Abstract
Risk factors for anastomotic leaks of pancreaticojejunostomy have been well described. We present a technique using indocyanine green dye (ICG) and a near-infrared (NIR) capable laparoscope to assess blood supply to the transected margin of the pancreas before pancreaticojejunal anastomosis. A 39-year-old female patient underwent a laparoscopic-assisted pancreaticoduodenectomy (Whipple's procedure) for an invasive ampullary adenocarcinoma. Before construction of the pancreaticojejunal anastomosis, the viability of the margin of the remnant pancreas was assessed with infrared scanning of injected ICG. The NIR identified an ischaemic segment of the margin, which was further resected. The patient had no postoperative evidence of a pancreatic leak and was discharged home on postoperative day 18. Ischaemia of the remnant pancreas is a risk factor for pancreaticojejunostomy leak. Infrared ICG testing might help to identify these ischaemic segments, which can be excised before anastomosis, and reduce failure rates.
INTRODUCTION
Surgical resection remains the only chance of cure for patients with pancreatic adenocarcinoma and periampullary cancers. The most commonly performed procedure for lesions in or around the head of the pancreas is a pancreaticoduodenectomy (Whipple's procedure). Although mortality associated with pancreaticoduodenectomy has decreased over the years, morbidity rates remain high. The most common complication associated with a Whipple's procedure is failure of the pancreatic anastomosis. Risk factors reported to increase the risk of pancreatic anastomotic failure include a soft pancreas and a small duct [1]. One other factor that has rarely been discussed in the literature is the association between ischaemia of the remnant pancreatic margin and pancreatic anastomotic failure. Strasberg et al. reported in 1998 that ensuring adequate blood supply to the remnant margin reduces the incidence of pancreatic anastomotic failure [2]. Here, we report a novel technique using indocyanine green dye (ICG) to assess blood flow at the pancreatic remnant margin before construction of the pancreatic anastomosis (pancreaticojejunostomy).
CASE REPORT
A 39-year-old female patient presented with symptoms of dyspepsia and jaundice. Investigations including endoscopy and computed tomography (CT) scans demonstrated an ampullary lesion in the duodenum. Biopsies taken at endoscopy were reported as a tubular adenoma with high-grade dysplasia. The initial treatment with ampullectomy demonstrated an infiltrating ampullary adenocarcinoma with incomplete excision on histopathology. In the presence of an incomplete resection, the patient subsequently underwent a laparoscopic-assisted Whipple's procedure. Before construction of the pancreaticojejunal anastomosis, the viability of the margin of the remnant pancreas was assessed with ICG and a near-infrared (NIR) laparoscopic camera (Olympus™, Hamburg, Germany).
Briefly, once the surgeon is prepared to start the construction of the pancreaticojejunal anastomosis, the anaesthetist injects 2 ml (0.5 mg) of Infracyanine™ (concentration was 0.25 mg/ml) intravenously via a peripheral vein. The infrared camera is then focussed on the transected margin of the pancreas. The ICG contrast can be visualized in the viable areas but not in the ischaemic areas (Fig. 1a). The ischaemic segment at the margin was further resected before anastomosis (Fig. 1b). A duct to mucosa single-layer pancreaticojejunal anastomosis was then constructed using continuous silk suture.
Figure 1:
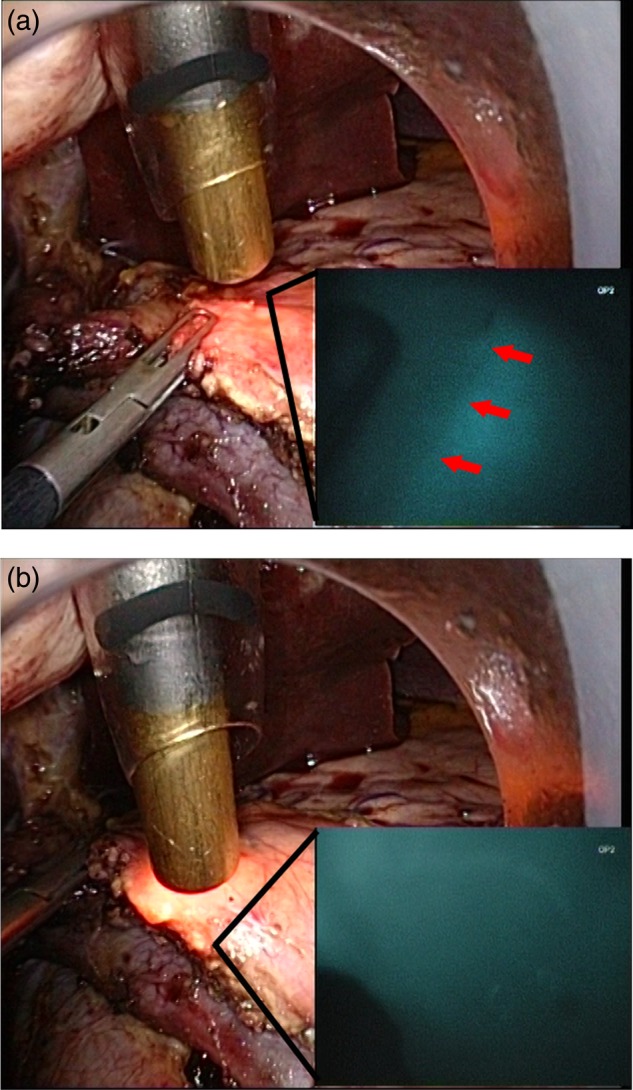
(a) The head of the pancreas has been resected laparoscopically. The pancreatic resection margin appears healthy to the naked eye with a small (<3 mm) pancreatic duct. ICG has been injected intravenously into the patient. The infrared camera is then switched on, and the ischaemic segment of the pancreatic margin demonstrating no ICG fluorescence can be visualized. (b) After resection of the ischaemic portion, the margin appears well perfused.
Histopathological examination of the resected specimen demonstrated a clear resection margin. The patient had no postoperative evidence of a pancreatic leak either clinically, on assessment of drain amylase or on CT imaging.
DISCUSSION
The development of pancreatic fistula after a Whipple's procedure remains a common complication. The definition proposed by the International Study Group for Pancreatic Fistula has graded the fistulas into A, B and C with Grade C accounting for significant morbidity and mortality [3]. A recent review by Denbo et al. reported an 18% incidence of pancreatic fistula with 15% of these being Grade C and suggested (rightfully so) that the focus should be on reducing this incidence [4].
Risk factors commonly reported for the development of a pancreatic fistula are a soft pancreas, small pancreatic duct and a fatty pancreas [5, 6]. While nothing can be done about the texture of the gland or the size of the duct, some studies have focussed on decreasing pancreatic exocrine function with the use of somatostatin analogues, using pancreatic stents, different forms of pancreatic anastomosis and gluing of the duct. The results are conflicting in the literature, but a recent review by Lermite et al. suggests that none of these demonstrated any significant advantage [7].
However, little has been published on pancreatic resection margin ischaemia and its association with the development of pancreatic fistula. The pancreatic head is supplied by the vascular arcade formed by the anastomosis of the superior and inferior pancreatico-duodenal arteries, while the body and tail are supplied by the splenic artery. The neck of the pancreas has been described as a watershed area with blood supply coming from either the head or the body [8]. Consequently, transection of the pancreas at the neck may result in an area of relative ischaemia and may contribute to anastomotic failure. Strasberg et al. reported that the assessment of blood supply to the transected margin of the pancreas visually and with Doppler ultrasound, with further transection if the blood supply is inadequate, resulted in decreased incidence of pancreatic fistula [2]. The technique by Strasberg et al. depends on the identification of good blood flow to the margin of the pancreas. Our technique differs from that of Strasberg et al. in that the objective was to identify ischaemic areas. Acute pancreatic ischaemia cannot be seen with the naked eye or with ultrasound, and fluorescence angiography is better than ultrasound in detecting tissue viability [9].
The use of ICG for NIR fluorescence angiography to demonstrate ischaemic tissue has been reported in animal models. In addition, Kudszus et al. demonstrated that NIR angiography using ICG and a detection video camera in colorectal surgery reduced the rate of anastomotic leaks [10]. The injection of ICG and assessment of its distribution with the NIR detection device allow the display of ischaemic zones, which can then be resected further. However, to our knowledge, this has never been used in pancreatic surgery. The disadvantage of Strasberg's technique is that the surgeon has to be skilled in intraoperative ultrasound assessment of the pancreas. The advantage of our technique is that no such skill is required, and the technique is simple and easy to learn. One disadvantage of this technique is the risk of an allergic reaction to ICG. However, compared with the morbidity and cost associated with pancreatic leaks in our opinion, this technique provides significant advantages.
The identification of an ischaemic transection margin during a Whipple's procedure allows further resection to viable tissue. This will improve the blood supply to the pancreatic anastomosis and may potentially help to decrease the incidence of pancreatic fistulas.
CONFLICT OF INTEREST STATEMENT
D.S., D.P., D.F. and B.G. have no conflict of interest. The NIR camera is supplied by Olympus™ (Tokyo, Japan) and is not yet available for commercial purchase.
REFERENCES
- 1.Poon RT, Lo SH, Fong D, Fan ST, Wong J. Prevention of pancreatic anastomotic leakage after pancreaticoduodenectomy. Am J Surg 2002;183:42–52. [DOI] [PubMed] [Google Scholar]
- 2.Strasberg SM, McNevin MS. Results of a technique of pancreaticojejunostomy that optimizes blood supply to the pancreas. J Am Coll Surg 1998;187:591–6. [DOI] [PubMed] [Google Scholar]
- 3.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 4.Denbo JW, Orr WS, Zarzaur BL, Behrman SW. Toward defining grade C pancreatic fistula following pancreaticoduodenectomy: incidence, risk factors, management and outcome. HPB (Oxford) 2012;14:589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg 2005;92:1117–23. [DOI] [PubMed] [Google Scholar]
- 6.Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg 2007;246:1058–64. [DOI] [PubMed] [Google Scholar]
- 7.Lermite E, Sommacale D, Piardi T, Arnaud JP, Sauvanet A, Dejong CH et al. Complications after pancreatic resection: diagnosis, prevention and management. Clin Res Hepatol Gastroenterol 2013;37:230–9. [DOI] [PubMed] [Google Scholar]
- 8.Skandalakis LJ, Rowe JS Jr, Gray SW, Skandalakis JE. Surgical embryology and anatomy of the pancreas. Surg Clin North Am 1993;73:661–97. [DOI] [PubMed] [Google Scholar]
- 9.Freeman DE, Gentile DG, Richardson DW, Fetrow JP, Tulleners EP, Orsini JA et al. Comparison of clinical judgment, Doppler ultrasound, and fluorescein fluorescence as methods for predicting intestinal viability in the pony. Am J Vet Res 1988;49:895–900. [PubMed] [Google Scholar]
- 10.Kudszus S, Roesel C, Schachtrupp A, Hoer JJ. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 2010;395:1025–30. [DOI] [PubMed] [Google Scholar]


