Abstract
The siderophore aerobactin is the dominant siderophore produced by hypervirulent Klebsiella pneumoniae (hvKP) and was previously shown to be a major virulence factor in systemic infection. However, strains of hvKP commonly produce the additional siderophores yersiniabactin, salmochelin, and enterobactin. The roles of these siderophores in hvKP infection have not been optimally defined. To that end, site-specific gene disruptions were created in hvKP1 (wild type), resulting in the generation of hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient). The growth/survival of these constructs was compared to that of their wild-type parent hvKP1 ex vivo in human ascites fluid, human serum, and human urine and in vivo in mouse systemic infection and pulmonary challenge models. Interestingly, in contrast to aerobactin, the inability to produce enterobactin, salmochelin, or yersiniabactin individually or in combination did not decrease the ex vivo growth/survival in human ascites or serum or decrease virulence in the in vivo infection models. Surprisingly, none of the siderophores increased growth in human urine. In human ascites fluid supplemented with exogenous siderophores, siderophores increased the growth of hvKP1ΔiucA, with the relative activity being enterobactin > aerobactin > yersiniabactin > salmochelin, suggesting that the contribution of aerobactin to virulence is dependent on both innate biologic activity and quantity produced. Taken together, these data confirm and extend a role for aerobactin as a critical virulence factor for hvKP. Since it appears that aerobactin production is a defining trait of hvKP strains, this factor is a potential antivirulence target.
INTRODUCTION
In the ongoing chess match between microbial pathogens and the human host, the pathogens as of late seem to be gaining the upper hand. Klebsiella pneumoniae is proving to be especially problematic and has evolved into two distinct epidemiologically and clinically defined pathotypes.
The first and best-known pathotype, best termed “classical” K. pneumoniae (cKP), is presently responsible for the majority of K. pneumoniae infections in Western countries, which primarily occur in hospitals and long-term-care facilities (1). Importantly, cKP strains have received increased notoriety due to their propensity for acquiring antimicrobial resistance determinants, primarily carbapenemases, that make treatment challenging. The spread of New Delhi metallo-β-lactamase (NDM-1)-containing strains from India that are associated with medical tourism and, more recently, the extremely drug-resistant K. pneumoniae (XDR-KP) outbreak at the Clinical Center Hospital on the NIH campus have captured the attention of physicians, scientists, and the press (2, 3). XDR-KP is spreading globally and is primarily responsible for the increase in infections due to carbapenem-resistant bacteria in the United States (4).
The second pathotype, which has been termed hypervirulent K. pneumoniae (hvKP) and is less appreciated, has taken a different direction and is undergoing global dissemination from the Asian Pacific Rim (5, 6). In contrast to the usual health care-associated venue for cKP infections in the West, hvKP causes serious life- and organ-threatening infections in young, healthy individuals from the community (5, 7–9). Although hvKP was initially described as causing pyogenic liver abscess in the absence of biliary tract disease, this represents just one of many primary infection syndromes (6, 10). A defining characteristic of hvKP compared to cKP and other members of the Enterobacteriaceae is the capacity for metastatic spread from a site of infection in the immunocompetent host with devastating sequelae (e.g., endophthalmitis and meningitis) (5, 7, 9). To date, the majority of hvKP strains have been relatively antimicrobial sensitive. However, the combination of the antimicrobial-resistant cKP and hypervirulent hvKP phenotypes appears to have already begun, with recent studies reporting that strains of hvKP have acquired extended-spectrum β-lactamases (ESBLs) and carbapenemases (11, 12). Even more disturbing is a 2014 report that described the successful conjugal transfer of a K. pneumoniae carbapenemase (KPC)-producing plasmid into an hvKP strain without a loss in biofitness (13). The prospect of a hypervirulent pathogen that can cause severe infections in healthy, ambulatory individuals is concerning enough; the widespread evolutionary confluence of the phenotypic features of hvKP that confer its hypervirulence with acquisition of drug resistance from cKP is a frightening prospect that calls for the immediate development of novel therapies directed against hvKP strains. Understanding the virulence factors responsible for conferring hvKP with their phenotype is a critical first step in this process.
The majority of studies that have focused on the pathogenesis of hvKP have focused on surface polysaccharides. A distinguishing factor of many hvKP strains is its hypermucoviscous phenotype (14). This phenotype has been established to be due to increased capsular polysaccharide (e.g., K1, K2 et al.) production (15–17). Although K1 and K2 capsular serotypes have been shown to contribute to the virulence of cKP strains in a mouse IP challenge model (18), increased expression of the K2 capsule (hypermucoviscous phenotype) has been shown to add to the virulence of hvKP in a mouse IP challenge model (15).
Recent studies by our group have established that iron acquisition is also a critical virulence factor for hvKP. It has long been known that bacteria require micromolar concentrations of iron for growth and that their ability to procure iron is requisite for growth and survival (19). To accomplish this goal, most bacteria produce chemically diverse siderophores (20), small-molecule chelators with remarkably high association constants for Fe3+, that enable them to acquire iron in iron-depleted environments, such as the human host. Interestingly, we have shown that hvKP is characterized by a 6- to 10-fold increase in siderophore activity compared to cKP strains (21, 22). Further, and surprisingly, aerobactin accounted for >90% of the siderophore activity despite the ability of hvKP to produce multiple siderophores (21). Lastly, and most importantly, aerobactin was a critical factor requisite for optimal growth in human ascites ex vivo and for virulence in an outbred mouse subcutaneous challenge model of systemic infection (21).
However, the majority of hvKP strains, including the model pathogen used in these studies, hvKP1, possess genes for the production of additional siderophores, namely, enterobactin, salmochelin, and yersiniabactin. Although these siderophores in aggregate accounted for less than 10% of the total siderophore production in hvKP1 and in two other hvKP strains assessed, their role if any in the pathogenesis of hvKP infection remains incompletely defined (21). Therefore, the goal of this study was to assess whether yersiniabactin, enterobactin, and salmochelin contributed to the virulence of hvKP ex vivo in clinically relevant human body fluids and in vivo in two mouse infection models.
MATERIALS AND METHODS
Strain description.
hvKP1 (ST86, K2 serotype, ampicillin resistant) was isolated from the blood and a liver abscess aspirate in a previously healthy 24-year-old male from Buffalo, NY, USA, with community-acquired pyogenic liver abscess and metastatic spread to the spleen (23). A bioinformatics analysis of the hvKP1 genome established the presence of the biosynthetic genes and their cognate receptors for the siderophores aerobactin, salmochelin, enterobactin, and yersiniabactin (24). Transcription of these genes under the iron-limiting conditions of M9 minimal medium and human ascites fluid ex vivo was confirmed by RT-qPCR. hvKP1 derivatives deficient in aerobactin (hvKP1ΔiucA, kanamycin resistant), salmochelin (hvKP1ΔiroB, kanamycin resistant), enterobactin and salmochelin (hvKP1ΔentB, kanamycin resistant), yersiniabactin (hvKP1Δirp2, kanamycin resistant), and enterobactin, salmochelin, and yersiniabactin (hvKP1ΔentBΔirp2, kanamycin and hygromycin resistant) were generated by allelic exchange of the majority of the target gene with an antibiotic resistance gene as described previously (25). Since salmochelin is a glycosylated derivative of enterobactin, hvKP1ΔentB is unable to produce enterobactin and salmochelin. Constructs were confirmed by sequence analysis of PCR-generated amplicons using primers outside the gene in question: iucA (plus, 5′-ATAAGGCAGGCAATCCAG-3′; minus, 5′-TAACGGCGATAAACCTCG-3′); iroB (plus, 5′-TGTGTGCTGTGGGTGAAAGC-3′; minus, 5′-ATGTTCGGTGAGATTCGCCAGT-3′); entB (plus, 5′-GCACCCATAACGATTACGA-3′; minus, 5′-ACCACAATCTCCCAGCTCT-3′); and irp2 (plus, 5′-GCATTTTCCGTATCGCTCT-3′; minus, 5′-GCTTCATAACCTGCCTGATG-3′). Polar effects had been previously excluded for hvKP1ΔiucA by a combination of downstream transcript identification and complementation as described previously (21). hvKP1ΔiroB, hvKP1ΔentB, and hvKP1Δirp2 did not require complementation, because the inability to produce enterobactin, yersiniabactin, or salmochelin did not decrease ex vivo growth/survival in human ascites fluid, serum, or urine, nor did it decrease virulence in in vivo infection models. Polar effects were excluded by identification of the expected transcript for the gene immediately downstream of each biosynthetic operon by RT-PCR (data not shown). For the enterobactin-biosynthetic operon, a putative carbon starvation protein (peg2489, accession no. AOIZ00000000) is immediately downstream whose transcript was identified by the primer pair 5′-ACTGGTTAAAGAGGAGATGGG-3′ (plus) and 5′-CTTTCACCACAATCATCGCC-3′ (minus), which generates a 107-nucleotide (nt) amplicon; for the yersiniabactin-biosynthetic operon, a putative hypothetical protein (peg4364) is immediately downstream whose transcript was identified by the primer pair 5′-TGAGTGGCAACATCCTACTAT-3′ (plus) and 5′-GGTCTCCCTCAAACTGCC-3′ (minus), which generates a 105-nt amplicon, and for the salmochelin-biosynthetic operon, a putative hypothetical protein (peg344) is immediately downstream whose transcript was identified by the primer pair 5′-CTGTTTTGCTGGAGTTTTTGG-3′ (plus) and 5′-CAATTAGAGGGAAGATGAGAAATAC-3′ (minus), which generates a 101-nt amplicon. Unlike in some hvKP strains (e.g., NTUH-K2044), only a single copy of iroB is present in hvKP1; therefore, only a single allelic exchange was needed for the construction of hvKP1ΔiroB. All strains were maintained at −80°C in 50% LB broth and 50% glycerol prior to use.
Media.
The procedures for obtaining human ascites fluid, serum, and urine were reviewed and approved by the Western New York Veterans Administration or the University at Buffalo-SUNY Institutional Review Board. Serum and urine were collected from healthy volunteers. Serum was used on the day of collection or stored at −80°C prior to use. Urine was used on the day of collection or stored at 4°C prior to use. Ascites fluid was collected from deidentified patients who were undergoing therapeutic paracentesis for symptoms due to abdominal distension. These individuals were not being treated with antimicrobials and were not infected with human immunodeficiency, hepatitis B, or hepatitis C virus. The ascites fluid was cultured to confirm sterility, divided into aliquots, and stored at −80°C. Each batch was obtained from a different patient and was designated by the date of removal. Ascites fluid batch 08/04/08 was used for the growth studies reported in Fig. 2. For various in vitro growth studies, ascites fluid, serum, or urine (90% fluid, 10% 1× phosphate-buffered saline (PBS) [pH 7.4]) was used. In some experiments, ascites fluid was supplemented with various concentrations of exogenous, purified siderophores (40 nM to 1.28 μM). Enterobactin, yersiniabactin, and salmochelin were obtained from a commercial source (Sigma-Aldrich), and aerobactin was purified from hvKP1-generated iron-poor conditioned minimal medium as described previously (21). To exclude the possibility that viable bacteria were present in ascites fluid that could not be cultured by traditional culture methods, FeCl3, Fe(NO3)3, or FeSO4 at 0.1 mM, 1.28 μM purified yersiniabactin or salmochelin, or a 1.28 μM concentration of a mixture of enterobactin, salmochelin, yersiniabactin (5% of total), and aerobactin (95% of total) was added to 1 ml of human ascites fluid, which was incubated overnight at 37°C. The next day, concentrated ascites fluid was plated on Todd-Hewitt plus 5% sheep blood agar plates. No growth was observed. Although we cannot exclude the possibility that noncultivable bacteria were present in the ascites fluid used in our experiments, they were not cultivated under the conditions utilized for the reported experiments, which in turn minimizes/excludes the consideration that such bacteria may play a role as confounders of the reported results.
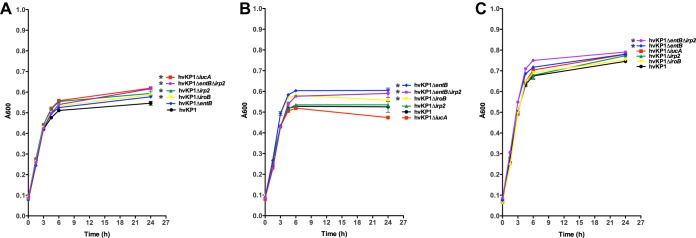
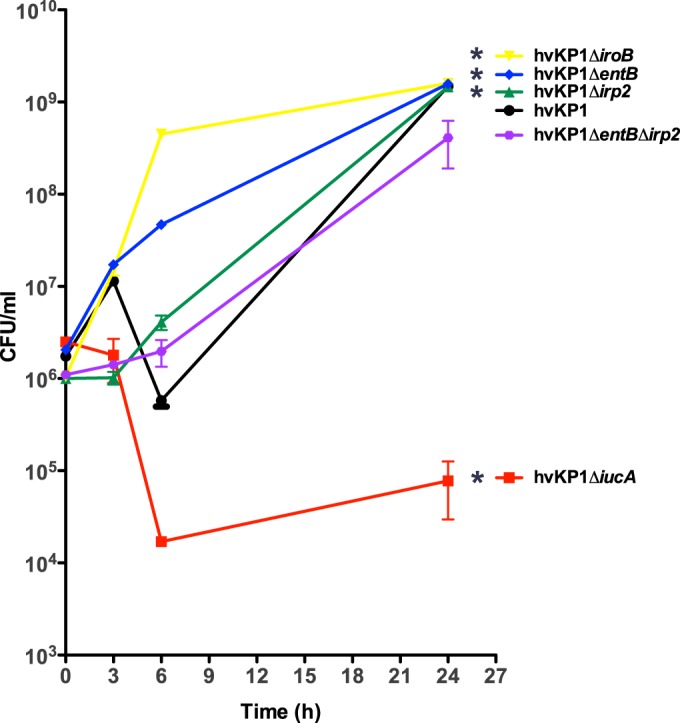
FIG 2.
Growth/survival of hvKP1 (wild-type), hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient) in human ascites fluid. The growth/survival of hvKP1, hvKP1ΔiucA, hvKP1ΔiroB, hvKP1Δirp2, hvKP1ΔentB, and hvKP1ΔentBΔirp2 was assessed by measurement of CFU at 0, 3, 6, and 24 h in 90% human ascites fluid–10% 1× PBS (pH 7.4). Data are means ± SEM; five to seven independent growth curves were performed for each strain. The growth/survival of hvKP1ΔiucA was significantly decreased compared to that of hvKP1 (*, P < 0.05/5, two-tailed unpaired t test).
Development of conditioned medium for use in siderophore assays.
Conditioned medium was generated as described previously (22) with the following modifications. For urine, all strains were initially grown overnight in LB medium. The next day, bacteria were washed in 1× PBS twice, diluted in urine to an A600 of 0.08, and then grown for 24 h. For ascites fluid, all strains were initially grown overnight in ascites fluid that had been heated at 56°C for 30 min (Δ56°) prior to use to inactivate complement so that the growth between strains would be similar. The next day, the overnight growth medium was removed, and strains were diluted in fresh Δ56° ascites fluid to an A600 of 0.2 and then grown for 24 h. For serum, all strains were initially grown overnight in M9 minimal medium supplemented with iron-chelated Casamino Acids (0.3%). The next day, the overnight growth medium was removed, and strains were diluted in 90% Δ56° serum–10% 1× PBS to an A600 of 0.13 to 0.16 and then grown for 24 h. Pilot experiments have demonstrated that these growth regimens result in maximum siderophore production. After the 24-h growth period in ascites fluid, serum, and urine, the supernatant was harvested and then filtered through a 0.2-μm syringe filter (Corning). Next, for ascites fluid and serum, the bacterium-free supernatant was processed through a 10-kDa centrifugal filter unit (Millipore) to remove residual color. For urine, a 3-kDa centrifugal filter unit was used.
Quantitative siderophore assay.
Quantitative siderophore assays were performed as described previously (21). For each strain grown in ascites fluid and urine, 5 independently generated conditioned media were assayed, whereas for serum, 3 independently generated conditioned media were tested. Two measurements were performed on each conditioned medium, and a mean value was generated.
In vitro growth in ascites fluid, serum, and urine.
Growth in these media was performed as described previously (26). For ascites fluid and serum, aliquots were removed for bacterial enumeration at various times, whereas for urine, A600 was measured.
Mouse SQ challenge and pneumonia models.
The mouse subcutaneous (SQ) challenge and pneumonia models have been described (21, 22, 27). Animal studies were reviewed and approved by the University at Buffalo—SUNY and Veterans Administration Institutional Animal Care Committee. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (28), and all efforts were made to minimize suffering. In brief, in the subcutaneous (SQ) challenge model outbred male CD1 mice (18 to 22 g) were injected SQ with various titers of the bacterial strain being assessed. Two independent experiments were performed in which groups of five mice were challenged by each of the six strains being evaluated (hvKP1, hvKP1ΔiucA, hvKP1ΔiroB, hvKP1ΔentB, hvKP1Δirp2, and hvKP1ΔentBΔirp2). Four different challenge titers at log intervals (3 × 102-5 to 5 × 102-5) were used per strain, resulting in a total of 10 animals per strain per titer. Data are presented as averages of the two titers for a given strain and log titer. In the pneumonia model, oropharyngeal aspiration in anesthetized CD1 mice (18 to 22 g) was used for intrapulmonary instillation of a single titer for hvKP1 or a mutant derivative ranging from 1.4 × 105 to 2.5 × 105 CFU. A total for 10 animals were challenged with each of the six strains in a single experiment. Animals were followed for 14 days, with an in extremis state or death being used as the study endpoint.
Statistical analyses.
Data are presented as means and standard errors of the means (SEM). P values of <0.05/n (n is the number of comparisons) are considered statistically significant based on the Bonferroni correction for multiple comparisons. Two-tailed unpaired t tests were used for comparison of quantitative siderophore data (Fig. 1). To normalize ex vivo growth/survival data (for Fig. 2 through 4), log10-transformed values were utilized, the area under each curve was calculated, and these areas were compared using two-tailed unpaired t tests (Prism 4 for Macintosh; GraphPad Software Inc.). A log-rank (Mantel-Cox) test was used for the analysis of the Kaplan-Meier plot (for Fig. 5 and 6) (Prism 4 for Macintosh; GraphPad Software Inc.).
FIG 1.

Quantitative measurement of siderophores (SP) in hvKP1 (wild type), hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient) grown in human ascites fluid (A), human serum (B), and human urine (C). Quantitative SP measurements were performed on bacterium-free supernatants harvested after overnight growth. The median SP concentrations for hvKP1ΔiucA, hvKP1ΔiroB, hvKP1Δirp2, hvKP1ΔentB, and hvKP1ΔentBΔirp2 were compared to that of hvKP1. Each symbol represents the mean concentration from an independent conditioned medium measured in duplicate. (A) The mean concentration for hvKP1ΔiucA, hvKP1Δirp2, hvKP1ΔentB, and hvKP1ΔentBΔirp2 was significantly decreased compared to that for hvKP1. (B) The mean concentration for hvKP1ΔiucA and hvKP1ΔentB was significantly decreased compared to that for hvKP1. (C) The mean concentration for hvKP1ΔiucA was significantly increased compared to that for hvKP1 (*, P < 0.05/5, two-tailed unpaired t test).
FIG 4.
Growth/survival of hvKP1 (wild-type), hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient) in human urine. The growth/survival of hvKP1, hvKP1ΔiucA, hvKP1ΔiroB, hvKP1Δirp2, hvKP1ΔentB, and hvKP1ΔentBΔirp2 was assessed by measurement of optical density at 0, 1.5, 3, 4.5, 6, and 24 h in 90% human urine–10% 1× PBS (pH 7.4). Urine from three different individuals was used (A to C). Data are means ± SEM; three independent growth curves were performed for each strain. (A) The growth/survival of hvKP1ΔiucA, hvKP1ΔiroB, hvKP1Δirp2, and hvKP1ΔentB was significantly increased compared to that of hvKP1. (B) The growth/survival of hvKP1ΔiroB, hvKP1ΔentBΔirp2, and hvKP1ΔentB was significantly increased compared to that of hvKP1. (C) The growth/survival of hvKP1ΔentBΔirp2, and hvKP1ΔentB was significantly increased compared to that of hvKP1 (*, P < 0.05/5, two-tailed unpaired t test).
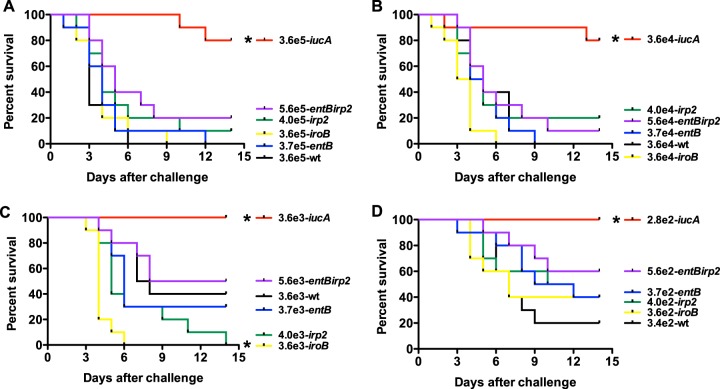
FIG 5.
Survival of outbred CD1 mice after subcutaneous (SQ) challenge with (wild-type), hvKPΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), and hvKP1ΔentBirp2 (enterobactin, salmochelin, and yersiniabactin deficient). (A) hvKP1, 3.6 × 105 CFU; hvKP1ΔiucA, 3.6 × 105 CFU; hvKPΔiroB, 3.6 × 105 CFU; hvKP1Δirp2, 4.0 × 105 CFU; hvKP1ΔentB, 3.7 × 105 CFU; hvKP1ΔentBirp2, 5.6 × 105 CFU. (B) hvKP1, 3.6 × 104 CFU; hvKP1ΔiucA, 3.6 × 104 CFU; hvKP1ΔiroB, 3.6 × 104 CFU; hvKP1Δirp2, 4.0 × 104 CFU; hvKP1ΔentB, 3.7 × 104 CFU; hvKP1ΔentBirp2, 5.6 × 104 CFU. (C) hvKP1, 3.6 × 103 CFU; hvKP1ΔiucA, 3.6 × 103 CFU; hvKP1ΔiroB, 3.6 × 103 CFU; hvKP1Δirp2, 4.0 × 103 CFU; hvKP1ΔentB, 3.7 × 103 CFU; hvKP1ΔentBirp2, 5.6 × 103 CFU. (D) hvKP1, 3.4 × 102 CFU; hvKP1ΔiucA, 2.8 × 102 CFU; hvKP1ΔiroB, 3.6 × 102 CFU; hvKP1Δirp2, 4.0 × 102 CFU; hvKP1ΔentB, 3.7 × 102 CFU; hvKP1ΔentBirp2, 5.6 × 102 CFU. Strains were grown overnight in LB medium. An in extremis state or death was scored as nonsurvival. The total number tested was 10 (n = 5 in each of 2 independent experiments) for each titer for each strain. The growth/survival of animals challenged with hvKP1ΔiucA was significantly increased compared to that of animals receiving hvKP1 at all challenge inocula as previously described (21). The growth/survival of animals challenged with hvKP1ΔiroB was significantly decreased compared to hvKP1 at a challenge inoculum of 3.6 × 103 CFU (C) (*, P < 0.05/5, log rank [Mantel-Cox] test).
FIG 6.
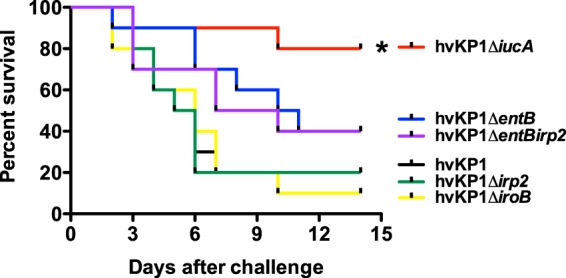
Survival of outbred CD1 mice after pulmonary challenge with (wild-type), hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), and hvKP1ΔentBirp2 (enterobactin, salmochelin, and yersiniabactin deficient). Animals underwent pulmonary challenge with either 2.1 × 105 CFU of hvKP1, 2.5 × 105 CFU of hvKP1ΔiucA, 1.8 × 105 CFU of hvKP1ΔiroB, 2.5 × 105 CFU of hvKP1ΔentB, 2.0 × 105 CFU of hvKP1Δirp2, or 1.4 × 105CFU of hvKP1ΔentBirp2. Strains were grown overnight in LB medium. An in extremis state or death was scored as nonsurvival. n = 10 for each strain. The growth/survival of animals challenged with hvKP1ΔiucA was significantly increased compared to hvKP1 (*, P < 0.05/5, log rank [Mantel-Cox] test).
RESULTS
Disruption of aerobactin synthesis results in the largest reduction of siderophore production.
The isogenic derivatives deficient in aerobactin production (hvKP1ΔiucA), salmochelin production (hvKP1ΔiroB), enterobactin and salmochelin production (hvKP1ΔentB), yersiniabactin production (hvKP1Δirp2), or enterobactin, salmochelin, and yersiniabactin production (hvKP1ΔentΔirp2) were generated by site-directed mutagenesis as described in Materials and Methods (see “Strain description”). Total siderophore production was measured in these strains in 3 clinically relevant human biologic fluids ex vivo: ascites fluid, urine, and serum (Fig. 1). As previously described in part (ascites fluid) (21), compared to the wild-type parent (hvKP1), disruption of iucA resulted in significant 95%, 94%, and 100% reductions in total siderophore production in ascites fluid, serum, and urine, respectively. The effects of disrupting entB, irp2, or iroB and the combination of entB and irp2 were quantitatively less and more variable. Disruption of entB resulted in a significant 18% reduction in total siderophore production in ascites fluid and a significant 27% reduction in serum. Disruption of irp2 resulted in a significant 45% reduction in total siderophore production only in ascites fluid. Disruption of the combination of entB and irp2 resulted in a significant 62% reduction in total siderophore production only in ascites fluid. Disruption of iroB did not have a significant effect on siderophore production. Taken together, these results demonstrate that the quantitatively greatest and only consistent reduction in total siderophore production was observed with the loss of aerobactin production.
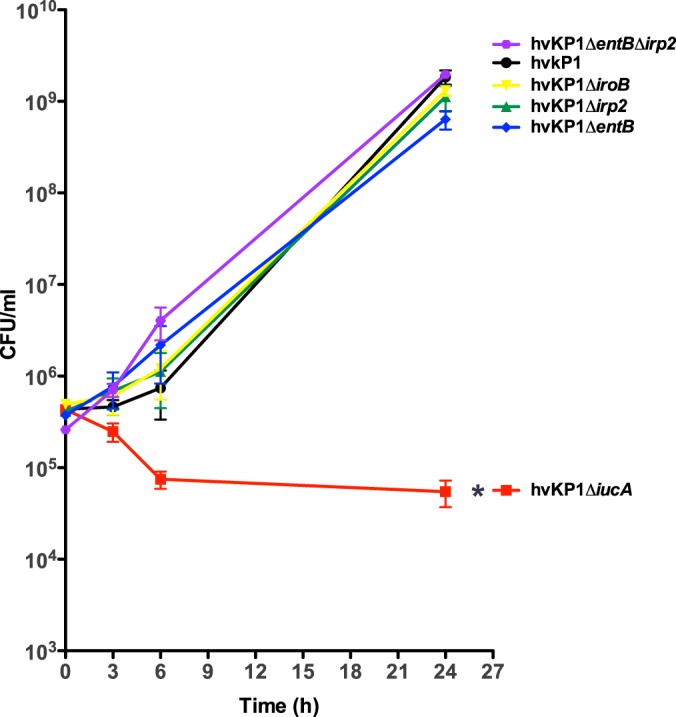
Disruption of aerobactin synthesis, but not enterobactin, salmochelin, or yersiniabactin synthesis, decreases growth/survival in human ascites fluid and serum ex vivo.
The growth/survival of hvKP1 (wild-type), hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient) was assessed in human ascites fluid and serum ex vivo. When strains were cultured in ascites fluid, the growth/survival of hvKP1, hvKP1ΔiroB, hvKP1ΔentB, hvKP1Δirp2, and hvKP1ΔentBΔirp2 as determined by the area under the curve (AUC) were similar (Fig. 2). In contrast, the growth/survival of hvKP1ΔiucA was significantly decreased compared to that of its wild-type parent, as previously described (P < 0.05/5, two-tailed unpaired t test) (21). When strains were grown in serum, the AUC growth/survival for hvKP1ΔiroB, hvKP1ΔentB, and hvKP1Δirp2 was significantly increased (P < 0.05/5, two-tailed unpaired t test) (not decreased) and that of hvKP1ΔentBΔirp2 was similar to that of hvKP1 (Fig. 3). In contrast, the AUC growth/survival of hvKP1ΔiucA was significantly decreased compared to that of its wild-type parent hvKP1 (P < 0.05/5, two-tailed unpaired t test) (Fig. 3). These data strongly support a role for aerobactin but not enterobactin, salmochelin, or yersiniabactin in increasing the growth/survival of hvKP in these clinically relevant environments.
FIG 3.
Growth/survival of hvKP1 (wild type), hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient) in human serum. The growth/survival of hvKP1, hvKP1ΔiucA, hvKP1ΔiroB, hvKP1Δirp2, hvKP1ΔentB, and hvKP1ΔentBΔirp2 was assessed by measurement of CFU at 0, 3, 6, and 24 h in 90% human serum–10% 1× PBS (pH 7.4). Data are means ± SEM; four independent growth curves were performed for each strain. The growth/survival of hvKP1ΔiucA was significantly decreased and the growth/survival of hvKP1ΔiroB, hvKP1Δirp2, and hvKP1ΔentB was significantly increased compared to that of hvKP1 (*, P < 0.05/5, two-tailed unpaired t test).
Disruption of aerobactin, enterobactin, salmochelin, or yersiniabactin synthesis does not decrease growth/survival in human urine ex vivo.
The growth of hvKP1 (wild type), hvKP1ΔiucA (aerobactin deficient), hvKP1ΔiroB (salmochelin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient) was assessed in three different human urine samples ex vivo. The growth/survival of hvKP1ΔiucA, hvKP1ΔiroB, hvKP1ΔentB, hvKP1Δirp2, and hvKP1ΔentBΔirp2 as determined by the AUC was variably increased (not decreased) in each of the three urine samples compared to that of hvKP1 (P < 0.05/5, two-tailed unpaired t test) (Fig. 4). These data do not support a role for aerobactin, enterobactin, salmochelin, or yersiniabactin in increasing the growth/survival of hvKP in human urine.
Disruption of aerobactin synthesis, but not enterobactin, salmochelin, or yersiniabactin synthesis, decreases virulence in a mouse SQ challenge infection model.
It has been previously demonstrated that aerobactin was an important factor in conferring virulence in an outbred mouse SQ challenge model (21). SQ challenge results in metastatic spread, making this model clinically relevant. This model was used to determine whether enterobactin, salmochelin, or yersiniabactin contributed to the hypervirulent phenotype of hvKP1 in this clinical setting. Four challenge inocula were used, ranging from 3.4 × 102 to 3.6 × 105 CFU, 3.6 × 102 to 3.6 × 105 CFU, 3.7 × 102 to 3.7 × 105 CFU, 4.0 × 102 to 4.0 × 105 CFU, and 5.6 × 102 to 5.6 × 105 CFU in approximate log10 intervals for hvKP1 (wild-type), hvKP1ΔiroB (salmochelin deficient), hvKP1ΔentB (enterobactin and salmochelin deficient), hvKP1Δirp2 (yersiniabactin deficient), and hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient), respectively. Previously published data on hvKP1ΔiucA were included for comparative purposes (21). The mortality of mice challenged with hvKP1ΔiroB, hvKP1ΔentB, hvKP1Δirp2, and hvKP1ΔentBΔirp2 was not decreased compared to that of mice challenged with hvKP1, as was previously observed with hvKP1ΔiucA (Fig. 5). These data do not support a role for salmochelin or yersiniabactin individually or the combination of enterobactin and salmochelin or of enterobactin, salmochelin, and yersiniabactin in increasing the virulence of hvKP1 in this systemic infection model.
Disruption of aerobactin synthesis, but not enterobactin, salmochelin, or yersiniabactin synthesis, decreases virulence in a mouse pneumonia model.
A mouse pulmonary challenge model was used to determine whether aerobactin, enterobactin, salmochelin, or yersiniabactin contributed to the hypervirulent phenotype of hvKP1 in this clinical setting. Outbred CD1 mice were challenged with 2.1 × 105 CFU (hvKP1, wild type), 2.5 × 105 CFU (hvKP1ΔiucA, aerobactin deficient), 1.8 × 105 CFU (hvKP1ΔiroB, salmochelin deficient), 2.5 × 105 CFU (hvKP1ΔentB, enterobactin and salmochelin deficient), 2.0 × 105 CFU (hvKP1Δirp2, yersiniabactin deficient), and 1.4 × 105 CFU of hvKP1ΔentBΔirp2 (enterobactin, salmochelin, and yersiniabactin deficient). The survival of mice challenged with hvKP1ΔiucA was significantly (P < 0.05/5, log rank [Mantel-Cox] test) increased compared to those challenged with hvKP1 (Fig. 6). In contrast, survival of mice challenged with hvKP1ΔiroB, hvKP1ΔentB, hvKP1Δirp2, and hvKP1ΔentBirp2 was similar to survival of those challenged with hvKP1 (Fig. 6). These data demonstrate that aerobactin increases the virulence of hvKP1 after pulmonary challenge. However, it does not support a role for enterobactin, salmochelin, or yersiniabactin in this setting.
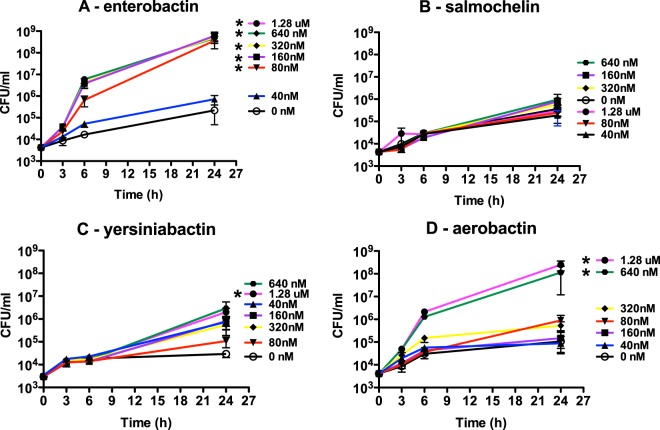
The growth/survival of hvKP1ΔiucA (aerobactin deficient) in human ascites fluid is maximized by chemical complementation with aerobactin and enterobactin.
The total amount of siderophore production by hvKP1ΔiucA in ascites fluid is 5% of the amount produced by its wild-type parent, hvKP1 (Fig. 1). This enabled us to test the effect of supplementing human ascites fluid with exogenous siderophores (40 nM to 1.28 μM) on the growth/survival of hvKP1ΔiucA. At a starting inoculum between 3.0 × 103 and 5.4 × 103 CFU/ml, the growth/survival of hvKP1ΔiucA is limited (Fig. 7). The addition of exogenous salmochelin had no effect on the growth/survival of hvKP1ΔiucA (Fig. 7B). The addition of 1.28 μM yersiniabactin significantly increased the growth/survival of hvKP1ΔiucA; however, a plateau density of only 2.0 × 106 CFU/ml was achieved (Fig. 7C). In contrast, the addition of 640 nM and 1.28 μM aerobactin and 80 nM, 160 nM, 320 nM, 640 nM, and 1.28 μM enterobactin significantly increased the growth/survival of hvKP1ΔiucA, with plateau densities of 1.1 × 108 to 2.5 × 108 CFU/ml and 3.6 × 108 to 6.1 × 108 CFU/ml achieved, respectively (Fig. 7A and D). These data support the concept that in human ascites fluid, the effect on growth/survival for these siderophores is not equivalent, with the relative biologic activity being enterobactin > aerobactin > yersiniabactin > salmochelin.
FIG 7.
Growth/survival of hvKP1ΔiucA (aerobactin deficient) in human ascites fluid with and without the addition of enterobactin, salmochelin, yersiniabactin, and aerobactin. The growth/survival of hvKP1ΔiucA, was assessed by measurement of CFU at 0, 3, 6, and 24 h in 90% human ascites fluid–10% 1× PBS (pH 7.4) with or without the addition of purified siderophores at the concentrations of 0 nM, 40 nM, 80 nM, 160 nM, 320 nM, 640 nM and 1.28 μM. The listed order of concentrations in each panel is based on plateau density. Data are means ± SEM; four to six independent growth curves were performed for each strain at each siderophore concentration. The growth/survival of hvKP1ΔiucA was significantly increased with the addition of 80 nM, 160 nM, 320 nM, 640 nM, and 1.28 μM enterobactin, with the addition of 1.28 μM yersiniabactin, and with 640 nM and 1.28 μM aerobactin compared to growth in the absence of exogenous siderophore (*, P < 0.05/6, two-tailed unpaired t test).
DISCUSSION
To date, increased capsule production and the siderophore aerobactin, which is produced at high levels, have been established as factors that increase the virulence of hvKP compared to cKP strains (15, 21). In this study, we extended previous observations on the importance of aerobactin in hvKP by demonstrating its importance for growth/survival in human serum ex vivo (Fig. 3) and its contribution to virulence in a mouse pulmonary challenge model of infection (Fig. 6). We also assessed whether the other siderophores produced in hvKP (enterobactin, yersiniabactin, and salmochelin) enhanced virulence. Interestingly, in contrast to aerobactin, the inability to produce enterobactin, salmochelin, or yersiniabactin individually or in combination did not decrease the ex vivo growth/survival in human ascites fluid or serum or decrease virulence in the mouse SQ challenge model, which results in systemic infection, or in the mouse pulmonary challenge model. These data do not support enterobactin, salmochelin, and yersiniabactin as factors that increase the virulence of hvKP in these clinically relevant settings. However, since competition experiments were not performed using siderophore receptor mutants, which would avoid the experimental challenge of siderophore cross-feeding, we cannot exclude a minor role for the nonaerobactin siderophores at these sites of infection. Further, whether these siderophores contribute to the pathogenesis of hvKP infection in other sites of infection remains to be determined. Lastly, the focus of this study was to assess the role of siderophores in systemic infection (i.e., after colonization and entry); however, some investigators have used an oral challenge model to study hvKP infection (29). Although it is beyond the scope of this study, that model could be used to study the role of siderophores in gastrointestinal colonization.
A critical unresolved question is whether the properties of aerobactin, its absolute amount, or the combination explains the apparent singular importance of this siderophore for hvKP infection. Data presented in this report demonstrate that enterobactin and aerobactin are the most biologically active siderophores in human ascites fluid, as measured by growth enhancement of the aerobactin-deficient strain hvKP1ΔiucA. Limited growth enhancement was observed with the addition of exogenous yersiniabactin, and none was observed with salmochelin supplementation. These data support the concept that both the quantity and biologic activity of aerobactin contribute to its role in virulence. These data also suggest that, at least in ascites fluid, even if yersiniabactin and salmochelin were produced in hvKP at high levels similar to those of aerobactin, they would not be functionally equivalent to aerobactin in the pathogenic process. In fact, the inability to produce salmochelin or yersiniabactin in human serum and urine and perhaps in the SQ challenge model resulted in enhanced virulence compared to that of their wild-type parent. These findings are consistent with a scenario in which the savings in metabolic costs associated with the production of these siderophores outweighs their contribution to growth/survival (30). Although it might appear surprising that enterobactin production was unable to compensate for the loss of aerobactin production in hvKP1ΔiucA, some combination of its low level of production (plus the fact that a portion of enterobactin produced is converted to salmochelin) and sensitivity to lipocalin 2 (to which aerobactin is resistant) is a likely explanation (31–34). Features that may contribute to the biologic activity of aerobactin despite a lower Fe association constant (Kf = 1022.9) (35) than enterobactin (Kf = 1052) (35), yersiniabactin (Kf = 1036) (36), and transferrin (∼1030) (37) include the facts that aerobactin is recycled (38), it transfers Fe from transferrin more efficiently than enterobactin (39), and, in contrast to enterobactin, this transfer is not impeded by albumin or immunoglobulins (40).
Findings from this study and previously published data (21) continue to demonstrate the importance of aerobactin in hvKP infection. These results are in contrast to a previous study in which an aerobactin deficient derivative of the hvKP strain NTUH-K2044 had a 50% lethal dose (LD50) similar to that of its wild-type parent after intraperitoneal and intragastric challenge in BALB/cByl mice, although decreased virulence was observed with an aerobactin-salmochelin-yersiniabactin-deficient derivative (41). We believe that the observed difference is most likely related to the mouse strains used; however, we cannot exclude a role for differences in the bacterial strains.
Our inability to demonstrate a role for salmochelin and yersiniabactin in hvKP infection after SQ and pulmonary challenge could also be considered surprising. Genes that encode yersiniabactin are more prevalent in hvKP (91%) than cKP (22%) (41), and genes that encode salmochelin production are present with aerobactin biosynthesis genes on a large virulence plasmid that appears to be critical for hvKP pathogenesis (24, 42–44). Further, previous studies using a cKP strain supported a role for these factors in pulmonary infection (31, 32, 45). However, the cKP strains assessed in those studies did not produce aerobactin, and in fact, the ability to produce aerobactin in combination with increased capsule production appears to be a defining hvKP trait (6, 21). In addition, salmochelin and yersiniabactin are not major contributors to the high siderophore levels observed in hvKP strains under Fe-poor conditions (Fig. 1) (21). Our findings are consistent with results obtained with the hvKP strain NTUH-K2044 in which the absence of salmochelin or yersiniabactin did not affect LD50s after intraperitoneal and intragastric challenge in BALB/cByl mice (41).
We were unable to demonstrate a role for any siderophore in increasing growth/survival in human urine. A similar result had been previously demonstrated for a cKP strain, but since that isolate did not produce aerobactin, its role was not assessed (32). Due to potential variability in urine composition, three different urine samples were evaluated. Further, increased levels of siderophores were measured in all of these urine samples (Fig. 1) compared to levels observed in an iron-replete medium (21). This is consistent with urine being an environment with low iron bioavailability in which iron acquisition is needed. Since siderophores are the dominant iron acquisition factors in K. pneumoniae, this finding was surprising. Potential explanations include a role for a siderophore-independent iron acquisition factors, or perhaps the functional role for siderophores in this setting is more egalitarian and the presence of just one or two siderophores at low concentrations is sufficient. Of course, growth in urine is not a complete assessment for a factor in uropathogenesis. It is possible that aerobactin or an alternative siderophore may be important for growth/survival in bladder or renal tissue or invasion of the bloodstream from these sites. Nonetheless, the loss of aerobactin production in urine does not have the same effect on the hvKP phenotype as in ascites fluid and serum. This finding supports an increasing body of data which demonstrates that selected siderophores are more important in certain settings (31, 46, 47).
In summary, our data confirm and extend the critical role for aerobactin in hvKP infection. In contrast, we were unable to establish a role for enterobactin, salmochelin, or yersiniabactin, at least under the ex vivo and in vivo conditions tested in this report. This appears to be due to a combination of the biologic properties of aerobactin and the high level of aerobactin production. These data have potential implications for the development of novel therapeutic strategies in the management of hvKP infection. This may be particularly important, since recent data support the idea that hvKP has the potential to acquire significant antimicrobial resistance (11), similar to what is now occurring with cKP (4). Ever-increasing antimicrobial resistance, especially in Gram-negative bacilli, has increased interest with regard to the development of antivirulence agents. The goal with this approach is not to kill bacteria but to target the ability of the pathogen to cause disease, thereby limiting the selective pressure for resistance. Iron deprivation would be a means to accomplish this. This study begins to address the issue of whether aerobactin biosynthetic enzymes will be suitable targets for an antivirulence treatment strategy.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grant 1R21AI088318-01A1 (T.A.R.), by a VA Merit Review from the Department of Veterans Affairs (T.A.R.), and by Telemedicine and Advance Technical Research Center (TATRC) cooperative agreement W81XWH-11-2-0218 (T.A.R.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Kuehn BM. 2013. “Nightmare” bacteria on the rise in US hospitals, long-term care facilities. JAMA 309:1573–1574. doi: 10.1001/jama.2013.2922. [DOI] [PubMed] [Google Scholar]
- 2.Moellering RC., Jr 2010. NDM-1—a cause for worldwide concern. N Engl J Med 363:2377–2379. doi: 10.1056/NEJMp1011715. [DOI] [PubMed] [Google Scholar]
- 3.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. 2013. Vital signs: carbapenem-resistant enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YC, Cheng DL, Lin CL. 1986. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med 146:1913–1916. doi: 10.1001/archinte.1986.00360220057011. [DOI] [PubMed] [Google Scholar]
- 6.Shon AS, Bajwa RP, Russo TA. 2013. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. 1991. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med 151:1557–1559. [PubMed] [Google Scholar]
- 8.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, McCormack JG, Yu VL. 2002. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 8:160–166. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, Wann SR, Lin HH. 1998. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 26:1434–1438. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. 2006. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med 259:606–614. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Sun G, Yu Y, Li N, Chen M, Jin R, Jiao Y, Wu H. 2014. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis 58:225–232. doi: 10.1093/cid/cit675. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Li XY, Wan LG, Jiang WY, Yang JH, Li FQ. 2014. Virulence and transferability of resistance determinants in a novel Klebsiella pneumoniae sequence type 1137 in China. Microb Drug Resist 20:150–155. doi: 10.1089/mdr.2013.0107. [DOI] [PubMed] [Google Scholar]
- 13.Siu LK, Huang DB, Chiang T. 2014. Plasmid transferability of KPC into a virulent K2 serotype Klebsiella pneumoniae. BMC Infect Dis 14:176. doi: 10.1186/1471-2334-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. 2008. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis 62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. 2010. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J Bacteriol 192:3144–3158. doi: 10.1128/JB.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu CR, Lin TL, Chen YC, Chou HC, Wang JT. 2011. The role of Klebsiella pneumoniae rmpA in capsular polysaccharide synthesis and virulence revisited. Microbiology 157:3446–3457. doi: 10.1099/mic.0.050336-0. [DOI] [PubMed] [Google Scholar]
- 17.Wacharotayankun R, Arakawa Y, Ohta M, Tanaka K, Akashi T, Mori M, Kato N. 1993. Enhancement of extracapsular polysaccharide synthesis in Klebsiella pneumoniae by RmpA2, which shows homology to NtrC and FixJ. Infect Immun 61:3164–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. 2009. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 4:e4982. doi: 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hider RC, Kong X. 2010. Chemistry and biology of siderophores. Nat Prod Rep 27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 21.Russo TA, Olson R, Macdonald U, Metzger D, Maltese LM, Drake EJ, Gulick AM. 2014. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun 82:2356–2367. doi: 10.1128/IAI.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo TA, Shon AS, Beanan JM, Olson R, Macdonald U, Pomakov AO, Visitacion MP. 2011. Hypervirulent K. pneumoniae secretes more and more active iron-acquisition molecules than “classical” K. pneumoniae thereby enhancing its virulence. PLoS One 6:e26734. doi: 10.1371/journal.pone.0026734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pomakova DK, Hsiao CB, Beanan JM, Olson R, Macdonald U, Keynan Y, Russo TA. 2012. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumoniae: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis 31:981–989. doi: 10.1007/s10096-011-1396-6. [DOI] [PubMed] [Google Scholar]
- 24.Russo TA, Gill SR. 2013. Draft genome sequence of the hypervirulent Klebsiella pneumoniae strain hvKP1, isolated in Buffalo, New York. Genome Announc 1:e0006513. doi: 10.1128/genomeA.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luke NR, Howlett AJ, Shao J, Campagnari AA. 2004. Expression of type IV pili by Moraxella catarrhalis is essential for natural competence and is affected by iron limitation. Infect Immun 72:6262–6270. doi: 10.1128/IAI.72.11.6262-6270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo TA, MacDonald U, Beanan JM, Olson R, MacDonald IJ, Sauberan SL, Luke NR, Schultz LW, Umland TC. 2009. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J Infect Dis 199:513–521. doi: 10.1086/596317. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarthy KV, Davidson BA, Helinski JD, Ding H, Law WC, Yong KT, Prasad PN, Knight PR. 2011. Doxorubicin-conjugated quantum dots to target alveolar macrophages and inflammation. Nanomedicine 7:88–96. doi: 10.1016/j.nano.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 29.Tu YC, Lu MC, Chiang MK, Huang SP, Peng HL, Chang HY, Jan MS, Lai YC. 2009. Genetic requirements for Klebsiella pneumoniae-induced liver abscess in an oral infection model. Infect Immun 77:2657–2671. doi: 10.1128/IAI.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv H, Hung CS, Henderson JP. 2014. Metabolomic analysis of siderophore cheater mutants reveals metabolic costs of expression in uropathogenic Escherichia coli. J Proteome Res 13:1397–1404. doi: 10.1021/pr4009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachman MA, Lenio S, Schmidt L, Oyler JE, Weiser JN. 2012. Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia. mBio 3:e00224-11. doi: 10.1128/mBio.00224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachman MA, Oyler JE, Burns SH, Caza M, Lepine F, Dozois CM, Weiser JN. 2011. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun 79:3309–3316. doi: 10.1128/IAI.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, Mak TW, Clifton MC, Strong RK, Ray P, Kolls JK. 2009. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol 182:4947–4956. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 35.Neilands JB. 1981. Microbial iron compounds. Annu Rev Biochem 50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 36.Perry RD, Balbo PB, Jones HA, Fetherston JD, DeMoll E. 1999. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology 145:1181–1190. doi: 10.1099/13500872-145-5-1181. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg ED. 1978. Iron and infection. Microbiol Rev 42:45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun V, Brazel-Faisst C, Schneider R. 1984. Growth stimulation of Escherichia coli in serum by iron(III) aerobactin. Recycling of aerobactin. FEMS Microbiol Lett 21:99–103. doi: 10.1111/j.1574-6968.1984.tb00193.x. [DOI] [Google Scholar]
- 39.Konopka K, Bindereif A, Neilands JB. 1982. Aerobactin-mediated utilization of transferrin iron. Biochemistry 21:6503–6508. doi: 10.1021/bi00268a028. [DOI] [PubMed] [Google Scholar]
- 40.Konopka K, Neilands JB. 1984. Effect of serum albumin on siderophore-mediated utilization of transferrin iron. Biochemistry 23:2122–2127. doi: 10.1021/bi00305a003. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT. 2008. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 197:1717–1727. doi: 10.1086/588383. [DOI] [PubMed] [Google Scholar]
- 42.Chen YT, Chang HY, Lai YC, Pan CC, Tsai SF, Peng HL. 2004. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 337:189–198. doi: 10.1016/j.gene.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Wu KM, Li LH, Yan JJ, Tsao N, Liao TL, Tsai HC, Fung CP, Chen HJ, Liu YM, Wang JT, Fang CT, Chang SC, Shu HY, Liu TT, Chen YT, Shiau YR, Lauderdale TL, Su IJ, Kirby R, Tsai SF. 2009. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol 191:4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nassif X, Fournier JM, Arondel J, Sansonetti PJ. 1989. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect Immun 57:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawlor MS, O'Connor C, Miller VL. 2007. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect Immun 75:1463–1472. doi: 10.1128/IAI.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bearden SW, Perry RD. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol 32:403–414. doi: 10.1046/j.1365-2958.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 47.Garcia EC, Brumbaugh AR, Mobley HL. 2011. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun 79:1225–1235. doi: 10.1128/IAI.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]