Abstract
The ability of certain species of Chlamydia to inhibit the biogenesis of phagolysosomes permits their survival and replication within macrophages. The survival of macrophage-adapted chlamydiae correlates with the multiplicity of infection (MOI), and optimal chlamydial growth occurs in macrophages infected at an MOI of ≤1. In this study, we examined the replicative capacity of Chlamydia muridarum in the RAW 264.7 murine macrophage cell line at different MOIs. C. muridarum productively infected these macrophages at low MOIs but yielded few viable elementary bodies (EBs) when macrophages were infected at a moderate (10) or high (100) MOI. While high MOIs caused cytotoxicity and irreversible host cell death, macrophages infected at a moderate MOI did not show signs of cytotoxicity until late in the infectious cycle. Inhibition of host protein synthesis rescued C. muridarum in macrophages infected at a moderate MOI, implying that chlamydial growth was blocked by activated defense mechanisms. Conditioned medium from these macrophages was antichlamydial and contained elevated levels of interleukin 1β (IL-1β), IL-6, IL-10, and beta interferon (IFN-β). Macrophage activation depended on Toll-like receptor 2 (TLR2) signaling, and cytokine production required live, transcriptionally active chlamydiae. A hydroxyl radical scavenger and inhibitors of inducible nitric oxide synthase (iNOS) and cathepsin B also reversed chlamydial killing. High levels of reactive oxygen species (ROS) led to an increase in cathepsin B activity, and pharmacological inhibition of ROS and cathepsin B reduced iNOS expression. Our data demonstrate that MOI-dependent TLR2 activation of macrophages results in iNOS induction via a novel ROS- and cathepsin-dependent mechanism to facilitate C. muridarum clearance.
INTRODUCTION
Infection of host epithelial cells by Chlamydia spp. sets in motion a cascade of signaling events that recruit multiple innate immune effectors to the infected site. Upon recognition of chlamydial pathogen-associated molecular patterns (PAMPs) and host danger signals, infiltrating leukocytes undergo transcriptional reprogramming to amplify the immune response by producing several cytokines and antimicrobial factors. The subsequent inflammatory process aids in bacterial clearance and primes elements of adaptive immunity while also contributing to the damaging pathology associated with chlamydial disease (1, 2).
Cells of the monocyte-macrophage lineage play critical roles in innate and adaptive immunity against chlamydial infections. Depletion of macrophages from mice prior to infection with Chlamydia muridarum and Chlamydia psittaci results in increased morbidity and pathogen burden (3, 4). Adoptive transfer of macrophages to RAG-1−/−/gamma interferon (IFN-γ)−/− mice has been shown to be sufficient for the control of Chlamydia pneumoniae in lung models of infection (5). Chlamydia trachomatis is rapidly rerouted to lysosomes in RAW macrophages, where the pathogen is killed (6). Macrophage killing and subsequent presentation of elementary body (EB) antigens plays a key role in priming CD4+ T-cell responses to chlamydial infection (7). However, macrophage activities may not lead to immediate pathogen clearance, since some species of Chlamydia can survive and undergo limited replication in these cells. For instance, lymphogranuloma venereum (LGV) biovar, but not oculogenital biovar, strains of C. trachomatis can productively infect macrophages (8–10). C. pneumoniae and C. psittaci have been similarly shown to replicate in macrophages by preventing maturation of the phagosome (11, 12). Chlamydial persistence or replication within phagocytic cells correlates with infection resolution and disease outcome. LGV strains are highly invasive and cause systemic infections by dissemination through the lymphatic system (13). These strains also require a longer antibiotic regimen for effective treatment than non-LGV strains (14). Persistent infection of macrophages by C. pneumoniae in several tissues has been associated with chronic inflammatory conditions, including atherosclerosis, reactive arthritis, and asthma (15, 16).
Intracellular replication of chlamydiae within macrophages in vitro is less efficient than in epithelial cells and is limited by the constitutive expression of perforin-2 in macrophages (17). Productive infection also appears to be contingent upon the macrophage activation state and multiplicity of infection (MOI). Macrophages classically activated by IFN-γ and lipopolysaccharide (LPS) or other microbial PAMPs switch to an M1 polarized state. This correlates with increased bacterial killing via proinflammatory cytokines, production of reactive oxygen species (ROS) and upregulation of inducible nitric oxide synthase (iNOS) (18). Several types of immune cells secrete IFN-γ in response to chlamydial infection, likely favoring M1 polarization (19). M2 anti-inflammatory macrophages are activated by interleukin 4 (IL-4) or IL-10 and participate in wound healing and fibrosis (18). None of the evaluated chlamydial species survive in M1 macrophages in vitro, but whether they selectively replicate in M2 or resting-macrophage (M0) reservoirs as opposed to M1 in vivo is unknown (20–23). Interestingly, a virulent strain of C. psittaci, 6BC, recruits M0 instead of activated macrophages in mice, and this correlates with increased morbidity (3). Other chlamydial species may also modulate the macrophage activation state or possess alternative macrophage subversion strategies.
Early work with macrophages isolated from various hosts and tissues indicated that recovery of chlamydial infectious particles is also dictated by the MOI (8, 12, 24). Optimal recoveries were obtained when macrophages were infected at an MOI of 1 or less. MOIs on the order of 10 to 250 of C. psittaci and C. trachomatis L2/434/Bu led to decreased EB recovery, and this was attributed to host cell cytotoxicity occurring within the first 6 to 10 h of infection.
In this study, we examined chlamydial recoveries from cells of the murine RAW 264.7 macrophage line (RAW macrophages) infected with the mouse pathogen C. muridarum at MOIs ranging from 0.5 to 100. We observed a decrease in chlamydial survival with increasing MOI, consistent with previous studies (12, 24). Interestingly, a majority of cells infected at an MOI of 10 remained healthy but failed to support chlamydial development. However, these cells produced high levels of proinflammatory cytokines, iNOS-derived nitric oxide (NO), and ROS, indicative of M1 polarization. Induction of iNOS expression depended on an increase in cathepsin B activity and ROS accumulation. Blocking iNOS, ROS, or cathepsin B restored chlamydial growth in moderately infected macrophages. Together, our results indicate that a novel ROS- and cathepsin B-dependent pathway for NO production controls the growth of C. muridarum in RAW macrophages. Importantly, our results also suggest that differing MOIs might explain some seemingly contradictory results concerning the relative importance of iNOS in the control of chlamydial infections reported previously.
MATERIALS AND METHODS
Cell lines and chlamydial propagation.
The murine RAW 264.7 macrophage line was a kind gift from Cheng Kao (Indiana University, Bloomington, IN). RAW cells were maintained in low-adhesion 10-cm bacterial petri dishes (VWR) in RPMI 1640 medium containing 2 mM l-glutamine (Life Technologies) supplemented with 10% fetal bovine serum albumin (FBS) (Atlanta Biologicals), 10 mM HEPES (Gibco), and 1 mM sodium pyruvate. Only low-passage-number (≤4) macrophages were used for experiments. C. muridarum (a generous gift from Harlan Caldwell, Rocky Mountain Laboratories, NIAID, NIH) (62) was propagated in McCoy mouse fibroblasts (American Type Culture Collection CRL-1696), and EBs were purified as previously described (25). The McCoy cells were cultured in Dulbecco's modified Eagle's medium (DMEM)–high-glucose medium with 4 mM l-glutamine, 110 mg/liter sodium pyruvate (HyClone), 10% FBS, 10 mM HEPES, and 100 μM nonessential amino acids (Gibco).
Infection of RAW cells and inclusion-forming unit (IFU) assays.
RAW cells were seeded in 24-well cell culture plates (Thermo Scientific) 48 h prior to infection. Chlamydial infections were performed on confluent monolayers in sucrose-phosphate-glutamic acid (SPG) buffer by centrifugation at 168 × g at room temperature (RT) for 1 h. Following infection, the SPG buffer was replaced with fresh culture medium, and the plates were incubated at 37°C in 5% CO2. In some experiments, RAW cells were treated 30 min prior to infection with one or more of the following reagents: 1 mM l-NG-monomethyl arginine citrate (l-NMMA), 2 mM l-NG-nitroarginine methyl ester (l-NAME) (Cayman Chemical), 15 mM N,N′-dimethylthiourea (DMTU) (Acros Organics), 50 or 100 ng/ml IL-1Ra, 25 μM acetyl (Ac)-YVAD-CHO (Peprotech), 25 μM Z-WEHD-FMK, and 25 μM CA-074 Me (kind gifts of Stanley Spinola, Indiana University School of Medicine, Indianapolis, IN). Treatment continued throughout the course of infection. The infected monolayers were frozen in 500 μl SPG buffer at 24 h postinfection (p.i.). Upon thawing, the cells were scraped from the wells and agitated with 3-mm glass beads to harvest EBs.
IFU assays were then performed by infecting McCoy cells in 96-well plates (Thermo Fisher) with serial dilutions of the harvests of experimental infections. At 24 h p.i., the cells were fixed with methanol and stained with mouse anti-chlamydial LPS monoclonal antibody (MAb) (EVIH1), followed by a secondary Alexa Fluor 488-conjugated MAb (Life Technologies). Chlamydial inclusions were imaged and counted using an Evos FL Auto Cell Imaging System (Life Technologies).
Cytotoxicity assay.
Lactate dehydrogenase (LDH) assays were performed according to the manufacturer's instructions (OPS Diagnostics). RAW cells were infected with C. muridarum at an MOI of 0.5, 10, or 100. Maximal LDH release was determined by treating cells in control wells with 10% Triton X-100 10 min prior to the assay. Supernatants were removed from the wells at the indicated time points after a brief spin to remove debris. In a 96-well plate, 25 μl of the supernatants was mixed with 75 μl of the dye-buffer solution. Following incubation at 37°C for 15 min, absorbance was measured at 490 nm.
Immunofluorescence microscopy.
RAW macrophages were grown on glass coverslips in 24-well plates. The cells were infected by centrifugation, fixed at 2 h p.i. with 4% formaldehyde, and permeabilized with 0.05% saponin. The cells were blocked in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) for 1 h, and then the chlamydial inclusions were stained with the primary MAb EVIH1, followed by an anti-mouse Alexa Fluor 488-conjugated secondary antibody (Life Technologies). The coverslips were mounted on glass slides using ProLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Life Technologies), and images were captured using a Leica DMI6000 B inverted microscope with a 63× oil immersion objective.
UV inactivation of C. muridarum EBs.
EB stocks were diluted in SPG buffer at a 1:10 ratio, pipetted onto a petri dish, and exposed to 1,200 J/cm2 twice in a UV-cross-linking cabinet (Spectralinker; Spectronics Corporation) (26, 27). The efficacy of the UV treatment was confirmed by IFU assays.
Cytokine analysis.
Culture supernatants were collected from infected RAW macrophages at the indicated times and centrifuged briefly to remove debris. The supernatants were assayed for IL-1β, IL-6, 1L-10, IL-12p40, tumor necrosis factor alpha (TNF-α), and IFN-γ (Milliplex) at the Bio-Plex core (IUPUI, Indianapolis, IN) according to the manufacturer's instructions. IFN-β levels were determined using a mouse IFN-β enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (BioLegend, San Diego, CA).
IFNAR1, TLR2, and TLR4 neutralization experiments.
RAW macrophages were incubated for 1 h with antibody (10 to 30 μg/ml) and then stimulated with C. muridarum (MOI = 10), conditioned medium, or C. muridarum (MOI = 0.5) with either 500 U/ml recombinant mouse IFN-β (R&D Systems) or 0.5 ng/ml E. coli LPS. Anti-IFNAR1 (clone MAR1.5A3), anti-Toll-like receptor 4 (TLR4)-MD2 (clone MTS510), anti-TLR2 (clone T2.5), and isotype-matched IgG1 and IgG2A antibodies were purchased from BioLegend (San Diego, CA). Cells were harvested for IFU assays at 24 h p.i., and supernatants from anti-TLR2-treated macrophages were assayed for IFN-β levels using an ELISA kit (BioLegend).
Griess assay.
Nitrite concentrations in culture media were measured with a commercial Griess assay kit (Biotium), and 150 μl of culture supernatant was removed from infected monolayers at 6, 12, and 24 h p.i. The samples were incubated with 20 μl Griess reagent [0.05% N-(1-naphthyl)ethylenediamine dihydrochloride, 0.5% sulfanilic acid, 2.5% phosphoric acid] in a 96-well plate at room temperature for 30 min. Absorbance was measured at 540 nm, and nitrite levels were calculated from a sodium nitrite standard curve.
Western blot analysis.
Infected RAW cells were treated with various reagents; washed with PBS at 6, 12, or 24 h p.i.; and then incubated in 50 μl lysis buffer (2% sodium dodecyl sulfate [SDS], 10% glycerol, 62 mM Tris, pH 6.8) containing a protease inhibitor minitablet (Pierce) for 10 min on ice. The cell lysates were boiled for 5 min, and protein samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated at 4°C overnight with rabbit iNOS MAb or rabbit GAPDH (glyceraldehyde-3-phosphate dehydrogenase) MAb and then incubated for 1 h with a secondary anti-rabbit–horseradish peroxidase conjugate. Antibodies were purchased from Cell Signaling Technology (Danvers, MA). Proteins were visualized using SuperSignal West Dura Chemiluminescent Substrate (Pierce) according to the manufacturer's instructions.
ROS assay.
ROS production of infected RAW cells in 96-well clear-bottom black plates (Corning) was assayed using the fluorogenic dye 2′,7′-dichlorofluorescein diacetate (DCFDA) (Abcam). Twenty micromolar DCFDA was mixed with culture media 45 min prior to measurement of fluorescence intensity (excitation and emission wavelengths, 485 nm and 535 nm).
Cathepsin B activity assay.
RAW macrophages were cultured on coverslips in 24-well plates. Infections and pharmacological treatments of RAW macrophages were performed. At 10 h p.i., reconstituted Magic Red cathepsin B substrate reagent MR-(RR)2 (Immunochemistry Technologies) was added directly to the culture media in the wells. After 30 min of treatment, nuclei were counterstained with Hoechst 33342 for 10 min. Coverslips were analyzed for cathepsin B activity by fluorescence microscopy (Leica DMI6000 B; 63× oil immersion objective). Fluorescence intensities were quantified from 10 images obtained from each of three independent experiments using ImageJ software (28).
Statistics.
Data were analyzed using Prism 6.0 software (GraphPad). For comparisons of multiple groups with two or more variables, data were subjected to log transformation and two-way analysis of variance (ANOVA) with Bonferroni posttest. Multiple comparisons for data with a single variable were analyzed by one-way ANOVA with Dunnett's posttest. Differences in values between two groups were determined using Student's t test. Differences were considered statistically significant when the P value was <0.05.
RESULTS
Productive C. muridarum infection of RAW macrophages is dependent on the multiplicity of infection.
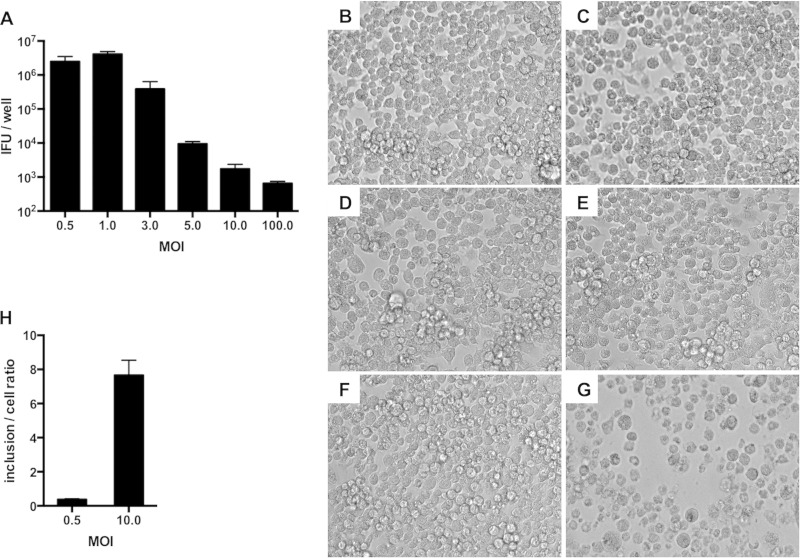
The effects of the MOI on C. muridarum IFU production were examined in RAW macrophages (Fig. 1A). At the lowest MOI (0.5), we observed a 2-fold increase in IFU recovery over input, which is in agreement with previous reports (24, 29). Maximal IFU recovery occurred when macrophages were infected at an MOI of 1. IFU recovery decreased at MOIs of 3 and higher. At moderate (10) and high (100) MOIs, IFU recoveries were approximately 0.001% and 0.0001% of input, respectively.
FIG 1.
C. muridarum is inhibited in RAW macrophages infected at a high MOI. (A) Macrophages were infected with C. muridarum at MOIs from 0.5 to 100. IFU assays were performed at 24 h p.i. (B to G) Light microscopy of macrophages either mock infected (B) or infected with C. muridarum at various MOIs (0.5 [C], 3 [D], 5 [E], 10 [F], or 100 [G]) at 18 h p.i. (H) Numbers of inclusions counted per cell in macrophages infected at different MOIs at 2 h p.i. The error bars represent standard deviations (SD).
To determine if reduced IFU recovery at moderate and high MOIs could be explained by host cell death, infected macrophages were examined by light microscopy (Fig. 1B to G). Cells infected at a high MOI exhibited rounding and cytotoxicity (Fig. 1G). However, most cells infected at a moderate MOI had normal morphologies (Fig. 1F) and released basal levels of LDH until late in the chlamydial developmental cycle (see Fig. S1 in the supplemental material). To test if low IFU recovery could be explained by reduced EB entry into macrophages, internalized EBs were quantified by fluorescence microscopy at 2 h p.i. The numbers of cytosolic EBs correlated with the input MOI (Fig. 1H). Overall, these findings suggested that moderate MOI infection triggered macrophage activation to inhibit chlamydial EB production.
Chlamydial inhibition in RAW macrophages requires de novo host protein synthesis.
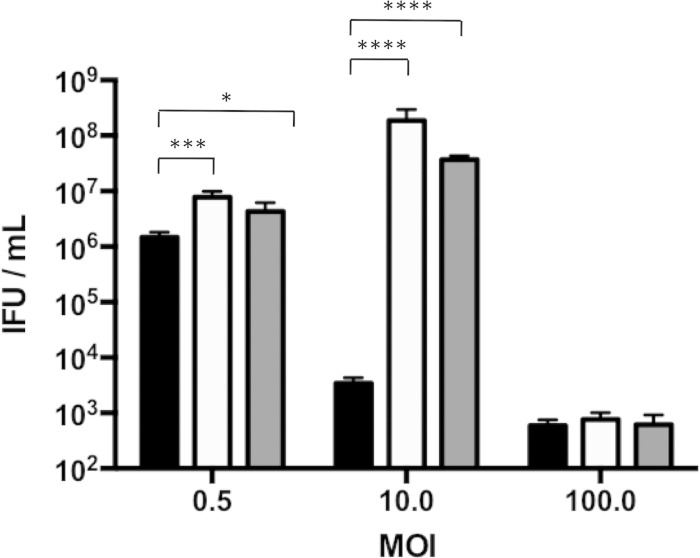
To elucidate the mechanism of C. muridarum growth inhibition in RAW macrophages, we initially tested if the antichlamydial factors were preformed. Infected macrophages were treated with cycloheximide to block protein synthesis either 4 h prior to or at the time of infection. Cycloheximide did not increase IFU production of macrophages infected at an MOI of 100 (Fig. 2). In contrast, cycloheximide treatment prior to and at the time of infection increased IFU production of macrophages infected at an MOI of 0.5 (low) by 3-fold and of macrophages infected at an MOI of 10 by more than 10,000-fold. These results implied that at low and moderate MOIs, C. muridarum induced macrophage defenses, whereas higher MOIs caused macrophage death.
FIG 2.
Inhibition of host protein synthesis rescues chlamydial growth in moderately infected macrophages. RAW macrophages were treated with 0.5 μg/ml cycloheximide 4 h before or at the time of infection at various MOIs. IFU assays were performed at 24 h p.i. Black bars, untreated; white bars, cycloheximide addition at 4 h prior to infection; gray bars, cycloheximide addition at 0 h p.i. ****, P < 0.0001; ***, P < 0.001; *, P < 0.05. Shown are means and SD.
Supernatants from RAW macrophages contain heat-sensitive antichlamydial factors.
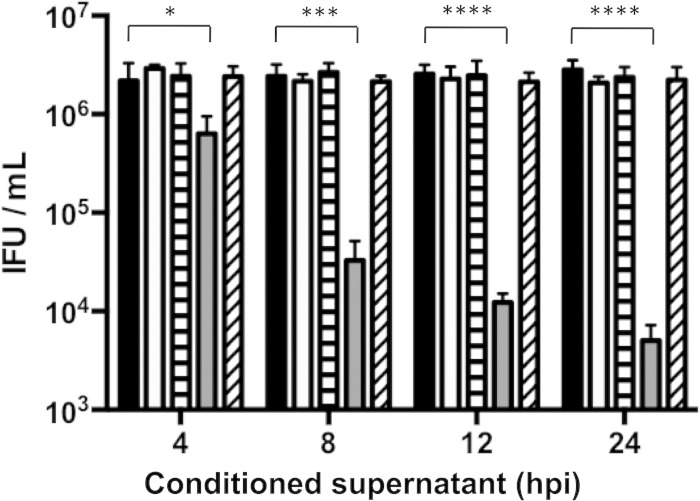
Activated macrophages release cytokines that can stimulate neighboring cells (30), so we tested if conditioned supernatants from infected macrophages could inhibit C. muridarum infection of newly infected macrophages. Conditioned supernatants from RAW cells infected at low or moderate MOIs were collected at various intervals postinfection. The conditioned supernatants were then transferred onto macrophages that had just been infected with C. muridarum at an MOI of 0.5 by centrifugation. Supernatants from low-MOI infections failed to inhibit chlamydial growth (Fig. 3). In contrast, supernatants from moderate-MOI infections strongly inhibited IFU production. The degree of this inhibition varied from 4-fold with 4-h p.i. supernatants to 1,000-fold with 24-h p.i. supernatants and was completely abolished if the supernatants were heated at 95°C for 5 min.
FIG 3.
Supernatants from moderately infected macrophages contain antichlamydial factors. RAW macrophages were infected with C. muridarum at an MOI of 0.5 or 10. Supernatants from these infections were removed at 4, 8, 12, or 24 h p.i. and transferred onto macrophages that had been freshly infected at an MOI of 0.5. Some supernatants were heated at 95°C for 5 min prior to transfer. IFU assays were performed on supernatant-treated macrophages at 24 h p.i. Black bars, mock treated; white bars, MOI = 0.5; horizontally hatched bars, MOI = 0.5 and heated; gray bars, MOI = 10; obliquely hatched bars, MOI = 10 and heated). ****, P < 0.0001; ***, P < 0.001; *, P < 0.05. Shown are means and SD.
Cytokine secretion from RAW macrophages varies with the C. muridarum EB dose and treatment.
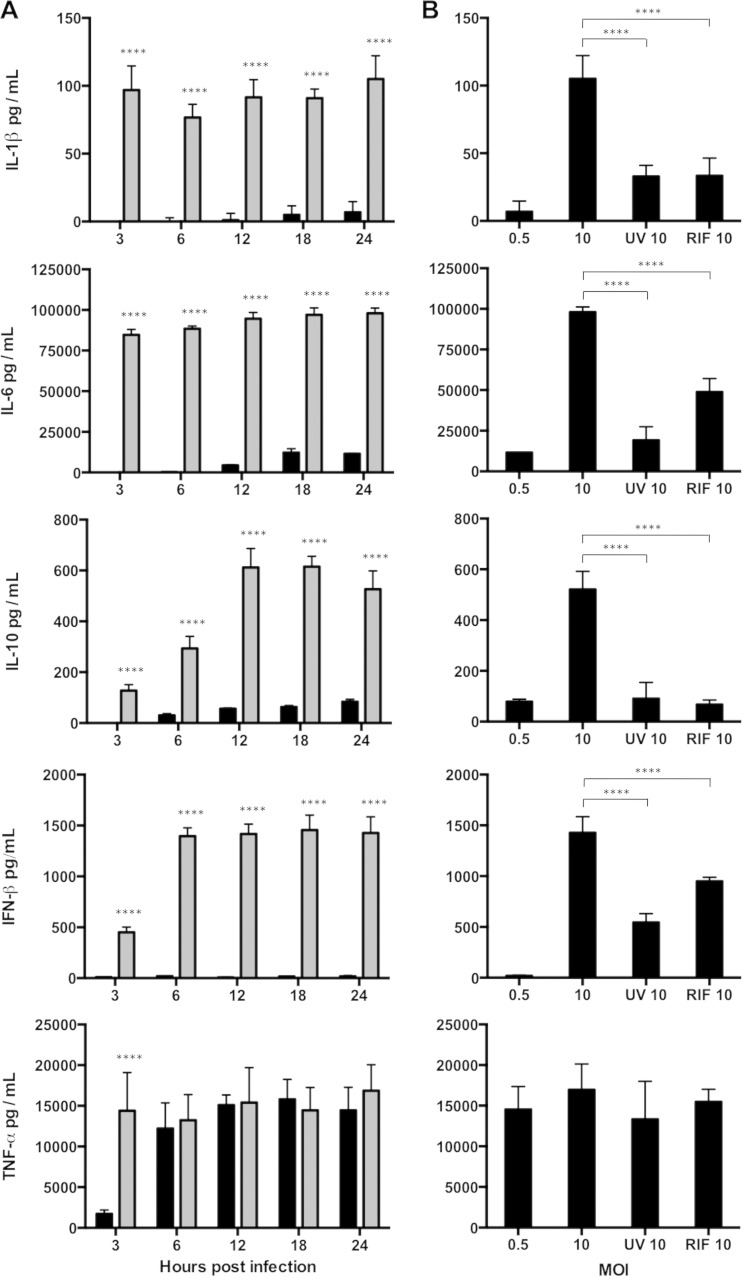
To attempt to identify the trans-acting inhibitory factor(s), we assayed cytokines in the conditioned supernatants of macrophages that had been infected at various MOIs. The levels of most of the cytokines produced by the moderately infected macrophages peaked by 3 h p.i. and were higher than those produced by the macrophages infected at low levels (Fig. 4A). High levels of TNF-α were detected at both MOIs, but the cytokine peaked earlier in supernatants from the macrophages infected at the higher MOI (Fig. 4A).
FIG 4.
Cytokine profiles of infected macrophages. (A) RAW macrophages were infected at an MOI of 0.5 (black bars) or 10 (gray bars). Supernatants were collected at 3, 6, 12, 18, and 24 h p.i. and assayed for cytokines. (B) Supernatants were collected at 24 h p.i. from RAW cells infected at an MOI of 0.5 or 10 with live EBs in the absence or presence of rifampin (RIF 10) or UV EBs (UV 10). ****, P < 0.0001. Shown are means and SD.
Since killed and viable EBs elicit dissimilar cytokine responses (31, 32), cytokines in supernatants from macrophages that were infected with nonreplicating chlamydiae at an MOI of 10 (wild-type EBs with rifampin or UV-inactivated EBs) were assayed. With the exception of TNF-α, cytokine levels were substantially lower than those observed in experiments with viable C. muridarum (Fig. 4B). Overall, the results of these experiments suggested that most cytokine secretion required an antigen not present in EBs and which was synthesized de novo by C. muridarum in the host macrophage.
IFN-β secreted by RAW macrophages induces antichlamydial responses.
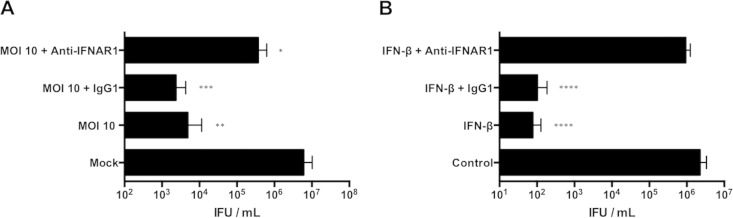
IFN-β has been previously shown to have an antichlamydial role in macrophages (33), so we tested if IFN-β secreted by moderately infected RAW macrophages stimulated host defense pathways. IFN-β signaling was blocked by treating RAW macrophages with an IFNAR1-neutralizing antibody (anti-IFNAR1), and these macrophages were then treated with conditioned medium from moderately infected macrophages. The anti-IFNAR1 antibody, but not an isotype control, increased the chlamydial yield 75-fold (Fig. 5A). Chlamydial inhibition mediated by the addition of exogenous IFN-β to macrophages infected at a low MOI was reversed by anti-IFNAR1, confirming the specificity of the antibody (Fig. 5B). Thus, our results indicated that IFN-β secretion in response to a moderate level of C. muridarum infection contributed to the paracrine upregulation of antichlamydial macrophage defense mechanisms.
FIG 5.
Secreted IFN-β inhibits C. muridarum in RAW macrophages. (A) IFU from macrophages infected with C. muridarum at an MOI of 0.5 and incubated with conditioned media from uninfected (mock) or moderately infected (MOI = 10) cells. Some wells were pretreated with anti-IFNAR1 or isotype control IgG1 antibody (Ab) (10 μg/ml). (B) IFU from macrophages infected with C. muridarum at an MOI of 0.5. Some wells treated with 500 U/ml IFN-β were preincubated with anti-IFNAR1 or isotype control IgG1 Ab. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05. Shown are means and SD.
Antichlamydial activity of RAW macrophages is mediated by TLR2, but not TLR4.
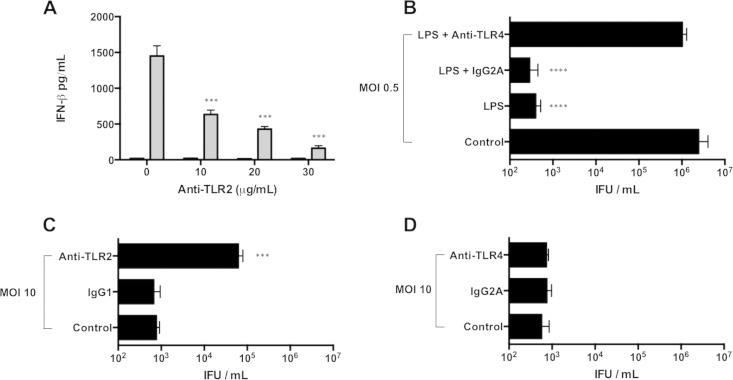
Chlamydial cell wall antigens and heat shock proteins induce TLR2 and, to a lesser extent, TLR4 signaling in phagocytes and epithelial cells (34, 35). To determine if TLR2 or TLR4 activation stimulated macrophage antichlamydial responses, we used antibodies to specifically block TLR2 (anti-TLR2) and TLR4 (anti-TLR4) responses. Control experiments were performed to establish that both antibodies effectively blocked cognate TLR function in RAW cells. Anti-TLR2 treatment of moderately infected macrophages reduced IFN-β production in a dose-dependent manner (Fig. 6A). Anti-TLR4 abolished chlamydial inhibition that was elicited by adding E. coli LPS to RAW macrophages infected with C. muridarum at a low MOI (Fig. 6B). However, only anti-TLR2 partially restored C. muridarum IFU production in macrophages infected at a moderate MOI (Fig. 6C and D). This indicated that the antichlamydial response of macrophages infected at moderate MOIs is primarily mediated by TLR2.
FIG 6.
TLR2, but not TLR4, mediates macrophage inhibition of C. muridarum at intermediate MOIs. (A) Macrophages pretreated with anti-TLR2 were infected at an MOI of 0.5 (black bars) or 10 (gray bars). IFN-β levels in supernatants at 24 h p.i. were measured by ELISA. (B) IFU from macrophages infected with C. muridarum at an MOI of 0.5 at 24 h p.i. Some wells exposed to E. coli LPS were treated with anti-TLR4 or IgG2A isotype control Ab (10 μg/ml). (C and D) IFU at 24 h p.i. from macrophages infected at an MOI of 10 treated with anti-TLR2 or IgG1 isotype control Ab (30 μg/ml) (C) and anti-TLR4 or IgG2A (D). ****, P < 0.0001; ***, P < 0.001. Shown are means and SD.
Chlamydial inhibition at moderate MOIs is mediated by iNOS and ROS.
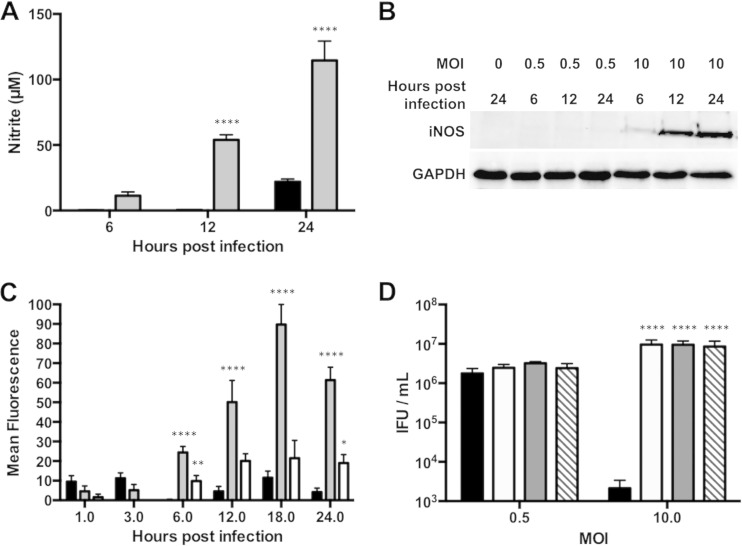
Macrophages activated by chlamydial infection upregulate expression of iNOS, an enzyme that catalyzes NO formation (29, 33, 36, 37). They can also accumulate high levels of ROS via the phagocyte NADPH oxidase complex (phox) or damaged mitochondria (38). To determine if iNOS was induced in RAW macrophages by C. muridarum infection, we measured the quantity of nitrites in culture supernatants. Higher nitrite levels were observed in supernatants from macrophages infected at a moderate MOI than from macrophages infected at a low MOI (Fig. 7A). The levels of iNOS protein measured by Western blotting correlated with supernatant nitrite levels (Fig. 7B). While iNOS was not detected in cells infected at a low MOI, iNOS was detected by 6 h p.i. in macrophages infected at a moderate MOI.
FIG 7.
Moderately infected RAW macrophages inhibit C. muridarum by producing nitric oxide and reactive oxygen species. (A) Supernatants and protein were collected at 6, 12, or 24 h p.i. from RAW cells infected at an MOI of 0.5 (black bars) or 10 (gray bars). Nitrite levels were quantified by Griess assay. (B) Western blotting for iNOS levels. (C) RAW cells infected at an MOI of 0.5 (black bars), 10 (gray bars), or 10 with DMTU (white bars) were assayed for ROS production at 1, 3, 6, 12, 18, and 24 h p.i. (D) RAW cells infected at an MOI of 0.5 or 10 were left untreated (black bars) or were treated with l-NMMA (white bars), l-NAME (gray bars), or DMTU (hatched bars). IFU assays were performed 24 h p.i. ****, P < 0.0001; **, P < 0.01; *, P < 0.05. Shown are means and SD.
The macrophage ROS response was assessed by incubating infected cells with the fluorogenic DCFDA. ROS production of macrophages infected at an MOI of 0.5 was low at all measured time intervals (Fig. 7C). However, macrophages infected at a moderate MOI produced strong fluorescence, which was almost entirely reversed by the addition of the hydroxyl radical scavenger DMTU.
To evaluate the chlamydicidal potential of macrophage-derived NO and ROS, infected macrophages were treated with the iNOS inhibitors l-NMMA and l-NAME or the hydroxyl radical scavenger DMTU. None of these compounds affected chlamydial recovery in low-MOI infections (Fig. 7D). However, both iNOS inhibitors and DMTU increased IFU production in the macrophages infected at a moderate MOI (39, 40). These results indicated that moderately infected RAW macrophages stimulated production of NO and reactive oxygen species that inhibited C. muridarum.
Ca-074Me, a cathepsin B inhibitor, rescues C. muridarum from antichlamydial macrophage responses.
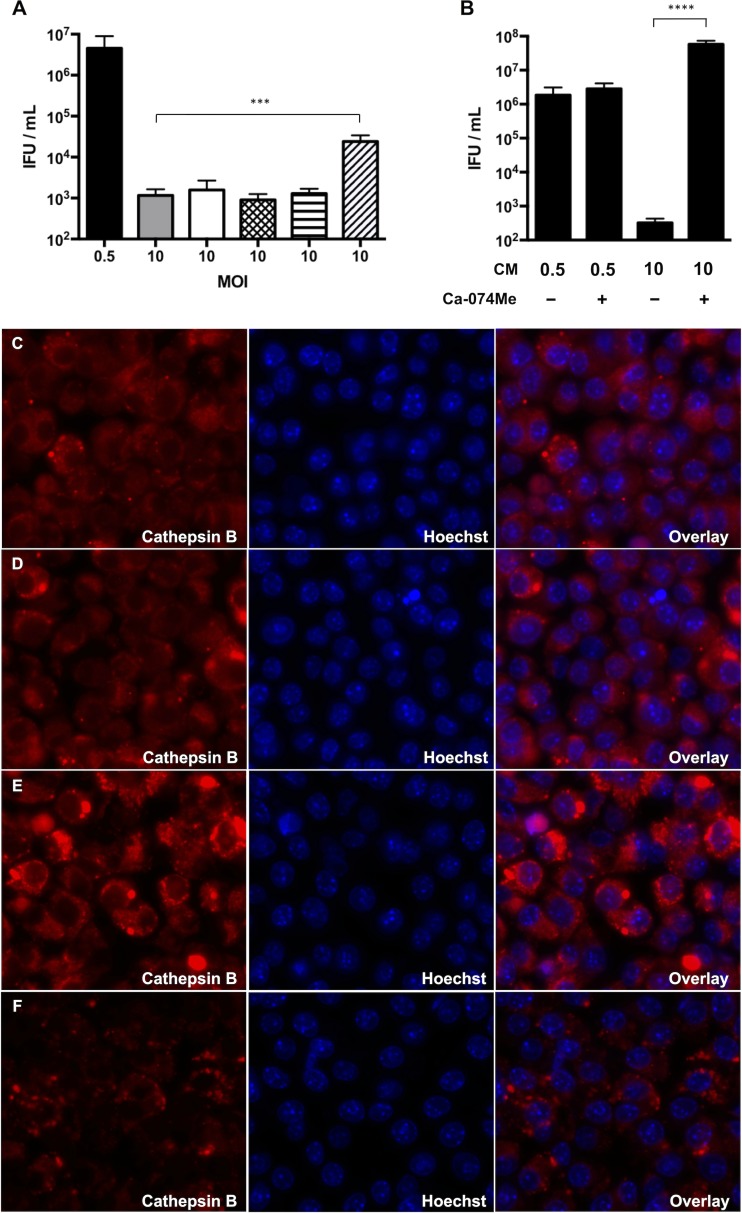
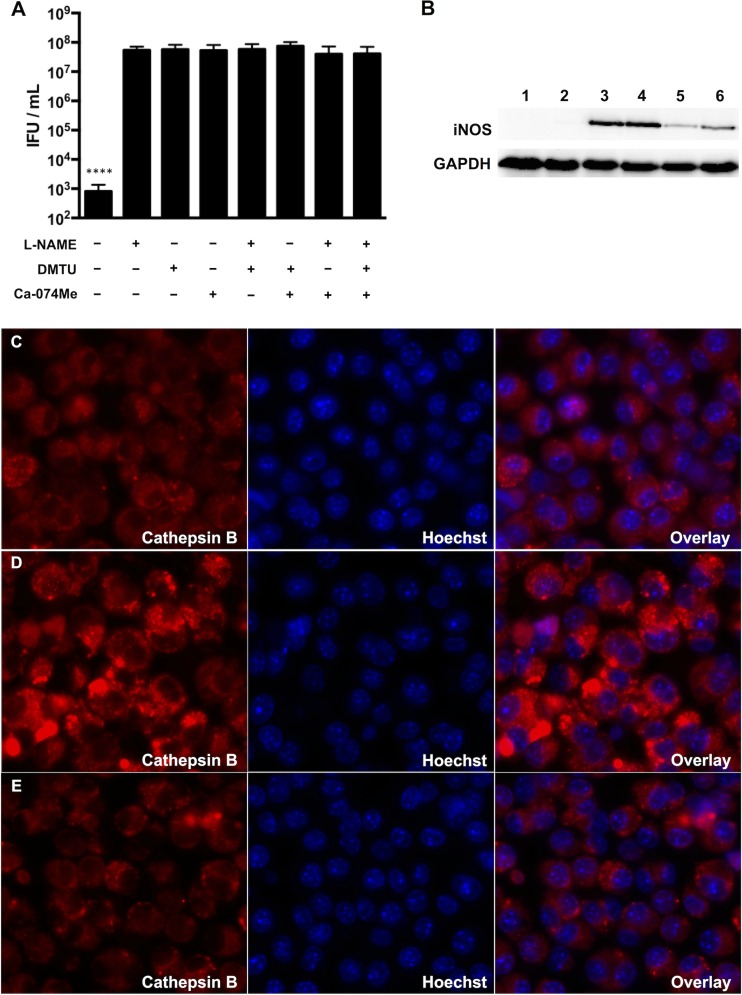
Many cytokines can stimulate macrophage iNOS expression, including caspase-1-dependent IL-1β (41–43). Since infected RAW macrophages secreted IL-1β, we evaluated its role, as well as that of caspase-1, in chlamydial inhibition using various inhibitors. Pretreatment of the macrophages with IL-1Ra, an IL-1β antagonist, did not influence chlamydial recovery (Fig. 8A). The caspase-1 inhibitor Ac-YVAD-CHO also failed to alter IFU production. However, the less specific caspase-1 inhibitor Z-WEHD-FMK increased IFU recovery in moderately infected macrophages approximately 10-fold. Z-WEHD-FMK can also inhibit lysosomal cathepsins (44), so we treated infected macrophages with the selective cathepsin B inhibitor Ca-074Me. Ca-074Me increased IFU production by 1,000-fold in moderately infected macrophages (Fig. 8B). Analysis of cathepsin B activity at 10 h p.i. using the fluorogenic substrate CV-(RR)2 indicated no difference in fluorescence intensity between mock-infected cells and cells infected at a low MOI (Fig. 8C and D; see Fig. S2 in the supplemental material). In contrast, cathepsin B activity was significantly increased in the macrophages infected at a moderate MOI, and this was reversed by the addition of Ca-074Me (Fig. 8E and F; see Fig. S2 in the supplemental material). Together, these results indicated that increased cathepsin B activity in RAW macrophages influences chlamydial IFU production.
FIG 8.
Ca-074Me, a cathepsin B inhibitor, rescues C. muridarum from macrophage inhibition. (A) RAW macrophages were infected at an MOI of 0.5 (black bar) or 10 (gray bar). Some cells infected at an MOI of 10 were treated with IL-1Ra at 50 μg/ml (white bar) or 100 μg/ml (crosshatched bar), Ac-YVAD-CHO (horizontally hatched bar), or Z-WEHD-FMK (obliquely hatched bar). (B) Treatment of macrophages infected at an MOI of 10 with CA-074Me. Shown are means and SD. (C to F) Representative live-microscopy images of cells at 10 h p.i. treated with the cathepsin B indicator dye MR-(RR)2 (red channel) and the nuclear stain Hoechst 33342 (blue channel). (C) Mock infected. (D) MOI = 0.5. (E) MOI = 10. (F) MOI = 10 with Ca-074Me. ****, P < 0.0001; ***, P < 0.001.
ROS and cathepsin B activities are necessary for maximal iNOS induction in C. muridarum-infected RAW macrophages.
Pharmacological inhibition of ROS, iNOS, and cathepsin B led to similar chlamydial recoveries. We asked if these effector mechanisms exerted additive effects, but combined inhibition did not improve chlamydial recovery beyond that observed with single-drug treatments (Fig. 9A). This suggested that they functioned in the same inhibitory pathway or that they played cooperative roles in chlamydial killing. To distinguish between these possibilities, we first analyzed iNOS protein and nitrite levels in moderately infected macrophages treated with l-NAME, DMTU, or Ca-074Me. l-NAME interferes with iNOS activity, but not expression. Western blots showed equal quantities of iNOS protein in untreated and l-NAME-treated cells, but nitrite levels were reduced in samples treated with l-NAME (Fig. 9B; see Fig. S3 in the supplemental material). However, both iNOS and nitrite levels were substantially lower in cells treated with DMTU or Ca-074Me. This indicated that ROS and cathepsin B controlled iNOS expression.
FIG 9.
iNOS expression in moderately infected macrophages is regulated by ROS and cathepsin B. (A) RAW macrophages infected at an MOI of 10 were treated with one of more of the following chemicals: l-NAME, DMTU, and Ca-074Me. Shown are means and SD. (B) Western blot for iNOS at 24 h p.i. from mock-infected cells (lane 1); cells infected at an MOI of 0.5 (lane 2), 10 (lane 3), or 10 with l-NAME (lane 4); cells infected at an MOI of 10 with DMTU (lane 5); and cells infected at an MOI of 10 with Ca-074Me (lane 6). (C to E) Representative live-microscopy images of cells at 10 h p.i. treated with the cathepsin B indicator dye MR-(RR)2 (red channel) and the nuclear stain Hoechst 33342 (blue channel). (C) Mock infected. (D) MOI = 10. (E) MOI = 10 with DMTU. ****, P < 0.0001.
We next investigated if ROS modulated cathepsin B activity or vice versa. Addition of DMTU to macrophages infected at a moderate MOI decreased cathepsin B activity to levels lower than those in mock-infected cells (Fig. 9C to E; see Fig. S4 in the supplemental material). Conversely, inhibition of cathepsin B with Ca-074Me in similarly infected macrophages caused a similar and small decrease in DCFDA fluorescence (see Fig. S5 in the supplemental material). However, since NO production was also inhibited in macrophages treated with Ca-074Me, we reasoned that the slight reduction in DCFDA intensity might be due to an absence of peroxynitrite. Indeed, DCFDA fluorescence levels did not differ between Ca-074Me- and l-NAME-treated macrophages (see Fig. S5 in the supplemental material). In summary, these results suggested that ROS production increased cathepsin B activity, which upregulated iNOS expression.
DISCUSSION
Members of the family Chlamydiaceae differ in their capacities to survive within macrophages, and this correlates with their ability to avoid fusion with lysosomes inside these cells (12). The ability to survive and replicate in macrophages has been linked to increased dissemination of C. trachomatis LGV strains, leading to systemic disease (13). Chlamydial persistence in macrophages can result in chronic inflammation and a delayed response to antibiotic treatment (14, 45). Thus, understanding the mechanisms that promote chlamydial survival in mononuclear phagocytes could have implications for the development of therapeutic strategies.
Chlamydial species that can evade phagolysosomal fusion within macrophages appear to do so only when host cells are infected at an optimal MOI. Several groups have reported reduced chlamydial recoveries from macrophages infected at MOIs of 100 or greater (12, 24). This correlated with immediate damage to the host cells and fusion of lysosomes with EB-containing phagosomes. In this study, we characterized the interactions of RAW 264.7 macrophages with C. muridarum and demonstrated that three different outcomes could be achieved in response to different MOIs. At an MOI of 1 or lower, RAW cells supported chlamydial replication. An MOI of 100 conferred immediate cytotoxicity, and few infectious chlamydial particles were recovered. Inhibition of host protein synthesis did not prevent chlamydial or host cell death. However, when RAW macrophages were infected at an intermediate MOI of 10, they did not succumb to early death and cleared C. muridarum infection by a process that could be reversed by cycloheximide.
Consistent with previous studies, we found that macrophage activation and subsequent clearance of C. muridarum were predominantly mediated by TLR2 (46, 47). The cognate chlamydial ligands for TLR2 have not been identified, but several candidates have been proposed in recent years. Recombinant chlamydial MIP-like protein can induce cytokine production via TLR2/TLR1/TLR6 and CD14 in human macrophages (48). Chlamydial LPS is less active than classic endotoxins but can elicit TLR2 signaling in vitro (49). Chlamydial Hsp60 induces inflammatory responses in mice in a TLR2- and TLR4-dependent fashion (34). Plasmid-deficient strains of C. trachomatis and C. muridarum exhibit impaired TLR2 responses, implying that one or more TLR2 antigens are either encoded or regulated by the plasmid (50, 51). Cytokine production via TLR2 signaling in epithelial cells also requires infection with live, replicating chlamydiae, indicating that the antigen is not present in EBs (32). Culture supernatants of RAW macrophages infected at an MOI of 10 were inhibitory and contained elevated levels of several cytokines, including IFN-β, which induced a paracrine antichlamydial pathway. Cytokine secretion by RAW cells required TLR2, as well as transcriptionally active chlamydiae.
Binding of microbial PAMPs to TLRs or other cytosolic pattern recognition receptors (PRRs) triggers cellular signaling events that culminate in the activation of NF-κB, mitogen-activated protein kinase (MAPK) or IRF3 pathways (52, 53). These in turn regulate the expression of genes that encode proinflammatory cytokines and iNOS. Aside from the direct induction of iNOS by PRR signaling, several cytokines can also regulate iNOS expression. Protective effects of nitric oxide in chlamydial infections have been well documented. Nitric oxide promotes IFN-γ-mediated eradication of chlamydiae and protects mice from the development of hydrosalpinx and infertility (54). Interestingly, TLR2/TLR4 double-deficient mice do not resolve C. pneumoniae lung infections because they do not induce iNOS, in spite of elevated IFN-γ secretion in these animals (55). This suggests that nitric oxide production relies on signaling via TLR2/TLR4 in the murine lung model of infection. We observed that RAW macrophages infected with C. muridarum at an MOI of 10 produced more iNOS and nitrite than cells infected an MOI of 0.5 and that this negatively impacted chlamydial survival. Our results are reminiscent of a study by Huang et al. in which the outcomes of low and high doses of intranasally inoculated chlamydiae in C57BL/6 mice were evaluated (56). Animals infected with higher numbers of C. psittaci remained healthier than those that were infected at low doses, and the accelerated bacterial clearance in heavily infected mice was linked to an increased NO production by macrophages. Comparable observations have been reported with C. muridarum infections of the murine genital tract, where high infectious doses result in less disease severity and fewer viable organisms in the oviducts (57). We speculate that similar macrophage activation programs control NO production in response to higher doses of infectious chlamydiae in both mice and cell culture.
We determined that iNOS induction in RAW macrophages was mediated by an increase in ROS production. Modulation of iNOS expression by ROS is not without precedent. High-MOI infection of macrophages with Propionibacterium acnes increases ROS levels, which in turn upregulate iNOS via NF-κB/AP-1 activation (58). Similar dependence on ROS for nitric oxide production has also been observed in mouse endothelial cells stimulated with IFN-γ and LPS (59). Multiple ROS sources exist within mammalian cells, including the NADPH oxidase complex and mitochondria. Whether ROS generated by RAW macrophages in our study was mitochondrion or NADPH oxidase derived was not determined. Several groups have reported a loss of mitochondrial membrane potential and integrity in cells invaded by pathogens (60). The subsequent mitochondrial ROS release can cause lysosomal membrane permeabilization (LMP), leading to the leakage of active lysosomal cathepsins into the cytosol. In agreement with these studies, we noted increased cathepsin B activity in RAW cells that was abolished by an ROS scavenger. However, a significant proportion of this cathepsin B activity at 10 h p.i. appeared to localize to large vesicular structures. Labeling with specific markers will be required to determine if these vesicles are lysosomes or also include EB-containing phagosomes that fused with lysosomes. Whether lysosomal cathepsin B leaks into the cytosol at later times due to ROS-mediated LMP requires further investigation. What is certain is that ROS affects cathepsin B activity in RAW macrophages. Surprisingly, the inhibition of cathepsin B led to a substantial decrease in iNOS and nitrite levels while also enabling chlamydial rescue. Pharmacological inhibition of iNOS did not greatly affect ROS levels or cathepsin B (unpublished observations) (see Fig. S5 in the supplemental material), indicating that chlamydial survival in RAW cells was dictated by the presence or absence of NO. However, we cannot rule out a direct role for ROS and cathepsin B in chlamydial inhibition, because chlamydiae damaged by reactive oxygen and nitrogen species might become more susceptible to lysosomal fusion and processing by cathepsin B.
Cathepsin B and ROS act in concert as innate immune effectors to activate NLRP3 inflammasome complexes, leading to IL-1β production (61). Inflammasome-derived IL-1β can stimulate iNOS-mediated nitric oxide production in macrophages to inhibit Leishmania spp. (42). Blocking IL-1β signaling in RAW macrophages did not reverse chlamydial killing or affect iNOS and nitrite levels (Fig. 8A and unpublished observations), so precisely how ROS and cathepsin B regulate iNOS expression in these cells remains unclear. A protective role for cathepsin B in immunity to C. muridarum disease in mice has not been described, and thus, future experiments will involve confirming our observations in primary macrophages and determining C. muridarum infection outcome in cathepsin B−/− mice.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant RO1AI099278 from the National Institutes of Health (D.E.N.).
We thank Stanley Spinola and Raymond Johnson for their experimental advice and critical review of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00382-15.
REFERENCES
- 1.Rusconi B, Greub G. 2011. Chlamydiales and the innate immune response: friend or foe? FEMS Immunol Med Microbiol 61:231–244. doi: 10.1111/j.1574-695X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 2.Darville T, Hiltke TJ. 2010. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 201(Suppl 2):S114–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyairi I, Laxton JD, Wang X, Obert CA, Tatireddigari VRRA, van Rooijen N, Hatch TP, Byrne GI. 2011. Chlamydia psittaci genetic variants differ in virulence by modulation of host immunity. J Infect Dis 204:654–663. doi: 10.1093/infdis/jir333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu H, Fan Y, Joyee AG, Wang S, Han X, Bai H, Jiao L, Van Rooijen N, Yang X. 2008. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J Immunol 181:2092–2102. doi: 10.4049/jimmunol.181.3.2092. [DOI] [PubMed] [Google Scholar]
- 5.Rothfuchs AG, Kreuger MR, Wigzell H, Rottenberg ME. 2004. Macrophages, CD4(+) or CD8(+) cells are each sufficient for protection against Chlamydia pneumoniae infection through their ability to secrete IFN-gamma. J Immunol 172:2407–2415. doi: 10.4049/jimmunol.172.4.2407. [DOI] [PubMed] [Google Scholar]
- 6.Sun HS, Eng EWY, Jeganathan S, Sin ATW, Patel PC, Gracey E, Inman RD, Terebiznik MR, Harrison RE. 2012. Chlamydia trachomatis vacuole maturation in infected macrophages. J Leukoc Biol 92:815–827. doi: 10.1189/jlb.0711336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell H, Pham OH, Li LX, Atif SM, Lee SJ, Ravesloot MM, Stolfi JL, Nuccio S-P, Broz P, Monack DM, Baumler AJ, McSorley SJ. 2014. Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity 40:213–224. doi: 10.1016/j.immuni.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CC. 1978. Cultures of Chlamydia trachomatis in mouse peritoneal macrophages: factors affecting organism growth. Infect Immun 20:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong EC, Chi EY, Kuo CC. 1987. Differential antimicrobial activity of human mononuclear phagocytes against the human biovars of Chlamydia trachomatis. J Immunol 139:1297–1302. [PubMed] [Google Scholar]
- 10.Manor E, Schmitz E, Sarov I. 1993. TNF and PGE(2) in human monocyte-derived macrophages infected with C. tracohmatis. Mediators Inflamm 2:367–371. doi: 10.1155/S0962935193000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold ES, Simmons RM, Petersen TW, Campbell LA, Kuo CC, Aderem A. 2004. Amphiphysin IIm is required for survival of Chlamydia pneumoniae in macrophages. J Exp Med 200:581–586. doi: 10.1084/jem.20040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyrick PB, Brownridge EA. 1978. Growth of Chlamydia psittaci in macrophages. Infect Immun 19:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EB, Custer RP. 1950. The histopathology of Lymphogranuloma venereum. J Urol 63:546–563. [Google Scholar]
- 14.de Vries HJC, Smelov V, Middelburg JG, Pleijster J, Speksnijder AG, Morre SA. 2009. Delayed microbial cure of Lymphogranuloma venereum proctitis with doxycycline treatment. Clin Infect Dis 48:E53–E56. doi: 10.1086/597011. [DOI] [PubMed] [Google Scholar]
- 15.Hoymans VY, Bosmans JM, Ieven MM, Vrints CJ. 2007. Chlamydia pneumoniae-based atherosclerosis: a smoking gun. Acta Cardiol 62:565–571. doi: 10.2143/AC.62.6.2024015. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschlag MR, Kohlhoff SA, Darville T. 2009. Chlamydia pneumoniae and Chlamydia trachomatis, p 27–52. In Fratamico PM, Smith JL, Brogden KA (ed), Sequelae and long-term consequences of infectious diseases. ASM Press, Washington, DC. [Google Scholar]
- 17.Fields KA, McCormack R, de Armas LR, Podack ER. 2013. Perforin-2 restricts growth of Chlamydia trachomatis in macrophages. Infect Immun 81:3045–3054. doi: 10.1128/IAI.00497-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Martinez FO, Gordon S. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rottenberg ME, Gigliotti-Rothfuchs A, Wigzell H. 2002. The role of IFN-gamma in the outcome of chlamydial infection. Curr Opin Immunol 14:444–451. doi: 10.1016/S0952-7915(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 20.Rothermel CD, Rubin BY, Murray HW. 1983. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol 131:2542–2544. [PubMed] [Google Scholar]
- 21.Shemer Y, Sarov I. 1985. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun 48:592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracey E, Inman RD. 2012. Chlamydia-induced ReA: immune imbalances and persistent pathogens. Nat Rev Rheumatol 8:55–59. doi: 10.1038/nrrheum.2011.173. [DOI] [PubMed] [Google Scholar]
- 23.Gracey E, Lin A, Akram A, Chiu B, Inman RD. 2013. Intracellular survival and persistence of Chlamydia muridarum Is determined by macrophage polarization. PLoS One 8:e69421. doi: 10.1371/journal.pone.0069421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo CC. 1978. Immediate cytotoxicity of Chlamydia trachomatis for mouse peritoneal macrophages. Infect Immun 20:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caldwell HD, Kromhout J, Schachter J. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun 31:1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayarapu K, Kerr MS, Katschke A, Johnson RM. 2009. Chlamydia muridarum-specific CD4 T-cell clones recognize infected reproductive tract epithelial cells in an interferon-dependent fashion. Infect Immun 77:4469–4479. doi: 10.1128/IAI.00491-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang DJ, Yang X, Berry J, Shen CX, McClarty G, Brunham RC. 1997. DNA vaccination with the major outer-membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis 176:1035–1040. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roshick C, Wood H, Caldwell HD, McClarty G. 2006. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect Immun 74:225–238. doi: 10.1128/IAI.74.1.225-238.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arango Duque G, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dessus-Babus S, Knight ST, Wyrick PB. 2000. Chlamydial infection of polarized HeLa cells induces PMN chemotaxis but the cytokine profile varies between disseminating and non-disseminating strains. Cell Microbiol 2:317–327. doi: 10.1046/j.1462-5822.2000.00058.x. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. 2006. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions—evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem 281:1652–1659. doi: 10.1074/jbc.M510182200. [DOI] [PubMed] [Google Scholar]
- 33.Rothfuchs AG, Gigliotti D, Palmblad K, Andersson U, Wigzell H, Rottenberg ME. 2001. IFN-alpha beta-dependent, IFN-gamma secretion by bone-marrow-derived macrophages controls an intracellular bacterial infection. J Immunol 167:6453–6461. doi: 10.4049/jimmunol.167.11.6453. [DOI] [PubMed] [Google Scholar]
- 34.Da Costa CUP, Wantia N, Kirschning CJ, Busch DH, Rodriguez N, Wagner H, Miethke T. 2004. Heat shock protein 60 from Chlamydia pneumoniae elicits an unusual set of inflammatory responses via Toll-like receptor 2 and 4 in vivo. Eur J Immunol 34:2874–2884. doi: 10.1002/eji.200425101. [DOI] [PubMed] [Google Scholar]
- 35.Ingalls RR, Rice PA, Qureshi N, Takayama K, Lin JS, Golenbock DT. 1995. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun 63:3125–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao SY, Ljunggren-Rose A, Stratton CW, Mitchell WM, Sriram S. 2001. Regulation by IFN-beta of inducible nitric oxide synthase and interleukin-12/p40 in murine macrophages cultured in the presence of Chlamydia pneumoniae antigens. J Interferon Cytokine Res 21:137–146. doi: 10.1089/107999001750133131. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Wang H, Ren J, Tang X, Jing Y, Xing D, Zhao G, Yao Z, Yang X, Bai H. 2012. IL-17A synergizes with IFN-gamma to upregulate iNOS and NO production and inhibit chlamydial growth. PLoS One 7:e39214. doi: 10.1371/journal.pone.0039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae YS, Oh H, Rhee SG, Do Yoo Y. 2011. Regulation of reactive oxygen species generation in cell signaling. Mol Cells 32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker NB, Berger EM, Curtis WE, Muldrow ME, Linas SL, Repine JE. 1985. Hydrogen peroxide causes dimethylthiourea consumption while hydroxyl radical causes DMSO consumption in vitro. J Free Radic Biol Med 1:415–420. doi: 10.1016/0748-5514(85)90155-2. [DOI] [PubMed] [Google Scholar]
- 40.Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. 2000. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol 58:1026–1034. [PubMed] [Google Scholar]
- 41.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, Vergnes L, Ojcius DM, Arditi M. 2011. Caspase-1 dependent IL-1 beta secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One 6:e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva ALN, Mineo TWP, Gutierrez FRS, Bellio M, Bortoluci KR, Flavell RA, Bozza MT, Silva JS, Zamboni DS. 2013. Inflammasome-derived IL-1 beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med 19:909–916. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 43.Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA. 1989. Identification of a monocyte specific pre-interleukin 1-beta convertase activity. Proc Natl Acad Sci U S A 86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman ZL, Leppla SH, Moayeri M. 2009. CA-074Me protection against anthrax lethal toxin. Infect Immun 77:4327–4336. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blasi F, Centanni S, Allegra L. 2004. Chlamydia pneumoniae: crossing the barriers? Eur Respir J 23:499–500. doi: 10.1183/09031936.04.00001004. [DOI] [PubMed] [Google Scholar]
- 46.Prebeck S, Kirschning C, Durr S, da Costa C, Donath B, Brand K, Redecke V, Wagner H, Miethke T. 2001. Predominant role of Toll-like receptor 2 versus 4 in Chlamydia pneumoniae-induced activation of dendritic cells. J Immunol 167:3316–3323. doi: 10.4049/jimmunol.167.6.3316. [DOI] [PubMed] [Google Scholar]
- 47.Darville T, O'Neill JM, Andrews CW, Nagarajan UM, Stahl L, Ojcius DM. 2003. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol 171:6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 48.Bas S, Lief L, Vuillet M, Spenato U, Seya T, Matsumoto M, Gabay C. 2008. The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J Immunol 180:1158–1168. doi: 10.4049/jimmunol.180.2.1158. [DOI] [PubMed] [Google Scholar]
- 49.Erridge C, Pridmore A, Eley A, Stewart J, Poxton IR. 2004. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via Toll-like receptor 2. J Med Microbiol 53:735–740. doi: 10.1099/jmm.0.45598-0. [DOI] [PubMed] [Google Scholar]
- 50.O'Connell CM, Ingalls RR, Andrews CW, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 51.O'Connell CM, Abdel Rahman YM, Green E, Darville HK, Saira K, Smith B, Darville T, Scurlock AM, Meyer CR, Belland RJ. 2011. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect Immun 79:1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchholz KR, Stephens RS. 2006. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell Microbiol 8:1768–1779. doi: 10.1111/j.1462-5822.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 53.Buchholz KR, Stephens RS. 2007. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect Immun 75:5924–5929. doi: 10.1128/IAI.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramsey KH, Miranpuri GS, Sigar IM, Ouellette S, Byrne GI. 2001. Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome. Infect Immun 69:5131–5137. doi: 10.1128/IAI.69.8.5131-5137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wantia N, Rodriguez N, Cirl C, Ertl T, Duerr S, Layland LE, Wagner H, Miethke T. 2011. Toll-like receptors 2 and 4 regulate the frequency of IFN gamma-producing CD4(+) T-cells during pulmonary infection with Chlamydia pneumoniae. PLoS One 6:e26101. doi: 10.1371/journal.pone.0026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang J, DeGraves FJ, Lenz SD, Gao DY, Feng P, Li D, Schlapp T, Kaltenboeck B. 2002. The quantity of nitric oxide released by macrophages regulates Chlamydia induced disease. Proc Natl Acad Sci U S A 99:3914–3919. doi: 10.1073/pnas.062578399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maxion HK, Liu W, Chang MH, Kelly KA. 2004. The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection. Infect Immun 72:6330–6340. doi: 10.1128/IAI.72.11.6330-6340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai HH, Lee WR, Wang PH, Cheng KT, Chen YC, Shen SC. 2013. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-kappa B and AP-1 activation in macrophages. J Dermatol Sci 69:122–131. doi: 10.1016/j.jdermsci.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Wu F, Tyml K, Wilson JX. 2008. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol 217:207–214. doi: 10.1002/jcp.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boya P, Kroemer G. 2008. Lysosomal membrane permeabilization in cell death. Oncogene 27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 61.Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD. 2013. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol 191:5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajaram K, Giebel AM, Toh E, Hu S, Newman JH, Morrison SG, Kari L, Morrison RP, Nelson DE. 2015. Mutational analysis of the Chlamydia muridarum plasticity zone. Infect Immun 83:2870–2881. doi: 10.1128/IAI.00106-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.