Abstract
T cell immunoglobulin- and mucin-domain-containing molecule 3 (Tim-3) has been regarded as an important regulatory factor in both adaptive and innate immunity. Recently, Tim-3 was reported to be involved in Th2-biased immune responses in mice infected with Schistosoma japonicum, but the exact mechanism behind the involvement of Tim-3 remains unknown. The present study aims to understand the role of Tim-3 in the immune response against S. japonicum infection. Tim-3 expression was determined by flow cytometry, and increased Tim-3 expression was observed on CD4+ and CD8+ T cells, NK1.1+ cells, and CD11b+ cells from the livers of S. japonicum-infected mice. However, the increased level of Tim-3 was lower in the spleen than in the liver, and no increase in Tim-3 expression was observed on splenic CD8+ T cells or CD11b+ cells. The schistosome-induced upregulation of Tim-3 on natural killer (NK) cells was accompanied by reduced NK cell numbers in vitro and in vivo. Tim-3 antibody blockade led to upregulation of inducible nitric oxide synthase and interleukin-12 (IL-12) mRNA in CD11b+ cells cocultured with soluble egg antigen and downregulation of Arg1 and IL-10, which are markers of M2 macrophages. In summary, we observed schistosome-induced expression of Tim-3 on critical immune cell populations, which may be involved in the Th2-biased immune response and alternative activation of macrophages during infection.
INTRODUCTION
Schistosomiasis is a human helminth infection that is estimated to afflict 200 million people worldwide (1). The pathogenesis of schistosomiasis is caused by egg-induced granuloma formation and subsequent fibrosis in hepatic and intestinal tissues, which is believed to be caused by the acquisition and accumulation of immune cells in the affected organs. The immune response that occurs during this process is complicated and poorly understood. During the early stages of infection, a type 1 T-helper-cell (Th1) response is induced by parasite migration and adult worms. As the eggs are released from the mature adult worms, the response is then polarized into a Th2 response 4 to 6 weeks after the initial infection. This is then followed by the development of granulomas around the eggs trapped in the liver. Macrophages surround the deposited eggs and play a pivotal role in the formation of fibrosis (2), since these cells are involved in Th2 development during schistosomiasis (3) and undergo either M1 or M2 activation (4–6). M2 macrophages are essential for host protection via the production of interleukin-10 (IL-10) but simultaneously promote progressive pathology due to granuloma formation (7, 8). Although the pathogenesis of schistosomal infection has long been known, the molecules and cells involved in immune triggering against the parasite remain enigmatic.
T cell immunoglobulin- and mucin-domain-containing molecule 3 (Tim-3) is an important regulatory factor in both adaptive and innate immunity (9). Tim-3 is preferentially expressed on activated Th1 cells but not Th2 cells (10). After binding to its ligand galectin-9, Tim-3 induces the death of Th1 cells and negatively regulates the Th1 response (11–13). This process plays critical roles in autoimmunity, allergy, viral infection, and transplant tolerance (9). Increased Tim-3 also leads to CD8+ T cell exhaustion, thereby abrogating proliferation, cytotoxicity, and cytokine production during viral infection (14, 15) and acute graft-versus-host disease (16). Apart from T cells, Tim-3 is also expressed on a variety of other immune cells, such as natural killer (NK) cells, monocytes, macrophages, and mast cells (17–19). Tim-3 was reported to inhibit NK cell cytotoxicity and induce NK cell apoptosis in chronic hepatitis B infection (20) and atherosclerosis (21).
The role of Tim-3 on macrophages is complex. In studies of pathogen infections (22), pregnancy (19), and murine experimental autoimmune encephalomyelitis (17), Tim-3 was found to promote macrophage activation. However, Tim-3 was also found to inhibit macrophage activation in many other conditions. For instance, Tim-3 decreases CD11b+-mediated inflammation in the heart during acute coxsackievirus B3-induced myocarditis in mice (18). Tim-3 expression decreases rapidly upon Toll-like receptor (TLR) stimulation, and blocking Tim-3 signaling or silencing Tim-3 expression leads to macrophage activation in peripheral blood CD14+ monocytes (23). Thus, the role of Tim-3 in macrophages is not fixed and may be controlled by different factors in various contexts.
Although immune cells regulated by Tim-3 are critical to the immune response against parasitic worms (24), few studies have investigated the role of Tim-3 in schistosomal diseases. Upon infection with Toxoplasma gondii, mice exhibit increased frequencies of Tim-3+ cells in the spleen and mesenteric lymph nodes (25). A recent study of Schistosoma japonicum infection discovered that blockade of the galectin-9/Tim-3 pathway remarkably reversed Th2-biased responses in splenic lymphocytes (26); however, the mechanism underlying this phenomenon has not been determined. In the present study, we examined the expression of Tim-3 on critical immune cell populations and further explored the mechanism of Tim-3 in the regulation of immunity against S. japonicum infection.
MATERIALS AND METHODS
Ethics statement.
All procedures performed on animals in the present study were conducted following animal husbandry guidelines of the Chinese Academy of Medical Sciences. This research was reviewed and approved by the Experimental Animal Committee of the Chinese Academy of Medical Sciences.
Animals and anti-TIM-3 treatment.
Oncomelania hupensis parasites were purchased from the Hunan Institute of Parasitic Diseases, Yueyang, China. Freshly released cercariae were harvested immediately. Six-week-old male C57BL/6 mice (specific pathogen free; Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were percutaneously infected with S. japonicum cercariae (20 parasites per mouse).
Preparation of soluble adult worm antigen (SWA) and soluble egg antigen (SEA).
Adult worms were obtained at 6 weeks postinfection by manual separation under a light microscope. Eggs were obtained from the liver and purified as previously described (27, 28). Briefly, S. japonicum-infected mice were euthanized 6 weeks postinfection, and their livers were removed, cut into pieces, and homogenized with a pestle. The homogenates were passed through a series of stainless steel sieves with pore sizes from 425 to 450 μm. The top sieve was rinsed continuously with 1.2% NaCl, and the sieves were agitated to ensure that most of the eggs passed through to the lowest sieve. The fluid that remained in the lowest sieve was collected and poured into a glass petri dish. The dish was gently swirled, and the eggs concentrated in the center of the vortex were collected. The enriched eggs were then poured into another glass petri dish, and the procedure was repeated. After three cycles, the resulting mature egg population was collected. The whole process was carried out on ice and with fresh cold 1.2% NaCl to keep the eggs from hatching into miracidia.
The SWA and SEA were prepared according to previously reported methods (28, 29), with minor modifications. Briefly, S. japonicum paired adult worms and purified eggs were washed in phosphate-buffered saline (PBS) five times to reduce contamination by host components. The worms were suspended in 4°C PBS at concentration of 200 worms/ml, and the eggs were suspended at concentration of 100,000 eggs/ml. The worms or eggs were then homogenized on ice for 10 to 15 min with 10 μg of protease inhibitor cocktail (Calbiochem, Darmstadt, Germany)/ml. The crude mixture was precentrifuged at 200 × g at 4°C for 5 min, and the supernatant was ultracentrifuged at 100,000 × g at 4°C for 90 min. The supernatant was then sterilized by passing through a 0.2-μm-pore-size filter. The SWA and SEA preparations were repeatedly treated with high-capacity endotoxin removal resin (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. Endotoxin was detected at levels of <0.005 EU/ml using a ToxinSensor Chromogenic LAL endotoxin assay kit (GenScript USA, Inc., Piscataway, NJ). Protein concentrations were determined by using a BCA protein assay kit (Pierce Biotechnology) according to the manufacturer's instructions.
Cell preparation, isolation, and culture.
Hepatic mononuclear cells (HMCs) were isolated by metrizamide gradient centrifugation as reported previously (30), with minor modifications. Briefly, murine livers were minced, pressed through a 200-gauge stainless steel mesh, and resuspended in PBS containing 2% fetal bovine serum (Gibco, Grand Island, NY). Red blood cells (RBCs) were depleted with an RBC lysis solution (155 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA, 170 mM Tris [pH 7.0]). Cells were washed twice with PBS and resuspended in a 40% Percoll solution (Pharmacia, Uppsala, Sweden). Hepatic parenchymal cells were removed by gradient centrifugation, and the HMC-containing pellet at the bottom of the centrifuge tube was harvested. Murine spleens were cut into pieces, minced, and pressed through a 200-gauge stainless steel mesh. RBCs were depleted with RBC lysis solution as described above. The cells were washed twice with PBS and then harvested for the following experiments. CD11b+ cells were isolated from the HMCs using magnetic beads (Miltenyi, Bergisch-Gladbach, Germany) according to the manufacturer's protocol. NK cells were isolated from splenic cells using an NK cell isolation kit (Miltenyi) according to the manufacturer's protocol.
S. japonicum antigen stimulation assay and anti-Tim-3 antibody treatment.
Spleen cells or CD11b+ cells were cultured in triplicate at 107 cells/ml in 96-well plates in RPMI 1640 medium (HyClone/Thermo, Beijing, China) supplemented with 10% fetal bovine serum (FBS). SWA or SEA was added to the culture medium with PBS as control. In addition, either 1 μg of anti-mouse TIM-3 purified antibody (catalog no. 14-5870; eBioscience, San Diego, CA) or purified rat IgG2α K isotype control (catalog no. 14-4321-85; eBioscience)/ml was simultaneously added. The cells were incubated for indicated time points and then collected for the following experiments.
Flow cytometry.
Cell concentrations were adjusted to 107 cells/ml and preincubated with rat IgG to reduce nonspecific binding. Cells were then incubated with the following specific antibodies or isotype-matched controls at the manufacturers' recommended concentrations at 4°C for 30 min: anti-mouse CD3e–phycoerythrin (PE)-Cy5, anti-mouse CD4–fluorescein isothiocyanate (FITC), anti-mouse CD8a–FITC, anti-mouse NK1.1–FITC, anti-mouse CD11b–FITC, anti-mouse TIM-3–PE, rat IgG2a K isotype control–PE-Cy5, rat IgG2a K isotype control–FITC, and rat IgG2a K isotype control–PE (all from eBioscience). The apoptosis was examined with an annexinV-FITC kit (NeoBioscience, Beijing, China). Cells were detected and analyzed using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA).
RNA isolation and quality control.
Total RNA was extracted from samples using an RNeasy minikit (Qiagen GmbH, Hilden, Germany), and genomic DNA was completely removed from the RNA samples using the Turbo DNA-free kit (Ambion, Austin, TX). RNA quantification and quality control were conducted using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) and denaturing agarose gel electrophoresis.
Real-time quantitative reverse-transcription PCR.
One microgram of total RNA was reverse transcribed into cDNA using the SuperScript III reverse transcriptase kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The primers for Arg1 (forward, TGAAAGGAAAGTTCCCAGATG; reverse, GTTCCCCAGGGTCTACGTCT), IL-10 (forward, GCTCTTACTGACTGGCATGAG; reverse, CGCAGCTCTAGGAGCATGTG), inducible nitric oxide synthase (iNOS; forward, TCCAGAAGCAGAATGTGACC; reverse, GGACCAGCCAAATCCAGTC), IL-12 (forward, CAATCACGCTACCTCCTCTTTT; reverse, CAGCAGTGCAGGAATAATGTTTC), and β-actin (forward, TGCGTGACATCAAAGAGAAG, reverse; TCCATACCCAAGAAGGAAGG) genes were used for real-time PCR, which was performed using a Brilliant II SYBR green QPCR master mix kit (Agilent, Santa Clara, CA). Each reaction was performed in a final volume of 25 μl containing 12.5 μl of 2× Brilliant II SYBR green QPCR master mix, 80 ng of cDNA, and 1 μl of 10 μM paired forward and reverse primers. The PCR program was performed for 40 cycles with denaturation at 95°C for 30 s, followed by annealing and extension at 60°C for 1 min. A dissociation step (95°C for 15 s, 60°C for 1 min, 95°C for 15 s, and 60°C for 15 s) was added to confirm the amplification specificity for each gene.
Histopathology and hematoxylin-and-eosin staining.
Mice were euthanized at 6 weeks postinfection. The livers were removed and embedded in OCT compound, and serial cryosections of 5 μm were obtained. The slides were fixed with 4% paraformaldehyde and stained with hematoxylin and eosin. After dehydration and mounting, the histological samples were observed using a Nikon microscope (Eclipse 80i; Nikon Instech Co. Ltd., Tokyo, Japan), and the granuloma areas were measured using an NIS-Elements BR 4.0.
Statistical analysis.
The data were analyzed using GraphPad Prism 5.0 and Microsoft Excel 2007. The Kruskal-Wallis nonparametric H test and Mann-Whitney nonparametric U test were used for comparison between groups. P values of <0.05 were considered significant.
RESULTS
Increased Tim-3 expression on both CD4+ and CD8+ T cells from S. japonicum-infected mice.
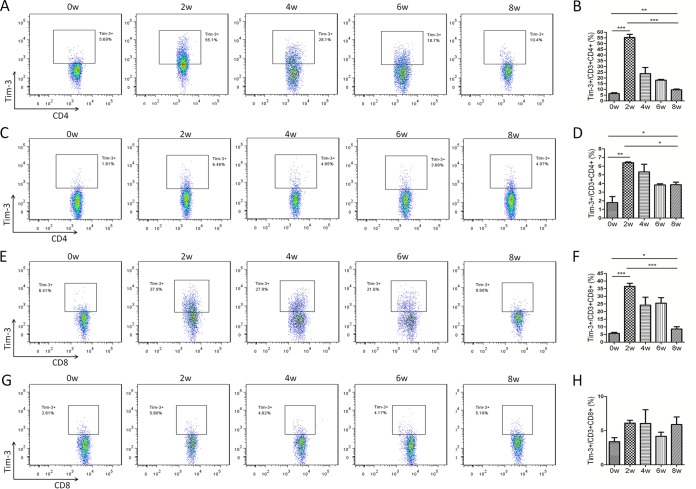
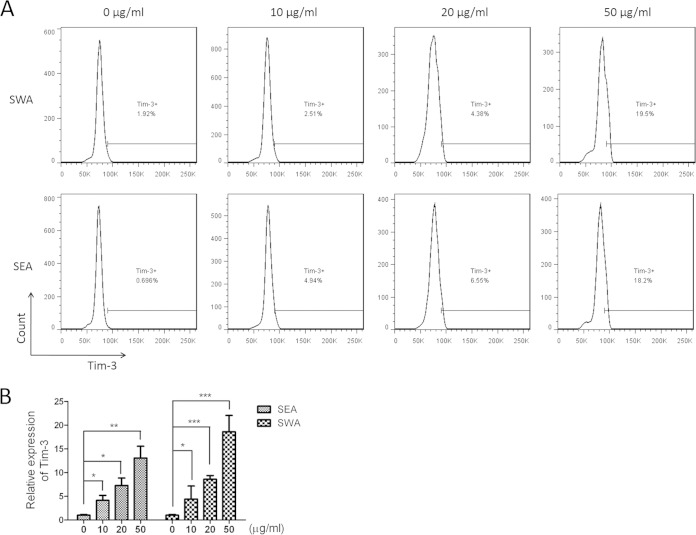
To gain a better understanding of the potential involvement of Tim-3 in schistosomiasis, we examined the expression levels of Tim-3 on immune cells from HMC and splenocyte preparations. Flow cytometric analysis revealed a substantial increase in Tim-3 expression on CD4+ T cells from both HMCs and splenic immune cells at 2 weeks postinfection, with a gradual decline in expression during weeks 2 to 8 (Fig. 1A to D). However, Tim-3 expression on CD4+ T cells at 8 weeks postinfection was still higher than that on cells from uninfected mice (Fig. 1A to D). Tim-3 expression levels on CD4+ T cells from HMCs were much higher than the levels on splenic immune cells (HMCs versus splenic immune cells: week zero, 6.40 ± 0.77 versus 1.80 ± 0.69, P = 0.0015; 2 weeks, 55.4 ± 2.72 versus 6.40 ± 0.92, P = 0.0002; 4 weeks, 23.8 ± 5.43 versus 5.34 ± 0.88, P = 0.0044; 6 weeks, 18.07 ± 0.71 versus 3.83 ± 0.14, P < 0.0001; 8 weeks, 9.67 ± 0.65 versus 5.27 ± 2.46, P = 0.6804; Fig. 1A to D), indicating that the Tim-3 response was asynchronous in both the liver and the spleen during schistosomiasis. For CD8+ T cells, Tim-3 expression showed a response pattern similar to that observed in CD4+ T cells in HMCs (Fig. 1E and F), but no obvious changes were observed in splenic immune cells (Fig. 1G and H). An in vitro assay was carried out with different concentrations of SWA or SEA. Flow cytometry results showed that Tim-3 expression on CD3+ cells was upregulated with the increase in schistosome antigen concentration and that SWA and SEA had equal effects (Fig. 2A). Real-time PCR with splenic immune cells yielded similar results (Fig. 2B).
FIG 1.
Increased Tim-3 expression on CD4+ and CD8+ T cells in S. japonicum-infected mice. HMCs (A, B, E, and F) and splenic immune cells (C, D, G, and H) from S. japonicum-infected mice were collected at 0, 2, 4, 6, and 8 weeks postinfection, and Tim-3 expression on T cells was detected by flow cytometry. (A, C, E, and G) Representative dot plots of Tim-3 expression on CD3+ CD4+ T cells (A and C) and CD3+ CD8+ T cells (E and G). The plots are representative of three independent experiments with five to seven mice in each group per experiment. (B, D, F, and H) Comparisons of the proportions of Tim-3+ cells in the CD3+ CD4+ T cell population (B and D) and in the CD3+ CD8+ T cell population (F and H) among groups of mice at 0, 2, 4, 6, and 8 weeks postinfection (means ± standard deviations [SD]; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
FIG 2.
Increased Tim-3 expression was associated with increased concentrations of schistosome antigens. Splenic immune cells from 6-week-old male C57BL/6 mice were collected and stimulated with 0, 10, 20, and 50 μg/ml SWA or SEA, respectively. After 24 h of incubation, cells were collected. (A) Representative histograms of Tim-3 expression on CD3+ cells detected by flow cytometry. The plots are representative of three independent experiments with five to seven mice in each group per experiment. (B) Comparisons of the relative expression of Tim-3 in the CD3+ cells under indicated antigen concentrations (means ± SD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Increased Tim-3 expression on NK cells results in NK cell loss during schistosomiasis.
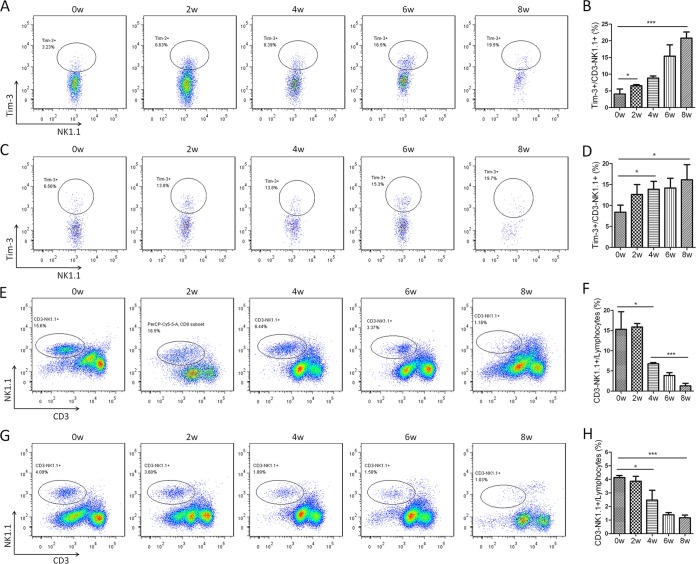
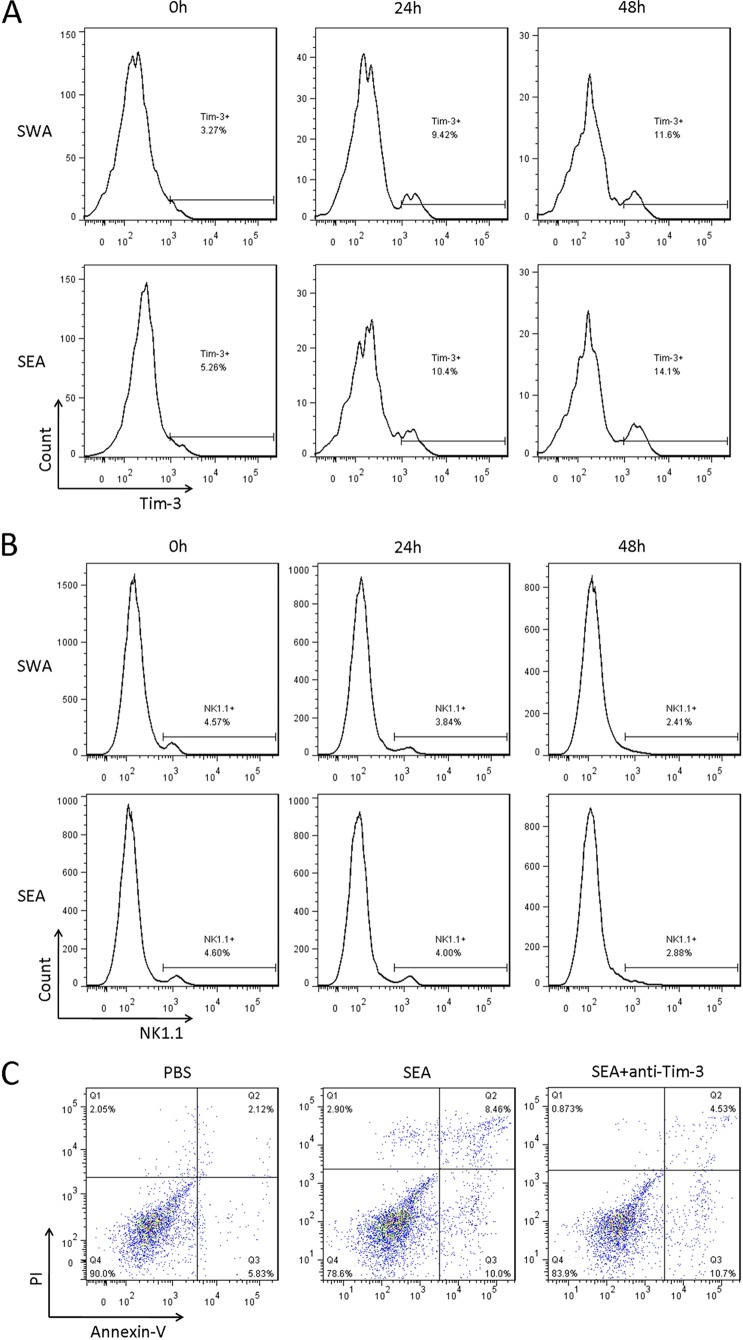
NK cells are the main source of the Th1 cytokine gamma interferon (IFN-γ) in schistosomiasis (31), and Tim-3 has been shown to inhibit IFN-γ production and induce NK cell apoptosis. Thus, the inhibitory effect of Tim-3 on NK cells during schistosomiasis was investigated in the present study. Flow cytometric analysis demonstrated that Tim-3 expression on CD3− NK1.1+ cells from both HMCs and splenic immune cells gradually increased from week zero to week 8 postinfection (Fig. 3A to D); however, there were no significant differences in Tim-3 expression between HMCs and splenic CD3− NK1.1+ cells (Fig. 3A to D and data not shown). As predicted, CD3− NK1.1+ cells gradually decreased from both HMC and spleen lymphocyte populations (Fig. 3E to H), and the proportion of NK1.1+ cells in HMC lymphocytes was much higher than in splenic lymphocytes (week zero, 15.3 ± 4.36 versus 4.12 ± 0.16, P = 0.0114; 2 weeks, 15.9 ± 0.85 versus 3.86 ± 0.37, P = 0.0029; 4 weeks, 6.78 ± 0.31 versus 2.48 ± 0.72, P = 0.0007; 6 weeks, 3.87 ± 0.70 versus 1.39 ± 0.16, P = 0.0395; 8 weeks, 1.27 ± 0.63 versus 1.17 ± 0.20, P = 0.8365; Fig. 3E to H). To investigate whether the increased expression of Tim-3 on NK cells was induced directly by S. japonicum infection, splenic immune cells were obtained from 6-week-old male C57BL/6 mice and treated with 20 μg of SWA or SEA/ml. Flow cytometric analysis showed that Tim-3 expression on NK1.1+ cells was increased in both SWA- and SEA-exposed (Fig. 4A) groups. Furthermore, in parallel with increased Tim-3 expression, the frequency of NK1.1+ cells in splenic lymphocytes decreased significantly after 48 h of treatment with 20 μg/ml SWA or SEA (Fig. 4B). Flow cytometry analysis showed that SEA treatment promoted NK1.1+ cell apoptosis or death and that anti-Tim-3 treatment reduced the proportion of the SEA-induced apoptotic or dead cells (Fig. 4C). These results indicate that Tim-3 upregulation by S. japonicum antigens may be responsible for NK cell loss during schistosomiasis.
FIG 3.
Increased Tim-3 expression and decreased proportion of NK1.1+ cells in S. japonicum-infected mice. HMCs (A, B, E, and F) and splenic immune cells (C, D, G, and H) from S. japonicum-infected mice were collected at 0, 2, 4, 6, and 8 weeks postinfection. (A to D) Tim-3 expression on CD3− NK1.1+ cells was detected by flow cytometry. (A and C) Representative dot plots of Tim-3 expression on CD3− NK1.1+ cells. (B and D) Comparisons of the proportions of Tim-3+ cells within the CD3− NK1.1+ cell population among groups of mice at 0, 2, 4, 6, and 8 weeks postinfection. (E to H) CD3− NK1.1+ cell proportions as detected by flow cytometry. (E and G) are representative plots of CD3− NK1.1+ cell proportions in lymphocytes. The dot plots are representative of three independent experiments with five to seven mice in each group per experiment. (F and H) Comparisons of the proportions of CD3− NK1.1+ cells in lymphocytes (means ± SD; *, P < 0.05, **, P < 0.01; ***, P < 0.001).
FIG 4.
Increased expression of Tim-3 induced by SWA and SEA led to NK cell loss. Splenic immune cells from wild-type C57BL/6 mice were treated with SWA and SEA. (A and B) Cells were collected at 0, 24, and 48 h and analyzed by flow cytometry. (A) Representative histograms of Tim-3 expression on CD3− NK1.1+ cells. (B) Representative histograms of CD3− NK1.1+ cell proportions in lymphocytes. (C) Cells were collected after 48 h of incubation, and NK cells were isolated using magnetic beads. The apoptosis of NK cells was detected by flow cytometry. The results are representative of two independent experiments.
Increased expression of Tim-3 is involved in macrophage polarization during schistosomiasis.
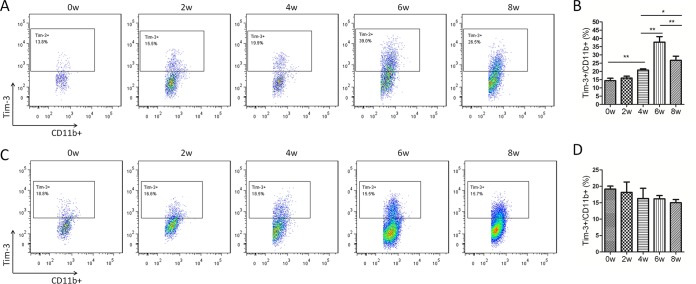
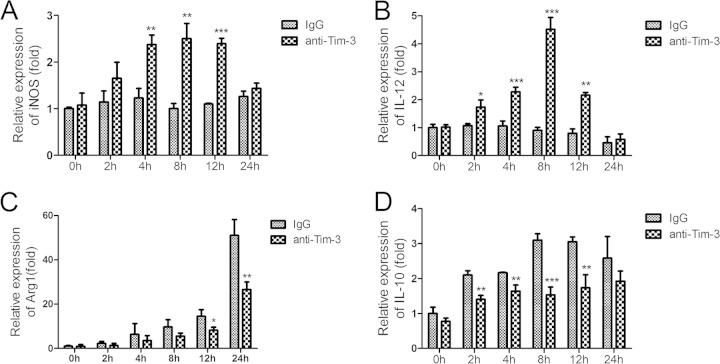
As a cell population critical for granuloma formation, macrophages play an important role in schistosomiasis pathogenesis (2). Flow cytometric analysis showed that Tim-3 expression on CD11b+ cells from HMCs of S. japonicum-infected mice gradually increased until 6 weeks postinfection, after which it started to decrease (Fig. 5A and B). However, Tim-3 expression on CD11b+ splenic immune cells of S. japonicum-infected mice did not vary over time (Fig. 5C and D). Because SEA is the dominant antigen released by the parasites in the liver, we used SEA to investigate the relationship between schistosome antigens and increased Tim-3 expression, as well as alternative macrophage activation. CD11b+ cells were isolated from HMCs of S. japonicum-infected mice at 6 weeks postinfection when the hepatic CD11b+ cells showed the highest levels of Tim-3 expression. An anti-Tim-3 antibody was used to block the Tim-3 pathway during SEA stimulation. Real-time reverse transcription-PCR (RT-PCR) showed that anti-Tim-3 treatment led to increased mRNA expression of iNOS (Fig. 6A) and IL-12 (Fig. 6B), which is characteristic of classically activated macrophages. As expected, mRNA expression of Arg1 (Fig. 6C) and IL-10 (Fig. 6D), the key markers of alternatively activated macrophages, was suppressed. Thus, Tim-3 blockade by anti-Tim-3 antibody polarized macrophages toward the M1 phenotype during schistosomiasis.
FIG 5.
Increased Tim-3 expression on CD11b+ cells in S. japonicum-infected mice. HMCs (A and B) and splenic immune cells (C and D) from S. japonicum-infected mice were collected at 0, 2, 4, 6, and 8 weeks postinfection, and Tim-3 expression on CD11b+ cells was detected by flow cytometry. (A and C) Representative dot plots of Tim-3 expression on CD11b+ cells. The dot plots are representative of three independent experiments with five to seven mice in each group per experiment. (B and D) Comparison of the proportions of Tim-3+ cells within the CD11b+ cell population among groups of mice at 0, 2, 4, 6, and 8 weeks postinfection (means ± SD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
FIG 6.
Tim-3 blockade polarized macrophages from the M2 to M1 phenotype. CD11b+ cells were isolated from HMCs of S. japonicum-infected mice euthanized at 6 weeks postinfection. Anti-Tim-3 antibody was used to block the Tim-3 pathway following SEA treatment, and rat IgG was used as a control. Cells were collected at 0, 2, 4, 6, and 8 h. Macrophage activation-related genes were quantified using real-time RT-PCR. (A) iNOS; (B) IL-12; (C) Arg1; (D) IL-10. Gene expression was normalized against β-tubulin and is presented as the fold change versus the expression of IgG group at week zero. The results are representative of two independent experiments (means ± SD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
DISCUSSION
Schistosome infection induces dramatic fluctuations in Th1 and Th2 responses, and the Th2 response becomes dominant after egg deposition begins, invoking severe fibrotic granulomatous responses in the host liver (2). However, the detailed mechanism underlying this Th1/Th2 transformation remains unclear. As an important immune regulator in both adaptive and innate immunity, Tim-3 has been reported to play a role during S. japonicum infection (26). In the present study, we investigated the potential role of Tim-3 in cellular immune regulation during the course of S. japonicum infection and discovered that increased expression of Tim-3 on critical immune cells is associated with Th2-biased responses and alternative macrophage activation.
Tim-3 has been shown to negatively regulate Th1 responses and induce Th1 cell apoptosis by binding to galectin-9 (13). This process plays a critical role in various diseases (9), and Tim-3 has also been implicated in parasitic disease (26). In the present study, we observed substantially increased expression of Tim-3 on CD4+ and CD8+ T cells, NK cells, and macrophages in the livers of S. japonicum-infected mice during the 8-week infection period (Fig. 1, 3, and 5). Tim-3 expression was also increased on CD4+ T cells and NK cells in the spleens of S. japonicum-infected mice, but this increase was lower than that observed in the livers. Furthermore, Tim-3 expression remained unchanged on CD8+ T cells and macrophages in the spleens of infected mice (Fig. 1 and 2). This observation may explain why Qi et al. (26) did not detect increased expression of Tim-3 in spleens of S. japonicum-infected mice by RT-PCR, since slightly increased Tim-3 expression in schistosome-infected spleen cells could be masked by large numbers of B cells that do not express Tim-3. In general, Tim-3 expression was much higher in hepatic immune cells than in splenic cells during schistosomiasis. Furthermore, an in vitro assay showed that increased Tim-3 expression on CD3+ cells occurred along with the increased concentration of schistosome antigen. It is postulated that, with the accumulation of schistosome eggs in the liver, the concentration of SEA would be increased in the organ. However, the concentration of SWA in the blood was much lower than that in the liver, which might explain the higher expression level of Tim-3 on the hepatic immune cells compared to splenic immune cells.
Increased Tim-3 expression promotes Th2-biased responses in schistosomiasis. First, increased Tim-3 expression regulates CD4+ T cell polarization. Tim-3 is specifically expressed on Th1 cells and induces Th1 cell death, thus negatively regulating Th1 responses in various diseases (11–13). Our study shows that Tim-3 expression dramatically increases on liver and splenic CD4+ T cells of S. japonicum-infected mice at 2 weeks postinfection, with a gradual decline during the following 6 weeks (Fig. 1A to D). It is well known that the Th1 response transforms to a Th2 response at 4 to 6 weeks after initial schistosomiasis infection (2); thus, Tim-3 expression on CD4+ T cells correlated well with the Th1 response, and its decline accompanied the progression of the Th2 response. Therefore, variations in Tim-3 expression mirrored the fluctuation of the Th1/Th2 response during schistosomiasis. Furthermore, the percentage of IFN-γ+ CD4+ Th1 cells was significantly increased in spleen lymphocytes from S. japonicum-infected mice cocultured with Tim-3-Fc fusion proteins throughout the 12-week infection period, but a decrease in IL-4+ CD4+ Th2 cells was observed (26). Thus, the increased expression of Tim-3 on CD4+ T cells could explain the Th2-biased response during schistosomiasis.
Second, it has been well-known that Tim-3 expression reduces or inhibits cells that produce IFN-γ, a powerful Th1 cytokine and mediator of defense against helminth infection. The results here showed that increased Tim-3 expression on hepatic and splenic NK cells was accompanied by decreased NK cell numbers in S. japonicum-infected mice (Fig. 3). In vitro experiments also showed that Tim-3 expression on splenic NK cells was upregulated by either SWA or SEA, and stimulation with either of the antigens led to NK cell apoptosis and reduced NK cell populations (Fig. 4). It has also been reported that Tim-3 upregulation resulted in the induction of NK cell apoptosis in atherosclerosis (21). Thus, the loss of NK cells observed in schistosomiasis was likely due to the increased cellular expression of Tim-3. NK cells have been shown to be the main source of IFN-γ in schistosomiasis (31). Thus, the Tim-3-induced loss of NK cells could lead to reduced IFN-γ production and played a role in promoting the Th1/Th2 transformation. Apart from its effects on NK cells, increased expression of Tim-3 has also been shown to induce CD8+ T cell exhaustion and decrease T cell cytokine production (14, 15). CD8+ T cells are also an important source of IFN-γ. Our study found increased Tim-3 expression on CD8+ T cells from HMCs of S. japonicum-infected mice (Fig. 1E and F). Thus, Tim-3 upregulation could reduce IFN-γ production in both NK cells and CD8+ T cells, and this might eventually promote Th2-biased responses during schistosomiasis.
Third, it has been known that Tim-3 engages in alternative macrophage activation, which promotes Th2-biased responses (8, 32). The polarized Th1 response is associated with increased expression of inducible nitric oxide synthase (iNOS) by M1-type macrophages, whereas the Th2 response is associated with M2 macrophage expression of arginase-1 (Arg1) (33). The upregulation of iNOS and downregulation of Arg1 by Tim-3 blockade in SEA-stimulated macrophages was shown in the present study (Fig. 5A and C). We also found upregulation of the M1-related cytokine IL-12 and downregulation of the M2-related cytokine IL-10 (Fig. 6B and D). Similar to our results, a study of TLR-stimulated human CD14+ monocytes showed that blocking Tim-3 signaling or silencing Tim-3 expression led to a significant increase in TLR-mediated IL-12 production and decreases in IL-10 production (23). Thus, increased Tim-3 expression on macrophages appears to promote alternative macrophage activation during S. japonicum infection and subsequently mediates Th2-biased responses through its regulation of macrophages.
Th2 responses lead to granuloma formation, which results in portal hypertension and hepatic fibrosis (2). Apart from the upregulation of M1 markers and downregulation of M2-related markers, anti-Tim-3 antibody treatment inhibited the production of Arg1 in SEA-stimulated macrophages (Fig. 6). The impact of M2 macrophages in granuloma formation is largely dependent on the metabolism of l-arginine into proline, a component of collagen, via Arg1 (34, 35). NK cells also play important roles in granuloma formation. NK cell depletion led to increased hepatic collagen content during late-stage granuloma formation in Schistosoma mansoni-infected mice and decreased levels of IL-12 mRNA expression in the liver (36). Another study found an increase of ca. 20% in the mean granuloma diameter in anti-NK1.1 antibody-treated mice infected with S. mansoni (37). Our study confirmed that increased Tim-3 expression on NK cells was responsible for the loss of NK cells in schistosomiasis (Fig. 3 and 4). Although in vivo experiments indicated anti-Tim-3 treatment was not strong enough to prevent schistosomal pathology, the areas of individual granulomas in anti-Tim-3 antibody-treated mice indeed showed a 32% reduction compared to the control group (see Fig. S1 in the supplemental material).
In summary, our study found increased Tim-3 expression on critical immune cell populations in S. japonicum-infected mice. Increased Tim-3 expression induced by schistosome infection promoted a Th2-biased response and alternative macrophage activation through the regulation of CD4+ and CD8+ T cells, NK cells, and macrophages.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grants 81270026 and 81301457), the National S&T Major Program (grant 2012ZX10004-220), and the Program for Changjiang Scholars and Innovative Research Team in University (grant IRT13007).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00517-15.
REFERENCES
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. 2009. Immunopathogenesis of human schistosomiasis. Parasite Immunol 31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 3.Kreider T, Anthony RM, Urban JF Jr, Gause WC. 2007. Alternatively activated macrophages in helminth infections. Curr Opin Immunol 19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. 2011. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. 2011. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem 286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. 2008. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab 7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, Fallon PG. 2004. Schistosoma mansoni worms induce anergy of T cells via selective upregulation of programmed death ligand 1 on macrophages. J Immunol 173:1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 8.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20:623–635. doi: 10.1016/S1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 9.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. 2010. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 11.Sakai K, Kawata E, Ashihara E, Nakagawa Y, Yamauchi A, Yao H, Nagao R, Tanaka R, Yokota A, Takeuchi M, Hirai H, Kimura S, Hirashima M, Yoshimura N, Maekawa T. 2011. Galectin-9 ameliorates acute GVH disease through the induction of T-cell apoptosis. Eur J Immunol 41:67–75. doi: 10.1002/eji.200939931. [DOI] [PubMed] [Google Scholar]
- 12.Bi S, Hong PW, Lee B, Baum LG. 2011. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc Natl Acad Sci U S A 108:10650–10655. doi: 10.1073/pnas.1017954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 14.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. 2011. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol 32:345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oikawa T, Kamimura Y, Akiba H, Yagita H, Okumura K, Takahashi H, Zeniya M, Tajiri H, Azuma M. 2006. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol 177:4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 17.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. 2007. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 18.Frisancho-Kiss S, Nyland JF, Davis SE, Barrett MA, Gatewood SJ, Njoku DB, Cihakova D, Silbergeld EK, Rose NR, Fairweather D. 2006. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol 176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Lei Z, Liu Y, Li B, Zhang L, Fang H, Song C, Wang X, Zhang GM, Feng ZH, Huang B. 2009. Human pregnancy upregulates Tim-3 in innate immune cells for systemic immunity. J Immunol 182:6618–6624. doi: 10.4049/jimmunol.0803876. [DOI] [PubMed] [Google Scholar]
- 20.Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W, Liang X, Gao L, Ma C. 2010. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol 52:322–329. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Hou N, Zhao D, Liu Y, Gao L, Liang X, Liu X, Gai X, Zhang X, Zhu F, Ni M, Zhang Y, Sun W, Ma C. 2012. Increased expression of T cell immunoglobulin- and mucin domain-containing molecule-3 on natural killer cells in atherogenesis. Atherosclerosis 222:67–73. doi: 10.1016/j.atherosclerosis.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. 2010. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med 207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ. 2012. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukoc Biol 91:189–196. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maizels RM, Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 25.Berrocal Almanza LC, Munoz M, Kuhl AA, Kamradt T, Heimesaat MM, Liesenfeld O. 2013. Tim-3 is differently expressed in genetically susceptible C57BL/6 and resistant BALB/c mice during oral infection with Toxoplasma gondii. Eur J Microbiol Immunol 3:211–221. doi: 10.1556/EuJMI.3.2013.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Y, Song XR, Shen JL, Xu YH, Shen Q, Luo QL, Zhong ZR, Wang W, Chu DY, Song WJ. 2012. Tim-2 upregulation and galectin-9-Tim-3 pathway activation in Th2-biased response in Schistosoma japonicum infection in mice. Immunol Lett 144:60–66. doi: 10.1016/j.imlet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Cai P, Bu L, Wang J, Wang Z, Zhong X, Wang H. 2008. Molecular characterization of Schistosoma japonicum tegument protein tetraspanin-2: sequence variation and possible implications for immune evasion. Biochem Biophys Res Commun 372:197–202. doi: 10.1016/j.bbrc.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 28.Tucker MS, Karunaratne LB, Lewis FA, Freitas TC, Liang YS. 2013. Schistosomiasis. Curr Protoc Immunol Chapter 103:Unit 19.1. doi: 10.1002/0471142735.im1901s103. [DOI] [PubMed] [Google Scholar]
- 29.Boros DL, Warren KS. 1970. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med 132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Z, Zhang J, Sun R, Wei H, Tian Z. 2007. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology 45:1400–1412. doi: 10.1002/hep.21597. [DOI] [PubMed] [Google Scholar]
- 31.Oswald IP, Caspar P, Jankovic D, Wynn TA, Pearce EJ, Sher A. 1994. IL-12 inhibits Th2 cytokine responses induced by eggs of Schistosoma mansoni. J Immunol 153:1707–1713. [PubMed] [Google Scholar]
- 32.Reyes JL, Terrazas LI. 2007. The divergent roles of alternatively activated macrophages in helminthic infections. Parasite Immunol 29:609–619. doi: 10.1111/j.1365-3024.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 33.Anthony BJ, Ramm GA, McManus DP. 2012. Role of resident liver cells in the pathogenesis of schistosomiasis. Trends Parasitol 28:572–579. doi: 10.1016/j.pt.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Wynn TA. 2004. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. 2001. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol 167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 36.Asseman C, Pancre V, Quatennens B, Auriault C. 1996. Schistosoma mansoni-infected mice show augmented hepatic fibrosis and selective inhibition of liver cytokine production after treatment with anti-NK1.1 antibodies. Immunol Lett 54:11–20. doi: 10.1016/S0165-2478(96)02634-X. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto A, Pincelli C, Fujioka A, Fukuyama K, Epstein WL. 1990. Relationship between NK cells and granulomatous inflammation in mice. J Clin Lab Immunol 33:41–47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.