Abstract
Staphylococcus aureus is a human bacterial pathogen causing a variety of diseases. The occurrence of multidrug-resistant strains of Staphylococcus aureus underlines the need for a vaccine. Defining immune correlates of protection may support the design of an effective vaccine. We used a murine Staphylococcus aureus infection model, in which bacteria were inoculated in an air pouch generated on the back of the animal. Analysis of the air-pouch content in mice immunized or not with an adjuvanted multiantigen vaccine formulation, four-component S. aureus vaccine (4C-Staph), prior to infection allowed us to measure bacteria, cytokines, and 4C-Staph-specific antibodies and to analyze host immune cells recruited to the infection site. Immunization with 4C-Staph resulted in accumulation of antigen-specific antibodies in the pouch and mitigated the infection. Neutrophils were the most abundant cells in the pouch, and they showed the upregulation of Fcγ receptor (FcγR) following immunization with 4C-Staph. Reduction of the infection was also obtained in mice immunized with 4C-Staph and depleted of neutrophils; these mice showed an increase in monocytes and macrophages. Upregulation of the FcγR and the presence of antigen-specific antibodies induced by immunization with 4C-Staph may contribute to increase bacterial opsonophagocytosis. Protection in neutropenic mice indicated that an effective vaccine could activate alternative protection mechanisms compensating for neutropenia, a condition often occurring in S. aureus-infected patients.
INTRODUCTION
Staphylococcus aureus is a human bacterial commensal which is asymptomatically carried in the nares of 20 to 50% of the population. The bacterium can occasionally turn into an opportunistic pathogen, causing a variety of community- and hospital-acquired pathologies, including skin diseases, osteomyelitis, septic arthritis, endocarditis, and bacteremia (1). Although invasive diseases are generally extremely acute and severe, the greatest burden of morbidity is due to skin and soft tissue infections, which either can be uncomplicated and easily treatable or can spread to deeper tissues and require hospitalization and sometimes surgery (2). The current emergence of strains which are resistant to multiple antibiotics, i.e., methicillin-resistant S. aureus strains (3), makes the treatment of S. aureus infections more difficult, underlining the medical need for an S. aureus vaccine, which is not yet available.
Increasing our knowledge of S. aureus-induced pathogenesis and host immune responses to colonization and infection and identifying immunological correlates of protection are key steps toward the development of an effective vaccine that can protect against a wide spectrum of diseases (4, 5). We recently described an aluminum hydroxide-adjuvanted vaccine formulation, four-component S. aureus vaccine (4C-Staph), which included five S. aureus antigens: a genetically detoxified derivative of the secreted alpha-toxin hemolysin (Hla), two surface-exposed antigens, FhuD2 and Csa1A, and EsxAB, a protein fusion of two secreted proteins, EsxA and EsxB. This formulation was able to protect mice from infection in different murine models, and induction of functional 4C-Staph-specific antibodies seemed to play a major role in achieving protection (6). The use of systemic infections (abscess, peritonitis, and pneumonia models) or skin infections resulting in dermonecrosis hampered a deeper analysis of immune humoral factors and cellular components possibly associated with protection at the site of infection. Therefore, this prompted us to use an infection model that would allow the concomitant evaluation in situ of different parameters related to both infection and host response.
Different animal models have been used to study host immune responses to bacterial infections and the protective efficacy of vaccine candidates. Among them, an “air-pouch” murine model has been extensively used to study inflammation (7–9). This model was adapted further to evaluate the effect of fibrinogen depletion on S. aureus infections (10), as well as to study the host responses to group A Streptococcus infection, and the reduction of infection in mice immunized with specific antigens (11–13). The model is based on two dorsolateral subcutaneous injections of air to generate a “pouch” in which bacteria are subsequently inoculated, mimicking a skin infection. Then, the content of the pouch can be retrieved, allowing the evaluation of multiple parameters, such as the number of bacteria, the recruitment of host live immune cells, and the presence of antigen-specific antibodies and cytokine release.
Here we report that immunization of mice with 4C-Staph significantly contained S. aureus infection and reduced the production of inflammatory cytokines at the site of infection. Importantly, immunization with 4C-Staph contained S. aureus infection even in neutropenic mice. This result is surprising given the important role played by neutrophils during S. aureus pathogenesis. We found that 4C-Staph vaccination in neutropenic mice resulted in an increased recruitment of macrophages and monocytes at the infection site, which might compensate for the lack of neutrophils. These findings may have important implications for vaccine development since neutropenia in humans is one of the pathological conditions that make patients most vulnerable to S. aureus infection.
MATERIALS AND METHODS
Mice.
Female 5- to 8-week-old C57BL/6 mice were used. All animal studies were carried out in compliance with current Italian legislation on the care and use of animals in experimentation (Legislative Decree 26/2014) and with the Novartis Animal Welfare Policy and Standards. Protocols were approved by the Italian Ministry of Health (authorization 185/2011-B) and by the local Novartis Animal Welfare Body (authorization AWB 201105). After infection, mice were monitored daily and euthanized when they exhibited defined humane endpoints that were pre-established in agreement with Novartis Animal Welfare Policies.
Bacterial strains.
S. aureus Newman strain was grown in tryptic soy broth from an initial optical density at 600 nm (OD600) of 0.05. Bacteria were incubated at 37°C with shaking at 250 rpm until reaching an OD600 of 2 and then centrifuged at 3,320 × g for 10 min. The pellet was washed with 50 ml of phosphate-buffered saline (PBS; pH 7.4; Invitrogen) and finally appropriately diluted in PBS to obtain the required infectious dose/mouse. Inocula were confirmed by spreading sample aliquots on tryptic soy agar and enumerating colony formation the day after the challenge experiment.
Air-pouch model and immunizations.
Two dorsolateral subcutaneous 3-ml injections of air (at days 0 and 3) were performed to generate a single pouch in which 107 CFU (500-μl volume) of the S. aureus Newman strain were inoculated at day 5. Mice were sacrificed 48 h after challenge, and the pouches were injected with 1 ml of PBS, which was immediately withdrawn with the same syringe to perform CFU counting, cytofluorimetric analyses, and antibody and cytokine titrations. Volumes recovered from different mice were comparable.
For protection experiments, 5-week-old C57BL/6 female mice were actively immunized by intramuscular injection of 100 μl (50 μl in each quadriceps) containing a total of 10 μg of each 4C-Staph antigen adsorbed to 200 μg of aluminum hydroxide (alum; 2 mg/ml) on days 0, 14, and 28. Control mice received an equal volume of alum alone. Animals were bled 1 to 2 days before the first immunization and 8 days after the third immunization (2 days before the infection) to measure the levels of IgG against the 4C-Staph antigens. At 10 days after the last immunization, mice were injected in the pouch with the required bacterial dose.
Immune sera from 4C-Staph-immunized rabbits were used for passive immunization. New Zealand rabbits were immunized by intradermal injection of 50 μg of each 4C-Staph protein adsorbed to alum on days 0, 21, and 35. Rabbit immune serum samples (150 μl) were injected into the tail vein of 8-week-old C57BL/6 mice 24 h prior to challenge with S. aureus. Control mice were injected with the same volume of serum from rabbits immunized with alum.
Cell recruitment analysis.
The material retrieved from the air-pouch lavages was centrifuged at 300 × g for 5 min, and the supernatant was sterilized by 0.22-μm-pore-size filtration and stored at −20°C for antibody titration and cytokine profiling. The cell pellet was treated with Fc block (BD Pharmingen) to avoid nonspecific binding of antibodies for 20 min at room temperature, as specified by the manufacturer's instructions, and subsequently incubated for 20 min with a mix of antibodies that bind known proteins present on one or more cellular populations. The mix was composed of the following antibodies conjugated with the relative fluorochrome: Ly6C-FITC, CD335-PerCP-Cy5.5, Ly6G-PE, and CD11b-APC (Pharmingen); F4/80-V450, CD3-PE-Cy7, MHC-II-A700, and CD11c-APC-eFluor 780 (eBioscience); and Live/Dead yellow (Invitrogen). In each experiment, we included a sample stained with the relative isotype to verify any nonspecific binding: rat IgG1-FITC, rat IgG2a-PerCP-Cy5.5, rat IgG2a-PE, and rat IgG2a-APC (Pharmingen); rat IgG2a-V450, Armenia hamster PE-Cy7, rat IgG2b-A700, and Armenia hamster IgG-isotype APC-eFluor 780 (eBioscience); and Live/Dead yellow (Invitrogen).
To determine their activation status, cells retrieved from the pouch lavages were stained using a mix composed of the following antibodies: Ly6G-PE-CF594, CD11b-BV510, CD80-PE, and Ly6C-FITC (Pharmingen); CD16/32-APC, CD21/35-PerCP-eFluor710, CD86-PE-Cy5, MHC-II-A700, and F4/80-eFluor450 (eBioscience); and Live/Dead yellow (Invitrogen). In each experiment, we included a sample stained with the relative isotype to exclude any nonspecific binding: rat IgG2a-PE-CF594, rat IgG2b-BV 510, hamster IgG2-PE, and rat IgG1-FITC (Pharmingen); rat IG2a-APC, rat IgG2a-PerCP-eFluor710, rat IgG2a-PE-Cy5, rat IgG2b-A700, and rat IgG2a-eFluor450 (eBioscience); and Live/Dead yellow (Invitrogen). For each antibody, the best working dilution was previously determined. In the activation experiments, treatment with Fc block was not performed.
Acquisition of labeled cells was performed using a FACS LSRII cytometer (Becton Dickinson). BD TruCount Absolute counting tubes were used to determine the absolute cell number for each population, according to the manufacturer's instructions. The data analysis was performed using the software Flow Jo 9.3.3.2, based on the expression of one or more of the cellular markers on the selected cell populations, following the gating strategy indicated in Fig. S1 in the supplemental material.
Specific IgG measurement and cytokine profiling.
The Luminex technology was used to evaluate the level of IgG specific for each antigen of the 4C-Staph in sera and pouch lavages. Antigens were covalently conjugated to the free carboxyl groups on microspheres according to the manufacturer's instructions. Pouch lavages were diluted 1:200, and sera were diluted 1:10,000 in PBS, followed by incubation with the microsphere for 30 min at room temperature. After washing, microspheres were incubated for 15 min at room temperature with phycoerythrin (PE)-conjugated anti-mouse IgG (1:200), washed, and suspended with 200 μl of PBS. Samples were analyzed by Luminex 200 (Merck-Millipore). The median fluorescence intensity (MFI) at 575 nm was directly proportional to the amount of antibody bound to the microspheres. The limit of detection (LOD) was calculated as the mean plus three standard deviations of the MFI values registered in the sample obtained from alum-immunized mice. The LOD values for HlaH35L, FhuD2, Csa1A, and EsxAB were, respectively, 25.6, 5.2, 7, and 1.6 in the pouch lavages and 31.8, 8.4, 6.7, and 26 in sera. Cytokine concentrations in the pouch lavages were determined using the Bio-Plex Pro mouse cytokine 23-plex immunoassay according to the manufacturer's instructions (Bio-Rad).
Neutrophil depletion.
One day before the infection, mice received a single intraperitoneal injection with 0.25 mg of endotoxin- and sodium azide-free rat-purified anti-mouse Ly6G (BD Pharmingen). Control mice received an equal amount of purified Rat IgG2ak isotype control (BD Pharmingen). Neutropenia was verified by fluorescence-activated cell sorting (FACS) analysis, according to the gating strategy depicted in Fig. S1 in the supplemental material, in all of the depletion experiments performed.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 5 software and the Mann-Whitney two-tailed test. P values of <0.05 were considered statistically significant.
RESULTS
Setup of a murine air-pouch model of S. aureus infection.
In initial experiments, we infected groups of C57BL/6 mice in the pouch with different doses of the S. aureus Newman strain, ranging from 5 × 106 to 1.5 × 109 CFU. Animal health condition, bacterial CFU, and recovery of live recruited immune cells were evaluated at 24 h, at 48 h, and at longer time points after infection. Eventually, an infectious dose of 107 and the 48-h time point were chosen, even though a high infection variability among mice was observed (Fig. 1A). Under these conditions, mice did not show any major sign of disease, and sufficiently high numbers of bacteria and live immune cells were recovered, which would allow CFU counting and cytofluorimetric analysis of the different immune cell populations. Using higher infectious doses decreased infection variability among mice (data not shown), but most of them showed severe symptoms or died; using lower bacterial doses resulted in the infection being naturally cleared by the immune system, in the absence of any vaccination (see Table S1 in the supplemental material). Time points later than than 48 h were excluded since skin lesions were progressing to ulceration and full recovery of CFU and of live immune cells were hampered (data not shown).
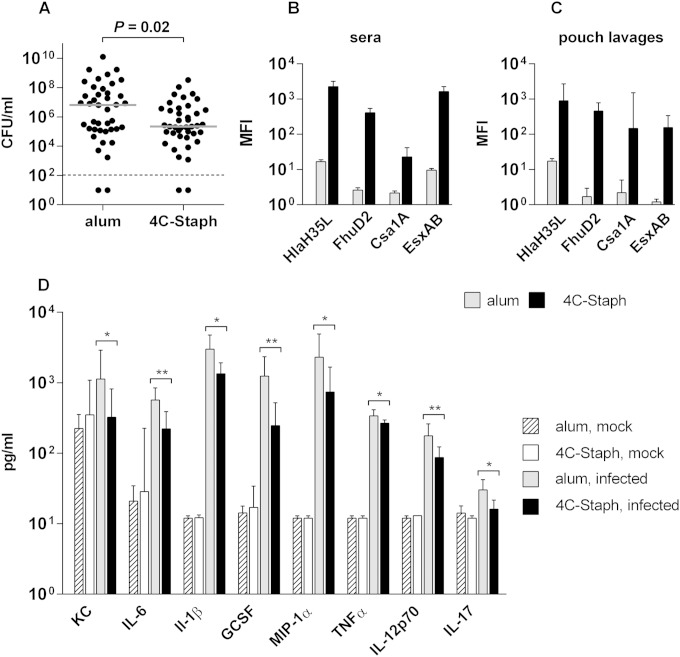
FIG 1.
4C-Staph-immunized mice show antigen-specific antibodies in the pouch and mitigate the infection and the inflammation in situ. (A) CFU measured in the pouch lavage 48 h postinfection. CFU values obtained for single mice are represented by black dots, while gray lines indicate the median values. The statistical significance was assessed with a two-tailed Mann-Whitney test, and the P value is indicated. (B and C) Bar graphs reporting the antibody responses elicited against each antigen of the 4C-Staph and measured in the sera (B) and air-pouch lavages (C) of animals immunized with either alum or 4C-Staph. Bars indicate the geometric mean fluorescence intensities (MFIs) with the 95% confidence intervals (CI) obtained from four independent experiments for a total of 63 mice for sera and one representative experiment with 6 mice for pouch lavages. Groups immunized with alum highlight the assay background threshold for each antigen. (D) Bar graph showing cytokine levels measured at 48 h postinfection in the pouch lavages of mice immunized with either alum or 4C-Staph and then either mock infected or infected with S. aureus Newman. Bars indicate the geometric mean (with 95% CI) for four S. aureus infection experiments (19 to 23 mice/group) and one representative experiment for mock-infected mice (6 mice/group). The statistical analysis was performed using the Mann-Whitney two-tailed test (*, P < 0.05; **, P < 0.01).
The protective efficacy of the 4C-Staph was then tested under the selected experimental conditions. Figure 1A shows the results obtained with two groups of C57BL/6 mice immunized with either 4C-Staph or alum alone, as a negative control, and infected in the air pouch with 107 CFU of the S. aureus Newman strain. At 48 h after infection, mice were sacrificed, and the number of CFU in the pouch lavage was evaluated, demonstrating a statistically significant reduction (P = 0.02) in mice immunized with 4C-Staph compared to the value for the animal control group immunized with alum. Therefore, S. aureus administration in the air pouch represents a model that is suitable for evaluating vaccine-induced protection against S. aureus infection and to study the immune mechanisms regulating this protection.
In situ analysis of the host immune response.
Having established that vaccination of mice resulted in reduction of CFU, we further investigated the host immune responses. Sera and pouch lavages were collected from immunized mice to measure 4C-Staph-specific antibodies and secreted cytokines. Figure 1B shows that immunization of mice prior to infection elicited detectable serum antibodies for each antigen and that significant levels of antigen-specific antibodies were measured in the air pouch (Fig. 1C). Cytokines, as well as host immune cells recruited in the pouch, were also evaluated in immunized and infected mice at 48 h postinfection. A set of 23 specific cytokines and chemokines involved in inflammation and immunity were analyzed. Although the levels of interleukin-12p70 (IL-12p70), IL-17, granulocyte colony-stimulating factor (G-CSF), gamma interferon, KC, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) increased in the pouch lavage specimens following bacterial infection compared to preinfection levels, immunization with 4C-Staph significantly reduced the in situ levels of some of these cytokines compared to alum alone (Fig. 1D).
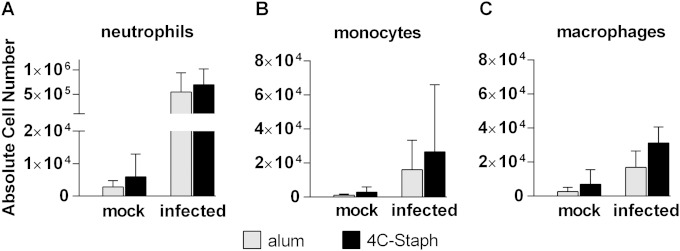
Recruitment in the pouch of host immune cells was also investigated. A basal level of live leukocytes could be detected in the pouch lavage specimens of noninfected animals independently of the vaccination status. When cells were stained for viability and for different cellular markers using the cytofluorimetric analysis strategy depicted in Fig. S1 in the supplemental material, multiple cell populations were identified, in particular neutrophils, eosinophils, macrophages, dendritic cells, natural killer, B and T cells. At 48 h after infection, the total number of live cells significantly increased in the pouch, with neutrophils, monocytes, and macrophages showing the sharpest increase (Fig. 2), in agreement with the role that these cell types have in the initial phases of the host response against the infection. Neutrophils were the most abundant cell population (ca. 90%), followed by monocytes, macrophages and eosinophils (see Fig. S2 in the supplemental material). The lymphocyte compartment represented only ca. 1% of the total pouch lavage fluid. No significant differences were observed between mice immunized with 4C-Staph and those immunized with alum.
FIG 2.
Host immune cells recruited in the pouch of infected mice. Bar graphs report the absolute cell numbers of neutrophils (A), monocytes (B), and macrophages (C) in mice immunized either with alum or with 4C-Staph and then either mock infected or infected with the Newman strain. Bars represent the geometric mean with the 95% CI from two to five independent experiments (12 to 30 mice/group).
Unraveling the role of different immune components in protection against S. aureus.
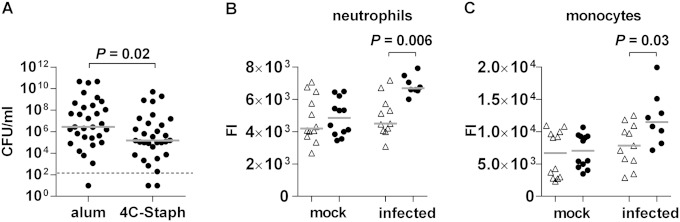
We have recently shown that passive administration of 4C-Staph-specific polyclonal antiserum protected mice from S. aureus infection in different disease models (6). The finding that antigen-specific antibodies were measured in pouch lavage specimens prompted us to assess the protective role of the humoral response in the air-pouch model as well. To this end, rabbit antisera raised against either 4C-Staph or alum alone were injected intravenously in naive mice 24 h before infection in the pouch with S. aureus, and CFU counts were assessed 48 h later. Passive administration of 4C-Staph-specific antiserum significantly reduced the number of CFU in the pouch lavage specimens (Fig. 3A), confirming that antibodies play a role in protecting mice from S. aureus infection in situ.
FIG 3.
4C-Staph elicits functional antibodies and increases the expression of FcγR III/II on neutrophils and monocytes. (A) CFU measured in the pouch lavage specimens of mice passively immunized with rabbit antisera raised against either alum or 4C-Staph. CFU counts in individual animals are represented by black dots (three independent experiments, 31 mice). Statistical analyses were performed using a two-tailed Mann-Whitney test. (B and C) Graphs showing the FACS fluorescence intensity signals (FI) detected with an anti-CD16/32 antibody directed against the FcγR III/II receptors on neutrophils and monocytes in the pouch lavage specimens of mice immunized either with alum (△) or with 4C-Staph (●) and then either mock infected or infected with the Newman strain. Gray lines indicate the median among independent experiments. Statistical analyses were performed using a Mann-Whitney two-tailed test.
On the other hand, the definition of a possible role, if any, in the early response to the infection of the immune cell types identified in the pouch was not immediately obvious. Cytofluorimetric analyses of the cellular repertoire in the pouch upon infection evidenced intense cell recruitment at the infection site, although no quantitative differences could be observed between 4C-Staph- and alum-immunized mice. We therefore investigated whether immunization with 4C-Staph might induce qualitative changes in the recruited immune cell populations contributing to a successful immune response. Levels of expression of different activation markers such as Fcγ receptor (FcγR) III/II (CD16/32), complement receptor (CR1/CR2) (CD35/21), and costimulatory molecules CD80 and CD86 were analyzed on the surface of monocytes, macrophages, and neutrophils, the three most abundant cell populations in the pouch. Neutrophils and monocytes showed a significantly higher expression of FcγR III/II that was dependent on immunization with 4C-Staph, followed by the infection (Fig. 3B and C). Increased levels of FcγR III/II expression were not observed in cells from the pouches of noninfected or of alum-vaccinated and infected mice. No changes in levels of expression were detected for CR1/CR2, CD80, and CD86 molecules, additional activation markers for the myeloid lineage (data not shown).
4C-Staph vaccination mitigates S. aureus infection in neutropenic mice.
Our observations that (i) neutrophils were the most abundant cell population recruited into the pouch and (ii) they were activated in 4C-Staph-immunized mice after infection well matched previous data reporting on the importance of neutrophils in controlling S. aureus infections. This encouraged us to confirm the key role of these cells in the air-pouch model by performing experiments with mice depleted of this cell population.
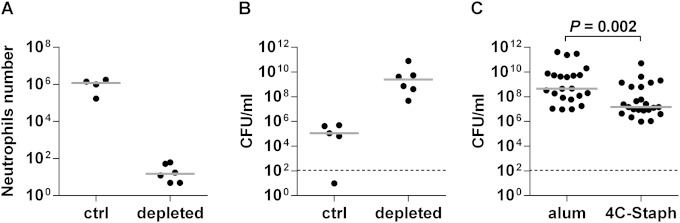
Neutrophil depletion (>99.8%) was obtained by treating mice once 24 h before infection with purified α-Ly6G antibody (Fig. 4A). Under these conditions, mice were more susceptible to S. aureus infection, as confirmed by the higher number of CFU measured in the pouch lavages (Fig. 4B) and were more evenly infected (compare with Fig. 1A), similar to what we previously observed in immunocompetent mice when infected with doses higher than 107 (data not shown).
FIG 4.
Immunization of neutrophil-depleted mice with 4C-Staph mitigates the infection in situ. (A) Number of neutrophils in the pouch lavage specimens of mice 3 days after treatment with either anti-Ly6G monoclonal antibody (depleted) or isotype-matched control (ctrl). (B) CFU at 48 h postinfection in the pouch of mice treated with anti-Ly6G or isotype-matched control. (C) CFU at 48 h postinfection in the pouch of anti-Ly6G-treated mice immunized either with alum or with 4C-Staph. Gray lines indicate the median either in single experiments (A and B) or from four independent experiments (C). The statistical significance was calculated using a two-tailed Mann-Whitney test.
In order to confirm the major role played by neutrophils in containing the infection, we immunized mice either with 4C-Staph or with alum, followed by neutrophil depletion and infection with S. aureus. Surprisingly, a significant reduction (P = 0.002) in the CFU count was observed in the pouch lavage specimens of neutropenic animals immunized with 4C-Staph compared to counts for the control group of neutropenic mice vaccinated with alum (Fig. 4C). The median decrease in bacterial load in the vaccinated group was ∼1.5 log, similar to what we had observed in immunocompetent mice (compare to Fig. 1A), although the median value obtained in neutropenic mice was higher due to their increased susceptibility to infection. Attenuation of the infection could not reflect differences in the depletion process of mock-immunized and 4C-Staph-immunized mice, since we confirmed a nonsignificant difference in the neutrophil numbers of the two groups (649 and 805 neutrophils per mouse, respectively, calculated as the geometric mean for 16 mice obtained from three independent experiments).
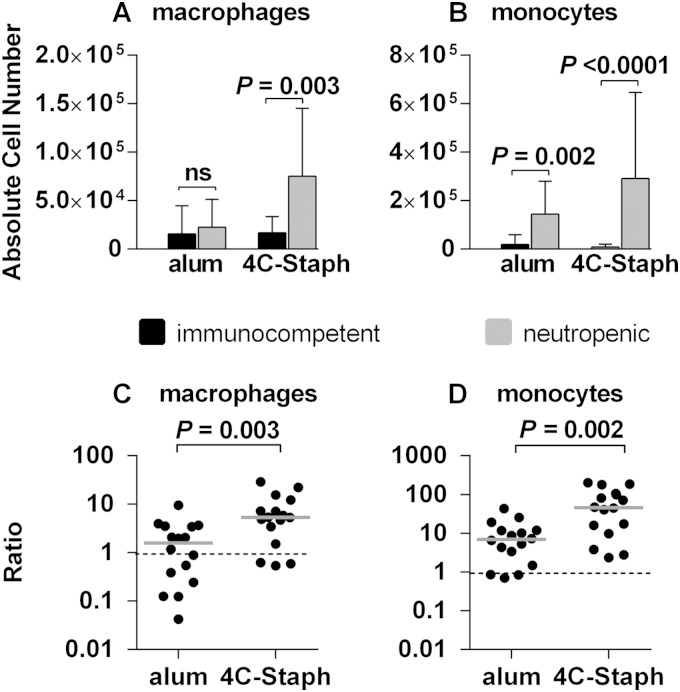
We hypothesized that in the absence of neutrophils, the immunization with 4C-Staph may have activated a compensatory protection mechanism(s) that mediated the control of the infection. In the attempt to support this hypothesis, we analyzed the panel of immune cells recruited in the pouch lavage specimens of neutropenic mice 48 h postinfection in both 4C-Staph- and alum-immunized mice, and we compared these results to those obtained in analogous groups of fully immunocompetent mice. Meaningful differences were measured for macrophages and monocytes. In fact, the absolute number of macrophages was significantly increased in neutropenic mice immunized with the vaccine (Fig. 5A), while monocytes of neutropenic mice increased both in mock-immunized and in vaccine-immunized animals (Fig. 5B). To better evaluate whether these increases could be specifically related to vaccination with 4C-Staph, we calculated for the two cell populations the ratio between the absolute cell number values recovered from each neutropenic mouse and the median of the absolute values of the same cell population recovered in the immunocompetent group, both for immunized and mock-immunized mice. Therefore, in Fig. 5C and D, a ratio higher than 1 indicated that the cell population was more abundant in neutropenic mice than in the immunocompetent counterpart. Infection of alum-immunized mice caused an increase in the number of monocytes (Fig. 5D) but not of macrophages (Fig. 5C). Interestingly, immunization with 4C-Staph resulted in significant increases of the monocytes and macrophages populations (C and D). These results suggested that monocytes and macrophages might compensate for neutrophil deficiency and mediate 4C-Staph-dependent protection.
FIG 5.
Immunization with 4C-Staph increases macrophage and monocyte recruitment in the pouch of neutropenic mice. (A and B) Absolute cell numbers of macrophages (A) and monocytes (B) in immunocompetent and neutropenic mice either immunized with 4C-Staph or mock immunized; (C and D) ratios between the absolute cell numbers of macrophages (C) and monocytes (D) of each neutropenic mouse and the medians of the absolute cell numbers of the same populations recovered in the corresponding immunocompetent groups. Ratios obtained for individual neutropenic mice are reported for groups immunized either with alum or with 4C-Staph. Gray lines indicate the median values of the ratios for each group (three independent experiments). The statistical significance was calculated using a two-tailed Mann Whitney test, and P values are indicated.
DISCUSSION
The approaches used thus far to develop a vaccine against S. aureus infections have been unsuccessful (14) and the definition of new strategies to achieve this goal is an urgent priority (4, 15). Development of an efficacious S. aureus vaccine appears to be a particularly challenging task since the pathogen has evolved to infect a variety of host microenvironments (1) and evade the immune system (16–18). Vaccine design is further complicated by the fact that the type of immune response which may be protective in humans and the possible correlates of protection from S. aureus infections are not known. In this context, development of suitable preclinical animal models can be powerful tools to address these issues (15, 19).
Here we describe a murine air-pouch skin model of S. aureus infection that we used to characterize the host immune responses to S. aureus infection, to evaluate the efficacy of an alum-adjuvanted multiprotein vaccine formulation (4C-Staph) that we recently described as protective in multiple animal models of S. aureus infection (6), and to identify immunological mechanisms stimulated by the vaccine that could possibly contribute to protection.
The observation that immunization of mice with 4C-Staph resulted in a 2-log reduction of the bacterial burden in the pouch (Fig. 1) confirmed that the model was appropriate to attempt the dissection of the different immune components contributing to the containment of the infection. Because of the evolution of the skin lesion over time, which resulted in damage of the pouch and hampered the full recovery of bacteria and immune cells, we restricted our observations to 48 h postinfection. Our analyses therefore concentrated on the early response to the infection, focusing on components of both innate and adaptive immunity (cytokines and chemokines, immune cells, and vaccine-specific antibodies), possibly highlighting interplay between them.
As expected, S. aureus infection caused an increase in a panel of innate cytokines, e.g., IL-1β, IL-6, IL-12p70, and TNF-α, and chemo-attractants, e.g., G-CSF, MIP-1α, KC, and IL-17, in the pouch (Fig. 1D) that were associated with the recruitment of neutrophils, monocytes, and macrophages (Fig. 2 and see Fig. 2S in the supplemental material). Interestingly, immunization with 4C-Staph significantly attenuated these increases, consistent with the reduction of the bacterial load in the pouch (Fig. 1A) and consequently with a lower inflammation status.
Evidence is available suggesting the importance of antibodies in controlling S. aureus infections: in vitro and in vivo antibody-dependent protection have been reported for S. aureus antigens (20–23), antibody titers against S. aureus have been shown to rise during infections (24), and immunoglobulin deficiencies were related to an increase in susceptibility to the pathogen (25). Nevertheless, limited correlations have been established between antibody levels and disease severity or protection (22, 26). The presence of 4C-Staph-specific antibodies in the pouch and the reduction in the bacterial load obtained by passive immunization of mice (Fig. 1C and 3A) suggested that antigen-specific antibodies may play an important role, contributing to early containment of the infection in the pouch.
In spite of the lack of knowledge on the mechanisms of protection against S. aureus infections, the role of polymorphonuclear neutrophils (PMNs) and antigen-specific antibodies is acknowledged. PMNs represent the initial and foremost defense against S. aureus, phagocytizing opsonized bacteria, killing them in the phagosome, and undergoing programmed cell death before being removed by macrophages, which contributes to a positive resolution of the infection (27, 28). Different animal models of S. aureus infections have confirmed the positive role played by PMNs, both in invasive (septicemia and septic arthritis) (29) and in cutaneous infections (30). The importance of PMNs has been further corroborated by the observation that neutrophil abscess formation is required for bacterial clearance in humans and that either a reduced number or an impaired functionality of PMNs can result in increased susceptibility to S. aureus infection in various tissues and organs (5, 31).
Consistent with these reports, we observed that PMNs represented 90% of the immune cells recruited in the pouch 48 h after the infection, followed by monocytes, macrophages and eosinophils (Fig. 2 and see Fig. 2S in the supplemental material). Recruitment of these cell populations is also in agreement with the increased levels of chemo-attractants detected in the pouch (Fig. 1D). Remarkably, no quantitative differences in immune cell recruitment were observed in mice immunized with 4C-Staph and in which bacterial load had significantly decreased compared to that in control mice. We then wondered whether immunization with 4C-Staph could immunologically activate one or multiple cell populations, making them more prone to compete with the infection. The fact that neutrophils and monocytes recovered from the pouch of immunized and infected mice showed an upregulation of the FcγR III/II molecules (Fig. 3B and C) might support our hypothesis. FcγR III/II, as well as other FcγRs, are key receptors on human and murine neutrophils, monocytes, dendritic cells, and macrophages, which recognize antibody-coated bacteria and infected cells and favor their elimination by phagocytosis and antibody-dependent cellular cytotoxicity (32, 33).
The scenario depicted thus far identified two major actors that might play a role in protecting mice from infection in situ: antibodies (adaptive immunity) and neutrophils (innate immunity). Whether and to what extent immunization with 4C-Staph contributed to modulate and bridge the action of these two immune components leading to containment of the infection remains to be established. Our initial attempts at measuring bacterial uptake by neutrophils isolated from the pouch after S. aureus infection failed due to the extreme fragility of these cells, which were recruited from the bloodstream to tissues and were activated by the interaction with the invading bacteria. Additional attempts will be necessary to confirm our hypothesis.
Immunization of mice with 4C-Staph, followed by infection with S. aureus, resulted in the upregulation of the expression of FcγR III/II on neutrophils, suggesting a transition from a resting to an activated state. At this stage, neutrophils, armed with functionally activated FcγR III/II complexes, would more efficiently take up the bacteria coated with antigen-specific antibodies, a process which accelerates neutrophil apoptosis (or phagocytosis-induced cell death) and their removal by macrophages. This would possibly counteract some of the evasion mechanisms exerted by S. aureus based on the modulation of phagocyte apoptosis, which favors bacterial survival in the host cells (28, 34). To our knowledge, this would be the first report of an S. aureus vaccine formulation contributing to the activation or potentiation of neutrophil killing mechanisms fighting the infection. In this specific model, this protection mechanism would likely cooperate with others, i.e., antibody-mediated neutralization of Hla, which was shown to play a role in skin infections (35).
Given the intense recruitment of neutrophils in the pouch, it was intriguing to observe that mice depleted of 99% of their neutrophils were still capable of significantly containing the infection when they had been previously immunized with 4C-Staph (Fig. 4C). Immunized neutropenic mice showed an increase of monocytes and macrophages (Fig. 5), suggesting that innate cells other than neutrophils may compensate for the neutropenia condition and contribute to protection from the infection. This result is consistent with the observation that an increase of monocytes has been observed in humans experiencing some neutropenic conditions (31) and significantly enriches previous observations reported for immunosuppressed mice immunized with a Pseudomonas aeruginosa live attenuated vaccine (36, 37).
The positive effect of 4C-Staph on neutropenic mice can be important when thinking of S. aureus-induced diseases in humans. S. aureus is one of the leading causes of nosocomial infections in patients who often have a weakened immune system. In this context, the availability of a multiantigen vaccine formulation that is able to trigger compensatory immune protective mechanisms under a neutropenic condition may be relevant for the development of novel strategies against S. aureus diseases in which active immunization with an effective vaccine is used in conjunction with traditional antibiotic therapy to ensure a better outcome against the disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Janulczyk for helpful discussions on the murine air-pouch model, Elena Amantini e Marco Tortoli for technical assistance with animal experiments, Claudia Facciotti and Vincenzo Nardi-Dei for protein antigens, and Simona Tavarini and Letizia Arcidiacono for support with FACS and serological analyses.
This study was supported by internal funding from Novartis Vaccines and Diagnostics, s.r.l.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00258-15.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Moellering RC. 2010. The problem of complicated skin and skin structure infections: the need for new agents. J Antimicrob Chemother 65:iv3–iv8. doi: 10.1093/jac/dkq250. [DOI] [PubMed] [Google Scholar]
- 3.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated methicillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown AF, Leech JM, Rogers TR, McLoughlin RM. 2014. Staphylococcus aureus colonization: modulation of host immune response and impact on human vaccine design. Front Immunol 4:507. doi: 10.3389/fimmu.2013.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnoli F, Fontana MR, Soldaini E, Mishra R, Fiaschi L, Cartocci E, Nardi-Dei V, Ruggiero P, Nosari S, De Falco MG, Lofano G, Marchi S, Galletti B, Mariotti P, Bacconi M, Torre A, Maccari S, Scarselli M, Rinaudo D, Inoshima N, Savino S, Mori E, Rossi-Paccani S, Baudner B, Pallaoro M, Swennen E, Liberatori S, Norais N, MMonaci E, Wardenburg JB, Schneewind O, Bensi G, Bertholet S, Rappuoli R, Grandi G. 2015. A vaccine composition formulated with a novel TLR-7 immunopotentiator induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A 112:3680–3685. doi: 10.1073/pnas.1424924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colville-Nash P, Lawrence T. 2003. Air-pouch models of inflammation and modifications for the study of granuloma-mediated cartilage degradation. Methods Mol Biol 225:181–189. [DOI] [PubMed] [Google Scholar]
- 8.Delano DL, Montesinos MC, Desai A, Wilder T, Fernandez P, D'Eustachio P, Wiltshire T, Cronstein BN. 2005. Genetically based resistance to the anti-inflammatory effects of methotrexate in the air-pouch model of acute inflammation. Arthritis Rheum 52:2567–2575. doi: 10.1002/art.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickerson-Nutter CL, Medvedeff ED. 1996. The effect of leukotriene synthesis inhibitors in models of acute and chronic inflammation. Arthritis Rheum 39:515–521. doi: 10.1002/art.1780390320. [DOI] [PubMed] [Google Scholar]
- 10.Rothfork JM, Dessus-Babus S, Van Wamel WJB, Cheung AL, Gresham HD. 2003. Fibrinogen depletion attenuates Staphylococcus aureus infection by preventing density-dependent virulence gene upregulation. J Immunol 171:5389–5395. doi: 10.4049/jimmunol.171.10.5389. [DOI] [PubMed] [Google Scholar]
- 11.Bensi G, Mora M, Tuscano G, Biagini M, Chiarot E, Bombaci M, Capo S, Falugi F, Manetti AGO, Donato P, Swennen E, Gallotta M, Garibaldi M, Pinto V, Chiappini N, Musser JM, Janulczyk R, Mariani M, Scarselli M, Telford JL, Grifantini R, Norais N, Margarit I, Grandi G. 2012. Multi-high-throughput approach for highly selective identification of vaccine candidates: the group A Streptococcus case. Mol Cell Proteomics 11:M111.015693. doi: 10.1074/mcp.M111.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiappini N, Seubert A, Telford JL, Grandi G, Serruto D, Margarit I, Janulczyk R. 2012. Streptococcus pyogenes SpyCEP influences host-pathogen interactions during infection in a murine air pouch model. PLoS One 7:e40411. doi: 10.1371/journal.pone.0040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiarot E, Faralla C, Chiappini N, Tuscano G, Falugi F, Gambellini G, Taddei A, Capo S, Cartocci E, Veggi D, Corrado A, Mangiavacchi S, Tavarini S, Scarselli M, Janulczyk R, Grandi G, Margarit I, Bensi G. 2013. Targeted amino acid substitutions impair streptolysin O toxicity and group A Streptococcus virulence. mBio 4(1):e00387-12. doi: 10.1128/mBio.00387-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botelho-Nevers E, Verhoeven P, Paul S, Grattard F, Pozzetto B, Berthelot P, Lucht F. 2013. Staphylococcal vaccine development: review of past failures and plea for a future evaluation of vaccine efficacy not only on staphylococcal infections but also on mucosal carriage. Expert Rev Vaccines 12:1249–1259. doi: 10.1586/14760584.2013.840091. [DOI] [PubMed] [Google Scholar]
- 15.Scully IL, Liberator PA, Jansen KU, Anderson AS. 2014. Covering all the basis: preclinical development of an effective Staphylococcus aureus vaccine. Front Immunol 5:109. doi: 10.3389/fimmu.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster TJ. 2005. Immune evasion by staphylococci. Nat Rev Microbiol 3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 17.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. 2014. Adhesion, invasion, and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. 2012. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HK, Missiakas D, Schneewind O. 2014. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods 410:88–99. doi: 10.1016/j.jim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown M, Kowalski R, Zorman J, Wang X-M, Towne V, Zhao Q, Secore S, Finnefrock AC, Ebert T, Pancari G, Isett K, Zhang Y, Anderson AS, Montgomery D, Cope L, McNeely T. 2009. Selection and characterization of murine monoclonal antibodies to Staphylococcus aureus iron-regulated surface determinant B with functional activity in vitro and in vivo. Clin Vaccine Immunol 16:1095–1104. doi: 10.1128/CVI.00085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebert T, Smith S, Pancari G, Clark D, Hampton R, Secore S, Towne V, Fan H, Wang X-M, Wu X, Ernst R, Harvey BR, Finnefrock AC, Wang F, Tan C, Durr E, Cope L, Anderson A, An Z, McNeely T. 2010. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum Antibodies 19:113–128. doi: 10.3233/HAB-2010-0235. [DOI] [PubMed] [Google Scholar]
- 22.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. 2013. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 56:1554–1561. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pancari G, Fan H, Smith S, Joshi A, Haimbach R, Clark D, Li Y, Hua J, McKelvey T, Ou Y, Drummond J, Cope L, Montgomery D, McNeely T. 2012. Characterization of the mechanism of protection mediated by CS-D7, a monoclonal antibody to Staphylococcus aureus iron-regulated surface determinant B (IsdB). Front Cell Infect Microbiol 2:36. doi: 10.3389/fcimb.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtfreter S, Kolata J, Broker BM. 2010. Towards the immune proteome of Staphylococcus aureus: the anti-S. aureus antibody response. Int J Med Microbiol 300:176–192. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Wood P, Stanworth S, Burton J, Jones A, Peckham DG, Green T, Hyde C, Chapel H, the UKPIN. 2007. Recognition, clinical diagnosis, and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol 149:410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dryla A, Prustomersky S, Gelbmann D, Hanner M, Bettinger E, Kocsis B, Kustos T, Henics T, Meinke A, Nagy E. 2005. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immunol 12:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauseef WM. 2007. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 28.Rigby K, DeLeo F. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdrengh M, Tarkowski A. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun 65:2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mölne L, Verdrengh M, Tarkowski A. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun 68:6162–6167. doi: 10.1128/IAI.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boxer LA. 2012. How to approach neutropenia. ASH Education Program Book 2012:174–182. [DOI] [PubMed] [Google Scholar]
- 32.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. 2014. The function of Fc g receptors in dendritic cells and macrophages. Nat Rev Immunol 14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 33.Selvaraj P, Fifadara N, Nagarajan S, Cimino A, Wang G. 2004. Functional regulation of human neutrophil Fcγ receptors. Immunol Res 29:219–229. doi: 10.1385/IR:29:1-3:219. [DOI] [PubMed] [Google Scholar]
- 34.Deleo FR. 2004. Modulation of phagocyte apoptosis by bacterial pathogen. Apoptosis 9:399–413. doi: 10.1023/B:APPT.0000031448.64969.fa. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy AD, Wardenburg JB, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamei A, Wu W, Traficante DC, Koh AY, Van Rooijen N, Pier GB, Priebe GP. 2013. Collaboration between macrophages and vaccine-induced CD4+ T cells confers protection against lethal Pseudomonas aeruginosa pneumonia during neutropenia. J Infect Dis 207:39–49. doi: 10.1093/infdis/jis657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarff JM, Goldberg JB. 2008. Vaccination against Pseudomonas aeruginosa pneumonia in immunocompromised mice. Clin Vaccine Immunol 15:367–375. doi: 10.1128/CVI.00419-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.