the muscle origin. This is a benefit of using three-dimensional modeling for analysis. Measure point 2 in the present study was determined by using a ruler tool (Maya) to determine precisely half the distance of the extravelar segment. At this point, the muscle increases slightly in circumference with a mean of 22.40 mm (Table 3) and is shaped like a flattened cylinder, with its greatest diameter in the anterior-to-posterior dimension. The mean anterior-to-posterior diameter was 8.7 mm (Table 4). Huang et al. (1997) reported the mean diameter (anterior-to-posterior) at the level of the torus tubarius of 8.14 mm (right muscle) and 8.11 mm (left muscle). Kuehn and Azzam (1978) obtained the muscle diameter at a level deep to the torus tubarius in which the muscle ranged from 5.5 mm to 11.4 mm (mean, 8.14 mm and 8.34 mm). Mean values reported across the literature are similar to those reported in the present study. It is possible that the minimal variations noted might be related to racial variations between Caucasians (present study) and those of Asian descent (Huang et al., 1997). Japanese and Caucasian individuals have been found to have statistically significant variations in craniometry, including difference in sella to nasion, anterior nasal spine to posterior nasal spine, and articulare to gnathion lengths (Chung and Kau, 1985; Chung et al., 1986). These differences might also be due to the arbitrary nature of past studies in identifying the points measured. For example, variations (although minimal) were observed among individuals in the location of the torus tubarius relative to the length of the muscle bundle.
The third measure point represented the midway point between measure 2 and the insertion of the levator muscle into the velum. At this point, the muscle remained nearly unchanged in shape, displaying a similar mean circumference and mean diameters to those obtained at the second measure point. Azzam and Kuehn (1977) reported the mean diameter of the left and right levator muscle bundle (as measured midway between the medial extent of the cartilage of the Eustachian tube and the levator insertion into the velum) at 9.1 mm and 9.9 mm (range between 7.1 mm and 12.6 mm). These values are quite similar to those observed in the present study, showing a mean anterior-to-posterior diameter of 9.2 mm and a range between 6.1 mm and 12.1 mm (Table 6). This point along the extravelar segment displayed the greatest circumference and anterior-to-posterior diameter.
The intravelar segment of the muscle is represented by measures 4, 5, and 6. Between measure points 4 and 5, the muscle remained fairly consistent with that observed in measure 3. At the midline, the muscle becomes thinner (smaller superior-to-inferior diameter) and broadens or diverges in the anterior-to-posterior dimension. The muscle is similar among subjects with regard to the extravelar segment. Inside the velum (intravelar segment), however, the variability of the muscle among subjects increases. At the midline of the velum, the mean muscle anterior-to-posterior diameter ranged between 4.6 mm and 12.6 mm (mean, 9.6 mm; SD, 1.1 mm). The circumference ranged from 13.61 mm to 30.27 mm (mean, 23.71 mm; SD, 6.5 mm). Although the muscle appears to increase in circumference compared with other points along the muscle length, the muscle appears to be more inconsistent and sparser (thinner in the superior-to-inferior diameter). This observation is consistent with previous findings. Numerous authors have reported a broad extension of the muscle fibers as they enter the velum (Dickson et al., 1974; Boorman and Sommerlad, 1985; Kuehn and Kahane, 1990; Carrasco, 1996; Huang et al., 1998). However, from the present study, it appears that the fibers do not broaden to the greatest extent until the midline of the velum (measure point 6), as opposed to the velar insertion point (measure point 4).
Kuehn and Moon (2005) reported the crossing of the levator muscle fibers at about 25% of the distance between the posterior nasal spine and the uvula. Through the mid-line, there was no septum separating the muscle bundles. Although the fibers were reported to overlap those of the opposing levator bundle, the midline was not marked by an increase in muscle fibers. In fact, the tissues were less sparse, with a slight accumulation of connective tissue in the midline of the levator sling. These findings are consistent with those of the present study. From the location where the levator muscle bundles insert into the body of the velum, the muscle becomes increasingly thinner in the superior-to-inferior diameter. This location is also referred to as the velar eminence, which is the point at which the velum is pulled superiorly and posteriorly to make contact with other velopharyngeal structures. During normal speech, velar bulging can be identified at the velar eminence where levator muscle fibers are sparse and the musculus uvulae fibers are present. In individuals with cleft palate, the velum postoperatively may remain thin or hypoplastic, which can be a functional disadvantage during velopharyngeal closure. It is not well understood whether individuals with cleft palate have a hypoplastic or absent musculus uvulae, as suggested by some authors (Piggot, 1969; Croft et al., 1978; Lewin et al., 1980). Examining the levator muscle and musculus uvulae using magnetic resonance imaging and three-dimensional technologies may provide further insight into the necessary amount of levator fiber overlap or alternative methods that might provide adequate velar bulging at the region of the velar eminence. In addition, postoperative magnetic resonance imaging studies are necessary to understand the nature of the muscles following primary palatoplasty.
The measures of the muscle length, extravelar length, intravelar length, and the distance between the locations where the two levator muscle bundles insert into the body of the velum are quite uniform across subjects and consistent with the previously reported literature. It is possible that the extravelar segment is more uniform across subjects because it is responsible for the primary movement of the velum. Because the levator muscle is sparse and irregular within the body of the velum (intravelar segment), it is hypothesized that its force contribution is less than that of the extravelar segment. The intravelar segment may serve merely as an attachment of the levator muscle to the body of the velum and therefore may have more variability across subjects. Physiologically, force is greatest at the region of the muscle belly. Based on this study, it is hypothesized that this force is greatest in the extravelar segment. Further research is needed to determine likely changes in levator circumference, as well as length changes, as the muscle contracts to elevate the velum during speech and swallowing maneuvers. What does appear consistent across all subjects regarding the intravelar segment is the continuous sling created through the midline. A septum or separation of the muscle fibers in the midline was never observed. This may be a critical feature for creating a strong connection between the levator muscle and the body of the velum. If the levator muscle had a septum or midline separation, the force of the muscle contraction might have a more lateral traction or pull on the velum, causing a restriction in the velar elevation. Because of a lack of data using muscle-imaging techniques (i.e., magnetic resonance imaging) following primary palatal surgery, it is not known what the fate is of the levator muscle sling. It may be possible that the muscle, following surgery, migrates toward a less favorable position (toward the original anterior hard palate insertion) and may even separate in the midline in some individuals (Kuehn et al., 2004). The amount of displacement or muscle separation may be dependent on the type of surgery. These are observations that only magnetic resonance imaging research in young children before and after primary palatoplasty will be able to examine. Such information may have an effect on the surgical outcomes related to speech.
Acknowledgments
This study was made possible by grant 1R03DC009676-01A1 from the National Institute of Deafness and Other Communicative Disorders. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health.
References
- Arnold WH, Nohadani N, Koch KHH. Morphology of the auditory tube and palatal muscles in a case of bilateral cleft palate. Cleft Palate Craniofac J. 2005;42:197–201. doi: 10.1597/03-138.1. [DOI] [PubMed] [Google Scholar]
- Azzam NA, Kuehn DP. The morphology of musculus uvulae. Cleft Palate J. 1977;14:78–87. [PubMed] [Google Scholar]
- Bell-Berti F. An electromyographic study of velopharyngeal function in speech. J Speech Hear Res. 1976;19:225–240. doi: 10.1044/jshr.1902.225. [DOI] [PubMed] [Google Scholar]
- Boorman JG, Sommerlad BC. Levator palati and palatal dimples: their anatomy, relationship and clinical significance. Br J Plast Surg. 1985;38:326–332. doi: 10.1016/0007-1226(85)90236-x. [DOI] [PubMed] [Google Scholar]
- Carrasco VN. Management of middle ear disease and malformations. In: Turvey TA, Vi KWL, Fonesca RJ, editors. Facial Clefts and Craniosynostosis. Philadelphia: WB Saunders; 1996. pp. 213–221. [Google Scholar]
- Chung C, Kau M. Racial differences in cephalometric measurements and incidence of cleft lip with or without cleft palate. J Craniofacial Genet Dev Biol. 1985;5:341–349. [PubMed] [Google Scholar]
- Chung C, Runck D, Bilben S, Kau M. Effects of interracial crosses on cephalometric measurements. Am J Phys Anthropol. 1986;69:465–472. doi: 10.1002/ajpa.1330690405. [DOI] [PubMed] [Google Scholar]
- Chung C, Runck D, Kau M. Racial differences in cephalometric measurements and incidence of cleft lip with or without cleft palate. J Craniofac Genet Dev Biol. 1985;5:341–349. [PubMed] [Google Scholar]
- Croft CB, Shprintzen RJ, Daniller A, Lewin ML. The occult submucous cleft palate and musculus uvulae. Cleft Palate J. 1978;15:150–154. [Google Scholar]
- Dickson DR, Dickson WM. Velopharyngeal anatomy. J Speech Hear Res. 1972;15:372–381. doi: 10.1044/jshr.1502.372. [DOI] [PubMed] [Google Scholar]
- Dickson DR, Grant JC, Sicher H, DuBrul EL, Palta J. Status of research in cleft palate anatomy and physiology. Cleft Palate J. 1974;11:471–492. [PubMed] [Google Scholar]
- Ettema SL, Kuehn DP. A quantitative histologic study of the normal human adult soft palate. J Speech Hear Res. 1994;37:303–313. doi: 10.1044/jshr.3702.303. [DOI] [PubMed] [Google Scholar]
- Ettema S, Kuehn D, Perlman A, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Craniofac J. 2002;39:130–144. doi: 10.1597/1545-1569_2002_039_0130_mriotl_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Talmi YP, Nachmani A. Levator veli palatini muscle and eustachian tube function. Plast Reconstr Surg. 1990;85:684–692. [PubMed] [Google Scholar]
- Fritzell B. Electromyography in the study of the velopharyngeal function: a review. Folia Phon. 1979;31:93–102. doi: 10.1159/000264155. [DOI] [PubMed] [Google Scholar]
- Fritzell B. The velopharyngeal muscles in speech. Acta Otolaryngol. 1969;250(suppl):1–81. [PubMed] [Google Scholar]
- Ha S, Kuehn D, Cohen M, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle in speakers with repaired cleft palate. Cleft Palate Craniofac J. 2007;44:495–505. doi: 10.1597/06-220.1. [DOI] [PubMed] [Google Scholar]
- Honjo I, Okazaki N, Noze T. The role of the tensor veli palatini in movement of the soft palate. Acta Otolaryngol. 1979;88:137–141. doi: 10.3109/00016487909137152. [DOI] [PubMed] [Google Scholar]
- Huang MH, Lee ST, Rajendran KA. Anatomic basis of cleft palate and velopharyngeal surgery: implications from a fresh cadaveric study. Plast Reconstr Surg. 1998;101:613–627. doi: 10.1097/00006534-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Huang MH, Lee ST, Rajendran KA. A fresh cadaveric study of the paratubal muscles: implications for eustachian tube function in cleft palate. Plast Reconstr Surg. 1997;100:833–842. doi: 10.1097/00006534-199709001-00003. [DOI] [PubMed] [Google Scholar]
- Johannsdottir B, Thordarson A, Magnusson T. Craniofacial skeletal and soft tissue morphology in Icelandic adults. Eur J Orthod. 2004;26:245–250. doi: 10.1093/ejo/26.3.245. [DOI] [PubMed] [Google Scholar]
- Kent RD, Carney PJ, Severeid LR. Velar movement and timing: evaluation of a model for binary control. J Speech Hear Res. 1974;17:470–488. doi: 10.1044/jshr.1703.470. [DOI] [PubMed] [Google Scholar]
- Konigsberg L, Algee-Hewitt B, Steadman D. Estimation and evidence in forensic anthropology: sex and race. Am J Phys Anthropol. 2009;139:77–90. doi: 10.1002/ajpa.20934. [DOI] [PubMed] [Google Scholar]
- Kuehn DP. A cineradiographic investigation of velar movement variables in two normals. Cleft Palate J. 1976;13:88–103. [PubMed] [Google Scholar]
- Kuehn D. Velopharyngeal anatomy and physiology. Ear Nose Throat J. 1979;58:316–321. [PubMed] [Google Scholar]
- Kuehn DP, Azzam NA. Anatomical characteristics of palatoglossus and the anterior faucial pillar. Cleft Palate J. 1978;15:349–359. [PubMed] [Google Scholar]
- Kuehn DP, Ettema SE, Goldwasser MS, Barkmeier JC. Magnetic resonance imaging of the levator veli palatini muscle before and after primary palatoplasty. Cleft Palate Craniofac J. 2004;41:584–592. doi: 10.1597/03-060.1. [DOI] [PubMed] [Google Scholar]
- Kuehn D, Folkins J, Cutting C. Relationships between muscle activity and velar position. Cleft Palate J. 1982;19:25–35. [PubMed] [Google Scholar]
- Kuehn DP, Kahane JC. Histologic study of the normal human adult soft palate. Cleft Palate J. 1990;27:26–34. doi: 10.1597/1545-1569(1990)027<0026:hsotnh>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Moon JB. Histologic study of intravelar structures in normal human adult specimens. Cleft Palate Craniofac J. 2005;42:481–489. doi: 10.1597/04-125r.1. [DOI] [PubMed] [Google Scholar]
- Lewin ML, Croft CB, Shprintzen RJ. Velopharyngeal insufficiency due to hypoplasia of the musculus uvulae and occult submucous cleft palate. Plast Recontr Surg. 1980;65:585–591. doi: 10.1097/00006534-198005000-00008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lowe A, Zeng X, Fu M, Fleetham J. Cephalometric comparisons between Chinese and Caucasian patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2000;117:479–485. doi: 10.1016/s0889-5406(00)70169-7. [DOI] [PubMed] [Google Scholar]
- Lubker J, Fritzell B, Lindquist J. Velopharyngeal function: an electromyographic study. Quarterly Progress Status Report-Speech Transmission Lab (Stockholm) 1970;4:9–20. [Google Scholar]
- Mehendale FV. Surgical anatomy of the levator veli palatini: a previously undescribed tendinous insertion of the anterolateral fibers. Plast Reconstr Surg. 2004;114:307–315. doi: 10.1097/01.prs.0000131869.45025.5a. [DOI] [PubMed] [Google Scholar]
- Moon J, Kuehn D. Communication Disorders Related to Cleft Lip and Palate. 5. Austin: Pro-Ed; 2004. Anatomy and physiology of normal and disordered velopharyngeal function for speech. [Google Scholar]
- Moon J, Smith A, Folkins J, Lemke J, Gartlan M. Coordination of velopharyngeal muscle activity during positioning of the soft palate. Cleft Palate Craniofac J. 1994;31:356–363. doi: 10.1597/1545-1569_1994_031_0045_covmad_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Perry J. Variations in velopharyngeal structures between supine and upright postures using open-type magnetic resonance imaging. Cleft Palate Craniofac J. 2011;48:123–133. doi: 10.1597/09-256. [DOI] [PubMed] [Google Scholar]
- Perry J, Kuehn D. Magnetic resonance imaging and computer reconstruction of the velopharyngeal mechanism. J Craniofac Surg. 2009;20:1739–1746. doi: 10.1097/SCS.0b013e3181b5cf46. [DOI] [PubMed] [Google Scholar]
- Perry J, Kuehn D. Three-dimensional computer reconstruction of the levator veli palatini muscle in situ using magnetic resonance imaging. Cleft Palate Craniofac J. 2007;69:214–216. doi: 10.1597/06-137.1. [DOI] [PubMed] [Google Scholar]
- Perry J, Kuehn D, Sutton B, Goldwasser M. Craniometric and velopharyngeal assessment of infants with and without cleft palate. J Craniofac Surg. 2011;22:499–503. doi: 10.1097/SCS.0b013e3182087378. [DOI] [PubMed] [Google Scholar]
- Piggot RW. The nasoendoscopic appearance of the normal palatopharyngeal valve. Plast Recontr Surg. 1969;43:19–24. [Google Scholar]
- Rood SR, Doyle WJ. Morphology of tensor veli palatini, tensor tympani, and dilator tubae muscles. Ann Otol Rhinol Laryngol. 1982;87:202–210. doi: 10.1177/000348947808700210. [DOI] [PubMed] [Google Scholar]
- Seaver E, Kuehn D. A cineradiographic and electromyographic investigation of velar positioning in nonnasal speech. Cleft Palate J. 1980;17:381–388. [PubMed] [Google Scholar]
- Seif S, Dellon AL. Anatomic relationships between the human levator and tensor veli palatini and the eustachian tube. Cleft Palate J. 1978;15:329. [PubMed] [Google Scholar]
- Shprintzen RJ, Croft CB. Abnormalities of the eustachian tube orifice in individuals with cleft palate. Int J Paediatr Otolaryngol. 1981;3:15. doi: 10.1016/0165-5876(81)90015-x. [DOI] [PubMed] [Google Scholar]
- Simpson P. Dynamic consequences of differences in male and female vocal tract dimensions. J Acoust Soc Am. 2001;109:2153–2164. doi: 10.1121/1.1356020. [DOI] [PubMed] [Google Scholar]
- Spauwen PHM, Hillen B, Lommen E, Otten E. Three-dimensional computer reconstruction of the eustachian tube and paratubal muscles. Cleft Palate J. 1991;28:217. doi: 10.1597/1545-1569_1991_028_0217_tdcrot_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Sudo M, Sando I, Suzuki C. Three-dimensional reconstruction and measurement study of human eustachian tube structures: a hypothesis of eustachian tube function. Ann Otol Rhinol Laryngol. 1998;107:547–554. doi: 10.1177/000348949810700701. [DOI] [PubMed] [Google Scholar]
- Swarts JD, Rood SR. The morphometry and three-dimensional structure of the adult eustachian tube: implications for function. Cleft Palate J. 1990;27:374. doi: 10.1597/1545-1569_1991_027_0374_tmatds_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Tian W, Yin H, Redett R, Shi B, Shi J, Zhang R, Zheng Q. Magnetic resonance imaging assessment of the velopharyngeal mechanism at rest and during speech in Chinese adults and children. J Speech Lang Hear Res. 2010;53:1595–1615. doi: 10.1044/1092-4388(2010/09-0105). [DOI] [PubMed] [Google Scholar]
- Tomoda K, Morii S, Yamahita T, Kumazawa T. Histology of the human eustachian tube: effect of aging. Ann Otol Rhinol Laryngol. 1984;93:17–24. doi: 10.1177/000348948409300105. [DOI] [PubMed] [Google Scholar]
- Yamawaki Y, Nishimura Y, Suzuki Y. Eustachian tube cartilage and medial movement of the lateral pharyngeal wall on phonation. Plastic Reconstr Surg. 1999;104:350–356. doi: 10.1097/00006534-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Yeong P, Huggare J. Morphology of Singapore Chinese. Eur J Orthod. 2004;26:605–612. doi: 10.1093/ejo/26.6.605. [DOI] [PubMed] [Google Scholar]
- Yuen S, Hwang J, Poon P. EMG power spectrum patterns of anterior temporal and masseter muscles in children and adults. J Dent Res. 1989;68:800–804. doi: 10.1177/00220345890680050901. [DOI] [PubMed] [Google Scholar]