Abstract
Severe malaria syndromes are precipitated by Plasmodium falciparum parasites binding to endothelial receptors on the vascular lining. This binding is mediated by members of the highly variant P. falciparum erythrocyte membrane protein 1 (PfEMP1) family. We have previously identified a subset of PfEMP1 proteins associated with severe malaria and found that the receptor for these PfEMP1 variants is endothelial protein C receptor (EPCR). The binding is mediated through the amino-terminal cysteine-rich interdomain region (CIDR) of the subtypes α1.1 and α1.4 to α1.8. In this study, we investigated the acquisition of anti-CIDR antibodies using plasma samples collected in four study villages with different malaria transmission intensities in northeastern Tanzania during a period with a decline in malaria transmission. We show that individuals exposed to high levels of malaria transmission acquire antibodies to EPCR-binding CIDR domains early in life and that these antibodies are acquired more rapidly than antibodies to other CIDR domains. The rate by which antibodies to EPCR-binding CIDR domains are acquired in populations in areas where malaria is endemic is determined by the malaria transmission intensity, and on a population level, the antibodies are rapidly lost if transmission is interrupted. This indicates that sustained exposure is required to maintain the production of the antibodies.
INTRODUCTION
Individuals in countries where malaria is endemic acquire immunity to febrile malaria episodes after years of exposure and repeated disease episodes (1, 2). The age at which protection is established depends on the malaria transmission intensity in the area of residence (3–6). Immunoglobulin G (IgG) targeting the asexual blood stages of the parasites is an important immunological effector mechanism mediating malaria immunity (7, 8), and several lines of evidence indicate that members of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) protein family are important targets for immunity (6, 9–13). PfEMP1s are large multidomain proteins consisting of two to nine Duffy binding-like (DBL) and cysteine-rich interdomain region (CIDR) domains, which based on sequence similarity can be divided into different subgroups (14, 15). The proteins are expressed on the surface of infected erythrocytes and mediate binding of these cells to receptors on the vascular lining (16–19). In this way, the infected erythrocytes are effectively sequestered, and they avoid splenic clearance. IgG recognizing PfEMP1 inhibits the binding between the infected erythrocytes and the endothelial cells, and parasites expressing a PfEMP1 targeted by binding inhibitory IgG will be killed in the spleen. However, in an evolutionary arms race each parasite genome has acquired about 60 var genes, encoding different PfEMP1s binding different endothelial receptors (20), and isogenic parasites can, depending on which PfEMP1 they express, bind different endothelial receptors. To multiply effectively, parasites are limited to expressing PfEMP1 types not targeted by binding inhibitory IgG, and exposed individuals slowly acquire malaria immunity as the anti-PfEMP1 antibody repertoire expands with repeated exposure to infections (21).
As opposed to immunity against uncomplicated febrile malaria attacks, immunity to severe malaria infections is acquired early in life after one to three episodes of life-threatening disease (22, 23). Interestingly, episodes of uncomplicated malaria occur prior to or in between the episodes of severe malaria, suggesting that uncomplicated malaria attacks do not always contribute to the acquisition of immunity to severe episodes (23). This has raised hopes that severe malaria is precipitated by parasites expressing a limited group of specific PfEMP1 molecules. Moreover, exposure to one or a few members of these PfEMP1s is thought to induce IgG that is widely cross-reactive to other members of the group, thereby protecting individuals who have acquired the antibodies against repeated attacks of severe disease. If this is true, it should be possible to protect children against severe malaria by inducing cross-reactive IgG by vaccination of infants. Analyses of var gene transcription and PfEMP1 expression in children suffering from severe malaria has indicated that parasites causing severe disease express a group of PfEMP1s binding endothelial protein C receptor (EPCR) (24–28). The binding is mediated by N-terminal CIDR domains, and only PfEMP1s containing CIDR domains of the subtypes α1.1 or α1.4 to α1.8 bind EPCR (29). Other subtypes (α2 to α5) of CIDR mediate parasite binding to CD36 (30), while yet others have not been associated with a binding phenotype. Since immunity to severe malaria is acquired during the first years of life in areas with high malaria transmission, antibodies against EPCR binding CIDR domains are predicted to be acquired early in life in populations in areas where malaria is endemic if they are mediators of immunity against severe malaria. We performed a comprehensive study on the acquisition of anti-CIDR antibodies and show (i) that antibodies to EPCR binding CIDR domains are acquired early in life and before antibodies to other CIDR domains, (ii) that the malaria transmission intensity has a profound influence on the speed by which these antibodies are acquired, and (iii) that on a population level these antibodies are rapidly lost if transmission is interrupted.
MATERIALS AND METHODS
Study population.
The study was conducted in the Tanga region of northeastern Tanzania in the villages of Mkokola and Kwamasimba of the Korogwe district and in the villages of Mapapayu and Magoda of the Muheza district. The region is characterized by marked variations in intensity of P. falciparum transmission, which is altitude dependent (31). Mapapayu, Magoda, and Mkokola villages are at low altitude (200 to 300 m), and Kwamasimba village is at intermediate altitude (800 to 1,200 m). Blood samples were collected either by finger prick or venipuncture after receiving written informed consent from the study participants or from their parents or legal guardians. Approximately 115 individuals between 0 and 60 years were randomly recruited at cross-sectional surveys conducted on an annual basis in May and June during 2004 to 2009 (32). For each of the 14 surveys (Mkokola and Kwamasimba in 2004 to 2009 and Magoda and Mapapayu in 2008), the target was to include 15, 25, 40, 20, and 15 individuals aged 0 to 1, 2 to 3, 4 to 7, 8 to 10, and 15 to 60 years, respectively, and these targets were largely met (Table 1). Plasma was collected after centrifugation at 2,000 rpm for 10 min and stored at −20°C until analysis. Both thick and thin blood slides were also prepared, stained with Giemsa and investigated for the presence of malaria parasites. During the study period, the area witnessed a dramatic decrease in malaria transmission (32). This was reflected in the prevalence of parasitemia among the study participants, which in Mkokola and Kwamasimba fell from 65.8 and 15.0% in 2004 to 6.0 and 0% in 2009, respectively. The point prevalence of parasitemia among study participants by age and year of sampling is shown in Table 2. The study protocols were approved by the Medical Research Coordinating Committee of the Tanzanian National Institute for Medical Research.
TABLE 1.
Sampling strategy comparing the target number of individuals in each age group for each survey with the number actually recruited
| Age range (yr) | Target no. per survey | Overall target no. for 14 surveys | Actual no. tested | Mean age (yr) ± SD |
|---|---|---|---|---|
| 0-1 | 15 | 210 | 173 | 1.5 ± 0.3 |
| 2-3 | 25 | 350 | 370 | 3.0 ± 0.6 |
| 4-7 | 40 | 560 | 492 | 5.3 ± 0.8 |
| 8-10 | 20 | 280 | 309 | 8.5 ± 0.9 |
| 15-60 | 15 | 210 | 178 | 21.1 ± 10.1 |
| Total | 115 | 1,610 | 1,522 |
TABLE 2.
Point prevalence of parasitemia among study participants in the four villages from 2004 to 2009
| Village | Parasitemia (% positive by microscopy of blood slides) in: |
|||||
|---|---|---|---|---|---|---|
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
| Kwamasimba | 15.0 | 8.3 | 1.9 | 6.8 | 3.3 | 0 |
| Mkokola | 65.8 | 52.0 | 33.0 | 26.8 | 8.0 | 6.0 |
| Magodaa | 50.8 | |||||
| Mapapayua | 34.3 | |||||
For Magoda and Mapapayu, only samples collected in 2008 were analyzed.
Protein expression, coupling to beads, and analysis of IgG reactivity.
Baculovirus protein expression, coupling of recombinant proteins to Microplex Microsphere beads, and measurements of IgG reactivity to multiplexed proteins were performed as described previously (33). Individuals were classified as having a measurable IgG response (i.e., as responders) if the measured antibody level was higher than the mean plus two standard deviations for 20 Danish controls. The individual IgG responses were quantified on the basis of the measured mean fluorescence intensity level.
ELISA.
Inhibition enzyme-linked immunosorbent assay (ELISA) was carried out as described previously (33) using 3 μg of EPCR/ml for coating, 1 μg of IT4var20 CIDRα1.1/ml, and 6% serum.
Statistics.
Demographic data were doubly entered and validated in Microsoft (MS) Access database and exported to STATA. The antibody levels measured as the mean fluorescence emission from the antibody-coupled beads were collected in MS Excel and exported to STATA12 for the statistical analyses. Individuals were categorized as responders or nonresponders for each of the CIDR domains based on the cutoff derived from the reactivity in plasma from the Danish controls. For each CIDR domain group (e.g., EPCR-binding CIDR domains) and in each individual, the percentage of domains to which an individual had antibodies was calculated for each CIDR grouping as the number of CIDR domains in the domain group recognized by IgG/number of CIDR domains tested in that domain group. For example, to compare the serological recognition of CIDR domains belonging to different groupings, the seropositivity of EPCR-binding domains (e.g., four domains recognized out of eight in individual X) was compared to the seropositivity of CIDR predicted to bind CD36 (e.g., two domains recognized out of thirteen tested in individual X). These types of comparisons were made in each of the 1,522 Tanzanian individuals, and the results were evaluated by paired t test.
To evaluate the acquisition of antibodies with age, Lowess mean smoothing values were generated for each individual for each of the antigenic groups using a bandwidth of 0.8 years. Lowess curves were compared statistically using paired comparisons of Lowess values for each antigenic group in each individual using a t test. Antibody levels across age groups and across samples collected at different years were evaluated using analysis of variance (ANOVA) reporting Bonferroni corrected P values. A multiple linear model was used to evaluate the relative influence of homestead, age group, and year of blood sampling on mean antibody levels. Homestead Kwamasimba, age 0 to 1 years, blood sampling groups in 2004 were used as reference groups.
RESULTS
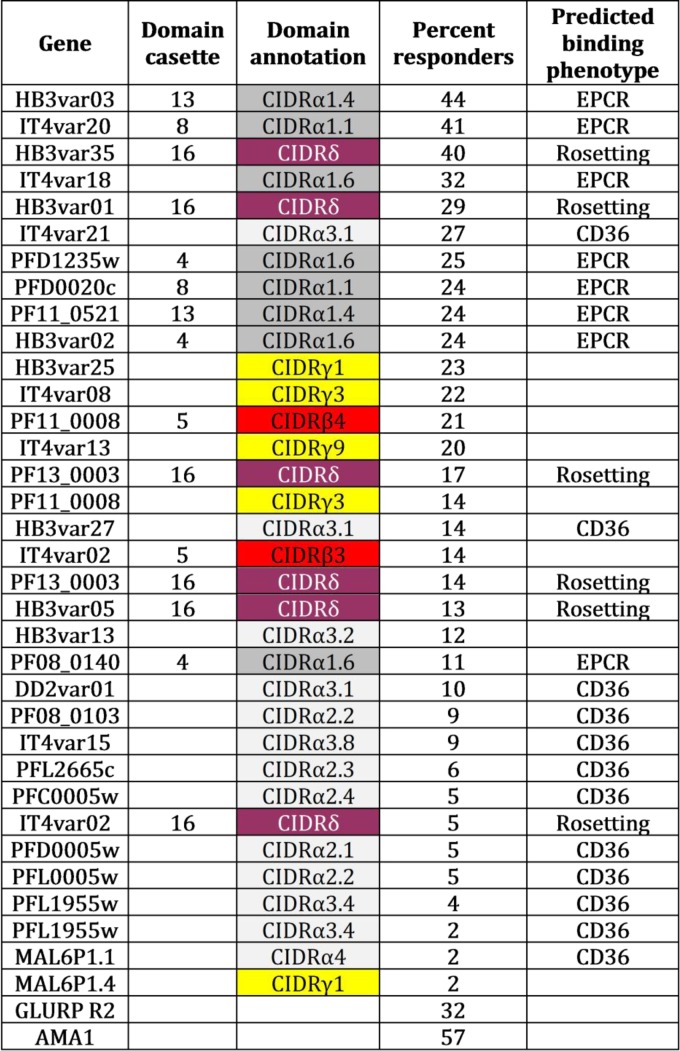
The IgG antibody response to 34 PfEMP1 CIDR domains (Fig. 1) was tested in plasma samples from 1,522 Tanzanian individuals using a multiplex system. Plasma IgG reactivity was scored as positive or negative based on a cutoff calculated using the reactivity in nonimmune plasma. The CIDR domains can be grouped according to phylogeny and for some subgroups the ligand for the domains can be predicted. Thus, we grouped the domains (Fig. 1) into those predicted to bind EPCR (CIDRα subgroups 1.1 and 1.4 to 1.8), those belonging to group CIDRδ possibly associated with PfEMP1 variants mediating rosetting (34), those predicted to bind CD36 (CIDR subgroups α2 to α5) (30), and those belonging to group CIDRγ or the CIDRβ3/4 subgroups (some of these are found in PfEMP1s binding PECAM1) (35).
FIG 1.
Description of recombinant proteins used in the study. The gene or protein name, domain cassette association (only shown if the domain is associated with a cassette [15]), annotation (color coded according to subtype [15]), percent responders (percentage among 1,522 Tanzanian individuals who had an IgG response above the cutoff), and predicted binding phenotype (as defined in Table 3) are presented.
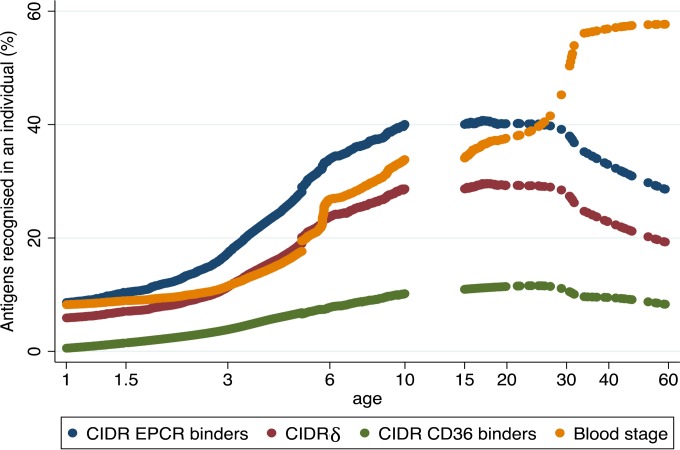
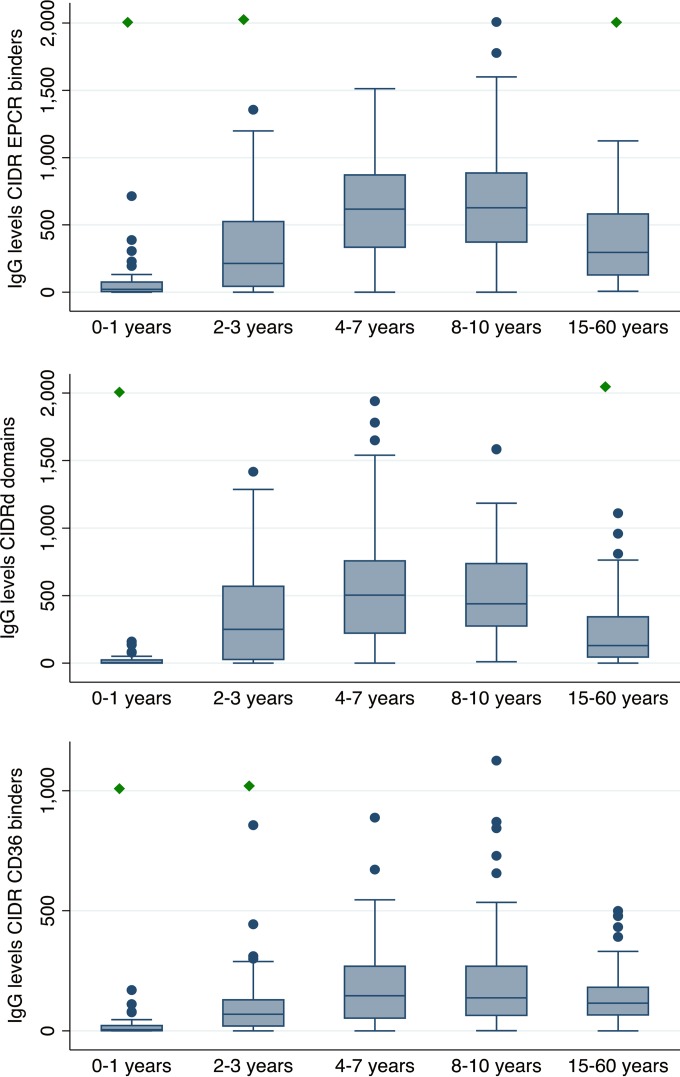
Individuals were categorized as responders or nonresponders for each of the 34 CIDR domains. For each CIDR domain group (e.g., the EPCR-binding CIDR domains) and in each individual, the proportion of CIDR domains to which the individual had antibodies was then calculated for each CIDR grouping. For example, individual “X” had antibodies to 4 of 8 EPCR-binding domains and 2 of 6 CIDRγ domains and therefore had a recognition of 0.50 of the EPCR-binding domains and 0.33 of the CIDRγ domains. This strategy allowed for a paired comparison between the recognition of each CIDR domain group in each individual, and it showed that recognition of EPCR-binding CIDR domains was more prevalent than recognition of CIDRγ domains and that CIDR domains binding CD36 were least likely to be recognized by IgG (Table 3). The two best-recognized domains bound EPCR (Fig. 1), and individuals were unlikely to possess IgG to other CIDR domains without having developed IgG against the two best-recognized domains (data not shown). These results show that binding phenotype predicts serological reactivity and suggest that individuals are exposed to EPCR-binding CIDR domains earlier in life than they are exposed to CIDR domains associated with other binding phenotypes. This notion was confirmed by analyzing the seropositivity by age (Fig. 2 and Table 4). Antibodies to CD36-binding domains were acquired slowly, and seropositivity plateaued at age 20 to 30 years. Antibodies to EPCR-binding domains were acquired rapidly, and seropositivity peaked at 10 years (seroreactivity was not measured in individuals aged 11 to 14 years). Seroreactivity was also measured to six antigenic constructs representing nonvariant blood-stage antigens (EBA-175, AMA-1, MSP3, and GLURPR) and, on average, these were acquired at a slower pace than antibodies to the EPCR-binding CIDR domains and on par with the antibodies against CIDRδ domains. Interestingly, the likelihood of having antibodies to blood-stage antigens continued to increase after the age of 15, whereas the seropositivity rates declined for the EPCR-binding CIDR domains and the CIDRδ domains. The same pattern was found when comparing domain-specific IgG levels in individuals of different ages (Fig. 3). For EPCR-binding CIDR domains and CIDRδ domains, the levels were highest in individuals aged 8 to 10 years and statistically significantly lower in individuals aged 15 years or older (P < 0.01, ANOVA, Bonferroni corrected).
TABLE 3.
Serological recognition of CIDR domain types among 1,522 individuals living in areas with different malaria endemicities
| CIDR domain type and predicted binding phenotypea | No. of domains | Median likelihood (95% CI) of responding to a domain of this type (n = 1,522) |
Pb |
|||
|---|---|---|---|---|---|---|
| CIDRα1.1-1.8 | CIDRδ | CIDRα2-5 | CIDRγ | |||
| CIDRα1.1 and CIDRα1.4-1.8 (EPCR binders) | 8 | 0.28 (0.27–0.30) | ||||
| CIDRδ (associated with rosetting PfEMP1) | 6 | 0.20 (0.18–0.21) | >5 × 10−5 | |||
| CIDRα2-5 (CD36 binders) | 13 | 0.08 (0.07–0.09) | >5 × 10−5 | >5 × 10−5 | ||
| CIDRγ (associated with rosetting PfEMP1) | 5 | 0.20 (0.19–0.21) | >5 × 10−5 | 0.41 | >5 × 10−5 | |
| CIDRβ3/4 (associated with PECAM1-binding PfEMP1) | 2 | 0.17 (0.16–0.19) | >5 × 10−5 | 0.001 | >5 × 10−5 | >5 × 10−5 |
Predicted binding phenotypes are indicated in parentheses. Binding was predicted from similar domain types binding to the indicated ligand. The binding phenotypes of CIDRδ and CIDRβ have not been tested extensively. Some CIDRδ domains are found in PfEMP1s-mediating rosetting. The CIDRβ3/4 domains are found in DC5 PfEMP1. DC5-expressing parasites have been reported to bind PECAM1, but the PfEMP1 domain mediating the interaction is unresolved.
A paired t test was used to compare the mean likelihood of responding to group X versus group Y, considering each individual as one natural experiment.
FIG 2.
Age-dependent serological reactivity to different groups of CIDR domains and blood-stage proteins. The mean percentage of malaria antigens within each grouping recognized by IgG according to age in plasma from 1,522 Tanzanian individuals. Antigen groupings: EPCR-binding CIDR domains (n = 8 domains), CIDRδ (n = 6 domains), CD36-binding CIDR (n = 13 domains), asexual blood-stage proteins (n = 6; EBA-175, AMA-1, MSP3, GLURP R0, GLURP R1, and GLURP R2). Curves were generated with Lowess mean smoothing with a bandwidth of 0.8. A statistical evaluation of age-related differences is presented in Table 4.
TABLE 4.
Age-related seropositivity (mean percentage) to different groups of CIDR domains and blood-stage antigens in 1,522 Tanzanian individuals
| Age group in yr (n) | Age-related seropositivity (mean %) and corresponding P valuesa |
||||||
|---|---|---|---|---|---|---|---|
| EPCR-binding CIDR (%) | CIDRδ |
CD36-binding CIDR |
Blood-stage antigensc |
||||
| % | Pb | % | Pb | % | Pb | ||
| 0–1 (172) | 4 | 2 | 0.047 | 1 | 0.0012 | 6 | 0.025d |
| 2–3 (370) | 14 | 11 | >5 × 10−5 | 4 | >5 × 105 | 11 | 0.0037 |
| 4–7 (598) | 32 | 23 | >5 × 10−5 | 8 | >5 × 105 | 21 | 5 × 10−5 |
| 8–10 (289) | 41 | 34 | >5 × 10−5 | 11 | >5 × 105 | 36 | 0.03 |
| 15+ (179) | 33 | 26 | >5 × 10−5 | 12 | >5 × 105 | 55 | 5 × 10−5d |
The CIDR groupings correspond to those given in Table 1.
P values were determined based on comparisons to the seropositivity of EPCR-binding CIDR (paired t test).
Six blood-stage antigens were tested (AMA1, EBA-175, MSP3, GLURPRO, GLURP-R1, and GLURP-R2).
Statistically significantly higher values than in EPCR-binding CIDR.
FIG 3.
Age-dependent acquisition of IgG to different types of CIDR domains. IgG levels (box plot: median with 25th and 75th percentiles, upper and lower adjacent values, and outliers) to EPCR-binding CIDR domains (upper panel), CIDRδ domains (middle panel), and CD36-binding CIDR domains (lower panel) in different age groups are shown. Individuals (n = 31, 82, 96, 60, and 45 for each age group, respectively) were subjected to high malaria transmission at the time of sampling (living in Mkokola in 2004 and 2005 and in Magoda). The green diamond indicates that the IgG levels were statistically significantly lower than the levels found in the age group 8 to 10 years (P < 0.001 ANOVA, Bonferroni corrected).
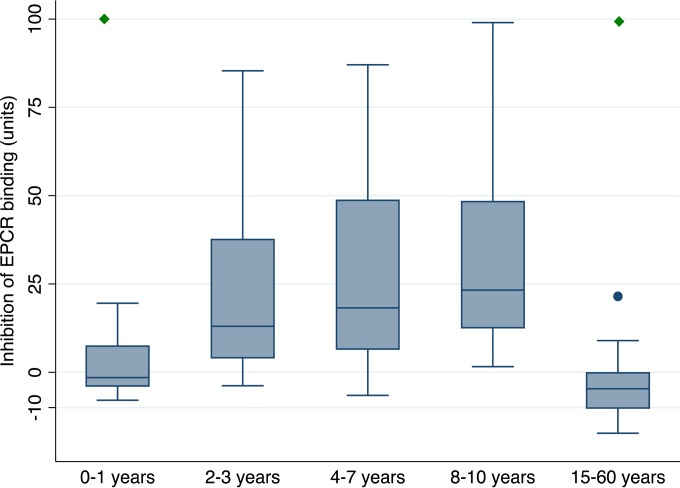
These data indicate that children are exposed to EPCR-binding domains early in life and that the exposure to these and CIDRδ domains declines during adulthood, despite a continued exposure to blood-stage antigens. The same pattern was seen when the abilities of plasma to inhibit the interaction between an EPCR-binding CIDR domain and EPCR were compared across age groups (Fig. 4).
FIG 4.
Binding inhibition assay. Inhibition of the binding between an EPCR-binding CIDR domain (IT4var20 CIDRα1.1) and EPCR by plasma collected in Mkokola in 2004 (n = 12, 26, 35, 17, and 13 in each age group, respectively). The green diamond indicates that the binding inhibition was statistically significantly lower than the binding inhibition in the age group 8 to 10 years (P < 0.004 ANOVA, Bonferroni corrected; correlation between anti-CIDRα1.1 antibody levels and inhibition [P < 0.0005, Spearman's rho = 0.49]). Box plots are as described in Fig. 3.
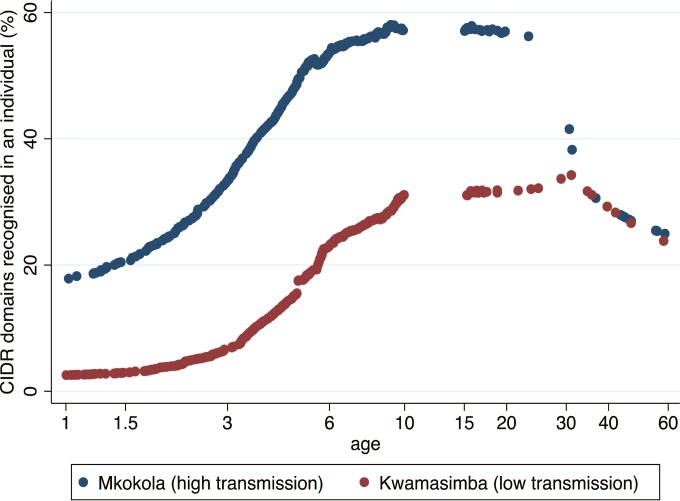
The plasma samples were collected from individuals living in four different villages characterized by a marked difference in malaria transmission and the samples were collected over a period of 6 years (2004 to 2009) during which the malaria transmission in the area plummeted. Linear regression models showed that age, village of residence, and year of sampling were predictors of plasma levels of antibodies to EPCR-binding CIDR domains (Table 5). This indicates that the level of malaria transmission has a strong influence on the pace at which antibodies to PfEMP1 are acquired. To analyze this in more detail, we compared the acquisition of antibodies against EPCR-binding domains in a high (Mkokola) and a low (Kwamasimba) transmission village using data collected during the first 3 years of the study (Fig. 5 and Table 6). Children living in Mkokola had higher antibody responses toward a broader range of EPCR-binding CIDR domains than children living in Kwamasimba, and antibodies were acquired at a faster pace in Mkokola. As noted earlier, the breadth of the antibody response diminished in adulthood, but interestingly, the peak occurred later in Kwamasimba than Mkokola. From the age of 30 years, the breadth of the response was similar in the two villages.
TABLE 5.
Multiple linear regression model predicting the anti-IgG plasma levels to EPCR-binding CIDR domains according to homestead, year of sampling, and subject agea
| Category | Coefficient | 95% CI | P |
|---|---|---|---|
| Village | |||
| Kwamasimba | Ref | ||
| Mkokola | 218 | 187 to 250 | >5 × 10−5 |
| Magoda | 373 | 307 to 439 | >5 × 10−5 |
| Mapapayu | 140 | 71 to 209 | >5 × 10−5 |
| Yr | |||
| 2004 | Ref | ||
| 2005 | 27 | –23 to 81 | 0.32 |
| 2006 | –36 | –91 to 19 | 0.20 |
| 2007 | –57 | –111 to –4 | 0.036 |
| 2008 | –84 | –136 to –31 | 0.002 |
| 2009 | –153 | –207 to –90 | >5 × 10−5 |
| Age group | |||
| 0–1 | Ref | ||
| 2–3 | 107 | 55 to 160 | >5 × 10−5 |
| 4–7 | 299 | 249 to 350 | >5 × 10−5 |
| 8–10 | 378 | 324 to 432 | >5 × 10−5 |
| 15–60 | 289 | 228 to 350 | >5 × 10−5 |
n = 1,522 individuals. Ref, reference.
FIG 5.
Age-dependent acquisition of anti-CIDR antibodies by individuals subjected to high or moderate malaria transmission. Mean percentage of eight EPCR-binding CIDR domains recognized by IgG in plasma from individuals living in a village with high (Mkokola) or low (Kwamasimba) malaria transmission. Curves were generated with Lowess mean smoothing with a bandwidth of 0.8. To avoid the effect of a general decline in transmission during the study, only samples collected from 2004 to 2006 were included (n = 652). A statistical evaluation of the age-related differences is presented in Table 4.
TABLE 6.
Age-related seropositivity to EPCR-binding CIDR in samples collected in Mkokola (high transmission) or Kwamasimba (moderate transmission)
| Age group (yr) | Mean % seropositivity |
Pa | |
|---|---|---|---|
| Mkokola | Kwamasimba | ||
| 0–1 | 4 | 3 | 0.047 |
| 2–3 | 24 | 5 | 5 × 10−5 |
| 4–7 | 45 | 18 | 5 × 10−5 |
| 8–10 | 49 | 27 | 5 × 10−5 |
| 15–60 | 35 | 27 | 0.07 |
| 30–60 | 23 | 16 | 0.55 |
Calculated using a Student t test that included 60 and 87, 142 and 175, 243 and 268, 90 and 89, 74 and 75, and 23 and 7 individuals for each age group in Mkokola and Kwamasimba, respectively.
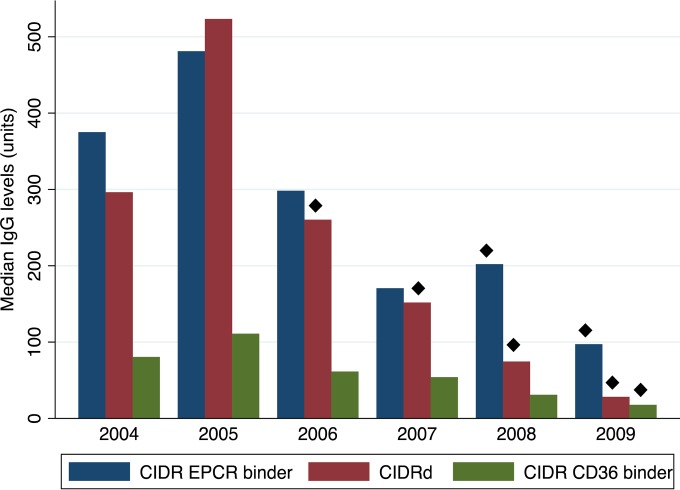
These data show how transmission influences antibody levels by comparing the situation in two geographical locations characterized by different transmission intensities. To analyze how a decline in transmission due to natural fluctuation or malaria intervention may influence antibody levels, we compared the median anti-CIDR antibody levels in plasma collected in Kwamasimba and Mkokola during the annual surveys from 2004 to 2009 (Fig. 6). For all CIDR groups (EPCR-binding, CIDRδ, and CD36-binding) the median antibody level peaked in 2005 and declined steadily thereafter. The decline was pronounced for EPCR-binding domains and CIDRδ domains, where the median 2009 levels were reduced to ca. 20 and 10% of the 2005 level, respectively. This was illustrated by comparing the mean levels of IgG to EPCR-binding domains in children aged 4 to 6, which were 711 U (95% confidence interval [95% CI] = 571 to 851) in 2005 and 271 U (95% CI = 151 to 391) in 2009 (P < 10−4 [t test]; n = 30 and n = 28 in 2005 and 2009, respectively). The corresponding levels for children aged 2 to 3 were 367 U (95% CI = 261 to 473) in 2005 and 64 U (95% CI = 23 to 105) in 2009 (P = 0.003 for the difference between 2005 and 2009 [t test]). The 2009 levels were on par with what is measured in nonendemic plasma (data not shown). These data indicate that the levels of antibodies against EPCR-binding CIDR domains are waning quickly in areas where transmission is declining and that children in such areas acquire these antibodies later in life.
FIG 6.
Comparison of anti-CIDR IgG levels in samples collected from 2004 to 2009. The median IgG levels among individuals from Mkokola and Kwamasimba to different groups of CIDR measured during the five study years were charted. Diamonds indicate that the IgG levels were statistically significantly lower than the levels found in 2005 (P < 0.01 ANOVA, Bonferroni corrected).
DISCUSSION
To complete asexual blood multiplication, late-stage P. falciparum has to sequester on cells of the vascular lining to avoid being killed in the spleen. The attachment is mediated by different members of the large family of PfEMP1 proteins (16–19), which can bind to receptors with nanomolar affinity and thereby anchor infected erythrocytes to endothelial cells and syncytiotrophoblasts (28, 36). Individuals exposed to malaria infections acquire IgG recognizing PfEMP1 expressed on the surface of infected erythrocytes, and some of these antibodies can inhibit the interaction between specific PfEMP1s and their binding partners (29, 37–42) (Fig. 4). Each parasite has the capacity to express about 60 different PfEMP1 molecules but usually only expresses one at a time (43–45). The data from experimental infections with a monoclonal parasite line indicate that among the hundreds of thousands of parasites that are released from the liver into the bloodstream, all PfEMP1 variants carried by the parasite are expressed (46, 47). This creates a situation where, in naive individuals, about 60 different isogenic but phenotypically distinct parasite populations compete to fill the ecological niche. In malaria-naive individuals, the multiplication rate of a parasite is influenced by how effectively the PfEMP1s expressed by this parasite mediate sequestration and prevent the parasite from being killed in the spleen. Thus, parasites expressing the most effective PfEMP1 binders will outgrow parasites expressing less effective binders and IgG responses will first be acquired by the domains constituting these PfEMP1s. We have previously shown that in populations in areas where malaria is endemic, the acquisition of anti-PfEMP1 IgG directed against DBL domains is not random (48, 49), but that antibodies to domains found in group A PfEMP1 and in group B PfEMP1 harboring domain cassette 8 are acquired early in life and before IgG to domains found in the remaining group B and group C PfEMP1s (24). In the absence of reliable data on the binding functionality of the domains tested, these earlier studies were limited by the fact that the grouping of domains was based on sequence analyses. Lately, it has become evident that PfEMP1 molecules can be divided by the binding capability of their N-terminal CIDR domains into those that bind EPCR (28) or CD36 (30) and variants with unknown binding capabilities, possibly linked to rosetting (15, 50). We compared levels of antibodies to a large number of CIDR domains in 1,522 individuals exposed to malaria and found pronounced and systematic differences in the IgG responses to the different groups of CIDR domains. Antibodies to EPCR-binding CIDR domains are most prevalent, most likely to be acquired first, and in areas of high malaria transmission likely to be acquired early in life (Table 3, Fig. 1 and 4). EPCR binding is associated with severe malaria, and parasites from patients suffering from severe malaria transcribe var genes encoding CIDR domains predicted to bind EPCR. The data presented here suggest not only that CIDR binding is associated with disease severity but that the first P. falciparum infections experienced by an individual are likely to be dominated by parasites expressing PfEMP1 types harboring EPCR-binding domains. We did not address whether the anti-CIDR antibodies induced during these early infections played a functional role in protection against severe malaria. However, in areas of high malaria transmission, children are at the highest risk of developing severe malaria when they are between 1 and 3 years of age. Most children only experience one, sometimes two, and very rarely three episodes of life-threatening malaria (51), and the combined evidence suggests that antibodies against EPCR-binding CIDR domains could play a role in protecting children against severe disease. We showed here that children acquire antibodies that block the binding between a CIDR and EPCR (Fig. 4). Moreover, we have previously shown that IgG affinity purified on a peptide corresponding to the CIDR domain's EPCR binding site of one CIDR domain are able to inhibit the EPCR binding of a broad selection of different CIDR domains (29). Since the amino acids mediating EPCR binding in some of these domains differed quite considerably from those of the peptide used for the affinity purification, the results indicated that individuals develop IgG that is functional and broadly cross-reactive between EPCR binding CIDR domains. Future longitudinal studies are needed to establish whether such antibodies play a role for the development of immunity.
Here we found that IgGs to CIDRδ domains were also acquired relatively early in life. No endothelial binding partner has been identified for CIDRδ, but these domains have been found in PfEMP1s mediating rosetting (50). Rosetting, the binding of uninfected erythrocytes to PfEMP1 expressed on infected erythrocytes, has previously been associated with severe malaria (37). Transcriptional analysis indicates that var genes encoding CIDRδ are highly transcribed in a significant but relatively small percentage of patients with severe malaria (24). The data presented here suggest that individuals are exposed to CIDRδ domains after relatively few infections. Our results also suggest that IgG response to CD36-binding CIDR domains begins to accumulate after IgG responses to EPCR-binding CIDR and CIDRδ domains have been established. This suggests that parasites expressing CIDR domains binding CD36 are at a disadvantage in the absence of an effective IgG response against parasites expressing EPCR-binding CIDR or CIDRδ.
The breadth and the level of the IgG response to EPCR-binding CIDR or CIDRδ domains peaked during childhood and declined markedly during adolescence (Fig. 2, 3, and 4). This indicates that the exposure to parasites expressing EPCR-binding CIDR or CIDRδ domains diminishes in these age groups despite a continued exposure to asexual blood-stage parasites and could be explained as a consequence of having an effective immune response against parasites expressing these phenotypes. It does not explain why older individuals not having high levels of these antibodies are not likely to develop severe malaria. It could be that these individuals are protected by other immune mechanisms or IgG with other specificities; alternatively, these individuals may have developed an immunological memory response enabling rapid production of IgG against critical CIDR domains.
The present study confirms earlier findings (48, 52) that the level of malaria endemicity in the area of habitation has a profound effect on the pace by which the anti-PfEMP1 antibody repertoire is developed (Fig. 5). Malaria transmission declined considerably during the 5-year study period (32), as reflected in a drop in point prevalence of parasitemia among the study individuals living in Mkokola from 65.8 to 6.0% between 2004 and 2009. During the same period there was a dramatic fall in the mean anti-CIDR IgG levels in all age groups (Fig. 6), indicating that a sustained exposure is required to maintain the production of these antibodies. Indeed, as a group, the children born during the last part of the study had antibody reactivity on par with those measured in unexposed Europeans. These results raise the question of malaria susceptibility in the population if the higher level of transmission is restored. To what extent will older children have lost malaria immunity, and will a broad age range of children born during the period with low transmission be at risk of developing severe disease? Unfortunately, these questions are not entirely academic, since there are indications that malaria transmission has increased in this region despite continued control efforts.
ACKNOWLEDGMENTS
We are grateful to the Tanzanian donors.
The study was funded by the Danish International, Development Agency, the Danish Council for Independent Research, Medical Sciences (T1333-00220), and the Sapere Aude program (DFF–4004-00624B).
REFERENCES
- 1.Macdonald G. 1957. The epidemiology and control of malaria. Oxford University Press, London, United Kingdom. [Google Scholar]
- 2.Bruce-Chwatt LJ. 1963. A longitudinal survey of natural malaria infection in a group of West African adults. I West Afr Med J 12:141–173. [PubMed] [Google Scholar]
- 3.Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P, Brahimi K, Faye O, Druilhe P, Pereira Da Silva L. 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg 51:123–137. [DOI] [PubMed] [Google Scholar]
- 4.Giha HA, Rosthoj S, Dodoo D, Hviid L, Satti GM, Scheike T, Arnot DE, Theander TG. 2000. The epidemiology of febrile malaria episodes in an area of unstable and seasonal transmission. Trans R Soc Trop Med Hyg 94:645–651. doi: 10.1016/S0035-9203(00)90218-9. [DOI] [PubMed] [Google Scholar]
- 5.Lusingu JP, Vestergaard LS, Mmbando BP, Drakeley CJ, Jones C, Akida J, Savaeli ZX, Kitua AY, Lemnge MM, Theander TG. 2004. Malaria morbidity and immunity among residents of villages with different Plasmodium falciparum transmission intensity in North-Eastern Tanzania. Malar J 3:26. doi: 10.1186/1475-2875-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodoo D, Staalsoe T, Giha H, Kurtzhals JA, Akanmori BD, Koram K, Dunyo S, Nkrumah FK, Hviid L, Theander TG. 2001. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect Immun 69:3713–3718. doi: 10.1128/IAI.69.6.3713-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S, Mc GI, Carrington S. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 8.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45:297–308. [DOI] [PubMed] [Google Scholar]
- 9.Marsh K, Howard RJ. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 10.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg 83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 11.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med 4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giha HA, Staalsoe T, Dodoo D, Roper C, Satti GM, Arnot DE, Hviid L, Theander TG. 2000. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol Lett 71:117–126. doi: 10.1016/S0165-2478(99)00173-X. [DOI] [PubMed] [Google Scholar]
- 13.Lusingu JP, Jensen AT, Vestergaard LS, Minja DT, Dalgaard MB, Gesase S, Mmbando BP, Kitua AY, Lemnge MM, Cavanagh D, Hviid L, Theander TG. 2006. Levels of plasma immunoglobulin G with specificity against the cysteine-rich interdomain regions of a semiconserved Plasmodium falciparum erythrocyte membrane protein 1, VAR4, predict protection against malarial anemia and febrile episodes. Infect Immun 74:2867–2875. doi: 10.1128/IAI.74.5.2867-2875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavstsen T, Salanti A, Jensen AT, Arnot DE, Theander TG. 2003. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar J 2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rask TS, Hansen DA, Theander TG, Gorm Pedersen A, Lavstsen T. 2010. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes–divide and conquer. PLoS Comput Biol 6:e1000933. doi: 10.1371/journal.pcbi.1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leech JH, Barnwell JW, Miller LH, Howard RJ. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med 159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. 1995. Cloning the Plasmodium falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 18.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101–110. doi: 10.1016/0092-8674(95)90056-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 20.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen MA, Staalsoe T, Kurtzhals JA, Goka BQ, Dodoo D, Alifrangis M, Theander TG, Akanmori BD, Hviid L. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J Immunol 168:3444–3450. doi: 10.4049/jimmunol.168.7.3444. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 23.Goncalves BP, Huang CY, Morrison R, Holte S, Kabyemela E, Prevots DR, Fried M, Duffy PE. 2014. Parasite burden and severity of malaria in Tanzanian children. N Engl J Med 370:1799–1808. doi: 10.1056/NEJMoa1303944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavstsen T, Turner L, Saguti F, Magistrado P, Rask TS, Jespersen JS, Wang CW, Berger SS, Baraka V, Marquard AM, Seguin-Orlando A, Willerslev E, Gilbert MT, Lusingu J, Theander TG. 2012. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A 109:E1791–E1800. doi: 10.1073/pnas.1120455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avril M, Brazier AJ, Melcher M, Sampath S, Smith JD. 2013. DC8 and DC13 var genes associated with severe malaria bind avidly to diverse endothelial cells. PLoS Pathog 9:e1003430. doi: 10.1371/journal.ppat.1003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, Andisi C, Bull PC, Mok S, Gupta AP, Wang CW, Turner L, Arman M, Raza A, Bozdech Z, Rowe JA. 2012. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A 109:E1772–E1781. doi: 10.1073/pnas.1120461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertin GI, Lavstsen T, Guillonneau F, Doritchamou J, Wang CW, Jespersen JS, Ezimegnon S, Fievet N, Alao MJ, Lalya F, Massougbodji A, Ndam NT, Theander TG, Deloron P. 2013. Expression of the domain cassette 8 Plasmodium falciparum erythrocyte membrane protein 1 is associated with cerebral malaria in Benin. PLoS One 8:e68368. doi: 10.1371/journal.pone.0068368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA, Magistrado P, Lusingu J, Smith JD, Higgins MK, Theander TG. 2013. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498:502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau CK, Turner L, Jespersen JS, Lowe ED, Petersen B, Wang CW, Petersen JE, Lusingu J, Theander TG, Lavstsen T, Higgins MK. 2015. Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe 17:118–129. doi: 10.1016/j.chom.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson BA, Welch TL, Smith JD. 2003. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analyzed across a parasite genome. Mol Microbiol 47:1265–1278. doi: 10.1046/j.1365-2958.2003.03378.x. [DOI] [PubMed] [Google Scholar]
- 31.Drakeley CJ, Carneiro I, Reyburn H, Malima R, Lusingu JP, Cox J, Theander TG, Nkya WM, Lemnge MM, Riley EM. 2005. Altitude-dependent and -independent variations in Plasmodium falciparum prevalence in northeastern Tanzania. J Infect Dis 191:1589–1598. doi: 10.1086/429669. [DOI] [PubMed] [Google Scholar]
- 32.Mmbando BP, Vestergaard LS, Kitua AY, Lemnge MM, Theander TG, Lusingu JP. 2010. A progressive declining in the burden of malaria in north-eastern Tanzania. Malar J 9:216. doi: 10.1186/1475-2875-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cham GK, Kurtis J, Lusingu J, Theander TG, Jensen AT, Turner L. 2008. A semi-automated multiplex high-throughput assay for measuring IgG antibodies against Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) domains in small volumes of plasma. Malar J 7:108. doi: 10.1186/1475-2875-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JD, Rowe JA, Higgins MK, Lavstsen T. 2013. Malaria's deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol 15:1976–1983. doi: 10.1111/cmi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger SS, Turner L, Wang CW, Petersen JE, Kraft M, Lusingu JP, Mmbando B, Marquard AM, Bengtsson DB, Hviid L, Nielsen MA, Theander TG, Lavstsen T. 2013. Plasmodium falciparum expressing domain cassette 5 type PfEMP1 (DC5-PfEMP1) bind PECAM1. PLoS One 8:e69117. doi: 10.1371/journal.pone.0069117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clausen TM, Christoffersen S, Dahlback M, Langkilde AE, Jensen KE, Resende M, Agerbaek MO, Andersen D, Berisha B, Ditlev SB, Pinto VV, Nielsen MA, Theander TG, Larsen S, Salanti A. 2012. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem 287:23332–23345. doi: 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, Wahlgren M. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336:1457–1460. doi: 10.1016/0140-6736(90)93174-N. [DOI] [PubMed] [Google Scholar]
- 38.Fried M, Duffy PE. 1998. Maternal malaria and parasite adhesion. J Mol Med (Berl) 76:162–171. doi: 10.1007/s001090050205. [DOI] [PubMed] [Google Scholar]
- 39.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med 200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vigan-Womas I, Lokossou A, Guillotte M, Juillerat A, Bentley G, Garcia A, Mercereau-Puijalon O, Migot-Nabias F. 2010. The humoral response to Plasmodium falciparum VarO rosetting variant and its association with protection against malaria in Beninese children. Malar J 9:267. doi: 10.1186/1475-2875-9-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bengtsson A, Joergensen L, Rask TS, Olsen RW, Andersen MA, Turner L, Theander TG, Hviid L, Higgins MK, Craig A, Brown A, Jensen AT. 2013. A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J Immunol 190:240–249. doi: 10.4049/jimmunol.1202578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams Y, Kuhnrae P, Higgins MK, Ghumra A, Rowe JA. 2014. Rosetting Plasmodium falciparum-infected erythrocytes bind to human brain microvascular endothelial cells in vitro, demonstrating a dual adhesion phenotype mediated by distinct P. falciparum erythrocyte membrane protein 1 domains. Infect Immun 82:949–959. doi: 10.1128/IAI.01233-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M. 1998. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J 17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joergensen L, Bengtsson DC, Bengtsson A, Ronander E, Berger SS, Turner L, Dalgaard MB, Cham GK, Victor ME, Lavstsen T, Theander TG, Arnot DE, Jensen AT. 2010. Surface coexpression of two different PfEMP1 antigens on single Plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog 6:e1001083. doi: 10.1371/journal.ppat.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang L, Mu J, Zhang Q, Ni T, Srinivasan P, Rayavara K, Yang W, Turner L, Lavstsen T, Theander TG, Peng W, Wei G, Jing Q, Wakabayashi Y, Bansal A, Luo Y, Ribeiro JM, Scherf A, Aravind L, Zhu J, Zhao K, Miller LH. 2013. PfSET vs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum. Nature 499:223–227. doi: 10.1038/nature12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang CW, Hermsen CC, Sauerwein RW, Arnot DE, Theander TG, Lavstsen T. 2009. The Plasmodium falciparum var gene transcription strategy at the onset of blood stage infection in a human volunteer. Parasitol Int 58:478–480. doi: 10.1016/j.parint.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Turner L, Wang CW, Lavstsen T, Mwakalinga SB, Sauerwein RW, Hermsen CC, Theander TG. 2011. Antibodies against PfEMP1, RIFIN, MSP3, and GLURP are acquired during controlled Plasmodium falciparum malaria infections in naive volunteers. PLoS One 6:e29025. doi: 10.1371/journal.pone.0029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cham GK, Turner L, Lusingu J, Vestergaard L, Mmbando BP, Kurtis JD, Jensen AT, Salanti A, Lavstsen T, Theander TG. 2009. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J Immunol 183:3356–3363. doi: 10.4049/jimmunol.0901331. [DOI] [PubMed] [Google Scholar]
- 49.Cham GK, Turner L, Kurtis JD, Mutabingwa T, Fried M, Jensen AT, Lavstsen T, Hviid L, Duffy PE, Theander TG. 2010. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infect Immun 78:4653–4659. doi: 10.1128/IAI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghumra A, Semblat JP, Ataide R, Kifude C, Adams Y, Claessens A, Anong DN, Bull PC, Fennell C, Arman M, Amambua-Ngwa A, Walther M, Conway DJ, Kassambara L, Doumbo OK, Raza A, Rowe JA. 2012. Induction of strain-transcending antibodies against group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathog 8:e1002665. doi: 10.1371/journal.ppat.1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goncalves BP, Fried M, Duffy PE. 2014. Parasite burden and severity of malaria in Tanzanian children. N Engl J Med 371:482. doi: 10.1056/NEJMc1406607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayor A, Rovira-Vallbona E, Srivastava A, Sharma SK, Pati SS, Puyol L, Quinto L, Bassat Q, Machevo S, Mandomando I, Chauhan VS, Alonso PL, Chitnis CE. 2009. Functional and immunological characterization of a Duffy binding-like alpha domain from Plasmodium falciparum erythrocyte membrane protein 1 that mediates rosetting. Infect Immun 77:3857–3863. doi: 10.1128/IAI.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]