Abstract
Objective
Magnetic resonance imaging studies of the levator veli palatini muscle have used small numbers of subjects and have not consistently controlled for sex, race, or age. The purpose of this study was to conduct a structural assessment using a large homogeneous sample to examine the sex differences in the levator muscle morphology.
Methods
Thirty white adult subjects (15 men and 15 women) were imaged using a 3 Tesla MRI system. A high-resolution SPACE (sampling perfection with application-optimized contrasts using different flip-angle evolution) sequence was used to acquire images of the velopharyngeal anatomy. Levator muscle measurements were obtained.
Results
Men displayed significantly greater levator extravelar segment length (P = .003), levator intravelar segment muscle length (P < .001), greater distance between levator insertion points (P < .001), and greater angles of origin (P= .008) compared with women. There was no statistically significant variation between men and women in the distance between points of origin at the base of the skull.
Conclusions
This study provides normative data to improve understanding of levator dysmorphology such as that in cleft palate muscle anatomy. Results of the study demonstrate significant differences between white men and women across several levator muscle measures. Variations in the relative size of the cranium or height of the individual were not proportionate to the variations observed in the levator muscle.
Keywords: imaging study, levator veli palatini muscle, MRI
Advances in magnetic resonance imaging (MRI) have enabled a substantial growth in the current understanding of the velopharyngeal structures and functions. Prior to the use of MRI, studies of the levator veli palatini (levator) muscle were restricted to anatomic dissection and histology. Although these studies have provided a basis for examining muscle tissue composition and morphology, they are limited because these methods are destructive in nature to the tissue and surrounding structures. MRI is the only imaging modality that provides visualization of the levator muscle in vivo (Kuehn et al., 2001).
Numerous studies have examined the effectiveness of using MRI for assessing the structure and function of the levator muscle among adults with normal anatomy (Ettema et al., 2002; Tian and Redett, 2009; Bae et al., 2011; Perry, 2011; Perry et al., in press-a), adults with repaired cleft palate (Ha et al., 2007), children and infants with normal anatomy (Tian et al., 2010c; Perry et al., 2011; Perry et al., in press-b), children and infants prior to cleft palate repair (Tian et al., 2010b), and children after cleft palate repair (Kuehn et al., 2004; Perry et al., 2011). Studies have examined traditional image planes including sagittal, oblique, and axial (Akgüner et al., 1998; Özgür et al., 2000; Kane et al., 2002; Beer et al., 2004; Atik et al., 2008; Kao et al., 2008; Drissi et al., 2011; Silver et al., 2011).
The levator muscle courses from the base of the skull anteriorly, medially, and inferiorly into the body of the velum and can only be visualized in its total length from an oblique coronal image plane. Analysis of the levator muscle using MRI in the oblique coronal image plane is critical in understanding the velopharyngeal mechanism. Studies have examined the oblique coronal image plane (e.g., Kuehn et al., 2001; Ettema et al., 2002; Kuehn et al., 2004; Ha et al., 2007; Tian and Redett, 2009; Tian et al., 2010a, 2010b; Tian et al., 2010c; Bae et al., 2011; Perry, 2011; Perry et al., 2011).
The velopharyngeal mechanism is a complex system involving several muscles and bony structures. The levator muscle plays the largest role in velar elevation and retraction during oral speech productions. The levator muscle is significantly altered in position and orientation in a child born with cleft palate, but normative data on the geometry and size of this muscle across race, sex, and age are not available to provide information on healthy controls. Normative data is essential to understanding the complexity of abnormal anatomy, such as that in children born with cleft palate. Numerous studies have demonstrated the impact of race, sex, and age on the bony craniofacial and velopharyngeal structures (e.g., Chung et al., 1985; Chung et al., 1986; Yuen et al., 1989; Liu et al., 2000; Simpson, 2001; Ettema et al., 2002; Johannsdottir et al., 2004; Yeong and Huggare, 2004). Because the velopharyngeal musculature is attached to these bony structures, it is reasonable to assume that significant differences exist as a function of age, race, and sex. MRI studies thus far have not controlled for age, race, and sex to determine the interaction among variables during a structural analysis of the levator muscle morphology. In addition, studies within a single race have had at most 12 subjects and often contain uneven male to female comparisons. The purpose of the current study was to conduct a structural assessment using a large homogeneous sample to examine the sex differences in the levator muscle morphology.
Studies of the velopharyngeal mechanism with emphasis on the levator muscle have varied in the type of imaging method used, number of subjects, sex, race, and selected age ranges. Ettema et al. (2002) used a 1.5 Tesla system and two-dimensional (2D) image sequences to examine the levator muscle structure among 10 adult subjects (five men and five women) between 21 and 53 years of age with normal anatomy. Measures of the levator muscle included the muscle length, distance between muscle origins, angles of origin, and muscle thickness. Results of the study demonstrated a nonsignificant effect on the muscle dimensions as a function of sex. A nonsignificant trend was noted in which men had larger measurements in the levator muscle dimensions. Bae et al. (2011) performed the measurements on a similar set of subjects with the use of a 3 Tesla system using three-dimensional (3D) sequences. The authors noted numerous benefits to using 3DMRI analyses on a 3 Tesla MRI system for sampling the levator muscle. Comparisons between the five white men and five white women demonstrated a significant difference in regard to muscle length in that men had longer levator muscle bundles compared with female subjects. Ettema et al. (2002) and Bae et al. (2011) examined sex differences after controlling for race (white).
Tian et al. (2010c) examined similar measures as Bae et al. (2011) and Ettema et al. (2002); however, the study did not aim to compare differences between men and women. Rather, the study compared data from 12 Chinese adults (five men and seven women) to data from nine Chinese children with normal anatomy. Adults demonstrated larger craniofacial and velopharyngeal structures compared with children. Ratios (e.g., anterior and posterior cranial base angles) at rest, however, were not significantly different between the groups. Perry et al. (in press-a) used MRI and 3D computer modeling to measure the levator muscle dimensions. Data (muscle length, thickness, and circumference measures) were obtained on 10 men of similar age (range, 20 to 32 years) and race (white). The levator muscle bundle length measure was divided into extravelar (muscle bundle running from origin to the point where it enters the velum) and intravelar (portion of the muscle contained within the velum) segments. Results demonstrated that the extravelar levator muscle length was consistent across individuals. However, the intravelar levator muscle segment was variable in muscle diameters and circumference across individuals. It was hypothesized that the extravelar segment may be more uniform across subjects due to its primary function in velar elevation as opposed to the intravelar segment (Perry et al., in press-a). Because intravelar levator muscle segments were sparse and irregular, they were hypothesized to have a weaker force contribution compared with the extravelar levator muscle segment.
The current study examines measures similar to those documented in previous studies, but within a sufficiently large, homogeneous population of subjects. Group sizes were informed by a power analysis to ensure sufficient statistical power to differentiate measures between groups. With this study, significant sex differences in levator muscle and cranial measures in a young adult white population can be examined.
METHODS
Subjects
A power analysis (assuming equal variance, α = .05, with at least 80% power; i.e., a sensitivity of 80% or a 20% false-negative rate) was performed using variances reported from previous studies (Tian et al., 2010c; Bae et al., 2011; Perry et al., in press-a). It was determined that 15 subjects per group were required to determine statistically significant comparisons. In accordance with the institutional review board, 30 healthy subjects between 19 and 32 years of age (23.3 ±4.1 years SD) were recruited to participate in the study. Fifteen subjects were men (23.6 ± 4.8 years SD) and 15 were women (22.9 ± 3.3 years SD). All subjects were white and were native English speakers. Subjects reported no history of swallowing, neurological, craniofacial, or musculoskeletal disorders. Sex and racial differences in craniometric measures, vocal tract dimensions, and levator muscle morphology have been reported (e.g., Chung et al., 1985; Chung et al., 1986; Yuen et al., 1989; Liu et al., 2000; Simpson, 2001; Ettema et al., 2002; Johannsdottir et al., 2004; Yeong and Huggare, 2004). These reported hard tissue variations indicate that further soft tissue variations may result from using diverse races, particularly in the area of the velopharyngeal mechanism. For this reason, only white subjects were recruited to control for racial influences.
All subjects were judged informally by a speech-language pathologist to have normal oral-to-nasal resonance balance. Men were on average 176 cm in height (SD = 7 cm) and 74 kg (SD = 11 kg). Women were on average 167 cm in height (SD = 7 cm) and 63 kg (SD = 10 kg). Body mass index (BMI) calculations (measured as body fat based on height and weight) and classifications were performed using guidelines from the National Heart, Lung, and Blood Institute (National Institutes of Health). All subjects had a BMI under 27 (23.2 ± 3.1 SD). Obesity (BMI > 30) has been related to variations in the pharyngeal airway (Mayer et al., 1996). In addition, larger individuals with very broad shoulders may have a difficult time fitting into the MRI scanning bore, making the experimental process uncomfortable. For these reasons, individuals with a BMI over 30 were excluded from the study.
Magnetic Resonance Imaging
Subjects were scanned in the supine position using a Siemens 3 Tesla Trio (Erlangen, Germany) and a 12-channel Siemens Trio head coil. During the 5-minute scan, each subject was instructed to breathe through the nose with the mouth closed. The velum was in a relaxed and lowered position. To minimize motion during the scanning session, a hook-and-loop–fastened elastic strap was placed around the subject’s head, passing at the level of the nasion and fastened to the head coil. Table 1 outlines the imaging protocol used in the present study. A high-resolution, T2-weighted turbo-spin-echo 3D anatomical scan called SPACE (sampling perfection with application optimized contrasts using different flip angle evolution) was used to acquire a large field of view covering the oropharyngeal anatomy (25.6 × 19.2 × 15.5 cm) with 0.8 mm isotropic resolution with an acquisition time of slightly less than 5 minutes (4:52). This imaging sequence has been described previously (Bae et al., 2011; Perry et al., in press-a).
TABLE 1.
Imaging Protocol for 3D MRI Scan Using 3 Tesla Scanner
| Static Three-Dimensional MRI Parameters | |
|---|---|
| Pulse sequence | SPACE: T2 turbo-spin-echo with variable flip angle |
| Field of view | 25.6 × 19.2 × 15.4 cm3 |
| Repetition time | 2500 ms |
| Echo time | 268 ms |
| Resolution | 0.8 mm isotropic |
| Length of scan | 4 min 52 s for 1 static image |
Image Analyses
Image processing methods were consistent with previously reported methods (Perry and Kuehn, 2007, 2009; Perry et al., 2011). In brief, magnetic resonance images were transferred into Amira 4 Visualization Volume Modeling software (Visage Imaging GmbH, Berlin, Germany), which has a built-in native Digital Imaging and Communication in Medicine (DICOM) support program. The DICOM support system enables the data to preserve their original geometry. The 3D MRI data were resampled to obtain an oblique coronal image plane that represented a clear view of the levator muscle from the origin to the insertion.
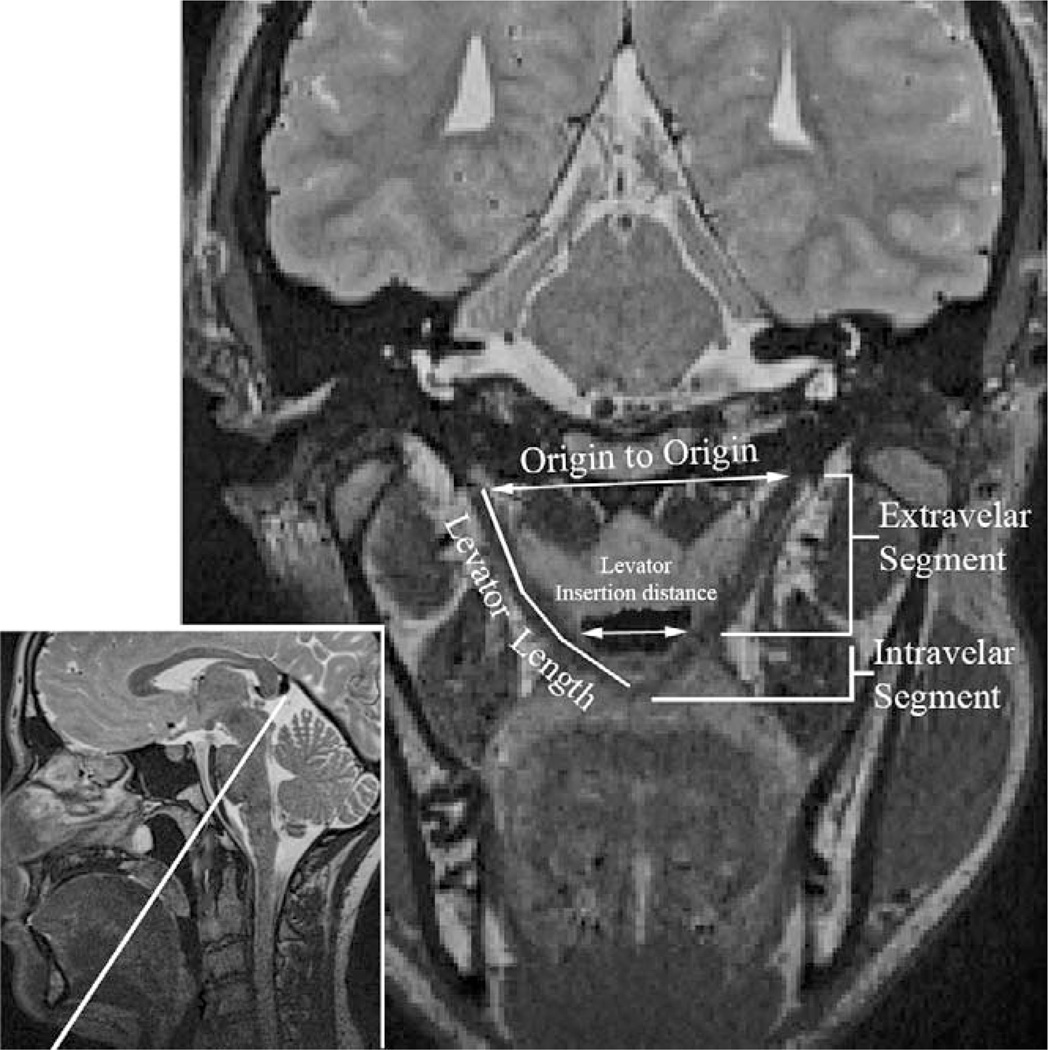
Quantitative measures were obtained to provide comparisons with previously reported MRI literature regarding the levator muscle (Ettema et al., 2002; Tian and Redett, 2009; Tian et al., 2010c, Bae et al., 2011; Perry et al., in press-a). These included entire muscle length, extravelar muscle length, intravelar muscle length, distance between the two locations where the levator muscle inserts into the velum, distance between origins, and oblique coronal angles of origin. Cranial measures were included to determine whether cranial size accounted for any observed levator muscle variations between men and women. Cranial measures including head circumference, palate width, palate height, palate length, cranial base measures (nasion to sella and sella to basion distance), face width, and face height (nasion to menton) were obtained on each subject. Descriptions of these measures are provided in Table 2 and displayed in Figure 1.
TABLE 2.
Description of Measurements
| Oblique Coronal Measures t of Levator Muscle | |
| Muscle length | Distance of the levator veli palatini muscle from the base of the skull (origin) through the midline of the muscle bundle to the midline insertion point in the velum. This measure was taken for the right and left muscle bundles and calculated as a combined mean value. |
| Extravelar muscle length | Distance of the levator veli palatini muscle from the base of the skull (origin) through the midline of the muscle bundle to the point where the muscle inserts into the body of the velum. This measure was taken for the right and left muscle bundles and combined for a mean value. |
| Intravelar muscle length | Distance of the entire levator veli palatini muscle that is contained within the body of the velum. A single measure is obtained that represents portion of the left and right levator muscle bundles. |
| Origin to origin | Distance between the two points of origin for the right and left levator muscle bundles. |
| Oblique coronal angle of origin | Angles created by the right and left muscle bundles as they descend from the base of the skull (origin to origin line). Right and left measurements combined for a mean value. |
| Cranial Measures | |
| Head circumference | Distance around the head measured at the point of maximal head diameter. |
| Palate width | Distance from the free lingual gingival margin of the second molar. |
| Palate height | Perpendicular line drawn from the palate width line to the roof of the hard palate. |
| Palate length | Distance from anterior nasal spine to the posterior nasal spine. |
| Nasion to sella | Distance from nasion to sella. |
| Sella to basion | Distance from sella to basion. |
| Face width | Bizygomatic distance as measured by the most lateral portion of the zygomatic arch as seen on a coronal magnetic resonance image. |
| Face height | Distance from the menton to the nasion. |
FIGURE 1.
Oblique coronal image displaying the measurements obtained in the present study.
Two trained raters, one primary and the other secondary, viewed the images in Amira to determine the coronal plane for reslicing and the reference locations for measures. The primary and secondary raters both have experience in measuring the structures of interest in this study. Prior to data collection, both raters made independent measurements using a previous dataset and then compared measurements to make clear definitions of the measurement boundaries. The primary and a secondary raters randomly selected and remeasured data from 12 subjects (40% of the subjects) 3 months after the first measures were obtained. Interrater and intrarater reliability measures were obtained using the Pearson product moment correlation (α = .05). Intrarater reliability ranged from r = .89 to r = .95 across the measures. Interrater reliability ranged from r = .80 to r = .94, with the lowest reliability (r = .80) for measures of the angles of origin.
Statistical Treatment
In order to control for errors based on multiple comparisons, a Hotelling T-square test was used to compare the effect of sex on six levator muscle measures. A Bonferroni correction was used to determine the significance of multiple two-sample t tests to assess each of the six muscle variables. The right and left muscle bundles showed small differences (from 0.1 mm to 1.2 mm); therefore, the right and left measures were combined to create a mean value. Perry et al. (2011) suggested that head circumference may be related to muscle measures. A regression analysis was conducted to evaluate the effect of head circumference (as an indicator of the size of the subject’s head) on the muscle morphology.
RESULTS
The Hotelling T-square test (Srivastava, 2002, p. 109) was used to determine whether there was a sex difference on the six muscle variables. The value of the test statistics was 6.415, the numerator degrees of freedom was 6, and the denominator degrees of freedom was 23. The P value for this method was .004, indicating a very significant sex difference with respect to the six variables measured. In order to gain further insight, two-sample t tests were conducted for each variable (Table 3). Even though these tests are for different characteristics, a very conservative Bonferroni level of significance (0.008) was used for each individual test. All characteristics showed statistically significant sex differences except for the distance between muscle origins. The mean for men minus the mean for women was used in the calculation; therefore, the positive values for the test statistics indicate that male measurements are larger than female measurements. The levator muscle length was measured to determine the overall length (mean of right and left levator muscle length) and to determine the extravelar and intravelar levator muscle lengths. The intravelar levator muscle length represents the entire portion of the muscle within the velum and is denoted as a single value. Extravelar segment lengths were obtained on the right and left muscle bundles and combined to create a mean value.
TABLE 3.
Mean Values Obtained Across the Levator Muscle Measures (Mean ± Standard Deviation) and Results of a Two-Sample t Test to Compare Between Groups
| Men | Women | P Value | |
|---|---|---|---|
| Muscle length | 49.4 ± 4.1 | 43.0 ± 2.9 | <.0001* |
| Extravelar length | 31.5 ± 3.4 | 27.8 ± 2.8 | .003* |
| Intravelar length | 35.7 ± 3.5 | 30.5 ± 3.2 | <.001* |
| Distance between velar insertion points of muscle | 26.5 ± 2.3 | 23.5 ± 2.3 | <.001* |
| Origin to origin | 58.3 ± 5.3 | 54.3 ± 5.6 | .06 |
| Oblique coronal angle of origin | 59.6 ± 2.7 | 56.4 ± 4.0 | .008* |
α < .05.
Comparisons between men and women demonstrated statistically significant differences between certain measures (Table 3). Results indicated a significant difference between men (49.4 ± 4.1 mm SD) and women (43.0 ± 2.9 mm SD) in the overall muscle length (P < .001). The levator muscle length for men is significantly longer compared with women (Table 3). Because male subjects are generally larger than the female subjects, using the characteristic of head circumference as an indicator for head size, a regression model was used on the six muscle measures to test the sex difference after adjusting for head circumference (Table 4). After removing the effect of head circumference, sex effect remained significant (P < .05; adjusted R2= 59.6%; df=2).
TABLE 4.
| Overall P Value |
P Value for Intercept |
P Value for Head Circumference |
P Value for Sex |
Adjusted R2 (%) | |
|---|---|---|---|---|---|
| Muscle length | <.0001 | .337 | .003 | .030 | 59.6 |
| Extravelar length | <.0001 | .283 | .012 | .291 | 39.4 |
| Intravelar length | .001 | .933 | .182 | .037 | 35.6 |
| Distance between velar insertion points of muscle | .002 | .958 | .111 | .089 | 33.2 |
| Oblique coronal angle of origin | .026 | .045 | .578 | .082 | 18.0 |
Origin-to-origin measure demonstrated no significant variation and therefore is not included in this statistical model.
α< .05.
The levator muscle was further analyzed to evaluate the extravelar and intravelar levator muscle segments. There was a very highly significant difference (P = .003) in the extravelar segment lengths and intravelar segment length (P < .001) between men and women. Men displayed significantly longer extravelar (31.5 ± 3.4 mm SD) and intravelar segment muscles (35.7 ± 3.5 mm SD) compared with the extravelar (27.8 ± 2.8 mm SD) and intravelar segments for women (30.5 ± 3.2 mm SD). After removing the effect of head circumference on extravelar segment length (Table 4), sex became nonsignificant (P = .29; adjusted R2 = 39.4 %; df= 2). This finding suggests that extravelar segment differences between groups is related to the head circumference (larger head = larger extravelar segment) as opposed to sex differences. After removing the effect of head circumference, sex differences in intravelar length remained significant (P= .037; adjusted R2= 35.6%; df=2).
The distance between the points where the levator muscle inserts into the velum were statistically significant (P < .001), in that men had significantly greater distance between these two points compared with women. After removing the effect of head circumference, sex differences were not significant (P =089; adjusted R2 = 33.2 %; df= 2). Men demonstrated a greater distance (58.3 ± 5.3 mm SD) between points of levator muscle origin compared with women (54.3 ± 5.6 mm SD). However, there was no statistically significant variation between men and women (P= .06) in the distance between points of origin at the base of the skull. There was a significant difference (P = .008) between the oblique coronal angle of origin measure between men (mean, 59.6°) and women (mean, 56.4°). Men demonstrated significantly larger angles of origin compared with women.
As seen in Table 5, men demonstrated a significantly greater (P < .001) head circumference (569.9 ± 13.3 mm SD) compared with women (544.9 ± 17.2 mm SD). Men had a significantly (P= .007) wider hard palate (38.5 ± 3.2 mm SD) compared with women (35.4 ± 2.6 mm SD); however, there was minimal difference (P > .05) in hard palate height and length between groups. Men demonstrated a significantly greater distance from the nasion to sella (70.3 ± 3.4 mm SD) compared with women (65.4 ±4.1 mm SD). Sella to basion measures were nearly the same between men (mean, 44.3 mm) and women (mean, 44.6 mm). Men demonstrated a significantly longer face (nasion to menton; P = .001); however, face width was not significantly different. Although men showed larger cranial measures (i.e., head circumference, palate width, nasion to sella distance, and face height), these differences were proportionately not as large as those for the levator muscle. Cranial measures in men were from 5% to 9% (mean, 6%) larger than those observed in women. The levator muscle measures in men, however, were from 7% to 17% (mean, 11%) larger than those observed in women. Men were, on average, 9 cm taller than the women. These differences (5% greater measures for men) were not proportionate to the differences (7% to 17% greater measures for men) observed in the levator muscle measures.
TABLE 5.
Results From a Two-Sample t Test Comparing Measures Across Men and Women; Measures (Standard Deviations in Parentheses) Are Reported in mm
| Men | Women | P Value | |
|---|---|---|---|
| Head circumference | 569.9 ± 13.3 | 544.9 ± 17.2 | <0.001* |
| Palate width | 38.5 ± 3.2 | 35.4 ± 2.6 | 007* |
| Palate height | 8.8 ± 2.8 | 8.0 ± 2.8 | 44 |
| Palate length | 60.5 ± 5.3 | 59.1 ± 4.9 | 48 |
| Nasion to sella | 70.3 ± 3.4 | 65.4 ± 4.1 | 001* |
| Sella to basion | 44.3 ± 2.5 | 44.6 ± 4.5 | 85 |
| Face width | 141.9 ± 5.1 | 123.4 ± 4.2 | 35 |
| Face height | 114.6 ± 7.5 | 105.7 ± 5.9 | 001* |
α=.05.
DISCUSSION
Previous studies have used small sample sizes and have not consistently controlled for race, sex, and age. Table 6 provides a comparison between the present study and similar studies that have reported data related to levator muscle morphology. Studies have varied in the number of subjects, age range, race, and group distribution. Using a power analysis, the statistical power was compared using varied sample sizes. A power of 88% can be achieved using groups of 15 subjects given the controlled nature of this study and the sizes of the differences between sexes that were expected. Previous studies examining the effect of sex on levator muscle morphology (Ettema et al., 2002; Bae et al., 2011) have used five subjects per group, which demonstrates a power of 33%. Variations in study findings may be attributed to these small sample sizes.
TABLE 6.
Comparisons of Variables Across Studies That Report Levator Muscle Measurements
| Current Study | Bae et al., 2011 | Perry et al, 2012 | Tian et al., 2010c | Tian & Redett, 2009 | Ettema et al., 2002 | |
|---|---|---|---|---|---|---|
| No. of adult Subjects | 30 | 10 | 10 | 12 | 17 | 10 |
| Age range, mean ± SD | 19–32 years, 23.3 ± 4.1 | 20–31 years 22.6 ± 3.60 | 20–32 years 23.8 ± 4.6 | 19–40 years, 28.1 ± 5.3 | 19–43 years, 30.0 (N/A) | 21–53 years, 27.2 ± 9.6 |
| Gender | 15 men, 15 women | 5 men, 5 women | 10 men | 5 men, 7 women | 5 men, 12 women | 5 men, 5 women |
| Race | White | White | White | Chinese | 11 White, 6 Chinese | White |
Studies have demonstrated variations in vocal tract regions including the nasopharynx and oropharyngeal spaces as a function of age (Macho, 1986; Fitch and Giedd, 1999; Xue and Hao, 2003). Vorperian et al. (2011) analyzed 605 head and neck images from subjects between 0 and 19 years of age and reported nonlinear growth patterns in the vocal tract with regions of the velopharynx showing large sex differences, particularly in length and growth rate. Macho (1986) demonstrated significant variations particularly in the length of the velum between selected age ranges. Studies (Table 6) using age ranges that extend across these limits proposed by Macho (1986) may be demonstrating differences that are a result of age effects on vocal tract shape as opposed to gender effects in the levator muscle morphology. The present study demonstrates a restricted age range to control for the effect of variations in upper vocal tract shape as a function of age.
Studies have varied in the type of MRI methods used. Few MRI studies of the velopharynx have examined the oblique coronal image plane (e.g., Kuehn et al., 2001; Ettema et al., 2002; Kuehn et al., 2004; Ha et al., 2007; Tian and Redett, 2009; Tian et al., 2010a, 2010b; Tian et al., 2010c; Bae et al., 2011; Perry, 2011; Perry et al., 2011). Prior to the use of 3D MRI sequences (Bae et al., 2011; Perry et al., in press-a), 2D acquisition along the length of the levator muscle required more time in the scanner and the levator muscle was more difficult to locate. With 3DMRI, data acquisition and analysis are significantly improved (Bae et al., 2011). The present study used 3DMRI scanning sequences and obtained data on a large number of subjects to perform comparisons between the muscle morphology of white men and women. Results of the study demonstrated significant differences between men and women across several levator muscle measures.
The difference in the length of the levator muscle between men and women has been previously examined and nonsignificant findings were demonstrated (Ettema et al., 2002). Bae et al. (2011) examined five men and five women and found a nonsignificant effect of sex on all levator muscle measures except for levator muscle length. Men had a significantly longer levator muscle length compared with female subjects; however, the levator muscle length was not examined for intravelar and extravelar segments. By increasing the number of subjects (power for analyses), a significant difference was observed in the present study in which men had significantly longer levator muscle lengths and greater angles of origin. Values obtained in the present study are similar to those obtained from similar studies (Table 7). The present study examined the length of the levator muscle to determine whether these statistically significant differences were due to intravelar or extravelar segment variations.
TABLE 7.
Comparisons of Variables Across Studies That Report Levator Muscle Measurements (Reported in mm with the Exception of Angle of Origin in Degrees)
| Current Study (15 Men, 15 Women) |
Bae et al., 2011 (5 Men, 5 Women) |
Perry et al, 2012 (10 Men) |
Tian et al., 2010c (5 Men, 7 Women)† |
Tian & Redett, 2009 (5 Men, 12 Women)† |
Ettema et al., 2002 (5 Men, 5 Women) |
|
|---|---|---|---|---|---|---|
| Muscle length | ||||||
| Men | 49.4 | 46.3 | 47.5 | 33.1 | 32.6 | 46.4 |
| Women | 43.0 | 38.7 | 44.1 | |||
| Extravelar length | ||||||
| Men | 31.5 | - | 30.3 | - | - | - |
| Women | 27.8 | - | - | - | - | |
| Intravelar length | ||||||
| Men | 35.7 | - | 34.3 | - | - | - |
| Women | 30.5 | - | - | - | - | |
| Distance between velar insertion points | ||||||
| Men | 26.5 | - | 26.2 | 24.9 | 24.3 | - |
| Women | 23.5 | - | - | |||
| Origin to origin | ||||||
| Men | 58.3° | 55.8° | 56.0° | 60.9° | 60.0° | 54.6° |
| Women | 54.3° | 50.4° | 52.6° | |||
| Angle of origin | ||||||
| Men | 59.6° | 59.9° | 56.0° | 56.7° | 56.3° | 60.4° |
| Women | 56.4° | 56.0° | 64.5° | |||
Values for right and left muscle measures (muscle length, extravelar muscle length, and angles of origin) are averaged to provide comparison to other similar reported studies.
Only averaged values for men and women reported.
Perry et al. (in press-a) demonstrated among 10 white male subjects that the levator extravelar segment was similar across individuals. More variability in the muscle diameter and circumference was observed within the intravelar segment. The authors hypothesized a different effect or contribution of the extravelar levator muscle segment compared to the intravelar muscle segment. It was hypothesized that the extravelar segment may be more uniform across subjects due to its primary function in velar elevation as opposed to the intravelar segment (Perry et al., in press-a). Intravelar levator muscle segments were sparse and irregular and were hypothesized to have a weaker force contribution compared with the extravelar levator muscle segment. These hypotheses were based on a sample size of 10 white men. The findings from the present study extend these hypotheses. A significant difference was observed in the levator muscle length between men and women. Consistent with Perry et al. (in press-a), the present study quantitatively observed differences in the shape of the intravelar segment. Although the length of the intravelar segment did not vary significantly within each group (men and women), the shape of the intravelar segment was not consistent. The fibers in the intravelar segment often were sparse and less dense (as evidenced by muscle thickness). All subjects demonstrated a fanning out of muscle fibers inside the velum (intravelar segment); however, some showed a greater fanning out than others. The intravelar segment appeared to be the region of the muscle in which variation across individuals was observed.
The distance between the points where the levator muscle inserts into the velum was measured and demonstrated a significant variation between men and women. Perry et al. (2011) provided magnetic resonance images of two infants (one boy and one girl) following primary palatoplasty and two infants without cleft palate for comparison. The male infant (Perry et al., 2011; fig. 4, p. 501) demonstrated a broader muscle insertion, displaying a greater distance between the insertion points of the levator bundles into the velum compared with the other subjects. The authors suggested these variations might be related to surgical procedures. It was suggested that a radical dissection around the hamulus might produce a smaller distance between muscle insertion points. No studies thus far have demonstrated sex variations in the distance between the points where the bundles insert into the velum. Given the findings from the present study, it is possible the observations by Perry et al. (2011) may have been related to sex variations.
Studies have observed males to have a longer and wider midface compared with females (Macho, 1986; White et al., 2004). It was expected that males would demonstrate a significantly greater distance between origins. The results of the present study demonstrated a nonsignificant difference (P = .06) in the distance between muscle origin in men and women. These variations were close to being statistically significant. A larger sample size may be needed for this measure to determine whether the differences between men and women are statistically significant. Men displayed significantly greater angles of origin compared with women, which may be explained by the significantly longer levator muscle, larger distance between origins (nonsignificant), and larger distance between muscle insertions into the velum observed among men compared with women. The greater distance between the insertions of the muscle into the velum is likely related to wider measures in the velum. Subject size was significant for determination of variations between groups but may not be sufficient in showing correlations between variables. Not all of the regression models showed stability, which may be attributed to the smaller sample sizes. Further studies should use larger sample sizes to determine whether these phenomena are consistent across larger datasets.
This study serves to provide normative data for white adult muscle anatomy. As a comparison to cleft palate anatomy, one white woman with repaired bilateral cleft lip and palate was analyzed using the same methodology. The subject reported a history of speech and language therapy from 3 to 10 years of age. At the time of the MRI (24 years of age), she was determined to have normal oral-to-nasal resonance. Cranial measures were within the mean and standard deviation of the female control group for palate width (35.9 mm), palate height (10.3 mm), palate length (54.7 mm), nasion to sella (69.2 mm), sella to basion (46.6 mm), and face width (119.1). The subject displayed a slightly greater face height (112.9 mm) compared with the normative group (mean, 105.7 mm). Levator muscle length (42.4 mm) was similar to that of the normative control group (mean, 43.0 mm); however, variations were noted within the extravelar muscle (24.2 mm) and intravelar muscle length (36.4 mm) compared with the normative female group (mean, 27.8 mm and 30.5 mm, respectively). Distance between muscle origins (54.2 mm) and oblique coronal angle of origins (54.2°) were similar to the normative group (mean, 54.3 mm and 56.4°, respectively). Ongoing studies in our laboratory are investigating muscle morphology in cleft palate anatomy to determine the impact of these noted variations on speech and resonance.
The findings from the present study may have clinical significance for individuals born with cleft palate. During primary palatoplasty, the levator muscle is reconstructed to create a levator sling that models normal muscle anatomy and function. However, it is not well understood what constitutes normal anatomy given variations such as race, sex, or craniometric morphology. The results suggest sex variations that should be explored in normative pediatric populations. Ha et al. (2007) demonstrated that adults with hypernasal speech also demonstrated the most variance from normal levator muscle arrangement. These findings suggest the importance of creating a normal levator sling to facilitate adequate velopharyngeal closure for speech. Studies on the levator muscle thus far have used small heterogeneous sample sizes resulting in conflicting findings.
Research on levator muscle morphology to date has not separated the muscle bundle into intravelar and extravelar segments to examine the muscle function on velar elevation. Ha et al. (2007) observed variations in the levator muscle morphology among adults with repaired cleft palate who demonstrated hypernasal speech compared with adults with repaired cleft palate and normal resonance. Future studies should examine the cleft palate population to determine whether velopharyngeal dysfunction is more likely related to variations in the extravelar muscle segment as opposed to the intravelar segment, as hypothesized by Perry et al. (in press-a). Primary palatoplasty is focused on dissection and reconstruction of the intravelar levator muscle segment. It may be of clinical value to evaluate the effect of surgical procedures on the extravelar segment as an indirect effect of intravelar dissection and repositioning. Sommerlad et al. (1994) and Cutting et al. (1995) proposed a radical dissection of the tensor tendon around the hamulus in order to release the tension applied to the anterior velar portion. The effect of surgical variations to the intravelar segment on the extravelar segment is not known.
CONCLUSION
Further MRI studies are needed to evaluate the clinical implications of these findings among individuals with cleft palate before and after primary palatoplasty. Kuehn et al. (2004) proposed additional questions about the fate of the muscle following surgery, which can only be assessed in living individuals through presurgical and postsurgical MRI evaluation. It is not known whether surgical procedures result in appropriate positioning or adequate mass of the levator muscle or whether the muscle migrates toward a more unfavorable position following surgery (Kuehn et al., 2004). Future studies should determine the impact of race and age on levator muscle morphology. Data from the present study may provide support for patient specific surgical planning based on sex. It is likely that race, age, and craniometry are additional contributing factors that may define the muscle morphology. In addition, these variables may be important factors in creating a functional velopharyngeal system following primary palatoplasty.
Acknowledgments
This study was made possible by grant number 1R03DC009676-01A1 from the National Institute of Deafness and Other Communicative Disorders. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Dr. Jamie L. Perry, Department of Communication Sciences and Disorders, East Carolina University, Greenville, North Carolina.
Dr. David P. Kuehn, Department of Speech and Hearing Science, University of Illinois at Urbana-Champaign, Champaign, Illinois.
Dr. Bradley P. Sutton, Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, Illinois.
Dr. Jinadasa K. Gamage, Department of Mathematics, Illinois State University, Illinois.
REFERENCES
- Akgüner M, Karaca C, Barutçu A, Özaksoy D, Yurt A, Vayvada H. Evaluation of velopharyngeal pathophysiology and velopharyngeal insufficiency with magnetic resonance imaging. Eur J Plast Surg. 1998;21:118–128. [Google Scholar]
- Atik B, Bekerecioglu M, Tan O, Etik O, Davran R, Arslan H. Evaluation of dynamic magnetic resonance imaging in assessing velopharyngeal insufficiency during phonation. J Craniofac Surg. 2008;19:566–572. doi: 10.1097/SCS.0b013e31816ae746. [DOI] [PubMed] [Google Scholar]
- Bae Y, Kuehn DP, Sutton BP, Conway CA, Perry JL. Three-dimensional magnetic resonance imaging of velopharyngeal structures. J Speech Hear Res. 2011;54:1538–1545. doi: 10.1044/1092-4388(2011/10-0021). [DOI] [PubMed] [Google Scholar]
- Beer JA, Hellerhoff P, Zimmermann A, Mady K, Sader R, Rummeny EJ, Hannig C. Dynamic near-real-time magnetic resonance imaging for analyzing the velopharyngeal closure in comparison with videofluoroscopy. J Magn Reson Imaging. 2004;20:791–797. doi: 10.1002/jmri.20197. [DOI] [PubMed] [Google Scholar]
- Chung C, Kau M. Racial differences in cephalometric measurements and incidence of cleft lip with or without cleft palate. J Craniofac Genet Dev Biol. 1985;5:341–349. [PubMed] [Google Scholar]
- Chung C, Runck D, Bilben S, Kau M. Effects of interracial crosses on cephalometric measurements. Am J Phys Anthropol. 1986;69:465–72. doi: 10.1002/ajpa.1330690405. [DOI] [PubMed] [Google Scholar]
- Cutting CB, Rosenbaum J, Rovati L. The technique of muscle repair in the soft palate. Oper Tech Plast Reconstr Surg. 1995;2:215–222. [Google Scholar]
- Drissi C, Mitrofanoff M, Talandier C, Falip C, Le Couls V, Adamsbaum C. Feasibility of dynamic MRI for evaluating velopharyngeal insufficiency in children. Eur Radiol. 2011;21:1462–1469. doi: 10.1007/s00330-011-2069-7. [DOI] [PubMed] [Google Scholar]
- Ettema SL, Kuehn DK, Perlman al, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Craniofac J. 2002;39:130–144. doi: 10.1597/1545-1569_2002_039_0130_mriotl_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Giedd J. Morphology and development of the human vocal tract: a study using magnetic resonance imaging. J Acoust Soc Am. 1999;106:1511–1522. doi: 10.1121/1.427148. [DOI] [PubMed] [Google Scholar]
- Ha S, Kuehn DP, Cohen M, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle in speakers with repaired cleft palate. Cleft Palate Craniofac J. 2007;44:495–505. doi: 10.1597/06-220.1. [DOI] [PubMed] [Google Scholar]
- Johannsdottir B, Thordarson A, Magnusson T. Craniofacial skeletal and soft tissue morphology in Icelandic adults. Eur J Orthod. 2004;26:245–250. doi: 10.1093/ejo/26.3.245. [DOI] [PubMed] [Google Scholar]
- Kane AA, Butman JA, Mullick R, Skopec M, Choyke P. A new method for the study of velopharyngeal function using gated magnetic resonance imaging. Plast Reconstr Surg. 2002;109:472–481. doi: 10.1097/00006534-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Kao DS, Soltysik DA, Hyde JS, Gosain AK. Magnetic resonance imaging as an aid in the dynamic assessment of the velopharyngeal mechanism in children. Plast Reconstr Surg. 2008;122:572–577. doi: 10.1097/PRS.0b013e31817d54d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC. Magnetic resonance imaging of the levator veli palatini muscle before and after primary palatoplasty. Cleft Palate Craniofac J. 2004;41:584–592. doi: 10.1597/03-060.1. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC, Wachtel JM. Magnetic resonance imaging in the evaluation of occult submucous cleft palate. Cleft Palate Craniofac J. 2001;38:421–431. doi: 10.1597/1545-1569_2001_038_0421_mriite_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lowe A, Zeng X, Fu M, Fleetham J. Cephalometric comparisons between Chinese and Caucasian patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2000;117:479–485. doi: 10.1016/s0889-5406(00)70169-7. [DOI] [PubMed] [Google Scholar]
- Macho GA. Cephalometric and craniometric age changes in adult humans. Ann Hum Biol. 1986;13:49–61. doi: 10.1080/03014468600008191. [DOI] [PubMed] [Google Scholar]
- Mayer P, Pepin JL, Bettega G, Veale D, Feretti G, Deschaux C, Levy P. Relationship between body mass index, age and upper airway measurements in snorers and sleep apnea patients. Eur Resp J. 1996;9:1801–1809. doi: 10.1183/09031936.96.09091801. [DOI] [PubMed] [Google Scholar]
- Özgür F, Tunçbilek G, Cila A. Evaluation of velopharyngeal insufficiency with magnetic resonance imaging and nasoendoscopy. Ann Plast Surg. 2000;44:8–13. doi: 10.1097/00000637-200044010-00002. [DOI] [PubMed] [Google Scholar]
- Perry JL. Variations in velopharyngeal structures between supine and upright postures using open-type magnetic resonance imaging. Cleft Palate Craniofac J. 2011;48:123–133. doi: 10.1597/09-256. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP. Magnetic resonance imaging and computer reconstruction of the velopharyngeal mechanism. J Craniofac Surg. 2009;20:1739–1746. doi: 10.1097/SCS.0b013e3181b5cf46. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP. Three-dimensional computer reconstruction of the levator veli palatini muscle in situ using magnetic resonance imaging. Cleft Palate Craniofac J. 2007;69:214–216. doi: 10.1597/06-137.1. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP. Morphology of the levator veli palatini muscle using magnetic resonance imaging. Cleft Palate Craniofac J. In press-a doi: 10.1597/11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Goldwasser M. Craniometric and velopharyngeal assessment of infants with and without cleft palate. J Craniofac Surg. 2011;22:499–503. doi: 10.1097/SCS.0b013e3182087378. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Wachtel J, Bailey J. Using magnetic resonance imaging for early assessment of submucous cleft palate: a case report. Cleft Palate Craniofac J. In press-b doi: 10.1597/10-189. [DOI] [PubMed] [Google Scholar]
- Silver AL, Nimkin K, Ashland JE, Ghosh SS, van der Kouwe AJ, Brigger MT, Hartnick C. Cine magnetic resonance imaging with simultaneous audio to evaluate pediatric velopharyngeal insufficiency. Arch Otolaryngol Head Neck Surg. 2011;137:258–263. doi: 10.1001/archoto.2011.11. [DOI] [PubMed] [Google Scholar]
- Simpson P. Dynamic consequences of differences in male and female vocal tract dimensions. J Acoust Soc Am. 2001;109:2153–2164. doi: 10.1121/1.1356020. [DOI] [PubMed] [Google Scholar]
- Sommerlad BC, Henley M, Birth M, Harland K, Moiemen N, Boorman JG. Cleft palate re-repair—a clinical and radiographic study of 32 consecutive cases. Br J Plast Surg. 1994;47:406–410. doi: 10.1016/0007-1226(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Srivastava MS. Methods of Multivariate Statistics. New York: John Wiley; 2002. [Google Scholar]
- Tian W, Redett R. New velopharyngeal measurements at rest and during speech: implications and applications. J Craniofac Surg. 2009;20:532–539. doi: 10.1097/SCS.0b013e31819b9fbe. [DOI] [PubMed] [Google Scholar]
- Tian W, Yin H, Li Y, Zhao S, Zheng Q, Shi B. Magnetic resonance imaging assessment of velopharyngeal motion in Chinese children after primary palatal repair. J Craniofac Surg. 2010a;21:568–577. doi: 10.1097/SCS.0b013e3181d08bd1. [DOI] [PubMed] [Google Scholar]
- Tian W, Yin H, Li Y, Zhao S, Zheng Q, Shi B. Magnetic resonance imaging assessment of velopharyngeal structures in Chinese children after primary palatal repair. J Craniofac Surg. 2010b;21:578–587. doi: 10.1097/SCS.0b013e3181d08bd1. [DOI] [PubMed] [Google Scholar]
- Tian W, Yin H, Redett RJ, Shi B, Shi J, Zhan R, Zheng Q. Magnetic resonance imaging assessment of the velopharyngeal mechanism at rest and during speech in Chinese adults and children. J Speech Hear Res. 2010c;53:1595–1615. doi: 10.1044/1092-4388(2010/09-0105). [DOI] [PubMed] [Google Scholar]
- Vorperian HK, Wang S, Schimek ME, Durtschi RB, Kent RD, Gentry LR, Chung MK. Developmental sexual dimorphism of the oral and pharyngeal portions of the vocal and pharyngeal portions of the vocal tract: An imaging study. J Speech Hear Res. 2011;54:995–1010. doi: 10.1044/1092-4388(2010/10-0097). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JE, Ayoub AF, Hosey M, Bock M, Bowman A, Bowman J, Siebert JP. Three-dimensional facial characteristics of Caucasian infants without cleft and correlation with body measurements. Cleft Palate Craniofac J. 2004;41:593–602. doi: 10.1597/03-069.1. [DOI] [PubMed] [Google Scholar]
- Xue SA, Hao GJ. Changes in the human vocal tract due to aging and the acoustic correlates of speech production. J Speech Hear Res. 2003;46:689–701. doi: 10.1044/1092-4388(2003/054). [DOI] [PubMed] [Google Scholar]
- Yeong P, Huggare J. Morphology of Singapore Chinese. Eur J Orthod. 2004;26:605–612. doi: 10.1093/ejo/26.6.605. [DOI] [PubMed] [Google Scholar]
- Yuen S, Hwang J, Poon P. EMG power spectrum patterns of anterior temporal and masseter muscles in children and adults. J Dent Res. 1989;68:800–804. doi: 10.1177/00220345890680050901. [DOI] [PubMed] [Google Scholar]