Abstract
In the past, reactive oxygen and nitrogen species (RONS) were shown to cause oxidative damage to biomolecules, contributing to the development of a variety of diseases. However, recent evidence has suggested that intracellular RONS are an important component of intracellular signaling cascades. The aim of this review was to consolidate old and new ideas on the chemical, physiological and pathological role of RONS for a better understanding of their properties and specific activities. Critical consideration of the literature reveals that deleterious effects do not appear if only one primary species (superoxide radical, nitric oxide) is present in a biological system, even at high concentrations. The prerequisite of deleterious effects is the formation of highly reactive secondary species (hydroxyl radical, peroxynitrite), emerging exclusively upon reaction with another primary species or a transition metal. The secondary species are toxic, not well controlled, causing irreversible damage to all classes of biomolecules. In contrast, primary RONS are well controlled (superoxide dismutase, catalase), and their reactions with biomolecules are reversible, making them ideal for physiological/pathophysiological intracellular signaling. We assume that whether RONS have a signal transducing or damaging effect is primarily defined by their quality, being primary or secondary RONS, and only secondly by their quantity.
Keywords: superoxide radical, hydrogen peroxide, hydroxyl radical, nitric oxide, mitochondria
1. Introduction
Reactive oxygen and nitrogen species (RONS) include two classes of chemically-reactive molecules containing oxygen (reactive oxygen species, ROS) and nitrogen (reactive nitrogen species, RNS). Both classes are referred to as RONS. The majority of RONS carries unpaired electrons and is called free radicals. In mammalians, a major function of specialized enzymes, such as NADPH-oxidase, myeloperoxidase and nitric oxide synthase (NOS), is the generation of RONS. The controlled generation of RONS in the extracellular space by these enzymes was developed evolutionarily as part of the innate immune system to kill bacteria. However, an overwhelming release of RONS may also induce deleterious effects, causing damage to host biological structures. Another group of enzymes release RONS intracellularly as a byproduct of metabolic processes. For instance, superoxide (O2•−) is released as a byproduct of mitochondrial respiration and monooxygenase activity of cytochrome p450. Intracellular RONS, as well as excessive release of extracellular RONS were thought to induce deleterious effects, causing oxidative damage to different kinds of biomolecules. These processes are referred to as “oxidative stress”.
Oxidative stress is believed to significantly contribute to the development of a number of diseases, particularly age-related diseases. However, more and more evidence suggest that intracellular generation of RONS is an important component of intracellular signaling cascades regulating several physiological functions, such as regulation of vascular tone, insulin synthesis, activation of hypoxia-inducible factor (HIF), cell proliferation, differentiation and migration. It took over 50 years for a clear understanding of the chemical basis of free radical/RONS biology to emerge. In the following 50 years, studies on the biological effects of free radicals with biological targets were undertaken. The aim of this review is to put together old and recent ideas on the chemical and pathophysiological role of RONS for a better understanding of their properties and specific activities. This review is predominantly based on selected reviews, elaborating on different aspects of RONS activity and thought to be a guide through a large body of literature existing on this topic.
2. Chemical Basics
The current knowledge on RONS biology is based on studies of free radical reactions conducted more than 100 years ago. Free radicals are defined as molecules having an unpaired electron. Their physical properties are similar to those of free electrons, giving a signal at g = 2.0023 in the electron paramagnetic resonance spectrum [1]. The chemical mechanisms underlying the formation and toxicity of free radicals were proposed by the British chemist Henry J. H. Fenton in 1894 and later developed by the Austrian chemist Joseph Weiss and the German chemist and Nobel Prize winner Fritz Haber in 1934. Henry J. H. Fenton showed that the formation of toxic hydroxyl radicals (•OH) from hydrogen peroxide (H2O2) is catalyzed by iron ions, called the “Fenton reaction” ([2], reviewed in [3]). He pointed out that iron ions are necessary to form toxic •OH radicals. Joseph Weiss and Fritz Haber discovered that O2•− can be converted into H2O2 and further to •OH, called the Haber–Weiss reaction ([4], reviewed in [5,6]). This reaction shows that one free radical can give rise to another secondary radical. Already in those days, the transformation of one ROS (O2•−) to another (•OH) was associated with the presence of iron ions as a catalyst (reviewed in [7]). Later, other transition metals, such as copper ions, were shown to generate toxic RONS.
Another important step in understanding the biological function of RONS was the discovery of free radical chain reactions. This was done in 1935 by the Russian chemist and Nobel Prize winner Nikolai Semenov. He described four types of free radical reactions, namely initiation, propagation, branching and termination [8]. The same reactions occur in biological membranes upon pathological conditions and are termed lipid peroxidation, the major mechanism of oxidative damage to biological membranes. Importantly, the branching chain reaction of lipid peroxidation, the cleavage of lipid peroxides, is catalyzed by ferrous ions similar to the Fenton reaction, which is a cleavage of H2O2 to •OH by ferrous ions. The branching chain reaction between lipid peroxides and iron ions accelerates lipid peroxidation [9], again suggesting that iron ions are the prerequisite for the toxic effects of lipid peroxidation.
In the 1950s, researchers started to associate free radical chemistry with biomedical questions. It has been suggested that most of the damaging effects of oxygen in living systems are due to the formation of free radicals (reviewed in [10]). This assumption promoted the application of the knowledge of free radical chemistry to biological systems.
3. Oxidative Stress
However, in 1968, a major breakthrough in the field of free radical biology was done by Irvin Fridovich who discovered superoxide dismutase (SOD), a specific enzyme catalyzing the transition of O2•− into H2O2 ([11,12], reviewed in [13]). A few years later, Chance and coauthors reported that mitochondria are the key generator of O2•− in cells ([14], reviewed in [15]). These two findings are crucial, as they show that free radicals, on the one hand, are produced in biological systems, and on the other hand, there is an enzymatic mechanism regulating their concentration. This clearly suggests that free radicals occur in biological systems and probably have a specific function. Since then, numerous studies have been performed to understand the biological function of free radicals.
Until the mid-1970s, the literature almost exclusively refers to free radicals. Later, it became evident that not only free radicals, but also non-radical products, such as H2O2 or hypochlorous acid (HOCl), which are also powerful oxidizing agents, participate in free radical reactions (reviewed in [16]). To take into account both the radical and the non-radical species, the more general term “reactive oxygen species” (ROS) was introduced. Later, nitric oxide (NO•) and peroxynitrite (ONOO−) were also shown to interact with ROS, and all of these species were termed RONS. Primarily, the toxic properties of RONS were of interest. It was shown that an excessive generation of RONS damaged almost all classes of biomolecules, such as lipids [17], proteins [18] and DNA (reviewed in [19,20]). In the 1970s and 1980s, the term “oxidative stress” was used for these deleterious processes. Later, “oxidative stress” was defined by the German biochemist Helmut Sies as an imbalance between oxidants and antioxidants in favor of the oxidants, potentially leading to damage ([21], reviewed in [22]). Evolutionarily, the induction of oxidative stress was possibly developed as an important part of the innate immune system as a defense mechanism against bacteria [23]. However, it was also shown that RONS, produced by the immune system, can also damage host cells [24].
Careful observation of oxidative damage reactions of biomolecules shows that primary RONS, such as O2•−, H2O2 or NO•, in most cases reversibly react with the target molecules. NO•, for instance, reversibly binds to heme proteins, whereas O2•− reacts with proteins, changing their redox state without damage to their structure. For instance, the reaction between O2•− and cytochrome c results in the reduction of heme, which is used to detect O2•− [25]. The damage is predominantly associated with secondary RONS, such as •OH, ONOO− and HOCl [26,27,28]. All of these toxic species are formed if more than one reactive species is present. Two major reactions leading to the formation of toxic RONS are: (i) the Fenton reaction between ferrous ions and H2O2 yielding •OH; and (ii) the reaction of O2•− with NO• yielding ONOO−. Furthermore, the formation of HOCl, formed in an enzymatic reaction from H2O2 and Cl−, is associated with damage to host tissues [28]. In addition, iron ions can directly react with organic peroxides, inducing lipid peroxidation. Moreover, the presence of iron and copper ions induces double-strand breaks of DNA, a DNA damage that is difficult to repair (reviewed in [29]). Some anticancer drugs are based on mechanisms causing double-strand breaks of DNA catalyzed by transition metals [30]. Oxidative damage to proteins is often associated with the reaction between amino acids and ONOO−, resulting in the formation of nitrated amino acids, such as nitrotyrosine. ONOO− is a secondary RONS formed in the reaction between NO• and O2•−. ONOO− and NO• may have quite opposite biological effects. For instance, NO• has an inhibitory effect on lipid peroxidation, while ONOO− activates this process (reviewed in [31]). Another important regulatory mechanism based on the interaction of NO• and O2•− is driven by decreased NO• levels, rather than by increased ONOO− levels (reviewed in [32,33]). The interaction between NO• and O2•− might also result in vasoconstriction by inactivation of prostacyclin synthase (reviewed in [34]).
The data described above suggest that primary ROS (O2•−, NO•, H2O2) only have a weak damaging potential, whereas secondary RONS are more toxic. Primary species are well controlled by SOD, catalase and NO synthases, while secondary species are less controllable, since there is no specific enzymatic system controlling their levels.
Interestingly, O2•−-controlling systems are different inside the cells and in extracellular fluids. SOD, the intracellular enzyme for removing O2•−, and the extracellular SOD (ecSOD) produce potentially dangerous H2O2. H2O2 itself is relatively inactive, but can lead to the formation of toxic •OH. In contrast, ceruloplasmin in the blood inactivates O2•−, yielding H2O [35]. However, extracellular SOD, which mainly occurs in tissue ([36,37]; reviewed in [38,39]), can also be found in plasma under specific pathological conditions [40] and contribute to the elimination of O2•−. The fact that SOD, not ceruloplasmin, occurs in cells indirectly suggests that H2O2 may have a physiological function inside cells, but not in extracellular fluids. The data gathered in the last few decades suggest that primary RONS formed in mitochondria (O2•− and H2O2) and NO• are associated with intracellular signaling cascades. Since NO-mediated signaling pathways have already been extensively reviewed ([41,42,43,44,45,46,47]), in this review, we focus on non-NO-mediated signaling pathways.
4. Signaling
There is a solid body of literature supporting the essential role of mitochondrial ROS in intracellular signaling. The data on the involvement of mitochondrial ROS in intracellular signaling pathways related to inflammation have been summarized in recent reviews [48,49,50]. In the last few years, the role of ROS in positive and negative regulation of insulin signaling has also been intensively studied and reviewed [51]. Furthermore, the role of mitochondrial ROS in activation of HIF has been intensively studied and debated (reviewed in [52,53]). In addition, the role of ROS has been demonstrated for NF-κB-dependent gene transcription and a number of other signaling cascades (reviewed in [54]). Notably, most of the publications on oxidative stress referred to specific types of RONS involved in oxidative damage, whereas data on signaling are predominantly addressed to ROS and RONS in general. This led to a large knowledge gap on the mechanisms of intracellular signaling concerning RONS, since is not clear whether all or only specific RONS contribute to these signaling pathways. In the following section, we will focus on studies where specific types of RONS contributing to intracellular signaling cascades were determined.
5. Superoxide Radical
A number of reports suggest O2•− as part of intracellular signaling cascades. This species is predominantly produced by mitochondrial complexes I and III (reviewed in [55]). Evidence of the involvement of mitochondrial O2•− in intracellular signaling cascades can be shown by:
Alteration of mitochondrial function, particularly of complexes I and III, by pharmacological or genetic modulation, which has an effect on signaling pathways.
The correlation of a certain O2•− level with an effective signaling cascade.
Application of mitochondria-targeted antioxidants (mtAOX) or radical scavengers has an effect on signaling pathways.
Genetic manipulation of mitochondrial SOD and cytoplasmic SOD decreases the efficiency of specific signaling cascades.
Mitochondrial O2•−, the primary mitochondrial ROS, was often associated with the regulation of inflammatory pathways, such as activation of the inflammasome, regulation of inflammatory cytokines synthesis and mechanisms of innate immunity. The involvement of mitochondrial O2•− in the activation of the inflammasome was suggested by Zhou et al. [56]. The authors showed that specific inhibition of mitochondrial complexes I and III, the major sources of ROS in mitochondria, significantly diminished the activation of the “nucleotide-binding domain, leucine-rich family and pyrin domain containing 3” (NLRP3) inflammasome, suggesting that mitochondrial O2•− is involved in this signaling cascade. Another important feature of inflammation is the release of cytokines. Bulua et al. [57] showed that LPS-stimulated IL-6 production could be reduced by treatment with MitoQ, a mitochondria-targeted radical scavenger. This effect was coincident with increased levels of mitochondrial O2•−, suggesting a key role of O2•− in this signaling pathway. Weidinger et al. demonstrated that mitochondria-targeted antioxidants reduce the expression of IL-6 and iNOS in a model of systemic inflammatory response induced by LPS [58]. In leukocytes, Kröller-Schön [59] showed that elevated mtROS formation activated NADPH-oxidase at the posttranslational level. Inhibition of the mitochondrial permeability transition pore, which is supposed to facilitate the transport of O2•− from mitochondria to the cytoplasm, prevented activation of NADPH-oxidase. In contrast, the deficiency of mitochondrial SOD intensified the stimulation of NADPH-oxidase, suggesting that this process is mediated by O2•−, rather than by H2O2. Applying specific mitochondrial inhibitors and direct detection of mtROS, Dikalov et al. suggested that mtROS, presumably O2•−, are able to activate NADPH-oxidase via activation of protein kinase C (PKC) [49]. These data suggest that mitochondrial O2•− orchestrate cellular ROS production upon inflammation. The same group has shown that stimulation of endothelial cells with angiotensin II elevates mitochondrial O2•− levels and simultaneously the activity of NADPH-oxidase in this non-immune cell type [60]. However, treatment with mitoTEMPO, a mitochondria-targeted antioxidant, or overexpression of mitochondrial SOD captured O2•− and decreased the activation of vascular NADPH oxidases [60]. NADPH oxidases, in turn, may regulate important cellular processes, such as cell migration [61], differentiation [62] and proliferation [63] (reviewed in [64]). These data again suggest that O2•− rather than H2O2 is involved in this signaling cascade. However, in the past, it was believed that O2•− does not participate in signaling, as it cannot exit mitochondria due to its polarity. Consequently, H2O2 formed from O2•− was considered a necessary intermediate of O2•−-mediated actions. H2O2 is a nonpolar molecule and can easily diffuse through the membranes. Recently, however, the situation has shifted. O2•− has been shown to leave mitochondria via the mitochondrial permeability transition pore [65] and anion channels [66]. We also showed that O2•− can be released from mitochondria by using O2•−-sensitive spin probes and electron spin resonance spectroscopy [67]. These data strongly support the postulation that O2•− can directly contribute to intracellular signal transduction pathways.
Nevertheless, other groups propose H2O2 being the RONS-based messenger in intracellular signaling cascades, as well. In contrast to O2•−, H2O2 is a neutral molecule and relatively inactive. Thus, H2O2 is able to cover relatively large distances, up to several cell diameters, before it reacts with its target or is catabolized [68]. Therefore, it is considered as a suitable ROS-dependent signaling component.
6. Hydrogen Peroxide
In the literature, the impact of H2O2 on intracellular signaling is supported by the following evidence:
Exogenous H2O2 has a direct effect on signaling cascades.
The H2O2 level correlates with the effectiveness of intracellular signal transduction.
Genetic manipulation of catalase has an effect on signaling.
Upregulation of MnSOD and/or Cu/ZnSOD activates signaling.
Treatment with H2O2 increased the proliferation of endothelial cells in a study of Chen et al., suggesting that H2O2 directly interferes with pathways regulating proliferation [69]. Wang et al. [70] showed that overexpression of the mitochondria-targeted catalase construct suppressed vascular endothelial growth factor (VEGF)-induced cell migration, suggesting the involvement of H2O2 in the regulation of cell migration. Schmidt et al. [71] demonstrated that overexpression of catalase in cell lines caused a deficiency in the activation of NF-κB in response to tumor necrosis factor (TNF), while the catalase inhibitor, aminotriazole, restored the induction of NF-κB. Overexpression of Cu/Zn-dependent SOD elevated NF-κB activation. These data suggest H2O2 rather than O2•− as the mediator of NF-kB pathway activation. Brunelle et al. [72] demonstrated that overexpressing glutathione peroxidase or catalase, but not SOD, stabilized HIF-1 in cells, suggesting that H2O2 acts as signaling molecule in the process of HIF-1 regulation. West et al. [73] showed that overexpressing catalase in mitochondria results in impaired bacterial killing by leukocytes, suggesting the predominant role of H2O2. Hoarau et al. [74] demonstrated that H2O2 plays an essential role in the development of β-cells, as it activates the ERK1/2 pathway. Other studies on H2O2-mediated signaling are summarized in several reviews [75,76,77].
7. Secondary RONS
The majority of papers on RONS-mediated intracellular signaling suggest either O2•− or H2O2 as the major signaling molecule. Only a few studies suggest that signaling molecules may be derivatives of H2O2. Garlid et al. [78] studied ROS-mediated opening of mitochondrial ATP-sensitive potassium channels and suggested an unknown derivative of H2O2 as a contributor to this pathway. Others suggested that HOCl may participate in extracellular, but not in intracellular signaling, for instance by interaction with TGF-ß1 [79]. A few more publications can be found on peroxynitrite-mediated signaling (reviewed in [80,81]). It has been assumed that peroxynitrite has an impact on pathways, which, under physiological conditions, are regulated by tyrosine phosphorylation and dephosphorylation. ONOO− causes tyrosine nitration, which blocks the respective signaling cascades. Tyrosine nitration seems to have a significant impact on a number of pathways, such as MAP kinase, STAT3, ERK and PKC-mediated pathways (reviewed in [80,81]). The fact that ONOO− irreversibly binds to proteins has a pathological impact on cellular function, rather than contributing to physiological intracellular signaling. This suggests that the biological impact of primary and secondary RONS is different. Primary RONS are predominantly associated with signaling, whereas secondary RONS are associated with oxidative stress. Primary RONS are regulated by SOD, catalase and peroxidases and have a specific physiological function for the regulation of intracellular signaling. The secondary RONS were evolutionarily developed for extracellular actions, predominantly as part of the innate immune system for killing bacteria. The intracellular release of such secondary RONS leads to deleterious consequences, as these are catalytically highly active, without a reliable control system for intracellular levels. We assume that, in evolution, the primary species were developed for intracellular physiological signaling and the secondary species for extracellular actions, such as killing of bacteria. However, at the same time, these species are able to cause damage to the cell.
8. Conclusions
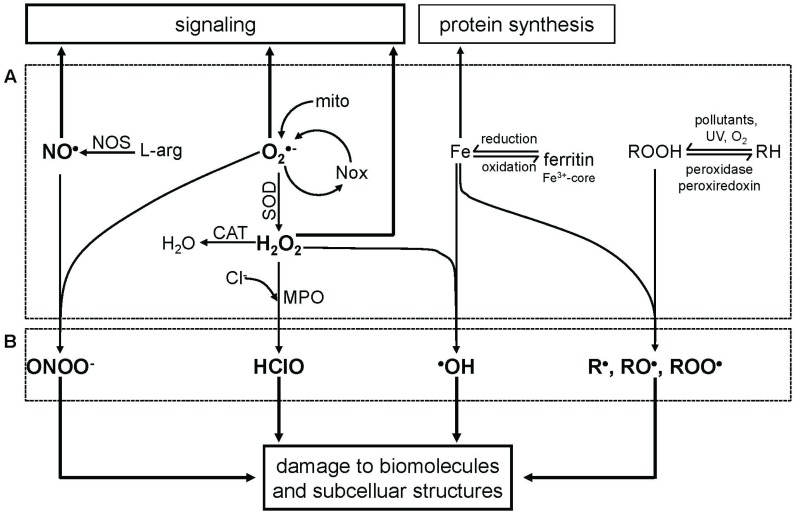
We assume that whether RONS have a beneficial or deleterious effect is primarily defined by their quality, being primary or secondary RONS, and only secondly by their quantity (Figure 1). Therefore, we think that the common statement that at low concentrations ROS regulate physiological processes and at high concentrations are deleterious is not completely correct. Critical consideration of the existing literature shows that deleterious effects, termed as oxidative stress, do not appear if only one primary species is present in a biological system, even at high concentrations. To develop deleterious effects, a primary species reacts with another or a transition metal, yielding highly reactive secondary species, such as ONOO− or •OH. The secondary RONS are catalytically very active, not tightly controlled and consequently may not act as signal transducers. In contrast, primary RONS are well controlled; their reactions with targets are reversible; and they do not damage target molecules. This makes them ideal for intracellular signaling processes. Unfortunately, the majority of papers on signaling refers to RONS without specifying their types. In this review, we highlight an approach allowing one to distinguish the contribution of different RONS. This can be used to define the origin of RONS contributing to intracellular signaling cascades in future studies.
Figure 1.
Scheme illustrating physiological and pathophysiological reactions of different reactive species. A, primary reactive species (NO•, O2•−, Fe, ROOH) and the products of the interaction of two identical reactive species (dismutation of O2 to H2O2) and transition metals (reactive oxygen, nitrogen and metal species = RONMS). B, secondary products of reactions between two different RONMS. Primary products predominantly contribute to physiological processes (e.g., signaling, protein synthesis); secondary products exert deleterious effects on diverse cell functions. Abbreviations: NO, nitric oxide; O2•−, superoxide; Fe, iron; ROOH, lipid peroxide; H2O2 hydrogen peroxide; RH, non-oxidized lipid; R•, RO•, ROO•, lipid radicals; NOS, nitric oxide synthase; l-arg, l-arginine; ONOO−, peroxynitrite; NOX, NADPH oxidase; mito, mitochondria; SOD, superoxide dismutase; CAT, catalase; H2O, water; Cl−, chloride ion; MPO, myeloperoxidase; HClO, hypochlorous acid; •OH, hydroxyl radical; UV, ultraviolet radiation.
Acknowledgments
The authors thank Asmita Banerjee for assistance in the preparation of this manuscript. Adelheid Weidinger was supported by FFG (Austria, COMET K-Project 825329, BioPersMed) and by FWF (Austria, Project P211221-B11).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wertz J.E., Bolton J.R. Electron Spin Resonance: Elementary Theory and Practical Applications. John Wiley & Sons; New York, NY, USA: 1972. [Google Scholar]
- 2.Fenton H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894;65:899–910. doi: 10.1039/ct8946500899. [DOI] [Google Scholar]
- 3.Koppenol W.H. The centennial of the Fenton reaction. Free Radic. Biol. Med. 1993;15:645–651. doi: 10.1016/0891-5849(93)90168-T. [DOI] [PubMed] [Google Scholar]
- 4.Haber F., Weiss J. The catalytic decomposition of hydrogen peroxide. Proc. R. Soc. 1934;147:332–351. doi: 10.1098/rspa.1934.0221. [DOI] [Google Scholar]
- 5.Koppenol W.H. The Haber-Weiss cycle—70 Years later. Redox Rep. 1994;6:229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 6.Kehrer J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 7.Ullrich V., Kissner R. Redox signaling: Bioinorganic chemistry at its best. J. Inorg. Biochem. 2006;100:2079–2086. doi: 10.1016/j.jinorgbio.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Semenov N.N. Some Problems in Chemical Kinetics and Reactivity. Princeton University Press; Princeton, NJ, USA: 1959. [Google Scholar]
- 9.Vladimirov Y.A., Olenev V., Suslova T., Cheremisina Z. Lipid peroxidation in mitochondrial membrane. Adv. Lipid Res. 1980;17:173–249. doi: 10.1016/b978-0-12-024917-6.50011-2. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert D.L. Oxygen and Living Processes: An Interdisciplinary Approach. Springer; New York, NY, USA: 1981. [Google Scholar]
- 11.McCord J.M., Fridovich I. The reduction of cytochrome c by hypoxanthine and xanthine oxidase. Biochem. J. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 12.Mccord J.M., Fridovich I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 13.Fridovich I. Superoxide anion radical, superoxide dismutases, and related matters. J. Biol. Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 14.Boveris B.A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem. J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B. Free radicals, reactive oxygen species and human disease: A critical evaluation with special reference to atherosclerosis. Br. J. Exp. Pathol. 1989;70:737–757. [PMC free article] [PubMed] [Google Scholar]
- 17.Azizova O.A., Panasenko O., Volnov T., Vladimirov Y.A. Free radical lipid oxidation affects cholesterol transfer between lipoproteins and erythrocytes. Free Radic. Biol. Med. 1989;7:251–257. doi: 10.1016/0891-5849(89)90132-9. [DOI] [PubMed] [Google Scholar]
- 18.Lyras L., Perry R.H., Perry E.K., Ince P.G., Jenner A., Jenner P., Halliwell B. Oxidative damage to proteins, lipids, and DNA in cortical brain regions from patients with dementia with Lewy bodies. J. Neurochem. 1998;71:302–312. doi: 10.1046/j.1471-4159.1998.71010302.x. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B., Aruoma O.I. DNA damage by oxygen-derived species. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 20.Cadet J., Douki T., Ravanat J.-L. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Sies H. Oxidative Stress. Academic Press; London, UK: 1985. Oxidative stress: Introductory remarks; pp. 1–8. [Google Scholar]
- 22.Sies H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 23.Rosen H., Klebanoff S.J., Wang Y., Brot N., Heinecke J.W., Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc. Natl. Acad. Sci. USA. 2009;106:18686–18691. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab L., Goroncy L., Palaniyandi S., Gautam S., Triantafyllopoulou A., Mocsai A., Reichardt W., Karlsson F.J., Radhakrishnan S.V., Hanke K., et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat. Med. 2014;20:648–654. doi: 10.1038/nm.3517. [DOI] [PubMed] [Google Scholar]
- 25.Arthur M., Kowalski-Saunders P., Gurney S., Tolcher R., Bull F., Wright R. Reduction of ferricytochrome C may underestimate superoxide production by monocytes. J. Immunol. Methods. 1987;98:63–69. doi: 10.1016/0022-1759(87)90436-4. [DOI] [PubMed] [Google Scholar]
- 26.Cadet J., Wagner J.R. Oxidatively generated base damage to cellular DNA by hydroxyl radical and one-electron oxidants: Similarities and differences. Arch. Biochem. Biophys. 2014;557:47–54. doi: 10.1016/j.abb.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Salgo M.G., Squadrito G.L., Pryor W.A. Peroxynitrite causes apoptosis in rat thymocytes. Biochem. Biophys. Res. Commun. 1995;215:1111–1118. doi: 10.1006/bbrc.1995.2578. [DOI] [PubMed] [Google Scholar]
- 28.Panasenko O.M., Evgina S.A., Driomina E.S., Sharov V.S., Sergienko V.I., Vladimirov Y.A. Hypochlorite induces lipoproteins and lipid peroxidation in blood phospholipid liposomes. Free Radic. Biol. Med. 1995;19:133–140. doi: 10.1016/0891-5849(94)00211-2. [DOI] [PubMed] [Google Scholar]
- 29.Chevion M. A site-specific mechanism for free radical induced biological damage: The essential role of redox-active transition metals. Free Radic. Biol. Med. 1988;5:27–37. doi: 10.1016/0891-5849(88)90059-7. [DOI] [PubMed] [Google Scholar]
- 30.Roy B., Hecht S.M. Hairpin DNA sequences bound strongly by bleomycin exhibit enhanced double-strand cleavage. J. Am. Chem. Soc. 2014;136:4382–4393. doi: 10.1021/ja500414a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Violi F., Milite M.T., Medica I.C., La Á. Nitric oxide and its role in lipid peroxidation. Diabetes Metab. Res. Rev. 1999;15:283–288. doi: 10.1002/(SICI)1520-7560(199907/08)15:4<283::AID-DMRR42>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 32.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular disease. The role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 33.Yung L.M., Leung F.P., Yao X., Chen Z.-Y., Huang Y. Reactive oxygen species in vascular wall. Cardiovasc. Hematol. Disord. Drug Targets. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 34.Schildknecht S., Ullrich V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch. Biochem. Biophys. 2009;484:183–189. doi: 10.1016/j.abb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Samokyszyns V.M., Miller D.M., Reif D.W., Austq S.D. Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J. Biol. Chem. 1989;264:21–26. [PubMed] [Google Scholar]
- 36.Marklund S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marklund S.L. Extracellular superoxide dismutase in human tissues and human cell lines. J. Clin. Invest. 1984;74:1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fattman C.L., Schaefer L.M., Oury T.D. Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 2003;35:236–256. doi: 10.1016/S0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 39.Fukai T., Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandstrom J., Nilsson P., Karlsson K., Marklund S.L. 10-Fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J. Biol. Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 41.Stamler J.S., Carolina N. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell. 1994;76:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 42.Foster M.W., McMahon T.J., Stamler J.S. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9:160–168. doi: 10.1016/S1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 43.Guikema B., Lu Q.I., Heuil D.J. Chemical considerations and biological selectivity of protein nitrosation: Implications for NO-mediated signal transduction. Antioxid. Redox Signal. 2005;7:593–606. doi: 10.1089/ars.2005.7.593. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed K.A., Sawa T., Akaike T. Protein cysteine S-guanylation and electrophilic signal transduction by endogenous nitro-nucleotides. Amino Acids. 2011;41:123–130. doi: 10.1007/s00726-010-0535-1. [DOI] [PubMed] [Google Scholar]
- 45.Piantadosi C.A. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim. Biophys. Acta. 2012;1820:712–721. doi: 10.1016/j.bbagen.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sha Y., Marshall H.E. S-nitrosylation in the regulation of gene transcription. Biochim. Biophys. Acta. 2012;1820:701–711. doi: 10.1016/j.bbagen.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawa T., Zaki M.H., Okamoto T., Akuta T., Tokutomi Y., Kim-Mitsuyama S., Ihara H., Kobayashi A., Yamamoto M., Fujii S., et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3',5'-cyclic monophosphate. Nat. Chem. Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- 48.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 49.Dikalov S.I., Nazarewicz R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulz E., Wenzel P., Münzel T., Daiber A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 52.Cash T.P., Pan Y., Simon M.C. Reactive oxygen species and cellular oxygen sensing. Free Radic. Biol. Med. 2007;43:1219–1225. doi: 10.1016/j.freeradbiomed.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klimova T., Chandel N.S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15:660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- 54.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 55.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 57.Bulua A.C., Simon A., Maddipati R., Pelletier M., Park H., Kim K.-Y., Sack M.N., Kastner D.L., Siegel R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) Exp. Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weidinger A., Müllebner A., Paier-Pourani J., Banerjee A., Miller I., Lauterböck L., Duvigneau J.C., Skulachev V.P., Redl H., Kozlov A.V., et al. Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats. Antioxid. Redox Signal. 2015;22:572–586. doi: 10.1089/ars.2014.5996. [DOI] [PubMed] [Google Scholar]
- 59.Kröller-Schön S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M., Xia N., Hausding M., Mikhed Y., Zinssius E., et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ullevig S., Zhao Q., Lee C.F., Seok Kim H., Zamora D., Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler. Thromb. Vasc. Biol. 2012;32:415–426. doi: 10.1161/ATVBAHA.111.238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J., Stouffs M., Serrander L., Banfi B., Bettiol E., Charnay Y., Steger K., Krause K.-H., Jaconi M.E. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and map kinase activation. Mol. Biol. Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crosas-Molist E., Bertran E., Sancho P., López-Luque J., Fernando J., Sánchez A., Fernández M., Navarro E., Fabregat I. The NADPH oxidase NOX4 inhibits hepatocyte proliferation and liver cancer progression. Free Radic. Biol. Med. 2014;69:338–347. doi: 10.1016/j.freeradbiomed.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 64.Crestani B., Besnard V., Boczkowski J. Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2011;43:1086–1089. doi: 10.1016/j.biocel.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Hou Y., Ghosh P., Wan R., Ouyang X., Cheng H., Mattson M., Cheng A. Permeability transition pore-mediated mitochondrial superoxide flashes. Neurobiol. Aging. 2014;35:975–989. doi: 10.1016/j.neurobiolaging.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lustgarten M.S., Bhattacharya A., Muller F.L., Jang Y.C., Shimizu T., Shirasawa T., Richardson A., van Remmen H. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. Biochem. Biophys. Res. Commun. 2012;422:515–521. doi: 10.1016/j.bbrc.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piskernik C., Haindl S., Behling T., Gerald Z., Kehrer I., Redl H., Kozlov A.V. Antimycin A and lipopolysaccharide cause the leakage of superoxide radicals from rat liver mitochondria. Biochim. Biophys. Acta. 2008;1782:280–285. doi: 10.1016/j.bbadis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Chen K. Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic. Biol. Med. 2003;35:117–132. doi: 10.1016/S0891-5849(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 69.Chen K., Thomas S.R., Albano A., Murphy M.P., Keaney J.F. Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J. Biol. Chem. 2004;279:35079–35086. doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Zang Q.S., Liu Z., Wu Q., Maass D., Dulan G., Shaul P.W., Melito L., Frantz D.E., Kilgore J.A., et al. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am. J. Physiol. 2011;301:C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt K.N., Amstad P., Cerutti P., Baeuerle P.A. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-κB. Chem. Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 72.Brunelle J.K., Bell E.L., Quesada N.M., Vercauteren K., Tiranti V., Zeviani M., Scarpulla R.C., Chandel N.S. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 73.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S., et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoarau E., Chandra V., Rustin P., Scharfmann R., Duvillie B. Pro-oxidant/antioxidant balance controls pancreatic β-cell differentiation through the ERK1/2 pathway. Cell Death Dis. 2014 doi: 10.1038/cddis.2014.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henriksen E.J. Effects of H2O2 on insulin signaling the glucose transport system in mammalian skeletal muscle. Methods Enzymol. 2013;528:269–278. doi: 10.1016/B978-0-12-405881-1.00016-1. [DOI] [PubMed] [Google Scholar]
- 76.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bretón-Romero R., Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garlid A.O., Jaburek M., Jacobs J.P., Garlid K.D. Mitochondrial reactive oxygen species: Which ROS signals cardioprotection? Am. J. Physiol. Heart Circ. Physiol. 2013;305:H960–H968. doi: 10.1152/ajpheart.00858.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng X.-X., Liu M., Yan W., Zhou Z.-Z., Xia Y.-J., Tu W., Li P.-Y., Tian D.-A. β3 Integrin promotes TGF-β1/H2O2/HOCl-mediated induction of metastatic phenotype of hepatocellular carcinoma cells by enhancing TGF-β1 signaling. PLOS ONE. 2013;8:e79857. doi: 10.1371/journal.pone.0079857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klotz L.-O., Schroeder P., Sies H. Peroxynitrite signaling: Receptor tyrosine kinases and activation of stress-responsive pathways. Free Radic. Biol. Med. 2002;33:737–743. doi: 10.1016/S0891-5849(02)00892-4. [DOI] [PubMed] [Google Scholar]
- 81.Szabó C., Ischiropoulos H., Radi R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]