Abstract
Attaching and effacing pathogens, including enterohemorrhagic Escherichia coli in humans and Citrobacter rodentium in mice, raise serious public health concerns. Here we demonstrate that interleukin-1 receptor (IL-1R) signaling is indispensable for protection against C. rodentium infection in mice. Four days after infection with C. rodentium, there were significantly fewer neutrophils (CD11b+ Ly6C+ Ly6G+) in the colons of IL-1R−/− mice than in wild-type mice. Levels of mRNA and protein of KC/CXCL1 were also significantly reduced in colon homogenates of infected IL-1R−/− mice relative to wild-type mice. Of note, infiltrated CD11b+ Ly6C+ Ly6G+ neutrophils were the main source of IL-22 secretion after C. rodentium infection. Interestingly, intestinal stromal cells isolated from IL-1R−/− mice secreted lower levels of KC/CXCL1 than stromal cells from wild-type mice during C. rodentium infection. Similar effects were found when mouse intestinal stromal cells and human nasal polyp stromal cells were treated with IL-1R antagonists (i.e., anakinra) in vitro. These results suggest that IL-1 signaling plays a pivotal role in activating mucosal stromal cells to secrete KC/CXCL1, which is essential for infiltration of IL-22-secreting neutrophils upon bacterial infection.
INTRODUCTION
Citrobacter rodentium is an enteric extracellular pathogen that serves as a mouse model of human infections with enterohemorrhagic and enteropathogenic Escherichia coli (1). C. rodentium colonizes the cecum and colon of mice after oral infection and targets epithelial cells by creating characteristic attaching and effacing lesions. Infection leads to weight loss, diarrhea, goblet cell loss, and inflammation by infiltration of macrophages, neutrophils, and mast cells primarily in the cecum and colon (2–4). The C. rodentium infection model is widely used for evaluating host immune responses against enteric bacterial pathogens in gut mucosal tissues (5–7).
Innate immune cells recognize pathogens via toll-like receptors (TLRs) and downstream signaling, most by way of MyD88-dependent signals (8, 9). TLRs have various isoforms and recognize specific ligands, bacterium-specific structures, and conserved structure motifs that include proteins, nucleic acids, and lipids. C. rodentium organisms produce abundant lipopolysaccharides, a known ligand for TLR4, and previous studies have shown that MyD88 and TLR4 signals are essential for protective immune responses (5, 10, 11).
Because the cytosolic recognition sectors of TLRs are similar to those of IL-1R, they are called the Toll/interleukin-1 (IL-1) receptor (IL-1R) domain (12). IL-1 is a key modulator for induction of innate immunity and inflammation, affects all types of cells, and is a major pathogenic mediator of autoimmune, inflammatory, and infectious diseases (13, 14). We and others have found clear evidence that IL-1 significantly contributes to host defense during respiratory and enteric bacterial infection (15, 16). In 2009, Lebeis et al. showed that IL-1R signaling plays an important role in inducing protective immunity in the gut against C. rodentium infection (17). Indeed, IL-1R−/− mice exhibited high mortality and severe colitis with severe epithelial cell damage compared to wild-type (WT) mice with intact IL-1R. They concluded that susceptibility to C. rodentium infection in the absence of IL-1R signaling is not caused by delayed responses to recruitment of innate immune cells, such as neutrophils (17), unlike the phenotype of MyD88−/− mice (11).
Intestinal stromal cells make diverse contributions to innate immunity in the gut and to the maintenance of gut homeostasis (18, 19). It is well known that intestinal stromal cells are critical for the expression of cytokines and chemokines and thus dynamically interact with innate immune cells. Previous studies revealed that human intestinal stromal cells strongly respond to IL-1 and IL-1R, with a variety of functional outcomes (20, 21). Recent murine data support a functional role for innate immune receptors on intestinal stromal cells, as NOD2-dependent CCL2 production by intestinal stromal cells plays a critical role in regulating inflammatory monocyte recruitment, which is essential for bacterial clearance during C. rodentium infection in the murine model (22). Despite recent advances in our understanding of the role intestinal stromal cells play in the regulation of pathogenesis of enteric bacteria, the underlying mechanisms are not understood.
In this study, we attempted to clarify the role of IL-1R in mouse intestinal stromal cells and development of protective immune responses against C. rodentium. We confirmed that IL-1R−/− mice are more susceptible than WT mice to C. rodentium infection, as reported previously (17). The most serious defect was in early defense mechanisms, with significantly reduced KC/CXCL1 in the large intestine 4 days after infection in the absence of IL-1R signaling. We found few IL-22-secreting neutrophils in the absence of IL-1R signaling. Of note, intestinal stromal cells were a primary regulator of the secretion of these chemokines. When our findings are considered together, they show that IL-1R in intestinal stromal cells is critical for recruitment of innate cells that play an essential role in clearance of C. rodentium.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Asan Biomedical Research Center (Seoul, Republic of Korea). All experiments were performed under anesthesia with a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg), and all effort was made to minimize suffering. The study that used human nasal polyps from patients with chronic rhinosinusitis was approved by the Institutional Review Board of the Asan Medical Center (Seoul, Republic of Korea) and signed written consent was obtained before sample collection (approval number 2011-0384).
Mice and bacterial strains.
C57BL/6 (B6) and IL-1R−/− mice were purchased from Charles River Laboratories (Orient Bio Inc., Seongnam, Republic of Korea) and Jackson Laboratory (Bar Harbor, ME), respectively. All mice were bred and maintained under specific-pathogen-free conditions in the experimental facility at the Asan Biomedical Research Center, where they received sterilized food and water ad libitum. C. rodentium (DBS100 strain) and the green fluorescent protein (GFP)-expressing strain were provided by B.A.V. Bacteria were grown in LB broth at 37°C overnight and reinoculated with 1% precultured bacteria in fresh medium (up to an optical density [OD] of ∼0.8 to 0.9). For oral infection, each mouse was administered 1 × 109 CFU of bacteria.
Intestinal permeability assays by FITC-dextran.
Translocation of intestinal fluorescein isothiocyanate (FITC)-dextran was measured as previously described (23). In brief, mice received 100 μl of FITC-dextran (4 kDa; Sigma-Aldrich, St. Louis, MO) by oral gavage in sterile phosphate-buffered saline (PBS) (80 mg/ml). Serum was collected 4 h later, and FITC levels were measured at 485 nm excitation and 535 nm emission with a fluorometer (PerkinElmer, Waltham, MA).
Histology and pathological score.
Whole colons were washed with PBS containing gentamicin and fixed in 4% formaldehyde for 1 h at 4°C. Tissues were dehydrated by gradually soaking in alcohol and xylene and then embedded in paraffin. The paraffin-embedded specimens were cut into 5-μm sections, stained with hematoxylin-eosin (H&E), and viewed with a digital light microscope (Olympus, Tokyo, Japan). Pathological scores were based on the following parameters: infiltration of inflammatory cells (on a scale of 0 to 3), epithelial integrity (0 to 3), submucosal edema (0 to 3), and crypt loss (0 to 4).
Measurement of CFU.
Tissues were removed and vigorously washed in PBS with gentamicin (50 μg/ml) to remove bacteria that were attached but had not invaded the intestine. Tissues were then mechanically homogenized in PBS (1 g/ml), diluted, and plated onto streptomycin-resistant LB agar. Colonies were counted after 18 h of culture at 37°C. To determine bacterial shedding, feces were suspended in PBS and plated onto LB agar plates containing streptomycin.
Immunohistochemical study.
The large intestines were fixed with 4% paraformaldehyde and dehydrated with 15% and 30% sucrose in PBS. Then dehydrated tissues were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Tokyo, Japan), frozen, and sliced into 5-μm sections. For staining, phycoerythrin (PE)-conjugated CD11b, Ly6G-FITC, and E-cadherin–FITC antibodies (BD Pharmingen, San Diego, CA), rabbit anti-Muc2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-rabbit IgG–PE antibody (GeneTex, San Antonio, TX) were used, and sections were viewed under a confocal laser microscope (Zeiss, Göttingen, Germany).
Real-time PCR.
Total RNA was extracted from large intestines using an RNeasy minikit (Qiagen, Venlo, Netherlands), and cDNA was synthesized using Superscript II reverse transcriptase and oligo(dT) primer (Invitrogen, Camarillo, CA). cDNA was used as the template for real-time PCR (RT-PCR) performed using SYBR green chemistry (Applied Biosystems, Foster, CA) on an ABI 7500 real-time PCR system (Applied Biosystems). Total RNA was extracted from large intestine using RNeasy minikit (Qiagen), and cDNA was synthesized using Superscript II reverse transcriptase and oligo(dT) primer (Invitrogen). cDNA was amplified with PCR primers for Cxcl1 (forward, 5′-GCTGGGATTCACCTCAAGAA-3′; reverse, 5′-TCTCCGTTACTTGGGGACAC-3′), Ccl2 (forward, 5′-TCTGGGCCTGCTGTTCACA-3′; reverse, 5′-CCTACTCATTGGGATCATCTTGCT-3′), Ccl3 (forward, 5′-ATCACTGACCTGGAACTGAATG-3′; reverse, 5′-CAAGTGAAGAGTCCCTCGATG-3′), Ccl4 (forward, 5′-CCCACTTCCTGCTGTTTCTC-3′; reverse, 5′-GAGGAGGCCTCTCCTGAAGT-3′), Ccl7 (forward, 5′-AAGCCCATCAGAAGTGGGTC-3′; reverse, 5′-AGCGGTGAGGAATTTTGCTT-3′), and Cxcl10 (forward, 5′-GGATGGCTGTCCTAGCTCTG-3′; reverse, 5′-ATAACCCCTTGGGAAGATGG-3′). RT-PCR was performed using SYBR green chemistry on an ABI 7500 real-time PCR system (both from Applied Biosystems). The levels of mRNA expression are displayed as the expression units of each target gene relative to the expression units of β-actin.
Isolation of polymorphonuclear leukocytes (PMNL).
Large intestines were opened longitudinally, and their contents were removed by shaking in cold PBS. Then, tissues were cut into 1- to 2-cm-long pieces. Intestinal epithelial cells and mucus were removed by shaking tissues in EDTA buffer (10 mM EDTA in PBS) for 30 min at 37°C. After being washed with prewarmed PBS, tissues were dissociated by RPMI containing 10% FBS, 0.5 mg/ml collagenase D (Sigma-Aldrich), and 100 μg/ml DNase I (Roche, Basel, Switzerland) twice for 30 min at 37°C. Cells were then enriched by a discontinuous density gradient containing 40% and 75% Percoll (Amersham Bioscience, Buckinghamshire, United Kingdom).
Flow cytometry analysis.
To assess phenotypes of isolated PMNL, we did flow cytometry analysis using antibodies for CD11b-FITC (clone M1/70; BD Biosciences, Franklin Lakes, NJ), Ly6C-APC (clone AL-21; BD Biosciences), and Ly6G-PE (clone RB6-8C5; eBioscience, San Diego, CA). To enumerate IL-22-secreting cells, intracellular staining was done using anti-IL-22 (clone 1H8PWSR; eBioscience). Isolated PMNL were stimulated with PMA (20 ng/ml) and ionomycin (1 μg/ml) for 4 h, stained, and then permeabilized with Cytofix/Cytoperm (BD Biosciences) according to the manufacturer's instructions. Flow cytometry data were determined by LSR II (BD Biosciences) or FACSCalibur flow cytometers (BD Biosciences), and files were analyzed using FlowJo software (Tree Star, Ashland, OR).
Depletion of neutrophils.
WT mice were injected intraperitoneally with 100 μg of either control anti-rat IgG (BioLegend, San Diego, CA) or anti-Gr1 (RB6-8C5, BioXcell, Lebanon, NH) antibodies 4 days after C. rodentium infection. As described elsewhere (24), dissociated tissues from middle and distal colon were retreated in vitro with anti-rat IgG or anti-Gr-1 antibodies and rabbit complement (AbD Serotec, Oxford, United Kingdom) for 6 h and washed with culture medium. Dissociated colon tissues were then further cultured for 3 days, and culture supernatant was harvested and analyzed for IL-22 protein levels.
Measurement of cytokines.
After whole colon tissue was weighed and homogenized with Tris-EDTA buffer (10 mM Tris-HCl, 1 mM EDTA, 0.05% sodium azide, 1% Tween 80, and protease inhibitor cocktail; Roche) and centrifuged at 11,000 × g for 10 min, supernatant was collected. Cytokine levels in the supernatant were measured with a cytometric bead array mouse inflammation kit (BD Biosciences) and a FlowCytomix kit (eBioscience) according to the manufacturers' instructions. Murine and human KC/CXCL1 and murine IL-17 and IL-22 were measured by commensal enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Isolation of stromal cells.
Stromal cells in mouse colon and human nasal polyp tissues were isolated by modification of a previously described protocol (25). In brief, tissues were dissociated by use of serum-free Dulbecco's modified Eagle medium (DMEM)/F-12 medium containing 1% penicillin-streptomycin (Life Technologies, Grand Island, NY) and 2 mg/ml collagenase-dispase (Sigma-Aldrich) for 2 h at 37°C. Thereafter, samples were passed through a 70-μm cell strainer. Isolated mononuclear cells were plated in a gelatin-coated culture dish with fibroblast growth factor (10 ng/ml) for 4 or 5 days. The attached fibroblast-like cells were then cultured an additional 7 days before use.
Chemotaxis assay.
PMNL were isolated from the bone marrow of naive B6 mice, and culture supernatants of intestinal stromal cells were obtained from WT and IL-1R−/− mice after infection. To evaluate the migration of PMNL by intestinal stromal cells, we used a chemotaxis assay as described previously (26). In brief, 5-μm Transwell inserts (Corning Costar, Tewksbury, MA) containing PMNL (1 × 106) were placed in the 24-well plates so as to make contact with 600 μl of the medium alone or culture supernatant of intestinal stromal cells. After 2 h, we removed the inserts, measured the populations that had migrated to the well bottoms, and determined cell phenotype by FACS analysis.
Statistics.
GraphPad Prism software (GraphPad, La Jolla, CA) was used for statistical analysis. Student's t test or analysis of variance (ANOVA) was used for comparisons. All results are expressed as means and standard deviations (SD) or standard errors of the means (SEM).
RESULTS
IL-1R−/− mice exhibit susceptibility to C. rodentium infection.
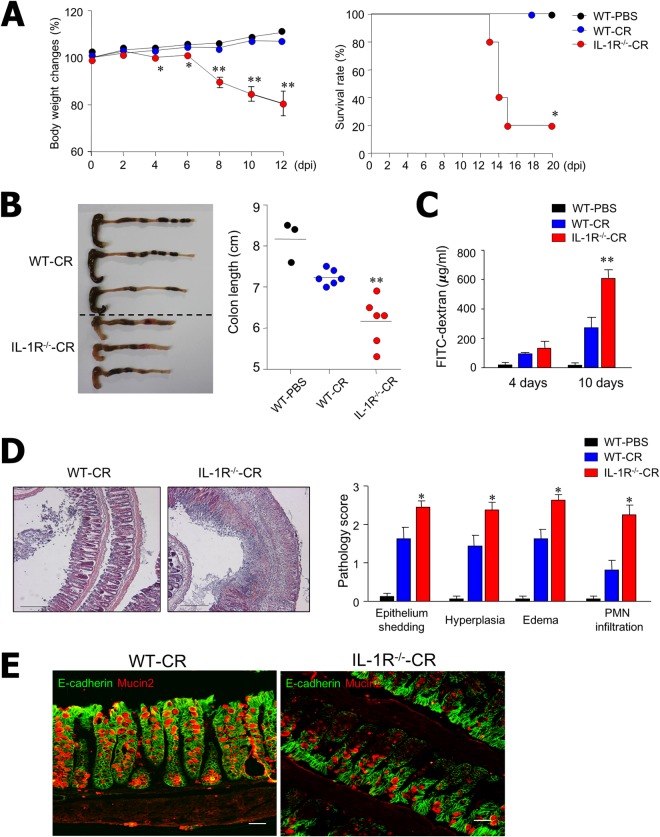
To determine the underlying mechanism of IL-1 on the control of enteric bacterial infection, we infected B6 and IL-1R−/− (B6 background) mice with C. rodentium (1 × 109) orally and measured body weight and survival rate every second day. There was continuous weight loss and reduced survival of IL-1R−/−-CR mice compared with WT-CR and WT-PBS mice beginning at day 3 (Fig. 1A). Mice infected with C. rodentium were then sacrificed on day 10, and changes in colon length and pathophysiological status were measured. As shown in Fig. 1B, IL-1R−/−-CR mice had significantly shorter colons and also showed signs of bleeding. Intestinal epithelial permeability was assessed by use of FITC-dextran translocation assays. The assays showed significantly higher levels of FITC-dextran in the serum of IL-1R−/−-CR mice compared to WT-CR mice 10 days after infection (Fig. 1C). Moreover, pathological scores for infiltrating inflammatory cells, epithelial integrity, submucosal edema, and crypt loss were much higher in the colon tissues of IL-1R−/−-CR mice than in WT-CR mice (Fig. 1D). Of note, there was significant reduction of E-cadherin and mucin 2 expression in the colon epithelium of IL-1R−/−-CR mice compared with WT-CR mice (Fig. 1E). These findings indicate that the IL-1R signaling pathway plays a critical role in host protection upon C. rodentium infection.
FIG 1.
IL-1R−/− mice are more susceptible to C. rodentium infection than wild-type (WT) mice. (A) WT (n = 12) and IL-1R−/− (n = 12) mice of B6 background were infected orally with C. rodentium (1 × 109 CFU) and monitored every other day for survival and body weight. Statistical significance for survival rate was determined by Kaplan-Meier analysis. (B) Macroscopic observation and colon measurements were done on postinfection day 10. Each dot represents an individual mouse. (C) Intestinal permeability was measured using FITC-dextran to determine migration from intestine to serum in WT (n = 7) or IL-1R−/− (n = 10) mice administered PBS or C. rodentium on days 4 and 10. Serum was collected at 4 h, and FITC levels were measured. (D) Representative H&E-stained samples and pathophysiologic scores of colon tissues from WT or IL-1R−/− mice on postinfection day 10. (E) Double immunostaining shows less E-cadherin (green) and mucin 2 (red) expressed in the colons of IL-1R−/− mice than in WT mice 4 days after C. rodentium infection. All data are representative of at least three experiments. *, P < 0.05; **, P < 0.01.
IL-1R signaling is required for protection against C. rodentium infection.
To clarify the role of IL-1 in the clearance of C. rodentium, we examined pathogen burdens in the colon, cecum, lymphoid tissues, and blood 10 days after infection. Although previous results showed severe inflammation and low survival rate of IL-1R−/− mice, we noted no overt differences in the numbers of bacterial colonies found in the colons and ceca of WT-CR and IL-1R−/−-CR mice (see Fig. S1A in the supplemental material). While there was no statistically significant difference in colony numbers in mucosal tissues, there were more colonies in spleens, mesenteric and iliac lymph nodes, and blood. Spleens were larger and weighed more in IL-1R−/−-CR than in WT-CR mice (see Fig. S1B in the supplemental material). In addition, we found higher numbers of GFP-expressing C. rodentium in spleen tissues of IL-1R−/−-CR mice (see Fig. S1C in the supplemental material). We further addressed cytokine levels in colon homogenates to clarify the severity of inflammation in the IL-1R-deficient condition. The levels of gamma interferon (IFN-γ), one of the major inflammatory cytokines, was significantly increased in IL-1R−/−-CR mice compared with WT-CR mice (see Fig. S2 in the supplemental material). No differences were found in other inflammatory cytokines (i.e., granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-23, and IL-10). Overall, the IL-1R signaling pathway contributed to both inflammation and clearance of C. rodentium bacteria in systemic tissues.
IL-1R signaling controls migration of innate immune cells.
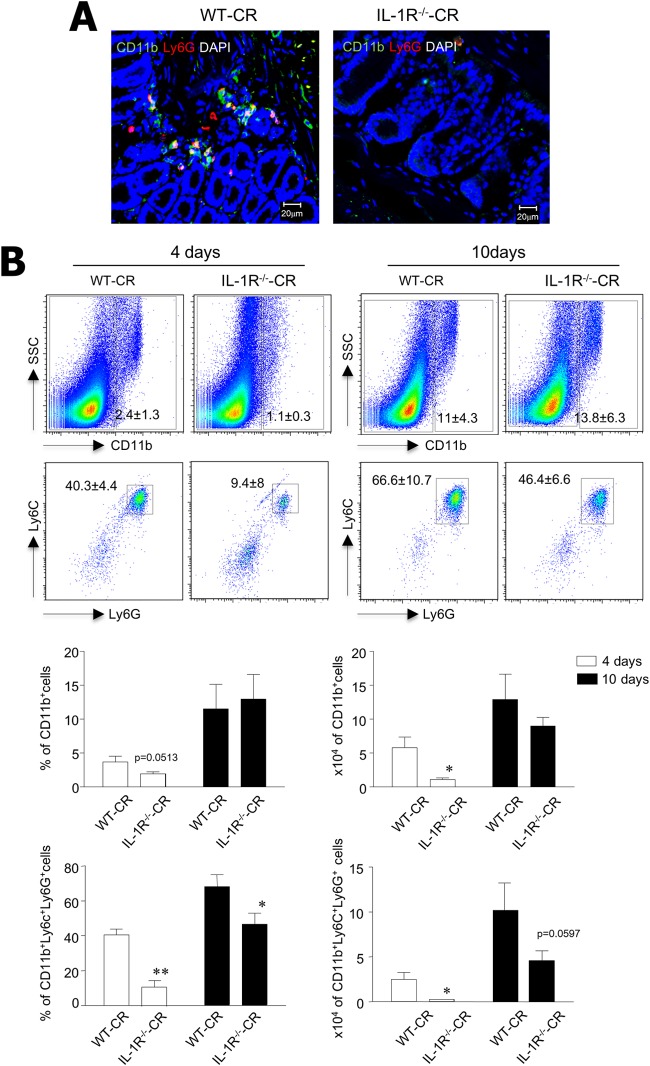
Innate immune cells such as myeloid lineage cells and neutrophils precede migration into the colon tissues upon exogenous stimuli, reflecting either infection or inflammation. To investigate whether IL-1 signaling controls migration of innate immune cells into the colon after C. rodentium infection, cell subsets were determined in colon tissues from WT-CR and IL-1R−/−-CR mice by immunohistochemical studies and FACS analysis. We found fewer CD11b+ Ly6G+ cells 4 days after infection in the colon tissues of IL-1R−/−-CR mice than in WT-CR mice (Fig. 2A). In support of the histology results, FACS analysis showed significantly fewer CD11b+ myeloid cells and CD11b+ Ly6C+ Ly6G+ neutrophils in the colons of IL-1R−/−-CR mice than in WT-CR mice 4 days after infection (Fig. 2B). Although there were significantly fewer infiltrating innate immune cells 4 days after infection, when analyzed by percentage and absolute cell numbers, there were no significant differences in infiltrated innate immune cells in the colon of both IL-1R-sufficient and -deficient mice at postinfection day 10 (Fig. 2B). Taken together, our findings indicate that the IL-1R signaling pathway plays an important role in the rapid migration of innate immune cells into the colon at an early time point after enteric bacterial infection.
FIG 2.
IL-1R is crucial for early recruitment of innate immune cells into the colon upon C. rodentium infection. (A) Immunohistochemical staining for CD11b (red), Ly6G (green), and DAPI (blue) in colon tissues of C. rodentium-infected WT and IL-1R−/− mice. (B) Innate immune cells were isolated from colon of WT (n = 7) and IL-1R−/− (n = 9) mice 4 and 10 days after infection and stained for CD11b, Ly6C, and Ly6G antibodies; percentages and absolute numbers of CD11b+ cells and Ly6C+ Ly6G+ in CD11b gated cells were determined. All data are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01.
IL-1R signaling controls chemokine secretion in the colon.
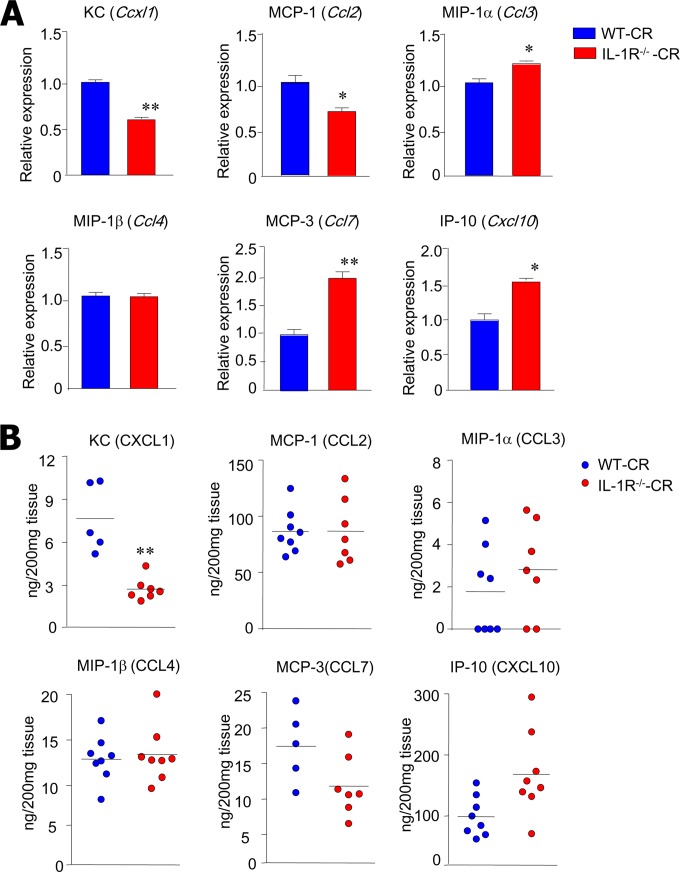
In order to identify the effects of IL-1R signaling on the secretion of chemokines that play a crucial role in the migration of innate immune cells such as CD11b+ Ly6C+ Ly6G+ neutrophils, we next measured mRNA and protein levels of KC/CXCL1, MCP-1, MIP-1α, MCP-3, MIP-1β, and CXCL10 in colon homogenates at postinfection day 4. We found that among the chemokines, the mRNA levels of KC/Cxcl1 were significantly reduced in the absence of IL-1R signaling (Fig. 3A). In addition, protein levels of KC/CXCL1 were significantly reduced in the supernatant of colon homogenates of IL-1R−/−-CR mice compared with WT-CR mice (Fig. 3B). However, there were no significant differences in the levels of other chemokines, indicating that IL-1R signaling might specifically regulate the KC/CXCL1 chemokine associated with neutrophil infiltration.
FIG 3.
IL-1R−/− mice have impaired KC/CXCL1 production during C. rodentium infection. (A) IL-1R−/− (n = 7) and WT (n = 5) mice were orally inoculated with C. rodentium (1 × 109 CFU), and mRNA expression was evaluated by real-time PCR for each chemokine. RNA was isolated from colon tissues collected 4 days after infection. (B) Quantification of chemokine protein levels in colon tissue homogenates of IL-1R−/− and WT mice 4 days after infection. Each dot represents an individual mouse. All data are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01.
Lack of IL-22-producing neutrophils in the colon of IL-1R-deficient mice.
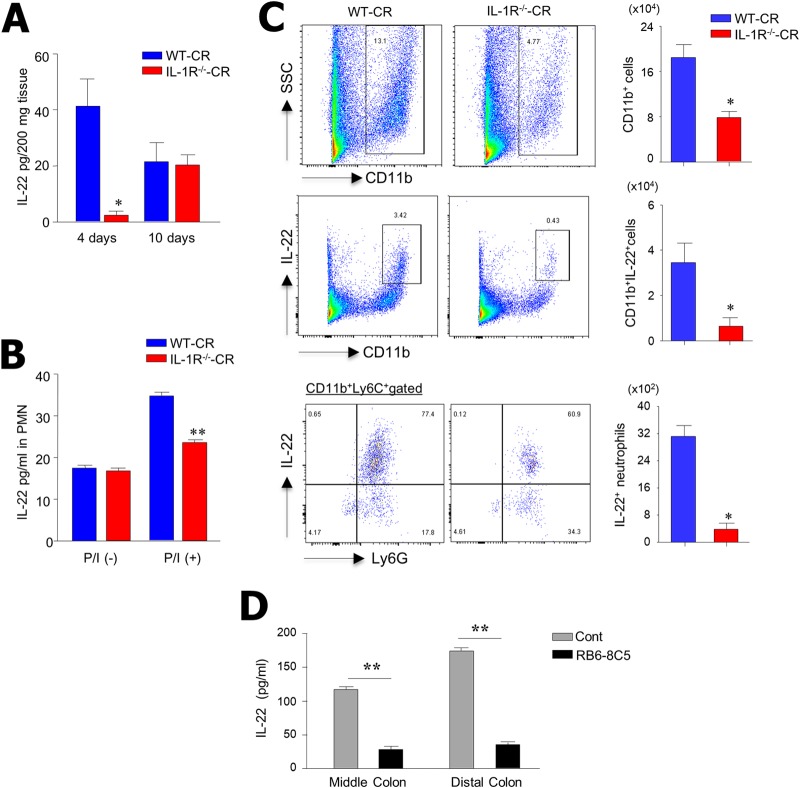
Because IL-22 is critical for host defense against several pathogens (27, 28), we examined secretion patterns of IL-22 in IL-1R-deficient mice. From colon homogenates of WT-CR and IL-1R−/−-CR mice, we measured IL-22 levels on postinfection days 4 and 10. Of note, IL-22 levels were significantly decreased at day 4 in the supernatant of colon homogenates of IL-1R−/−-CR mice compared with those of WT-CR mice (Fig. 4A). No differences were found at postinfection day 10. We subsequently isolated PMNL in the colon to enumerate IL-22-secreting cells upon stimulation with PMA-ionomycin. As shown in Fig. 4B, significantly less IL-22 was detected in colon PMNL isolated from IL-1R−/−-CR mice than from WT-CR mice. We next addressed whether neutrophils are the source of IL-22 in C. rodentium-infected mice. Of interest, we found that CD11b+ Ly6C+ Ly6G+ neutrophils were the main cell subset that secreted IL-22 in the colon in response to C. rodentium infection (Fig. 4C). To further confirm a role for neutrophils in IL-22 secretion, we depleted neutrophils by using the anti-Gr-1 neutralizing antibody (RB6-8C5) during C. rodentium infection. We found that neutrophil depletion resulted in significant reduction in IL-22 production in the middle and distal colon compared with neutrophil-intact colon (Fig. 4D). This suggests that IL-1R signaling regulates infiltration of CD11b+ Ly6C+ Ly6G+ neutrophils, the primary source of IL-22 production in the colon upon C. rodentium infection.
FIG 4.
IL-1R−/− mice have impaired production of IL-22 in response to C. rodentium infection at early infection times. (A) ELISA measurement of IL-22 in colon tissue homogenates of WT and IL-1R−/− mice on postinfection days 4 (n = 5) and 10 (n = 7). (B) PMNL were isolated from colon tissue of infected IL-1R−/− and WT mice. Cells (1 × 105) were stimulated with PMA-ionomycin for 24 h, and IL-22 levels were assessed by ELISA. (C) PMNL were isolated from the colon of WT and IL-1R−/− mice on 4 days after infection, stained for IL-22, and assessed by FACS analysis by gating for CD11b+ and CD11b+ Ly6C+ Ly6G+ cells. (D) To deplete neutrophils, wild-type mice were injected intraperitoneally with anti-rat IgG as a control or anti-Gr-1 antibodies at 4 days after C. rodentium infection. Colonic tissues from the middle and distal regions were retreated in vitro with anti-rat IgG or anti-Gr-1 antibodies and rabbit complement for 6 h and washed with culture medium. Dissociated colon tissues were then cultured for an additional 3 days, and culture supernatant was harvested and analyzed for IL-22 protein levels. Results are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01.
Mucosal stromal cells impair KC/CXCL1 in the IL-1R-deficient condition.
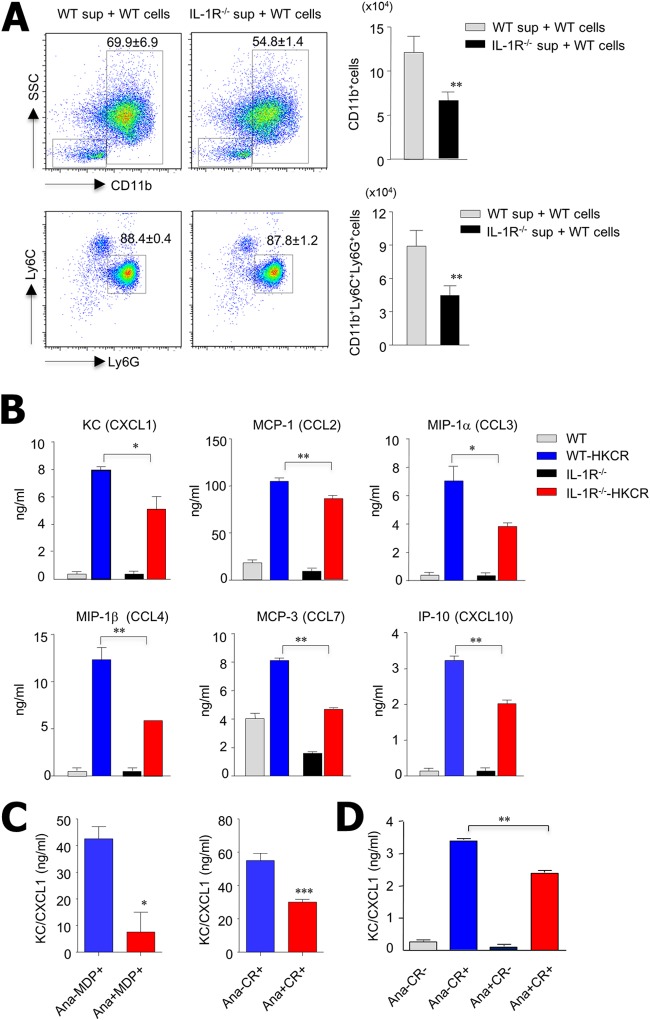
Because a recent study demonstrated an important role for mucosal stromal cells as regulators of cytokines and chemokines (22), we isolated intestinal stromal cells to assess their role in chemokine secretion during C. rodentium infection. After intestinal stromal cells from WT-CR and IL-1R−/−-CR mice were cultured for 4 days, we performed chemotactic assays using the culture supernatant of the intestinal stromal cells and bone marrow cells from naive B6 mice. A lower percentage of CD11b+ cells migrated into the culture supernatant of IL-1R−/−-CR mouse intestinal stromal cells than into that of WT-CR mouse intestinal stromal cells (54.8% ± 1.4% versus 69.9% ± 6.9%). Similarly, there were also lower absolute cell numbers for the two mouse groups (6.5 × 104 versus 12.1 × 104) (Fig. 5A). As for CD11b+ Ly6C+ Ly6G+ cells, there were no changes in the ratio of migrated cells (87.8% ± 1.2% versus 88.4% ± 0.4%), but there were significantly lower absolute cell numbers in the culture supernatant of intestinal stromal cells from IL-1R−/−-CR than in that from WT-CR mice (4.5 × 104 versus 8.9 ×104) (Fig. 5A). We also assessed bone marrow cells of naive IL-1R−/− mice (see Fig. S3 in the supplemental material) and found that significantly fewer absolute CD11b+ Ly6C+ Ly6G+ neutrophils from IL-1R−/− mice migrated into the culture supernatant of IL-1R−/−-CR mouse intestinal stromal cells than into that of WT-CR mouse cells (5.6 × 104 versus 10.6 ×104). These results indicate that IL-1R signaling on the neutrophils did not influence the migration of neutrophils. We subsequently identified the chemokine levels in the culture supernatant of intestinal stromal cells from WT and IL-1R−/− mice in the absence and presence of heat-killed C. rodentium during in vitro culture. Although overall chemokine levels were decreased in the stromal cells of IL-1R−/− mice compared with those of WT mice in the presence of heat-killed C. rodentium, KC/CXCL1 levels were also significantly reduced (Fig. 5B). To further clarify the role of IL-1R in impaired chemokine secretion by intestinal stromal cells, we adopted the anakinra (IL-1R antagonist) in vitro culture system. In order to stimulate intestinal stromal cells in vitro, we used heat-killed C. rodentium or muramyl dipeptide (MDP), which can activate IL-1α and IL-1β secretion through inflammasome activation (29). Intestinal stromal cells of naive B6 mice secreted high levels of KC/CXCL1 after stimulation with heat-killed C. rodentium or MDP in vitro; however, treatment with anakinra significantly inhibited their KC/CXCL1 secretion (Fig. 5C). To further clarify the role of IL-1R on chemokine secretion in human tissues, we used human polyp stromal cells from chronic rhinosinusitis patients. These cells secreted high levels of KC/CXCL1 in the presence of heat-killed C. rodentium in vitro; however, treatment with anakinra significantly inhibited KC/CXCL1 secretion (Fig. 5D). These results were identical in both mouse and human stromal cells and suggest that the IL-1–IL-1R interaction is important for chemokine secretion by mucosal stromal cells and contributes to the migration of IL-22-secreting innate immune cells against enteric bacterial infections in both mice and humans.
FIG 5.
Mucosal stromal cells are impaired in KC/CXCL1 production during C. rodentium infection in the absence of IL-1R signaling. Stromal cells isolated from the colons of WT and IL-1R−/− mice 3 days after infection were seeded into 24-well plates (2 × 105 cells/well). Culture supernatants were harvested 4 days later. (A) Mononuclear cells isolated from bone marrow of naive B6 mice were suspended in complete medium and placed in the upper chambers of Transwell plates (106 cells/well). Lower chambers contained medium alone or stromal-cell-cultured supernatant. After incubation for 2 h, cells that had migrated were harvested from the lower chambers and stained for CD11b, Ly6C, and Ly6G antibodies. (B) Protein levels of each chemokine in culture supernatant of intestinal stromal cells in the absence and presence of heat-killed C. rodentium (HKCR) were analyzed by ELISA. (C) Intestinal stromal cells isolated from naive B6 mice and stimulated with heat-inactivated C. rodentium (2 × 107 CFU) or muramyl dipeptide (MDP) as positive controls with and without anakinra (Ana; 50 ng/ml). KC/CXCL1 levels in culture supernatants were measured by ELISA (C and D). (D) Human stromal cells isolated from nasal polyp tissue of patients with chronic rhinosinusitis and stimulated with heat-inactivated C. rodentium (2 × 107 CFU) in vitro in the presence and absence of Ana (50 ng/ml). All data are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
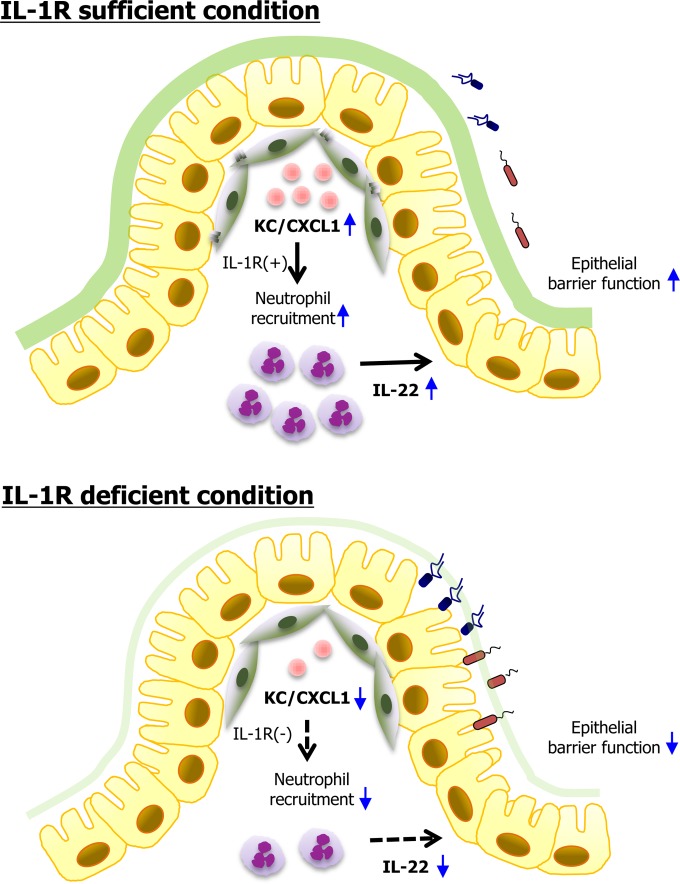
IL-1 is a central mediator of innate immunity, and thus IL-1R-mediated signaling is an important defense mechanism against infection and inflammation. In this study, we found evidence that IL-1R-dependent signaling plays a critical protective role during C. rodentium infection, in agreement with earlier studies (17, 30). The most unusual finding in the present study was that infiltration of CD11b+ Ly6G+ Ly6C+ neutrophils, the main source of IL-22 secretion, was significantly delayed in the colon at an early time point during C. rodentium infection. Of note, intestinal stromal cells were found to be critical for secretion of KC/CXCL1 in an IL-1R-dependent manner. We demonstrated the importance of IL-1–IL-1R stimulation on intestinal stromal-cell maturation, which directly influences the infiltration of IL-22-secreting innate immune cells after enteric bacterial infection (Fig. 6).
FIG 6.
Proposed model of the role of IL-1R signaling for migration of IL-22-producing neutrophils by intestinal stromal cells. In the presence of sufficient IL-1R signaling, attaching and effacing pathogens such as C. rodentium infect outer and inner mucus layers and access colon epithelium and stromal cells. Following infection of stromal cells, the KC/CXCL1 chemokine is induced and attracts IL-22-secreting neutrophils, which enhance barrier function of epithelial cells. In the absence of IL-1R signaling, gut stromal cells impair KC/CXCL1 secretion, and subsequently, the lack of recruitment of IL-22-secreting neutrophils leads to a less than optimal host defense system.
Innate immune cells, including dendritic cells, macrophages, granulocytes, and innate lymphoid cells, provide the first-line defense in the gut where there is continuous exposure to endogenous and exogenous microbes. Migration of innate immune cells into local mucosal tissues is fully dependent on specific chemokines such as CXCL1, MCP-1, MIP-1α, MCP-3, MIP-1β, and CXCL10 (31, 32). Earlier studies showed that neutrophils are indispensable for the clearance of pathogens in vivo (33). For instance, a CXCR2-dependent mucosal neutrophil influx contributes to colitis-associated diarrhea caused by C. rodentium (34). Among the chemokines, KC/CXCL1 is the major chemoattractant for recruiting neutrophils (35). Others have reported that IL-1 stimulates cytokine-induced neutrophil chemoattractant 1 (CINC-1), CXCL2, and RANTES production in vitro as well as in vivo (36, 37). Another study showed that neutrophil recruitment by IL-1R is mainly mediated by MyD88 signaling during infection with C. rodentium (38). In the present study, we found that protein and mRNA levels of KC/CXCL1 were significantly reduced in the colon tissues of IL-1R−/− mice early after C. rodentium infection (Fig. 3). Accordingly, neutrophil migration was delayed in the absence of the IL-1R signal pathway (Fig. 2). Our findings reveal a previously unrecognized role of IL-1R in the regulation of chemokine secretion and infiltration of innate immune cells in the colon in response to an enteric pathogen that occurs in a KC/CXCL1-dependent manner.
IL-22 plays an important role in gut epithelial cell homeostasis. While innate lymphoid cells have been identified to produce IL-22, other studies have demonstrated that IL-17+CD4+ T cells and natural killer cells also produce IL-22 in both humans and mice (37–40). Most recently, neutrophils were found to produce IL-22 in the colon during colitis (24). These IL-22-secreting neutrophils activate colonic epithelial cells to secrete antimicrobial peptides (i.e., RegIIIβ and S100A8) and protect against epithelial damage from dextran sulfate sodium-induced acute colitis (24). We found that IL-22 mRNA expression in colon tissues and IL-22 secretion by colonic PMNL were significantly reduced in IL-1R−/− mice 4 days after C. rodentium infection (Fig. 4A). Additionally, CD11b+ Ly6G+ Ly6C+ neutrophils were the main cell subset to secrete IL-22 in the colon (Fig. 4C and D). Although the role of neutrophils in host defense remains controversial, our results imply that migration of IL-22-secreting neutrophils in the colon upon enteric bacterial infection is impaired in the absence of an IL-1R signal pathway.
Aside from driving antimicrobial responses, IL-22 has been shown to mediate other changes in epithelial cell function, since its receptor is expressed exclusively by intestinal epithelial cells. IL-22 also induces an increase in epithelial cell proliferation as well as mucin production/secretion (41). While we did not note any overt changes in epithelial cell proliferation, the IL-1R−/− mice did have reduced mucin staining (Fig. 1E), suggesting that control over the mucus barrier may be one mechanism by which IL-1R-induced neutrophil recruitment may protect this intestine during C. rodentium infection.
Intestinal stromal cells include myofibroblasts and fibroblasts (42, 43) and can produce proinflammatory mediators, including cytokines, chemokines, and metabolites in response to various TLR ligands (44). Intestinal stromal cells dynamically interact with multiple hematopoietic immune cell populations and associated cytokines (18). Furthermore, intestinal stromal cells have a cell-intrinsic role in bacterial sensing at the intestinal barrier (18). One recent study supports a functional role for stromal expression of innate immune receptors in vivo, as NOD2-dependent CCL2 production by intestinal stromal cells was key for regulating inflammatory monocyte recruitment and thus pathogen clearance during C. rodentium infection (22). In addition, gut stromal cells strongly respond to the proinflammatory cytokines IL-1α and IL-1β (20, 21). For these reasons, we speculate that intestinal stromal cells might be critical regulators of the migration of innate immune cells. As expected, we found that intestinal stromal cells mainly produce KC/CXCL1 during C. rodentium infection (Fig. 5). To our knowledge, these findings are the first to show that the IL-1R signal pathway plays an important role for development of innate immune responses during enteric bacterial infection via colon stromal-cell-derived-KC/CXCL1-dependent migration of IL-22-secreting neutrophils. These findings thus provide a better understanding of the critical role played between IL-1R-mediated signals and intestinal stromal cells.
As expected, patients with IL-1R-associated kinase (IRAK) deficiency are highly susceptible to invasive and noninvasive bacterial infections (45). In IRAK-deficient patients, S. pneumoniae was involved in 54% of documented invasive episodes, whereas other Gram-negative enteric bacteria, such as Shigella sonnei and Clostridium septicum, were found in fewer than 7% of invasive episodes. Despite the narrow susceptibility of enteric bacteria in IRAK-deficient patients, it is important to note that the first bacterial infection occurred before the age of 2 years in 90% of IRAK-deficient patients.
Anakinra, an IL-1R antagonist, is used to treat rheumatoid arthritis patients (46–48). Other IL-1R-blocking reagents are used to treat inflammatory disorders (49). However, in our study, blockade of the IL-1R-mediated pathways resulted in impaired innate immune cell infiltration, a crucial step during early host defense in both mice and humans. Studies regarding future infectious and inflammatory disease therapies using blockade of the IL-1R pathways need to consider the possibility that the results may be contradictory.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Korean Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea HI12C06870000 (A120770) and HI13C0016, and the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP) (no. 2010-0029133 and 2012-0000805), as well as funding from the Canadian Institutes of Health Research and Crohn's and Colitis Canada (to B.A.V.).
We have no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00670-15.
REFERENCES
- 1.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. 2005. Citrobacter rodentium of mice and man. Cell Microbiol 7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 2.Luperchio SA, Schauer DB. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect 3:333–340. doi: 10.1016/S1286-4579(01)01387-9. [DOI] [PubMed] [Google Scholar]
- 3.Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. 2004. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun 72:3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wales AD, Woodward MJ, Pearson GR. 2005. Attaching-effacing bacteria in animals. J Comp Pathol 132:1–26. doi: 10.1016/j.jcpa.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W, Finlay BB, Vallance BA. 2006. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect Immun 74:2522–2536. doi: 10.1128/IAI.74.5.2522-2536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergstrom KS, Sham HP, Zarepour M, Vallance BA. 2012. Innate host responses to enteric bacterial pathogens: a balancing act between resistance and tolerance. Cell Microbiol 14:475–484. doi: 10.1111/j.1462-5822.2012.01750.x. [DOI] [PubMed] [Google Scholar]
- 7.Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, Niess JH. 2013. CX3CR1+ macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol 6:177–188. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Janeway CA, Medzhitov R. 2002. Innate immune recognition. Annu Rev Immunol 20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 10.Gibson DL, Ma C, Bergstrom KS, Huang JT, Man C, Vallance BA. 2008. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol 10:618–631. doi: 10.1111/j.1462-5822.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 11.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. 2007. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol 179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 12.Bowie A, O'Neill LA. 2000. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol 67:508–514. [DOI] [PubMed] [Google Scholar]
- 13.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, Booty M, Estes JD, Sandler NG, Plass N, Stone DL, Turner ML, Hill S, Butman JA, Schneider R, Babyn P, El-Shanti HI, Pope E, Barron K, Bing X, Laurence A, Lee CC, Chapelle D, Clarke GI, Ohson K, Nicholson M, Gadina M, Yang B, Korman BD, Gregersen PK, van Hagen PM, Hak AE, Huizing M, Rahman P, Douek DC, Remmers EF, Kastner DL, Goldbach-Mansky R. 2009. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med 360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, Hessner MJ, Verbsky J. 2009. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med 360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. 2011. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol 186:7080–7088. doi: 10.4049/jimmunol.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, Ko HJ, Yang JY, Kim JJ, Seo SU, Park SG, Choi SS, Seong JK, Kweon MN. 2013. Interleukin-1 promotes coagulation, which is necessary for protective immunity in the lung against Streptococcus pneumoniae infection. J Infect Dis 207:50–60. doi: 10.1093/infdis/jis651. [DOI] [PubMed] [Google Scholar]
- 17.Lebeis SL, Powell KR, Merlin D, Sherman MA, Kalman D. 2009. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun 77:604–614. doi: 10.1128/IAI.00907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owens BM, Simmons A. 2013. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol 6:224–234. doi: 10.1038/mi.2012.125. [DOI] [PubMed] [Google Scholar]
- 19.Pinchuk IV, Mifflin RC, Saada JI, Powell DW. 2010. Intestinal mesenchymal cells. Curr Gastroenterol Rep 12:310–318. doi: 10.1007/s11894-010-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Mari JF, Mifflin RC, Adegboyega PA, Saada JI, Powell DW. 2003. IL-1α-induced COX-2 expression in human intestinal myofibroblasts is dependent on a PKCζ-ROS pathway. Gastroenterology 124:1855–1865. doi: 10.1016/S0016-5085(03)00399-8. [DOI] [PubMed] [Google Scholar]
- 21.Okuno T, Andoh A, Bamba S, Araki Y, Fujiyama Y, Fujiyama M, Bamba T. 2002. Interleukin-1β and tumor necrosis factor-α induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand J Gastroenterol 37:317–324. doi: 10.1080/003655202317284228. [DOI] [PubMed] [Google Scholar]
- 22.Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. 2011. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Napolitano LM, Koruda MJ, Meyer AA, Baker CC. 1996. The impact of femur fracture with associated soft tissue injury on immune function and intestinal permeability. Shock 5:202–207. doi: 10.1097/00024382-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, Chaplin DD, Weaver CT. 2013. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A 110:12768–12773. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J, Virshup DM. 2014. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 26.Bowman EP, Kuklin NA, Youngman KR, Lazarus NH, Kunkel EJ, Pan J, Greenberg HB, Butcher EC. 2002. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med 195:269–275. doi: 10.1084/jem.20010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, Ouyang W. 2011. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol 12:941–948. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 29.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2; implications for Crohn's disease. J Biol Chem 278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 30.Sham HP, Yu EY, Gulen MF, Bhinder G, Stahl M, Chan JM, Brewster L, Morampudi V, Gibson DL, Hughes MR, McNagny KM, Li X, Vallance BA. 2013. SIGIRR, a negative regulator of TLR/IL-1R signalling promotes microbiota dependent resistance to colonization by enteric bacterial pathogens. PLoS Pathog 9:e1003539. doi: 10.1371/journal.ppat.1003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffith JW, Sokol CL, Luster AD. 2014. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 32.Esche C, Stellato C, Beck LA. 2005. Chemokines: key players in innate and adaptive immunity. J Investig Dermatol 125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- 33.Conlan JW. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun 65:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spehlmann ME, Dann SM, Hruz P, Hanson E, McCole DF, Eckmann L. 2009. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol 183:3332–3343. doi: 10.4049/jimmunol.0900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritzman AM, Hughes-Hanks JM, Blaho VA, Wax LE, Mitchell WJ, Brown CR. 2010. The chemokine receptor CXCR2 ligand KC (CXCL1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect Immun 78:4593–4600. doi: 10.1128/IAI.00798-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calkins CM, Bensard DD, Shames BD, Pulido EJ, Abraham E, Fernandez N, Meng X, Dinarello CA, McIntyre RC Jr. 2002. IL-1 regulates in vivo C-X-C chemokine induction and neutrophil sequestration following endotoxemia. J Endotoxin Res 8:59–67. doi: 10.1177/09680519020080010601. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Fujieda S, Yanagi S, Yamamura H, Inatome R, Yamamoto H, Igawa H, Saito H. 2001. IL-1 induced chemokine production through the association of Syk with TNF receptor-associated factor-6 in nasal fibroblast lines. J Immunol 167:283–288. doi: 10.4049/jimmunol.167.1.283. [DOI] [PubMed] [Google Scholar]
- 38.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. 2006. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. 2006. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res 16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 40.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner JE, Stockinger B, Helmby H. 2013. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog 9:e1003698. doi: 10.1371/journal.ppat.1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith RS, Smith TJ, Blieden TM, Phipps RP. 1997. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol 151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 43.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. 1999. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 277:C183–C201. [DOI] [PubMed] [Google Scholar]
- 44.Otte JM, Rosenberg IM, Podolsky DK. 2003. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology 124:1866–1878. doi: 10.1016/S0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 45.Picard C, Casanova JL, Puel A. 2011. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBα deficiency. Clin Microbiol Rev 24:490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinarello CA. 2000. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 47.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. 1999. IL-1 α/β blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-α blockade only ameliorates joint inflammation. J Immunol 163:5049–5055. [PubMed] [Google Scholar]
- 48.Mertens M, Singh JA. 2009. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol 36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 49.Dinarello CA. 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.