Abstract
Severe invasive infectious diseases remain a major and life-threatening health problem. In serious cases, a systemic activation of the coagulation cascade is a critical complication that is associated with high mortality rates. We report here that streptokinase, a group A streptococcal plasminogen activator, triggers the activation of the human contact system. Activation of contact system factors at the surface of the Streptococcus pyogenes serotype M49 is dependent on streptokinase and plasminogen. Our results also show that secreted streptokinase is an efficient contact system activator, independent from a contact surface. This results in the processing of high-molecular-weight kininogen and the release of bradykinin, a potent vascular mediator. We further investigated whether the ability of 50 different clinical S. pyogenes isolates to activate the contact system is associated with an invasive phenotype. The data reveal that isolates from invasive infections trigger an activation of the contact system more potently than strains isolated from noninvasive infections. The present study gives new insights into the mechanisms by which S. pyogenes triggers the human contact system and stresses the function of soluble and surface located plasmin exploited as a group A streptococcal virulence factor through the action of streptokinase.
INTRODUCTION
Streptococcus pyogenes, referred to as group A streptococcus (GAS), is a Gram-positive major human pathogen responsible for uncomplicated throat and skin infections such as pharyngitis and impetigo. Infections can occasionally develop into serious and life-threatening conditions, of which streptococcal toxic shock syndrome and necrotizing fasciitis are associated with high morbidity and mortality (1). Although GAS virulence factors have been studied intensively, the mechanisms by which local infections progress to severe systemic infections are not yet fully understood. The systemic activation of host immune responses has been reported to account for several symptoms seen in septic patients, i.e., hypotension, and coagulation abnormalities such as disseminated intravascular coagulation, edema formation, and multiorgan failure. Previous studies have shown that the systemic activation of the human contact system plays an important role in invasive GAS infections (for a review, see reference 2). The contact system comprises four plasma proteins, circulating as zymogens in the bloodstream or being assembled on various cell types: the serine proteases factor XII (FXII), factor XI (FXI), and prekallikrein (PKK) and the nonenzymatic cofactor high-molecular-weight kininogen (HK). The latter forms equimolar complexes with plasma kallikrein (PK) or FXI. The cascade is initiated upon contact to a negatively charged surface and starts with the limited autoactivation of FXII due to a conformational change. FXIIa then activates PKK to PK, which recruits further active FXII molecules in a positive-feedback loop. Moreover, PK cleaves its complex partner HK to release the vasoactive and proinflammatory peptide bradykinin. The nonapeptide has a very short half-life (a matter of seconds) and exhibits its functions via the B1 and B2 receptors (3). Generating other mediators such as nitric oxide, prostaglandins, and leukotrienes, bradykinin is involved in the regulation of blood pressure, the induction of fever and pain, vascular leakage, and the chemotaxis of immune cells (4). In addition, further processing of HK may also lead to the generation of antimicrobial peptides (5), implicating a critical role of the human contact system in the early defense against microbial invaders (6).
Pathogens have evolved various mechanisms to manipulate the host immune system and interference with contact factors has been described for a number of bacterial species (for a review, see reference 7). In the case of S. pyogenes, the secreted streptococcal cysteine protease SpeB was found to cleave HK directly (8). In addition, the surface-bound M protein, one of the classical virulence determinants of GAS, has been demonstrated to bind HK, followed by bradykinin generation (9, 10). Binding and assembly of contact system factors on the bacterial surface are thought to be sufficient to activate this proteolytic system (6); however, additional mechanisms must exist, since Sriskandan et al. documented that streptococcal culture supernatants were able to activate PK in vitro (11).
Many pathogenic streptococci secrete streptokinase in order to accelerate the conversion of human plasminogen to plasmin (12). Lacking enzymatic activity itself, streptokinase evolves its function by forming a stoichiometric 1:1 complex with plasminogen or a trimolecular complex with plasminogen and fibrinogen. The activator complexes can bind at the bacterial surface via host factor receptors and convert other plasminogen molecules to plasmin (13). Plasmin is a broad-spectrum serine protease and mainly dissolves fibrin clots, but as early as 1995 Ewald and Eisenberg proved plasmin also to initiate the contact system cascade (40). This prompted us to investigate if the streptococcal plasminogen activator streptokinase might contribute to contact system activation by S. pyogenes.
Here, we report that the human contact system is activated by the action of streptokinase. The role of secreted and surface-bound streptokinase in this process was investigated by comparing an M49 S. pyogenes wild-type strain with its isogenic Δska mutant, which is unable to trigger plasmin activity in human plasma. Moreover, we analyzed contact activation by a set of clinical GAS strains isolated from invasive and noninvasive cases at the Rostock Medical Center. Our results show a thus-far-undescribed mechanism by which S. pyogenes triggers the human contact system. This adds another important and prominent virulence mechanism and function to a growing list of streptokinase activities.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
GAS serotype M49 strain 591 was obtained from R. Lütticken (Aachen, Germany). GAS M1 strain 90-226 was obtained from the World Health Organization Center for Reference and Research on Streptococci at the University of Minnesota. It was originally isolated from the blood of a patient with sepsis. The construction of the M1-protein-lacking mutant strain from GAS M1 has been described previously (14). The different clinical M serotype GAS strains used in the present study have been described by Köller et al. (15). A streptokinase-deficient mutant (Δska) in the GAS M49 591 chromosomal background was generated previously by insertional inactivation of the ska gene (16). The construction of the epf, emm49, and prtF2 mutant strains from GAS M49 591 has been described previously (17–20). The GAS strains were cultured in Todd-Hewitt broth (THB; Invitrogen) at 37°C under a 5% CO2 and 20% O2 atmosphere.
Materials.
Pooled normal plasma and plasma deficient in FXII or PKK were provided by George King Bio-Medical, Inc. (Overland Park, KS). Fresh frozen plasma samples from healthy individuals were obtained from the blood bank at Rostock University Hospital, Rostock, Germany, and kept frozen at 80°C until use. Plasminogen-deficient plasma was purchased from Haemochrom Diagnostica (Germany). Purified streptokinase (pSK) from beta-hemolytic streptococci was derived from Sigma-Aldrich.
Measurement of PK/FXIIa activity.
PK/FXIIa activity on bacterial surfaces exposed to normal or plasminogen-deficient plasma was measured using chromogenic substrate S-2302 (H-D-Pro-Phe-Arg-pNA·2HCl; Chromogenix). Ten-milliliter overnight cultures (THB) of the S. pyogenes M49 wild type and its isogenic knockout mutants were washed three times with 50 mM Tris (pH 7.5) buffer and diluted (final concentration, 3 × 107 CFU/ml) in 50 mM Tris. Then, 100 μl of bacterial suspension was mixed with 100 μl of plasma or buffer (control), followed by incubation at 37°C for 30 min. After centrifugation, the pellets were washed three times, centrifuged, and resuspended in 200 μl of buffer containing a 1 mM concentration of the substrate. After 60 min, the samples were centrifuged, and the absorbance of the supernatants was measured at 405 nm in an enzyme-linked immunosorbent assay (ELISA) reader. Control values (bacteria incubated in buffer) were used as a blank. No endogenous proteolytic activity was measured when S-2303 was incubated with bacteria in the absence of plasma.
PK/FXIIa activity in normal and plasminogen-deficient plasma incubated with supernatants from overnight cultures was measured by adding 100 μl of supernatant to 100 μl of plasma and 100 μl of 50 mM Tris (pH 7.5) buffer containing a 1 mM concentration of the chromogenic substrate. The samples were incubated at 37°C for 60 min, and the absorbance was determined at 405 nm. Plasma incubated with medium instead of culture supernatant served as a control and was subtracted from the sample values. When required, the plasmin inhibitor d-Val-Phe-Lys chloromethyl ketone dihydrochloride (Merck) was added to a final concentration of 0.5 μg/ml after an initial incubation time of 15 min prior to substrate addition.
Measurement of plasmin activity.
To measure the plasmin activity on bacterial surfaces exposed to plasma, bacteria were grown overnight in 10 ml of THB, washed three times with phosphate-buffered saline (PBS), and diluted (final, concentration 5 × 107 CFU/ml) in PBS. Next, 200-μl bacterial suspensions were mixed with 200 μl of plasma or buffer, followed by incubation for 3 h in human plasma. After three further washing steps with PBS, the pellet was suspended in Tris-NaCl buffer (19.2 mM/1.062 M; pH 7.5) containing 20 μg/ml of the chromogenic substrate S-2251 (H-D-Val-Leu-Lys-pNA·2HCl; Sigma), followed by an incubation for 60 min at 37°C. Samples were centrifuged, and the absorbance of the supernatants was measured at 405 nm in an ELISA reader.
Plasmin activity in plasma treated with bacterial supernatants was determined as follows. Portions (50 μl) of plasma were mixed with 70 μl of culture supernatant from a 10-ml THB overnight culture. The plasmin-specific chromogenic substrate was added, and the mixture was incubated at 37°C. After 3 h, the absorbance at 405 nm was measured. Instead of the supernatant, 70 μl of medium or 30 μl of pSK (10 U/μl) served as negative and positive controls, respectively.
Electrophoresis and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Neville (21). Proteins from plasma samples were separated on gels of 10% total acrylamide with 3% bisacrylamide. In this way, untreated plasma or plasma treated with kaolin, THB, or H2O for 240 min served as controls. Plasma was diluted 1/100 in sample buffer containing 2% (wt/vol) SDS and 5% (vol/vol) β-mercaptoethanol. Before loading, the samples were boiled for 5 min. Western blot analyses were performed with sheep antibodies against HK (Affinity Biologicals) and its degradation products, as described previously (22).
Bradykinin measurement.
Purified SK or plasmin was incubated with normal human plasma for 15 min, and the bradykinin content was measured as described earlier (22, 23). Kaolin-treated plasma samples served as positive controls. Bradykinin release in H2O-treated plasma was used as a negative control.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism (v6.0). The P value was determined by using the unpaired t test (comparison of two groups). All samples were analyzed in triplicates, and all experiments were performed at least three times, if not otherwise declared. The bars in the figures indicate the standard deviations (SD).
RESULTS
Activation of PK and FXII at the surface of S. pyogenes M49 is dependent on streptokinase and plasminogen.
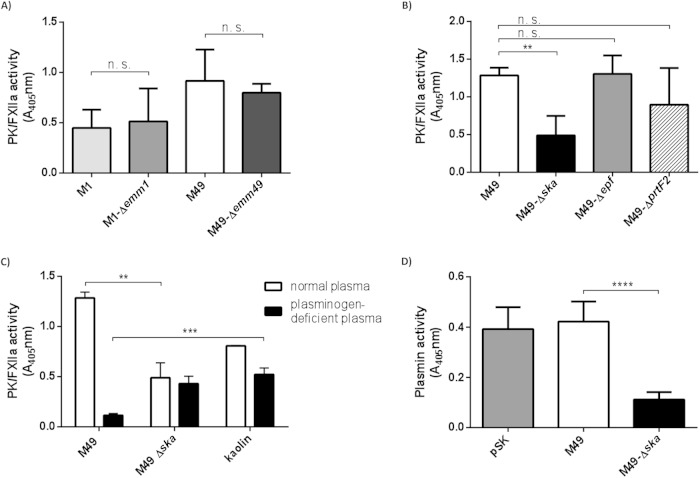
The S. pyogenes M1 strain has previously been shown to bind and activate contact factors on its surface (24, 25). To test whether S. pyogenes M49 and M1 strains trigger contact activation on their surfaces, the bacteria were incubated with human plasma. After 30 min, unbound plasma proteins were removed by centrifugation and washing steps. A chromogenic substrate (S-2302), specific for PK and FXIIa, was used to measure contact activation. Both strains exhibited PK/FXIIa activity on their surfaces (Fig. 1A). Interestingly, compared to the wild types, knockout of the M protein in these strains did not change PK/FXIIa activity significantly (Fig. 1A), suggesting that activation of contact system factors occurs independently of the M protein.
FIG 1.
PK/FXIIa and plasmin activity detected on bacterial surfaces. Overnight cultures were washed and incubated in plasma or buffer for 30 min. Unbound plasma proteins were removed by washing and the chromogenic substrate S-2302, specific for PK/FXIIa, was added. After incubation, bacteria were removed and hydrolysis of the substrate was measured at 405 nm. M1 and M49 wild type and corresponding mutant strains lacking the M protein (Δemm) (A) and M49 mutant strains lacking streptokinase (Δska), extracellular protein factor (Δepf), or protein F2 (ΔprtF2) (B) were tested for surface PK/FXIIa activity after incubation with normal human plasma. (C) The PK/FXIIa activity at the surfaces of M49 wild-type and Δska mutant strains was examined after exposure to normal or plasminogen-deficient plasma. Kaolin served as a positive control for plasmin-independent contact system activation. (D) Plasmin activity at the surfaces of M49 wild-type and Δska mutant strains was examined after exposure to normal plasma. Overnight cultures were washed and incubated in plasma or in buffer for 3 h. After washing, the chromogenic substrate S-2251, specific for plasmin, was added. After incubation, the bacteria were removed, and hydrolysis of the substrate was measured at 405 nm. As a positive control, purified SK (pSK) was incubated in plasma, and the substrate was added directly. The results are shown as the means of at least three independent experiments with fresh frozen plasma from different donors ± the SD. n.s., not significant; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Several respective deletion mutants with M49 background were subsequently tested for their ability to activate the contact system at their surfaces. Strains deficient in adhesins, such as protein F2 (ΔprtF2) (18) or Epf (Δepf) (26), were not significantly impaired in PK/FXIIa activity (Fig. 1B). In contrast, the mutant strain deficient in streptokinase (Δska) showed a significantly decreased PK/FXIIa activity on its surface compared to the wild-type strain (Fig. 1B).
Streptokinase (SK), secreted by group A, C, and G streptococci, binds and activates human plasminogen (27) to plasmin, the key serine protease in fibrinolysis. To test whether PK/FXIIa activity is dependent on plasminogen, the M49 wild type and its Δska mutant were incubated in normal or plasminogen-deficient plasma for 30 min. After incubation, unbound plasma proteins were removed and PK/FXIIa activity determined. Kaolin, a potent contact system activator, was used as a positive control (Fig. 1C). After incubation of M49 wild-type bacteria in plasminogen-deficient plasma, no PK/FXIIa activity was detectable (A405 = 0.11) compared to normal plasma (A405 = 1.28). The PK/FXIIa activity of the Δska mutant in plasminogen-deficient plasma (A405 = 0.43) was as low as in normal plasma (A405 = 0.48). This indicates that contact system activation occurs at the surface of the Δska mutant, although to a significantly lower extent compared to the M49 wild type (Fig. 1C). Since the Δska mutant shows no detectable surface plasmin activity (Fig. 1D), the surface contact activation of this mutant seems to occur independently from plasminogen. This is in contrast to the M49 wild type, as our data clearly show that surface-bound SK and plasminogen are important factors for contact system activation in this strain.
When the M49 strain was incubated in FXII- or PKK-deficient plasma, it retained surface PK/FXIIa activity (data not shown). In a purified system, plasmin was shown to activate both FXII (28) and PK (29), and these findings are supported by our data.
Surface-independent contact activation by secreted SK.
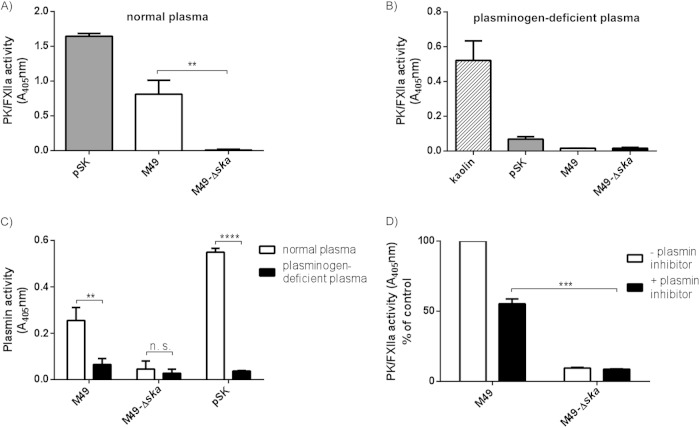
SK is secreted by S. pyogenes into the surrounding area. We therefore tested bacterial culture supernatants from M49 wild-type and Δska mutant bacteria for contact system activation. Human plasma was incubated with bacterial culture supernatants and PK/FXIIa activity determined, using the specific substrate. Purified SK (pSK) was used as a positive control, and THB medium served as a negative control, which was subtracted from the sample values. Culture supernatants from the M49 wild type, as well as pSK, induced strong PK/FXIIa activity in human plasma (Fig. 2A). In contrast, no activity was observed when plasma was incubated with the supernatant from Δska mutant bacteria (Fig. 2A).
FIG 2.
PK/FXIIa and plasmin activity induced by bacterial culture supernatants. (A and B) Supernatants from overnight cultures of M49 wild-type (M49) and Δska mutant (Δska) strain or purified streptokinase (pSK) were incubated with normal human plasma (A) or plasma deficient in plasminogen (B). The chromogenic substrate S-2302, specific for PK/FXIIa, was added and, after incubation, hydrolysis was determined at 405 nm. (C) Plasmin activity was detected in normal and plasminogen-deficient plasma after incubation with supernatants from overnight cultures of M49 wild-type (M49) or Δska mutant (Δska) strains or purified streptokinase (pSK). The chromogenic substrate S-2251, specific for plasmin, was added and, after incubation, hydrolysis was determined at 405 nm. (D) Supernatants from overnight cultures of M49 wild-type or Δska mutant strain were incubated with normal human plasma. After incubation, but prior to substrate addition, free plasmin and SK-plasmin complexes were inhibited by a specific plasmin inhibitor to avoid cross-reaction with the substrate. Hydrolysis was determined at 405 nm, and the M49 sample without inhibitor was set as 100%. The results are shown as means of at least three independent experiments with fresh frozen plasma from different donors (A) or pooled normal plasma (C and D) ± the SD. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To test whether the observed PK/FXIIa activity is connected to the presence of plasminogen, supernatants, THB (control), kaolin, or pSK was incubated with plasminogen-deficient plasma and the chromogenic substrate for PK/FXIIa. Neither the supernatants nor pSK induced an activation of PK/FXII in the absence of plasminogen, which was, in contrast to kaolin, a potent activator of the contact system via FXII (Fig. 2B).
When measuring plasmin activity in normal or plasminogen-deficient plasma, the M49 supernatant as well as pSK induced activity in normal plasma but not in plasminogen-deficient plasma (Fig. 2C). The supernatant from the Δska mutant did not induce plasmin activity (Fig. 2C). The results indicate that secreted SK is a potent contact system activator, exhibiting its function independently from a contact surface.
The chromogenic substrate S-2302 is specific for PK/FXIIa but is also sensitive to plasmin. We therefore measured the PK/FXIIa activity after addition of chloromethyl-ketone d-Val-Phe-Lys, which is an efficient inhibitor of plasmin (30) and the SK-plasminogen complex (data not shown). In the presence of the plasmin inhibitor, the PK/FXIIa activity in plasma induced by M49 wild-type supernatant was reduced by ca. 50% (Fig. 2D). This shows that a substantial part of the measured activity is assigned to PK/FXIIa, but also that plasmin hydrolyzes the substrate.
Degradation of HK and release of bradykinin by secreted SK.
To investigate whether treatment of plasma with SK results in the degradation of HK, pSK, plasmin, or kaolin was incubated with normal plasma and investigated by Western blot analysis (Fig. 3A). Samples were analyzed with an antibody directed against HK. Ponceau S staining of blotted plasma proteins was performed to confirm equal plasma amounts per lane (data not shown). Figure 3A depicts intact HK at 120 kDa in the control sample. Treatment of plasma with pSK or kaolin initiated complete HK cleavage within 240 min of incubation (Fig. 3A). Interestingly, incubation with plasmin had no obvious effect on HK cleavage (Fig. 3A), suggesting that free plasmin is rapidly inhibited by the plasma component α2-antiplasmin (13). In addition, no cleavage of HK was observed when plasminogen-deficient plasma was incubated with pSK for up to 240 min (Fig. 3B). We further incubated plasma with bacterial supernatants. Purified SK and kaolin were used as positive controls (Fig. 3C). Incubation of normal plasma with M49 wild-type supernatant, pSK, or kaolin degraded HK, whereas intact HK was detected after incubation of plasma with medium alone (THB-ctrl.) or Δska supernatant (Fig. 3C). Incubation of plasminogen-deficient plasma with bacterial supernatants, pSK, or plasmin left HK intact, whereas treatment with kaolin degraded HK (Fig. 3D), indicating that HK cleavage by bacterial supernatants is mainly triggered by the action of plasmin. This was supported when a PKK-deficient plasma was used (Fig. 3E), revealing a cleavage of HK by M49 wild-type supernatant, pSK, and partially by kaolin, but not by the Δska supernatant.
FIG 3.
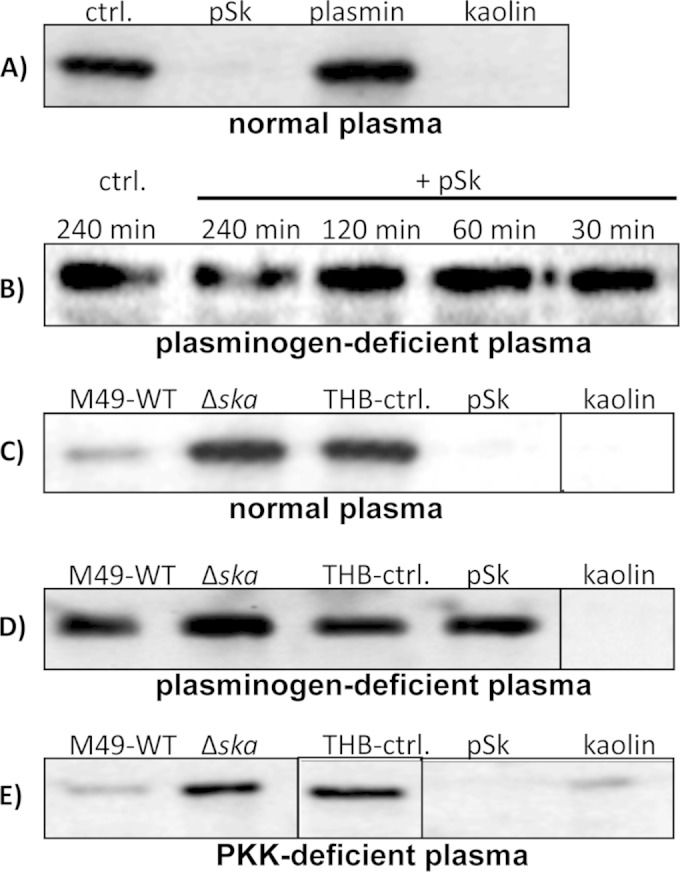
SK and SK-containing bacterial supernatants initiate plasmin-mediated HK degradation. Human pooled plasma was incubated for 240 min (if not otherwise indicated) at 37°C with pSK, plasmin, kaolin, or bacterial supernatants from THB overnight cultures of M49 wild type (M49-WT) or the Δska mutant strain (Δska) and analyzed for 120-kDa single-chain HK by immunoblotting. (A) pSK, plasmin, or kaolin was incubated in normal plasma, and plasma treated with buffer served as a negative control. (B) Plasma deficient in plasminogen was incubated with pSK for up to 240 min and analyzed for HK cleavage. Bacterial supernatants from M49 wild type (M49-WT) and the Δska mutant strain (Δska) were incubated in normal plasma (C), plasminogen-deficient plasma (D), or PKK-deficient plasma (E). SK, kaolin, and THB served as positive and negative controls, respectively.
We further investigated whether SK-activated plasmin is a source of bradykinin release in plasma, and pSK or plasmin was incubated with normal human plasma. Bradykinin release was measured via an ELISA. Purified SK triggered massive bradykinin release after 15 min, whereas bradykinin amounts in plasma treated with plasmin were significantly lower (Fig. 4).
FIG 4.
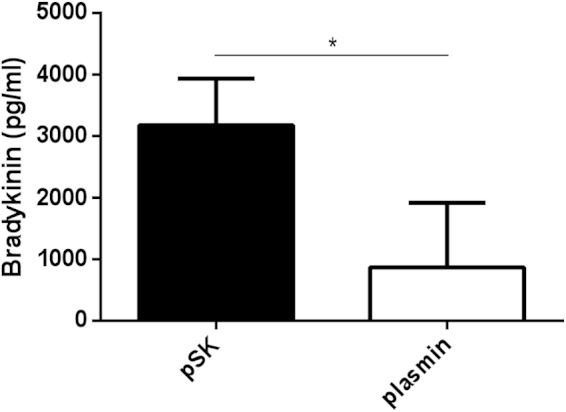
SK induces bradykinin release in plasma. Bradykinin release in diluted plasma samples was measured after 15 min of incubation with either pSK (100 U) or plasmin (10 μg) using an ELISA kit. The bradykinin concentration in plasma samples treated with equal volumes of H2O was set as background and subtracted from the obtained values. The results are shown as means ± SD of three technical replicates with fresh frozen plasma from different donors. *, P < 0.05.
Taken together, these results demonstrate that degradation of HK is triggered by SK, followed by the release of bradykinin. Moreover, HK degradation can be induced independently from PK.
Contact system activation by clinical isolates.
To test whether contact activation is associated with an invasive GAS phenotype, clinical isolates from invasive (n = 23) and uncomplicated (n = 27) GAS infections, collected at a North-East German center for tertiary care (15), were compared for their ability to activate PK/FXIIa in normal pooled plasma. PK/FXIIa activity on bacterial surfaces was measured for all isolates, but no significant differences between isolates from invasive and noninvasive cases were determined (Fig. 5A). In contrast, the PK/FXIIa activity induced by bacterial supernatants was significantly higher in isolates from invasive cases (Fig. 5B).
FIG 5.
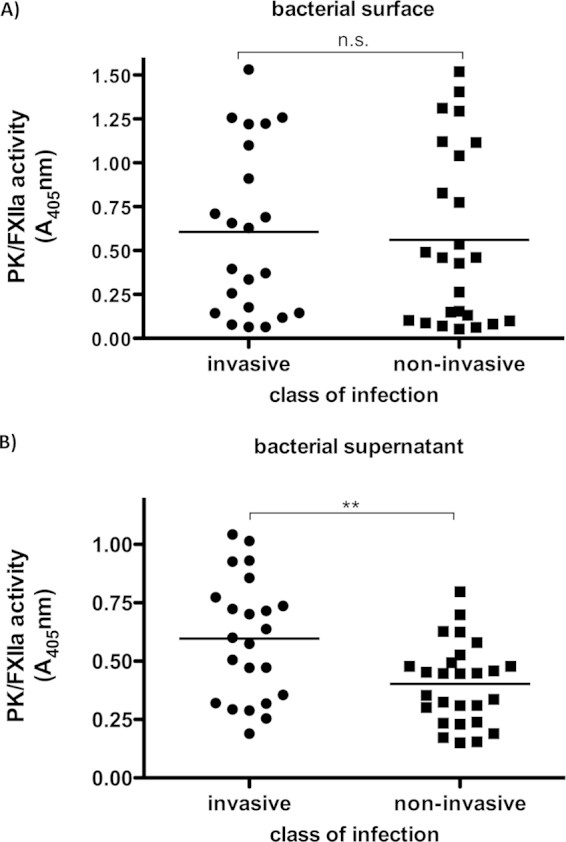
PK/FXIIa activity detected on bacterial surfaces or triggered by culture supernatants from clinical S. pyogenes isolates. (A) Bacterial overnight cultures were washed and incubated in pooled normal plasma or buffer (negative control) for 30 min. Unbound plasma proteins were removed by washing, and the PK/FXIIa-specific chromogenic substrate S-2302 was added. After incubation, the bacteria were removed and hydrolysis of the substrate was measured at 405 nm. (B) Supernatants from overnight cultures were incubated with normal human plasma. After incubation but prior to substrate addition, free plasmin and SK-plasmin complexes were inhibited by a specific plasmin inhibitor to avoid cross-reaction with the substrate. Hydrolysis was determined at 405 nm. Each plot shows the mean value of three independent experiments conducted for each strain. n.s., not significant; **, P < 0.01.
Among all strains, 13 different emm genotypes were identified in the former study (15), and the data from at least three strains of one serotype were plotted against the emm type (Fig. 6). The figure shows that M49 strains (n = 7) induced significantly more PK/FXIIa activity than did M1 (n = 10), M3 (n = 7), M6 (n = 4), or M89 strains (n = 3) (Fig. 6A). M12 (n = 7) and M28 (n = 10) strains showed a high variability in PK/FXIIa activity. The M type-specific differences in contact activation were observed at bacterial surfaces (Fig. 6A), as well as with bacterial supernatants (Fig. 6B) in all strains except for the M89 strains. All three M89 strains showed low PK/FXIIa surface activity and high PK/FXIIa supernatant activity (Fig. 6B).
FIG 6.
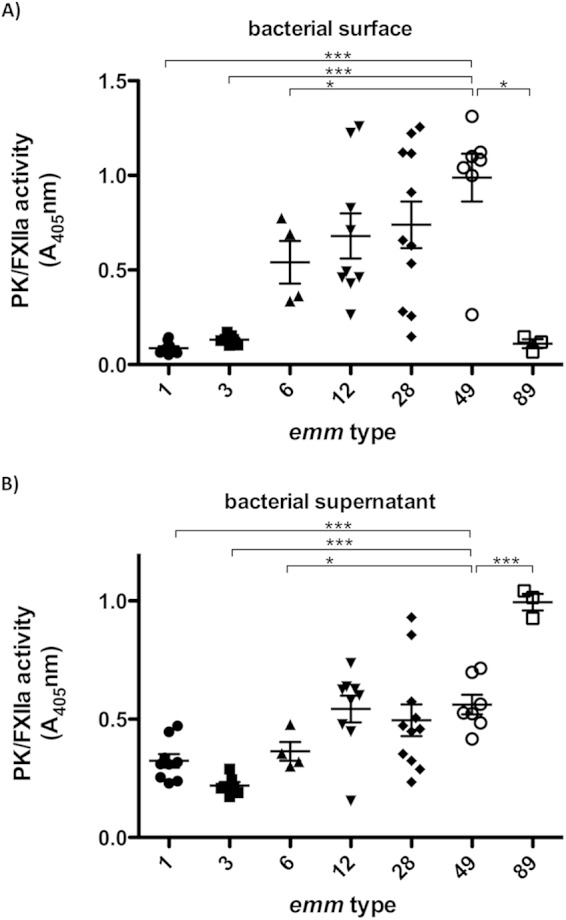
emm-type-specific PK/FXIIa activity of different S. pyogenes serotypes. (A) Hydrolysis of the PK/FXIIa specific chromogenic substrate was measured at the surface of bacteria, as described in Fig. 5A. (B) PK activation by bacterial supernatants was examined as described above (see Fig. 5B). Prior to substrate addition, free plasmin was inhibited by a specific plasmin inhibitor to avoid cross-reaction with the substrate. Each plot shows mean values of three independent experiments in normal pooled plasma conducted for each strain. Statistical significances are depicted compared to the M49 group. n.s., not significant; *, P < 0.05; ***, P < 0.001.
Plasmin activity in plasma triggered by bacterial supernatants was also determined, but no difference was found between isolates from invasive and noninvasive infections (Fig. 7A). Furthermore, there was no correlation between PK/FXIIa and plasmin activity (data not shown), suggesting that additional components in streptococcal supernatants influence contact activation in different strains. The plasmin activities from M1, M3, and M6 culture supernatants, as well as from M28 culture supernatants, were significantly lower compared to the M49 strains (Fig. 7B).
FIG 7.
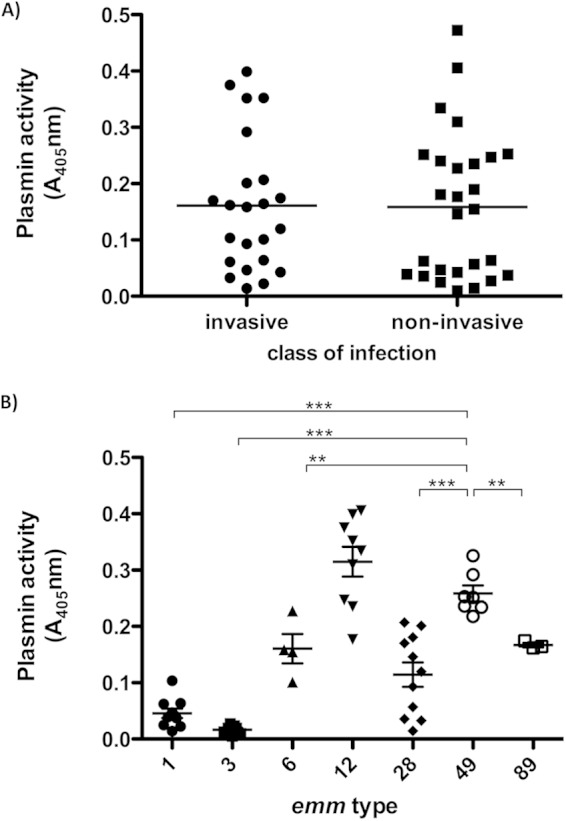
Plasmin activity triggered by streptococcal culture supernatants. Supernatants from overnight cultures were incubated with pooled normal human plasma. After incubation the substrate S-2251, specific for plasmin, was added and hydrolysis was determined at 405 nm. Each plot shows the mean value of three independent experiments conducted for each strain in pooled normal plasma. (A) Comparison of strains isolated from noninvasive and invasive infections. (B) emm-type-specific plasmin activity of different S. pyogenes serotypes. Statistical significances are depicted in comparison to the M49 group. **, P < 0.01; ***, P < 0.001.
Collectively, our data show that bacterial supernatants of clinical isolates from invasive diseases activate the contact system more potently compared to isolates from noninvasive diseases.
DISCUSSION
The contribution of the human contact system to the early innate immune defense against bacteria is supported by a recent study demonstrating that triggering the intrinsic pathway of coagulation promotes entrapment and killing of bacteria in a fibrin clot (31). The cleavage of HK results in the liberation of the nonapeptide bradykinin, which is involved in the regulation of inflammatory processes, vascular permeability, and blood pressure. Another consequence of contact system activation is the release of antimicrobial peptides from HK (5, 32), disturbing the integrity of the bacterial membrane and recruiting further elements of the immune system. Even so, bacteria use different mechanisms to activate the contact system specifically and in turn manipulate the host immune response (2, 7, 11, 33).
In the present study, we found that S. pyogenes M49 activates the contact system by the streptococcal plasminogen activator SK, leading to degradation of HK and the release of bradykinin. Contact activation occurs at the bacterial surface of S. pyogenes M49, and we showed for the first time that this activation is dependent on SK and plasminogen. Deletion of the M protein in two different serotypes (M49 and M1) did not influence PK/FXIIa activity at the bacterial surface, suggesting that the M protein is not important for the activation of contact factors. We further showed that the deletion of the important streptococcal adhesins Epf and PrtF2 from GAS M49 also had no effect on contact system activation; thus, the components responsible for surface binding of contact factors in GAS M49 remain to be investigated.
The term “contact system” is related to its mode of action, since binding (contact) and assembling of the contact factors to a negatively charged surface trigger the activation of the system. Using streptococcal supernatant from an isogenic ska knockout strain, we found that SK is an important component activating the contact system independently from a bacterial surface through the generation of plasmin. Many invasive pathogens exploit plasmin as a virulence factor to degrade fibrin clots, overcome tissue barriers, and evade peptide-derived host immune defenses (34, 35). Under normal conditions, soluble plasmin is immediately inhibited by α2-antiplasmin (6, 22, 32, 36); however, the SK-plasmin complex is protected from this inhibitor and promotes uncontrolled plasmin activity. We found that the degradation of HK by SK generates high levels of bradykinin and can be induced independently of PK. SK accumulates as soluble component in the culture supernatants of S. pyogenes strains, and during bacteremia the distribution of SK via the bloodstream may occur, as antibody titers against streptokinase have been used in the serodiagnosis of streptococcal infections (37). Consequently, an interaction of the SK-plasmin complex with various plasma proteins such as contact factors is more than likely. This mechanism could explain the in vivo contact activation and bradykinin liberation seen during streptococcal invasive infections (2, 11, 33, 38). Local and controlled activation of the contact system has been discussed to promote bacterial invasive spread via bradykinin-induced vascular leakage, since inflowing nutrient-rich plasma to the infected tissue site might serve as a route for the disseminating pathogen (6, 22, 36, 39). On the other hand, the systemic activation of the contact cascade is supposed to contribute to the pathophysiology of severe invasive infections. The consumption of contact factors, as well as high bradykinin levels, was detected in patients with sepsis or toxic shock syndrome caused by S. pyogenes and correlated with the clinical manifestations observed under these conditions: an exaggerated inflammatory response, tissue damage and multiorgan failure, hypotension, edema formation, and coagulopathy (2).
Interestingly, similar findings were revealed when patients who had been treated with SK for acute myocardial infarction were analyzed. In these patients, re-occlusion after thrombolytic therapy and irreversible hypotension were observed, and this was associated with a hypothesized plasmin-mediated contact phase activation that results in massive thrombin generation (38). Ewald and Eisenberg previously reported that plasmin-mediated activation of the contact system appears to account, at least in part, for increased procoagulant activity in patients treated with fibrinolytic agents (40). Coagulopathy is a well-recognized clinical feature of invasive streptococcal infections (39), and dysregulation of the tightly regulated hemostasis by contact system activation may represent another virulence mechanism for SK.
The host plasminogen activation system is thought to play a key role in GAS, switching from localized to systemic infections. Epidemiological studies suggest that GAS strains associated with invasive diseases bind plasminogen more avidly than those associated with benign infections (for a review, see references 1 and 5). We have tested different clinical GAS isolates regarding their ability to activate contact factors and plasmin. Our results show that strains isolated from patients with an invasive infection trigger PK/FXIIa activity more potently than do strains isolated from noninvasive infections. Intriguingly, this difference was not observed when looking at the plasmin activities of the different strains. Thus, other components released by the bacteria into the surrounding, such as SpeB, probably influence contact activation in different GAS strains.
In light of the emm types, our data show that strains of M1 or M3 serotypes induce significantly less activity of contact factors, compared to M49 strains (Fig. 6B and 7B). The meaning of this finding is currently not clear. Nevertheless, the streptococcal inhibitor of complement SIC could play a role, since it interferes with the activation of the contact system. It was shown that SIC is secreted by all isolates of the M1 serotype, binding to HK and inhibiting its antibacterial activity (41).
Hence, activation of the contact system adds another level of complexity to the interaction between pathogen and host during infections. SK is one streptococcal component responsible for contact system activation. It may be responsible for the in vivo contact activation and bradykinin liberation seen in streptococcal sepsis, and SK inhibition may be an alternative therapeutic approach for the treatment of severe GAS infections.
ACKNOWLEDGMENTS
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (project OE 547/2-1 [S.O.-H.]) and by a grant from the Medical Faculty of the University of Rostock in the framework of the FORUN program 2012.
We thank Jana Normann for excellent technical assistance.
REFERENCES
- 1.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. 2014. Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev 27:264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oehmcke S, Herwald H. 2010. Contact system activation in severe infectious diseases. J Mol Med 88:121–126. doi: 10.1007/s00109-009-0564-y. [DOI] [PubMed] [Google Scholar]
- 3.Colman RW, Schmaier AH. 1997. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood 90:3819–3843. [PubMed] [Google Scholar]
- 4.Renné T. 2012. The procoagulant and proinflammatory plasma contact system. Semin Immunopathol 34:31–41. doi: 10.1007/s00281-011-0288-2. [DOI] [PubMed] [Google Scholar]
- 5.Frick I-M, Åkesson P, Herwald H, Mörgelin M, Malmsten M, Nägler DK, Björck L. 2006. The contact system: a novel branch of innate immunity generating antibacterial peptides. EMBO J 25:5569–5578. doi: 10.1038/sj.emboj.7601422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frick I-M, Björck L, Herwald H. 2007. The dual role of the contact system in bacterial infectious disease. Thromb Haemost 98:497–502. doi: 10.1160/TH07-01-0051. [DOI] [PubMed] [Google Scholar]
- 7.Nickel KF, Renné T. 2012. Crosstalk of the plasma contact system with bacteria. Thromb Res 130(Suppl 1):S78–S83. doi: 10.1016/j.thromres.2012.08.284. [DOI] [PubMed] [Google Scholar]
- 8.Herwald H, Collin M, Müller-Esterl W, Björck L. 1996. Streptococcal cysteine proteinase releases kinins: a virulence mechanism. J Exp Med 184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben Nasr AB, Herwald H, Müller-Esterl W, Björck L. 1995. Human kininogens interact with M protein, a bacterial surface protein and virulence determinant. Biochem J 305(Pt 1):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oehmcke S, Shannon O, Mörgelin M, Herwald H. 2010. Streptococcal M proteins and their role as virulence determinants. Clin Chim Acta 411:1172–1180. doi: 10.1016/j.cca.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Sriskandan S, Kemball-Cook G, Moyes D, Canvin J, Tuddenham E, Cohen J. 2000. Contact activation in shock caused by invasive group A Streptococcus pyogenes. Crit Care Med 28:3684–3691. doi: 10.1097/00003246-200011000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Shannon O, Herwald H, Oehmcke S. 2013. Modulation of the coagulation system during severe streptococcal disease. Curr Top Microbiol Immunol 368:189–205. doi: 10.1007/82_2012_283. [DOI] [PubMed] [Google Scholar]
- 13.Parry MA, Zhang XC, Bode I. 2000. Molecular mechanisms of plasminogen activation: bacterial cofactors provide clues. Trends Biochem Sci 25:53–59. doi: 10.1016/S0968-0004(99)01521-2. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerlein B, Park H-S, Li S, Podbielski A, Cleary PP. 2005. The M protein is dispensable for maturation of streptococcal cysteine protease SpeB. Infect Immun 73:859–864. doi: 10.1128/IAI.73.2.859-864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köller T, Manetti AGO, Kreikemeyer B, Lembke C, Margarit I, Grandi G, Podbielski A. 2010. Typing of the pilus-protein-encoding FCT region and biofilm formation as novel parameters in epidemiological investigations of Streptococcus pyogenes isolates from various infection sites. J Med Microbiol 59:442–452. doi: 10.1099/jmm.0.013581-0. [DOI] [PubMed] [Google Scholar]
- 16.Siemens N, Patenge N, Otto J, Fiedler T, Kreikemeyer B. 2011. Streptococcus pyogenes M49 plasminogen/plasmin binding facilitates keratinocyte invasion via integrin-integrin-linked kinase (ILK) pathways and protects from macrophage killing. J Biol Chem 286:21612–21622. doi: 10.1074/jbc.M110.202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreikemeyer B, Nakata M, Köller T, Hildisch H, Kourakos V, Standar K, Kawabata S, Glocker MO, Podbielski A. 2007. The Streptococcus pyogenes serotype M49 Nra-Ralp3 transcriptional regulatory network and its control of virulence factor expression from the novel eno ralp3 epf sagA pathogenicity region. Infect Immun 75:5698–5710. doi: 10.1128/IAI.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreikemeyer B, Oehmcke S, Nakata M, Hoffrogge R, Podbielski A. 2004. Streptococcus pyogenes fibronectin-binding protein F2: expression profile, binding characteristics, and impact on eukaryotic cell interactions. J Biol Chem 279:15850–15859. doi: 10.1074/jbc.M313613200. [DOI] [PubMed] [Google Scholar]
- 19.Woischnik M, Buttaro BA, Podbielski A. 2000. Inactivation of the cysteine protease SpeB affects hyaluronic acid capsule expression in group A streptococci. Microb Pathog 28:221–226. doi: 10.1006/mpat.1999.0341. [DOI] [PubMed] [Google Scholar]
- 20.Fiedler T, Kreikemeyer B, Sugareva V, Redanz S, Arlt R, Standar K, Podbielski A. 2010. Impact of the Streptococcus pyogenes Mga regulator on human matrix protein binding and interaction with eukaryotic cells. Int J Med Microbiol 300:248–258. doi: 10.1016/j.ijmm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Neville DM. 1971. Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem 246:6328–6334. [PubMed] [Google Scholar]
- 22.Mattsson E, Herwald H, Cramer H, Persson K, Sjöbring U, Björck L. 2001. Staphylococcus aureus induces release of bradykinin in human plasma. Infect Immun 69:3877–3882. doi: 10.1128/IAI.69.6.3877-3882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oehmcke S, Mörgelin M, Malmström J, Linder A, Chew M, Thorlacius H, Herwald H. 2012. Stimulation of blood mononuclear cells with bacterial virulence factors leads to the release of pro-coagulant and proinflammatory microparticles. Cell Microbiol 14:107–119. doi: 10.1111/j.1462-5822.2011.01705.x. [DOI] [PubMed] [Google Scholar]
- 24.Oehmcke S, Shannon O, von Koeckritz-Blickwede M, Morgelin M, Linder A, Olin AI, Björck L, Herwald H. 2009. Treatment of invasive streptococcal infection with a peptide derived from human high-molecular-weight kininogen. Blood 114:444–451. doi: 10.1182/blood-2008-10-182527. [DOI] [PubMed] [Google Scholar]
- 25.Ben Nasr A, Herwald H, Sjöbring U, Renné T, Müller-Esterl W, Björck L. 1997. Absorption of kininogen from human plasma by Streptococcus pyogenes is followed by the release of bradykinin. Biochem J 326(Pt 3):657–660. [PMC free article] [PubMed] [Google Scholar]
- 26.Linke C, Siemens N, Oehmcke S, Radjainia M, Law RHP, Whisstock JC, Baker EN, Kreikemeyer B. 2012. The extracellular protein factor Epf from Streptococcus pyogenes is a cell surface adhesin that binds to cells through an N-terminal domain containing a carbohydrate-binding module. J Biol Chem 287:38178–38189. doi: 10.1074/jbc.M112.376434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen LR, Macleod CM. 1945. A proteolytic enzyme of serum: characterization, activation, and reaction with inhibitors. J Gen Physiol 28:559–583. doi: 10.1085/jgp.28.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan AP, Austen KF. 1971. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med 133:696–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogt W. 1964. Kinin formation by plasmin, an indirect process, mediated by activation of kallikrein. J Physiol (Lond) 170:153–166. doi: 10.1113/jphysiol.1964.sp007320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collen D, Lijnen HR, De Cock F, Durieux JP, Loffet A. 1980. Kinetic properties of tripeptide lysyl chloromethyl ketone and lysyl p-nitroanilide derivatives toward trypsin-like serine proteinases. Biochim Biophys Acta 615:158–166. doi: 10.1016/0005-2744(80)90019-4. [DOI] [PubMed] [Google Scholar]
- 31.Loof TG, Mörgelin M, Johansson L, Oehmcke S, Olin AI, Dickneite G, Norrby-Teglund A, Theopold U, Herwald H. 2011. Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood 118:2589–2598. doi: 10.1182/blood-2011-02-337568. [DOI] [PubMed] [Google Scholar]
- 32.Cederholm-Williams SA, De Cock F, Lijnen HR, Collen D. 1979. Kinetics of the reactions between streptokinase, plasmin, and α2-antiplasmin. Eur J Biochem 100:125–132. doi: 10.1111/j.1432-1033.1979.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 33.Linder A, Johansson L, Thulin P, Hertzen E, Mörgelin M, Christensson B, Björck L, Norrby-Teglund A, Åkesson P. 2010. Erysipelas caused by group A streptococcus activates the contact system and induces the release of heparin-binding protein. J Investig Dermatol 130:1365–1372. doi: 10.1038/jid.2009.437. [DOI] [PubMed] [Google Scholar]
- 34.Law RHP, Abu-Ssaydeh D, Whisstock JC. 2013. New insights into the structure and function of the plasminogen/plasmin system. Curr Opin Struct Biol. 23:836–841. doi: 10.1016/j.sbi.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. 2004. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- 36.Bengtson SH, Phagoo SB, Norrby-Teglund A, Påhlman L, Mörgelin M, Zuraw BL, Leeb-Lundberg LMF, Herwald H. 2006. Kinin receptor expression during Staphylococcus aureus infection. Blood 108:2055–2063. doi: 10.1182/blood-2006-04-016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisno A, Brito M, Collins C. 2003. Molecular basis of group A streptococcal virulence. Lancet Infect Dis 3:191–200. doi: 10.1016/S1473-3099(03)00576-0.. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmeister HM, Ruf M, Wendel HP, Heller W, Seipel L. 1998. Streptokinase-induced activation of the kallikrein-kinin system and of the contact phase in patients with acute myocardial infarction. J Cardiovasc Pharm 31:764–772. doi: 10.1097/00005344-199805000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Olsen RJ, Shelburne SA, Musser JM. 2009. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol 11:1–12. doi: 10.1111/j.1462-5822.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 40.Ewald GA, Eisenberg PR. 1995. Plasmin-mediated activation of contact system in response to pharmacological thrombolysis. Circulation 91:28–36. doi: 10.1161/01.CIR.91.1.28. [DOI] [PubMed] [Google Scholar]
- 41.Frick I-M, Shannon O, PÅkesson Mörgelin M, Collin M, Schmidtchen A, Björck L. 2011. Antibacterial activity of the contact and complement systems is blocked by SIC, a protein secreted by Streptococcus pyogenes. J Biol Chem 286:1331–1340. doi: 10.1074/jbc.M110.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]