Abstract
Pregnant and postpartum women with a history of bariatric surgery are at risk of micronutrient deficiencies as a result of the combination of physiologic changes related to pregnancy and iatrogenic postoperative alterations in the absorption and metabolism of crucial nutrients. This systematic review investigates micronutrient deficiencies and related adverse clinical outcomes in pregnant and postpartum women after bariatric surgery. A systematic approach involving critical appraisal was conducted independently by 2 researchers to examine deficiencies of phylloquinone, folate, iron, calcium, zinc, magnesium, iodide, copper, and vitamins A, D, and B-12 in pregnant and postpartum women after bariatric surgery, together with subsequent outcomes in the neonates. The search identified 29 relevant cases and 8 cohort studies. The quality of reporting among the case reports was weak according to the criteria based on the CARE (CAse REporting) guidelines as was that for the cohort studies based on the criteria from the Cohort Study Quality Assessment list of the Dutch Cochrane Center. The most common adverse neonatal outcomes related to maternal micronutrient deficiencies include visual complications (vitamin A), intracranial hemorrhage (phylloquinone), neurological and developmental impairment (vitamin B-12), and neural tube defects (folate). On the basis of the systematically collected information, we conclude that the evidence on micronutrient deficiencies in pregnant and postpartum women after bariatric surgery and subsequent adverse neonatal outcomes remains weak and inconclusive.
Keywords: maternal nutrition, pregnancy, micronutrients, bariatric surgery, obesity surgery, neonatal complications, gestation, early postpartum
Introduction
Pursuing an adequate maternal nutritional status during pregnancy is crucial to optimize maternal, fetal, and neonatal health. The avoidance of nutritional deficiencies during pregnancy is one of the key messages in the “1000 day” concept. This concept highlights the importance of a lasting foundation for health through adequate nutrition from the start of pregnancy until a child’s second birthday (1). The obstetric field is now adressing a clinical concern of nutritional deficiencies in pregnant and postpartum women with previous bariatric surgery (2), who represent a growing population as a consequence of the worldwide obesity epidemic. The complexity of physiologic changes related to the surgery combined with the physiologic changes related to pregnancy may predispose these women to depleted micronutrient concentrations or clinical deficiencies (2).
Restrictive bariatric procedures limit the size of the gastric pouch by the use of a band [laparoscopic adjustable gastric banding (LAGB)]11 or staples (sleeve gastrectomy). The intestinal continuity is kept intact, resulting in a normal digestion and absorption of nutrients. Depleted micronutrient concentrations or deficiencies, however, may occur because of restricted food intake, low nutrient-dense food choices, poor eating habits, and food intolerance (3, 4). Malabsorptive procedures [biliopancreatic diversion (BPD) or Scopinaro procedure] are characterized by bypassing the duodenum and jejunum. The amount of intestine available for absorption is reduced. In mixed procedures [Roux-and-Y gastric bypass (RYGB)], both energy intake and absorption are limited. Food intake is reduced by the creation of a gastric pouch, and less intestine, gastric acid, bile, and pancreatic enzymes are available for absorption (4). Frequently reported deficiencies after LAGB are folate and thiamin deficiencies; after BPD are vitamin A, D, and E and phylloquinone deficiencies; and after RYGB are iron, thiamin, vitamin D, folate, and calcium deficiencies (4). Pregnancy is mainly characterized by an increased maternal distribution volume and the increased need for nutrients allowing for the development and growth of the placenta and fetus (5, 6). Maternal kidney function adapts to the clearance of fetal and maternal metabolic waste, resulting in an increased urine excretion of water-soluble vitamins (5). In normal pregnancy, therefore, the need for most micronutrients is increased. Serum concentrations of the water-soluble vitamin B-6, vitamin B-12, folate, and thiamin, together with the fat-soluble vitamin A, phylloquinone, and the inactive form of vitamin D (25-hydroxyvitamin D [25(OH)D]) decrease, whereas vitamin E serum concentrations and the active metabolite of blood vitamin D increase. The need for minerals and trace elements is most often increased during pregnancy. An overview table showing the percentage increase in dietary intakes for micronutrients in pregnant women compared with nonpregnant women is presented in the article by Ladipo (5).
To the best of our knowledge, there are no systematic reviews on micronutrient deficiencies and related adverse outcomes in pregnancies after bariatric surgery, despite a growing clinical concern for the nutritional monitoring and follow-up of these high-risk pregnancies. Therefore, a systematic review was performed that aimed to identify original research reporting on micronutrient deficiencies in pregnant and postpartum women with bariatric surgery, together with the clinical impact on the neonate.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines have been applied appropriately. An internal review protocol was developed to make the review process applicable for 2 independent researchers (GJ and CM).
Criteria for considering studies for inclusion.
The target population consisted of pregnant and postpartum women with a history of bariatric surgery. The postpartum period is defined as the period beginning immediately after birth and extending for ∼6 wk. The intervention under study was bariatric surgery. This includes a variety of surgical procedures for the treatment of obesity, including the purely restrictive, the purely malabsorptive, and the combined procedures. The primary outcomes are depleted maternal concentrations or deficiencies of phylloquinone, folate, iron, calcium, zinc, magnesium, iodine, copper, and vitamins A, D, and B-12. The choice for these micronutrients was based on a nonsystematic descriptive overview study by Kaska et al. (7), with the exception of vitamin D, which was added because of the relevant prevalence of deficiencies postoperatively and its important role in pregnancy (8). Secondary outcomes were fetal and neonatal complications related to low maternal micronutrient concentrations or deficiencies after bariatric surgery. Original research studies, including nonrandomized and randomized controlled trials, cohort studies, case-control studies, and case series, were considered for this review. Case reports were also considered as relevant because the overview study by Kaska et al. (7) referred to many case-report studies.
Search methods for identification of studies.
First, a database search was conducted in PubMed, Embase, and the Cochrane Library by using the following search terms: (vitamins OR vitamin* OR micronutrient* OR folate OR cobalamin OR zinc OR magnesium OR iodide OR iron OR copper) AND (pregnancy OR pregnan* OR gestation*) AND [bariatric surgery OR gastroenterostomy OR (obesity AND surgery) OR gastric by-pass]. Second, the reference lists of relevant publications identified in the first step of the search strategy were screened for additional relevant publications (“snowballing”). The last search was performed on 12 March 2015.
Data collection and analysis.
All studies identified were screened for the following inclusion criteria: 1) reported concentrations of folate, phylloquinone, iron, calcium, zinc, magnesium, iodine, copper, and vitamins A, D and B-12; 2) performed in pregnant and postpartum women with previous bariatric surgery; 3) published in English; 4) published during the past 25 y (1990 to March 2015); and 5) were original research studies.
Critical appraisal of the selected studies.
Because no relevant specific scoring system for case reports was available to our knowledge, a self-developed scoring system was used to critically evaluate the quality of the case reports. The reporting guidelines for case reports [CARE (CAse REporting) guidelines] were first consulted (9). From there, it was decided to formulate 6 specific criteria that seemed to be clinically relevant and necessary in order to obtain a clear and comprehensive view on the cases. The following criteria were formulated and assessed for the specific reports: time between procedure and pregnancy, micronutrient status and supplement intake before pregnancy, supplement intake during pregnancy, laboratory values, and any clinical follow-up in case of adverse pregnancy outcome.
For the cohort studies, the Cohort Study Quality Assessment list of the Dutch Cochrane Center was used (10). The criteria were sample description, selection bias, definition of the exposure, definition of outcomes, length of follow-up, selective loss to follow-up, and confounding factors. Two independent authors (GJ and CM) were responsible for the search and quality appraisal. In case of disagreement or doubt, a third (AB) and fourth (RD) reviewer were consulted.
Results
Search strategy
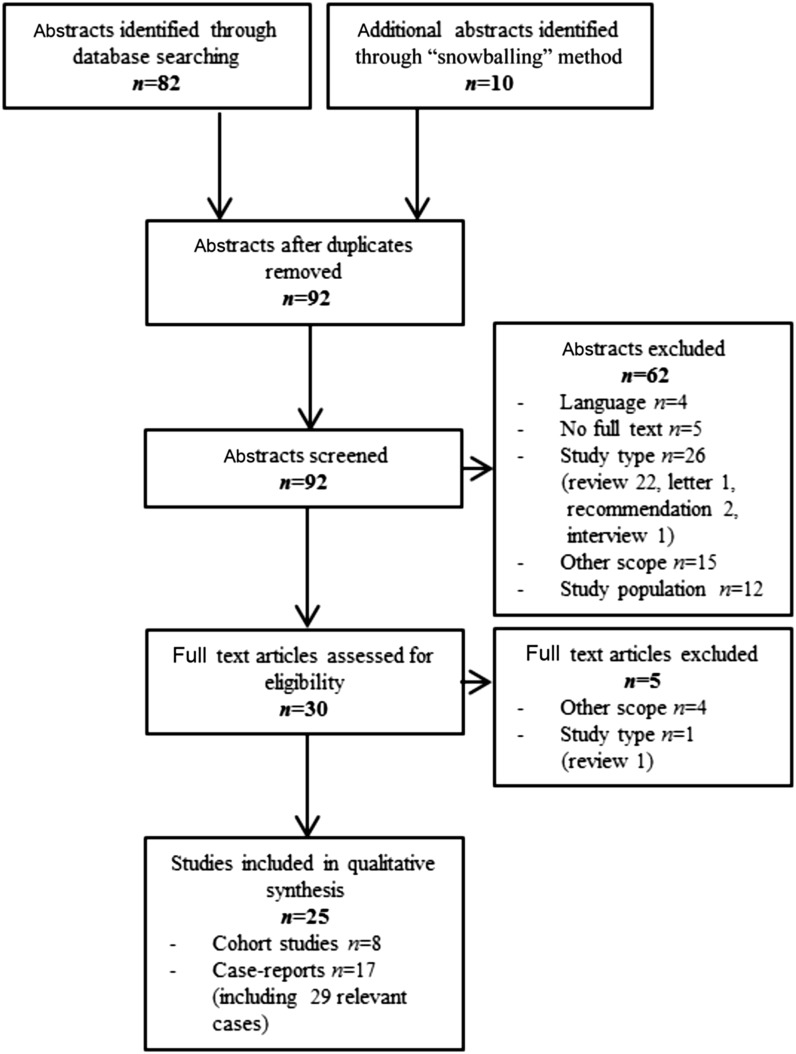
Figure 1 presents the flow chart of the literature search and study selection. The review included 25 articles: 17 case-report studies presenting 29 relevant cases and 8 cohort studies.
FIGURE 1.
Participant flow chart.
Quality appraisal of the selected studies
Case reports.
Data presented by Pelizzo et al. (11, 12) appear to derive from the same case in their 2 reports [2013 (case 2) and 2014 (case 1)]. The time interval between procedure and conception is not reported in 5 of 29 cases. More than half of the cases do not provide information on micronutrient serum concentrations (n = 21 of 30 cases) and supplement intake (n = 17 of 30 cases) before pregnancy. Supplement intake is often summarized without specifications about dosage and time frame. Information about supplement intake during pregnancy is provided more often and is more detailed (n = 24 of 30 cases), as are laboratory values (n = 22 of 30 cases). Clinical follow-up in case of deficiencies and adverse outcomes is often restricted to neonates only.
Cohort studies.
Four cohort studies had a retrospective design (13–16), 2 included both retrospective and prospective data (17, 18), and 2 included only prospective data (19, 20). It is not clear whether the study by Nomura et al. (15) was performed prospectively or retrospectively. The 2 prospective cohort studies were based on the same research population. The background characteristics of the study populations were mostly clearly described, but the inclusion and exclusion criteria of the historical cohorts was not always clearly defined (n = 6 of 8 studies). The type of surgery is well defined in most studies (n = 7 of 8 studies); however, in the study by Gadgil et al. (16), the authors had insufficient knowledge to judge whether insurance claims deviated from the standard classification of bariatric procedures. The Materials and Methods sections in 7 of 8 cohort studies do not provide clear information on the biochemical variables that were determined, the methods used for analyzing, and the reference values used to define a deficiency. Numbers and reasons for drop-out were specified in only 3 of 8 studies (13, 19, 20), indicating that these were the only studies in which a selective loss to follow-up can be excluded. The adjustment for potential confounding factors is only well specified in the study by Gadgil et al. (16) (n = 1 of 8 studies).
Primary and secondary outcome measures
Intracranial hemorrhages have been reported in neonates after BPD (21, 22) and LAGB (22, 23), possibly related to low phylloquinone serum concentrations and disturbed clotting function. These bleedings resulted in lifelong consequences, including psychomotor and mental retardation, and in 4 cases in perinatal death. A prospective follow-up study reported no adverse outcomes in 49 pregnant women with bariatric surgery in the short term, although almost 88% of the women during the first pregnancy trimester and ∼50% of the women at birth had low phylloquinone concentrations (<0.8 nmol/L) (19).
The prevalence of maternal vitamin A deficiency at birth was found to be 58% in the study by Devlieger et al. (20). Cases of women who were deficient in vitamin A have been reported mainly after BPD procedures and resulted in maternal night blindness, preterm birth, and vision complications in the neonate (24–27). Cabano et al. (28) also presented a case of neonatal aortic dilatation that was linked to depleted neonatal vitamin A concentrations.
The prevalences of maternal vitamin B-12 and folate deficiency at birth were found to be 48% and 0%, respectively, in the cohort study by Devlieger et al. (20). One pregnancy was terminated after diagnosis of a neural tube defect (NTD). In the cohort study by Bebber et al. (13), which consisted of 39 pregnancies after RYGB, the prevalences of vitamin B-12 and folate deficiency at birth were found to be 53% and 16%, respectively. No adverse clinical outcomes were reported. Vitamin B-12 deficiency in pregnancy after bariatric surgery did not always result in complications in the mother and child (29). Maternal vitamin B-12 deficiency can result in clinical neurological disease and developmental delay in the neonate (11, 12, 24, 30–34). Maternal deficiency in vitamin B-12 may affect the amount of vitamin B-12 in breast milk, as reported in 2 exclusively breastfed children of mothers with a history of RYGB (30, 31). Pelizzo et al. (11, 12) described 4 NTDs, of which 3 presented in a folate-deficient woman with RYGB and 1 in a folate-deficient woman with BPD. Another case of depleted maternal folate and vitamin B-12 concentrations accompanied with the presence of NTDs was described by Moliterno et al. (34). Low iron concentrations were found in ∼10 cases independently of the type of procedure, and no significant maternal and neonatal adverse outcomes were reported (11, 12, 24, 25, 33, 35).
No studies were found on depleted calcium, zinc, magnesium, iodine, and copper concentrations in pregnant and postpartum women with previous bariatric surgery. One case report found no significant nutritional deficiencies in a pregnant patient after RYGB (36).
Dao et al. (14) compared 21 pregnancies within the first year after RYGB with 13 pregnancies after the first year after RYGB. No significant differences were found in maternal nutritional status. On the other hand, a higher need for intravenous iron therapy was seen in women who became pregnant ≥4 y after surgery than in women who became pregnant <4 y after surgery (15) (Tables 1 and 2).
TABLE 1.
Overview of case reports: maternal nutrient deficiencies after bariatric surgery and short- and long-term effects on the neonate1
| Study (reference) | Procedu re | Maternal Nutrientdeficiency | Maternal laboratory values2 | Supplementation | Neonatal complications | Long-term outcome |
| Bersani et al. (21) | BPD | Coagulopathy | PT: 72.3 (10.15) s | 2 × 10 mg/d Konakion (Roche Products Ltd) | Severe coagulopathy | Uncomplicated |
| INR: 5.7 (0.8 – 1.2) | ||||||
| Low vitamin K factors | F II: 19.1% (70–140%) | |||||
| F VII: 34.8% (70–140%) | ||||||
| F IX: 20% (70–140%) | ||||||
| F X: 10% (70–140%) | ||||||
| Protein C 50% (70–140%) | ||||||
| Protein S 33% (54–110%) | ||||||
| Cabano et al. (28) | BPD | None | No vitamin A | Preterm birth | Normal aortic measurements | |
| Bilateral microphthalmia | ||||||
| Respiratory distress | ||||||
| Enterocolitis | ||||||
| Aortic dilatation | ||||||
| Campbell et al. (29) | RYGB | Vitamin B-12 | 138 (228–1514) pg/mL | Stopped (side effects) | Vitamin B-12 deficiency | Uncomplicated |
| Celiker et al. (30) | RYGB | Vitamin B-12 | 84 pg/mL (unknown) | Oral multivitamin | Vitamin B-12 deficiency | Delayed gross motor |
| Delayed speech | ||||||
| Cools et al. (24) | BPD | Vitamin A | Not reported | Multivitamins | Congenital abnormalities | Retardation |
| Fe, Ca, Zn | Not reported | Fe | Preterm birth | Epilepsy | ||
| Packed cells | Severe anemia | Blind | ||||
| Deaf | ||||||
| Cools et al. (24) | BPD | Vitamin A | Not reported | Multivitamins | Preterm birth | Uncomplicated |
| Vitamin D | Not reported | Fe | ||||
| Fe, Zn, Ca, P | Not reported | Zn | ||||
| Cools et al. (24) | BPD | Vitamin B-12 | Not reported | Parenteral vitamins | Preterm birth | Uncomplicated |
| Vitamin A | Not reported | Packed cells | ||||
| Vitamin D | Not reported | TPN | ||||
| Ca, Se, Fe | Not reported | |||||
| Cools et al. (24) | BPD | Fe | Not reported | Vitamin B-12 | Cerebral cystic zones | Epilepsy |
| Fe | ||||||
| Eerdekens et al. (22) | AGB | Coagulopathy | PT: 46.8% (70–150%) | TPN | Preterm birth | Perinatal death |
| aPTT: 29.3 (24–31) s | Vitamin K deficiency bleeding | |||||
| Low vitamin K factors | F II: 56% (70–130%) | |||||
| F V: 121% (70–130%) | ||||||
| F VII: 40% (70–130%) | ||||||
| F IX: 75% (70–130%) | ||||||
| F X: 27% (70–130%) | ||||||
| Phylloquinone | 0.2 (0.8–5.3) nmol/L | |||||
| Eerdekens et al. (22) | AGB | None | NA | Not reported | Preterm birth | Perinatal death |
| Vitamin K deficiency bleeding | ||||||
| Eerdekens et al. (22) | AGB | None | NA | Not reported | Subdural hematoma | Delayed gross motor |
| Cerebral palsy | ||||||
| Eerdekens et al. (22) | BPD | None | NA | Not reported | Subarachnoidal bleeding | Mental retardation |
| Cerebral palsy | ||||||
| Eerdekens et al. (22) | BPD-D | Phylloquinone | 0.0008 (0.8–5.3) nmol/L | Not reported | Preterm birth | Perinatal death |
| Congenital abnormalities | ||||||
| Gilchrist et al. (25) | BPD | Vitamin A | 0.1 (1.6–2.3) μmol/L | Vitamin A (oral) | Bilateral microphthalmia | Ocular malformations |
| Vitamin D | 30 (50–300) nmol/L | Fe i.v. | Inferior adherent leukoma | Light-sensitive child | ||
| Vitamin K | Unknown | Optic nerve hypoplasia | ||||
| Fe | 3 (10–33) μmol/L | |||||
| Gomez-Lobo et al. (36) | RYGB | None | Not reported | Prenatal vitamins | None | None |
| Ferrous sulfate, 325 mg/d | ||||||
| Folic acid | ||||||
| Grange and Finlay (32) | BPD | Vitamin B-12 | 73 pmol/L (unknown) | Daily prenatal vitamin | Failure to thrive | Uncomplicated |
| incl 8 μg vitamin B-12 | Anemia | |||||
| Neutropenia | ||||||
| Gurewitsch et al. (33) | RYGB | Vitamin B-12 | 120 (226–966) pg/mL | Prenatal multivitamin | Uncomplicated | Uncomplicated |
| Fe | 10 (50–150) μg/dL | Ferrous sulfate, 325 mg/d | ||||
| Fe saturation | 2% (15–45%) | vitamin B-12 i.m. | ||||
| Huerta et al. (26) | BPD | Vitamin A | <0.2 (0.3–0.9) mg/L | Not during pregnancy | Premature birth | Retinal damage |
| Vitamin D | 6 (15–57) g/L | PP: multivitamin | Vitamin A deficiency | |||
| Vitamin B-12 | 153 (160–840) pg/mL | PP: ferrous sulfate, 325 mg/d | ||||
| Zn | 45 (65–256) μg/dL | |||||
| Iavazzo et al. (35) | RYGB | Fe | Not reported | Fe | Preterm birth | Unknown |
| Iavazzo et al. (35) | AGB | Fe | Not reported | Fe | Preterm birth | Unknown |
| Iavazzo et al. (35) | AGB | Folate | Not reported | Nutritional supplements | Unknown | Unknown |
| Moliterno et al. (34) | RYGB | None | NA | Folic acid | Open NTD | Uncomplicated |
| Vitamin B-12 | ||||||
| Pelizzo et al. (11) | RYGB | Folate | Not reported | None | NTD | Unknown |
| Vitamin B-12 | Fetal malformations | |||||
| Severe anemia | ||||||
| Pelizzo et al. (11) | RYGB | Folate | Not reported | None | NTD | Unknown |
| Vitamin B-12 | Fetal malformations | |||||
| Severe anemia | ||||||
| Pelizzo et al. (12) | RYGB | Vitamin B-12 | 201 (243–894) pg/mL | 22 wk: vitamins, minerals | NTD | Unknown |
| Vitamin A | 0.24 (0.25–0.86) μg/mL | Ventricular dilatation | ||||
| 1,25(OH)2D | 44.6 (48–110) pmol/L | |||||
| Fe | 16 (25–156) μg/dL | |||||
| Ferritin | 2 (18–440) μg/L | |||||
| Pelizzo et al. (12) | RYGB | Vitamin B-12 | <15 (243–894) pg/mL | 20 wk: vitamins, minerals | NTD | Unknown |
| Vitamin A | 0.1 (0.25–0.86) pg/mL | Ventricular dilatation | Unknown | |||
| 1,25(OH)2D | 40.3 (48–110) pmol/L | |||||
| Fe | 73 (25–156) μg/mL | |||||
| Ferritin | 10 (18–440) μg/dL | |||||
| Pelizzo et al. (12) | BPD | Not confirmed | Unknown | None | NTD | Failure to thrive |
| Bilateral microphthalmia | ||||||
| Sensorineural deafness | ||||||
| Skeletal dysplasia | ||||||
| Short limbs | ||||||
| Smets et al. (27) | BDP | Vitamin A | 17 (30–80) μg/dL (16 wk) | Multivitamin i.v. | Bilateral microphthalmia | Unknown |
| 14.5 μg/dL (30–80) (22 wk) | Vitamin B-12 | |||||
| Fe | ||||||
| Folic acid | ||||||
| Van Mieghem et al. (23) | AGB | Coagulopathy | PT: 12 s (not reported) | Water-soluble vitamins | Preterm birth | Perinatal death |
| aPTT: 29 s (not reported) | TPN | Cerebral hemorrhage | ||||
| Low vitamin K factors | F II: 56% (not reported) | |||||
| F VII: 40% (not reported) | ||||||
| F X: 27% (not reported) | ||||||
| Phylloquinone | 0.2 (0.8–5.3) nmol/L | |||||
| Vitamin A | 72 (300–650) μg/L | |||||
| Wardinsky et al. (31) | RYGB | None | NA | Not reported | Macrocytic anemia | Developmental delay |
| Vitamin B-12–deficient breast milk | Improvement at 12 mo | |||||
| Vitamin B-12 deficiency | ||||||
| Folate deficiency |
AGB, adjustable gastric banding; aPTT, activated partial thromboplastin time; BPD, biliopancreatic diversion; BDP-D, biliopancreatic diversion with duodenal switch; Ca, calcium; F, factor; Fe, iron; incl, including; INR, International Normalized Ratio; NA, not applicable; NTD, neural tube defect; P, phosphorus; PP, postpartum; PT, prothrombin time; RYGB, Roux-en-Y gastric bypass; Se, selenium; T, total parenteral nutrition; Zn, zinc; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Maternal laboratory values are presented as measured maternal values; reference intervals in parentheses.
TABLE 2.
Overview of cohort studies: main findings1
| Study (reference) | Procedure | Study population | Main findings |
| Bebber et al. (13) | RYGB | 39 pregnancies (9 miscarriages) | Vitamin B-12: low (<175 pg/mL) in 53% (n = 16/31) of the women in T1 |
| Folic acid: low (4–17 μg/L) in 16% (n = 5/31) of the women in T1 | |||
| Dao et al. (14) | RYGB | 21 early pregnancies (<1 y after surgery) | Iron: deficiency in 1 patient of early group |
| 13 late pregnancies (≥1 y after surgery) | |||
| Devlieger et al. (20) | Mixed | 49 pregnancies | Vitamin A: deficiency (<300 μ/L) in 19% (T1) and 58% (T3) of the women |
| Vitamin B-12: deficiency (<191 ng/L) in 35% (T1) and 41% (T3) of the women | |||
| Folic acid: deficiency (<252 μg/L) in 3% (T1) and 0% (T3) of the women | |||
| Thiamin: deficiency (<70 nmol/L) in 2% (T1) and 17% (T3) of the women | |||
| 25(OH)D: severe deficiency (<7 μg/L) in 14% (T1) and 6% (T3) of the women | |||
| 25(OH)D: mild deficiency (7–20 μg/L) in 26% (T1) and 36% (T3) of the women | |||
| Ferritin: deficiency (<30 μg/L) in 27% (T1) and 42% (T3) of the women | |||
| No association between supplement intake and deficiencies | |||
| One women was diagnosed with an NTD | |||
| Dixon et al. (17) | AGB | 79 consecutive first pregnancies | No specific nutritional deficiencies |
| 84% of the women reported daily intake of multivitamin | |||
| Faintuch et al. (18) | RYGB | 14 pregnancies | Iron: occasional low values in T2 (78 ± 50 μg/dL; RV: 37–145 μg/dL) |
| Vitamin B-12: occasional low values in T2 (193 ± 102 μg/dL; RV 180–914 μg/dL) | |||
| Gadgil et al. (16) | Mixed | 456 pregnancies after surgery | Vitamin B-12: deficiency in 12% after surgery vs. 2% before surgery |
| 338 pregnancies before surgery | Folate: deficiency in 7% after surgery vs. 1% before surgery | ||
| Vitamin D: deficiency in 3% after surgery vs. 0% before surgery | |||
| Iron: deficiency in 9% after surgery vs. 2% before surgery | |||
| Jans et al. (19) | Mixed | 49 pregnancies | Phylloquinone: lower in surgical (0.44 nmol/L) vs. nonsurgical (0.65 nmol/L) group in T1 |
| 27 pregnancies without surgery | For study group higher in T3 (4.7 nmol/L) vs. T1 (0.42 nmol/L) | ||
| Nomura et al. (15) | RYGB | 17 pregnancies <4 y after RYGB | Anemia: defined as hemoglobin <11 g/dL |
| 13 pregnancies ≥4 y after RYGB | More i.v. iron in late (30.8%) vs. early (0%) group (P = 0.026) |
AGB, adjustable gastric banding; NTD, neural tube defect; RYGB, Roux-en-Y gastric bypass; RV, reference value; T1, first trimester of pregnancy; T2, second trimester of pregnancy; T3, third trimester of pregnancy; 25(OH)D, 25-hydroxyvitamin D.
Discussion
This systematic review, which is, to our knowledge, the first on this subject, revealed that the literature on micronutrient concentrations during pregnancy after bariatric surgery is limited to 29 relevant cases and a small number (n = 8) of retrospective and prospective cohort studies. The overall level of evidence was low, and studies often did not provide sufficient information for a comprehensive understanding. Globally, depleted maternal concentrations and deficiencies of vitamins A and B-12, phylloquinone, and folate, together with low iron concentrations, have been reported. The most common adverse fetal and neonatal outcomes related to these deficiencies include visual complications (vitamin A), intracranial hemorrhage (phylloquinone), neurological and developmental impairment (vitamin B-12), and NTDs (folate).
Our findings are in line with those of Maggard et al. (37), who found minimal evidence of nutritional adverse events in pregnancies after bariatric surgery. The main reasons for this were inappropriate study designs and the fact that most studies monitored supplement adherence rather than nutritional serum concentrations. A study by Johansson et al. (38) measured birth outcomes, including large-for-gestational age, small-for-gestational age, preterm birth, neonatal death, and major congenital malformation, in pregnancy after bariatric surgery. Although nutritional status of the mothers and infants was not measured, because this was not a focus of the study, the outcomes measured could be associated with malnutrition during pregnancy. In fact, the possibility of nutritional deficiency in iron, vitamin B-12, and folate was acknowledged in the article.
Important adverse maternal, fetal, and neonatal outcomes were revealed by the search, mainly in the large number of case reports. Publication bias certainly must be taken into account. For a variety of reasons, adverse outcomes are unlikely to be reported and published. It can therefore be anticipated that the published cases represent only a fraction of the clinical practice. On the other hand, the fact that adverse events are highlighted may affect readers’ judgment of actual risk. Therefore, these cases should be interpreted with caution. Case-report studies are seen as a research design with the greatest chance of bias in outcomes and are situated low in the hierarchy of level of evidence (39). Cohort studies are also known to have low internal validity and are therefore situated in the middle of the levels of evidence. Moreover, the lack of inclusion and exclusion criteria for the historical cohorts may suggest a certain degree of selection bias, because a cohort could be determined retrospectively with knowledge about a certain outcome or only a part of the initial cohort might have been selected (10). The cohort studies within this systematic review mainly showed nutritional deficiencies. Only the prospective cohort mentioned 1 termination of pregnancy after diagnosis of an NTD in a folate-deficient woman with a history of bariatric surgery (19, 20). It is impossible to know to which extent the deficiency in folic acid was the direct cause of the congenital malformation because NTDs are also more frequent in overweight and obese women than in normal-weight women (40). Most women after bariatric surgery are still obese, as was the woman in this cohort [BMI (in kg/m2): 36.0].
The scoring systems used for the quality appraisal shed light on the incompleteness of reporting details for both cohort and case-report studies, although specific reporting guidelines do exist [e.g., CARE and STROBE (Strengthening The Reporting of OBservational studies in Epidemiology) guidelines]. The heterogeneity in the findings makes it hard to generate an overall picture of the current literature. This heterogeneity is reflected by the following aspects: 1) studies used different cutoff values to define micronutrient deficiencies; 2) studies reported on all types of surgery, including the rarely performed malabsorptive procedures; and 3) the time between surgery and conception varied markedly between the different studies. The first year after surgery is known to be a period of rapid weight loss and is believed to be harmful for both mother and child (41). For this reason, it is recommended to delay pregnancy until 12–18 mo after surgery (42). Surprisingly, only a limited number of the case studies presented data on micronutrient deficiencies and adverse events in women who became pregnant within 18 mo after surgery (12, 22, 24, 34, 35). In addition, the mean period between surgery and conception in the cohort studies varied between 18 and 36 mo, with no or very limited information on the critical period of the first 18 mo after surgery. From these limited data, however, no indication was found of seriously adverse events from depleted micronutrient concentrations in pregnancies within the first 18 mo after surgery. Stronger evidence is needed before the recommendation of delaying pregnancy 12–18 mo after surgery can be re-evaluated.
The lack of adverse health outcomes due to iron deficiency in this population could be explained by the short follow-up period in the different studies and by missing information on ferritin, hemoglobin, or hepcidin, which are better indicators of iron depletion or iron anemia. Adverse effects due to iron deficiency have a time lag before the developing infant is affected. During pregnancy, different mechanisms evolve that ensure adequate iron status for the developing child, even if this occurs to the detriment of the mother (e.g., an increase in the percentage of iron transfer from mother to developing infant) (43).
To increase the level of knowledge, large cohort studies are needed to obtain prevalence and incidence rates of micronutrient deficiencies in pregnancies after bariatric surgery and to document the effect of supplementation regimens because the optimal regimens and micronutrient requirements are unclear. Comparative studies are needed in obese and lean pregnant women without bariatric surgery, because it has been shown that most micronutrient serum concentrations seem to be lower in obese pregnant women than in their lean counterparts (44). The cause of nutritional deficiencies in obese pregnant women is not completely known but might be related to poorer dietary patterns (45–47) and differences in requirements of and physiologic responses to critical nutrients (47). A poor dietary pattern has been shown in pregnant women with previous bariatric surgery (48), and this combined with their gastrointestinal modifications may theoretically increase the risk of micronutrient deficiencies. Moreover, pregnant women with previous bariatric surgery often still enter pregnancy with overweight or obesity. Therefore, prepregnancy BMI remains an important variable that should be incorporated in these comparative analyses. Studies on breast-milk composition could be of value, because case reports showed vitamin deficiencies and negative outcomes in exclusively breastfed children in postpartum women with previous bariatric surgery. Finally, counseling women who may become pregnant or those who are pregnant needs to be evaluated to increase the adherence to vitamin supplements, because individuals post–bariatric surgery may not be compliant in taking vitamins (49).
Strong recommendations for the clinical field cannot be formulated until more research is performed. On the basis of the weak evidence gained from this systematic review and from our clinical experience with earlier and current research projects in this population (50), we tentatively present some suggestions for the screening and monitoring of micronutrient concentrations in the preconception and pre- and postnatal periods of women with a history of bariatric surgery (Table 3).
TABLE 3.
Proposed micronutrient screening in pregnancy after bariatric surgery
| Who | All women regardless of type of surgery | |
| When | Preconception period | Every 6 mo |
| Pregnancy | Every trimester of pregnancy | |
| Additional screening if low concentrations despite supplement | ||
| Postpartum | At 6–8 wk, especially breastfeeding mothers | |
| What | Weak evidence | Vitamins A, D, and B-12; folate; phylloquinone; iron |
| No evidence | Zinc, calcium, magnesium, iodine | |
| Note | Intention of becoming pregnant | Prenatal multivitamin with vitamin B-12 and folate |
| Caution with | Hyperemesis gravidarum, gestational weight loss |
A limitation of this systematic review is that it relies only on case reports and cohort studies. This review did not capture the complete “1000 day” concept because the search and results were limited to the early postpartum period; however, we reported several severe complications with long-term to permanent health consequences reaching far beyond the second year of life. A strength of this study is that all of the evidence was collected in a systematic manner. In addition, rigorous quality assessment was undertaken by 2 independent reviewers. Until larger prospective cohort studies have been performed, this systematic review can conclude that the evidence on micronutrient deficiencies and related adverse outcomes in pregnancy after bariatric surgery is relatively weak, most probably due to the variable quality of the research published so far.
Acknowledgments
GJ, CM, and RD designed the research and had primary responsibility for final content; GJ and CM conducted the research and analyzed the data; and GJ wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BPD, biliopancreatic diversion; LAGB, laparoscopic adjustable gastric banding; NTD, neural tube defect; RYGB, Roux-en-Y gastric bypass; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Horton R. Maternal and child undernutrition: an urgent opportunity. Lancet 2008;371:179. [DOI] [PubMed] [Google Scholar]
- 2.Guelinckx I, Devlieger R, Vansant G. Reproductive outcome after bariatric surgery: a critical review. Hum Reprod Update 2009;15:189–201. [DOI] [PubMed] [Google Scholar]
- 3.Valentino D, Sriram K, Shankar P. Update on micronutrients in bariatric surgery. Curr Opin Clin Nutr Metab Care 2011;14:635–41. [DOI] [PubMed] [Google Scholar]
- 4.Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS allied health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis 2008;4(5, Suppl):S73–108. [DOI] [PubMed] [Google Scholar]
- 5.Ladipo OA. Nutrition in pregnancy: mineral and vitamin supplements. Am J Clin Nutr 2000;72(1, Suppl):280S–90S. [DOI] [PubMed] [Google Scholar]
- 6.Berti C, Decsi T, Dykes F, Hermosa M, Koletzko B, Massari M, Morena LA, Serra-Majem L, Cetin I. Critical issues in setting micronutrient recommendations for pregnant women: an insight. Matern Child Nutr 2010;6(Suppl 2):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaska L, Kobiela J, Abacjew-Chmylko A, Chmylko L, Wojanowska-Pindel M, Kobiela P, Walerzak A, Makarewicz W, Proczko-Markuszewska M, Stefaniak T. Nutrition and pregnancy after bariatric surgery. International Scholarly Research Notices 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros M, Saunders C, Chagas CB, Pereira SE, Saboya C, Ramalho A. Vitamin D deficiency in pregnancy after bariatric surgery. Obes Surg 2013;23:1679–84. [DOI] [PubMed] [Google Scholar]
- 9.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D.; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014;67:46–51. [DOI] [PubMed] [Google Scholar]
- 10.Dutch Cochrane Center. Tool to evalute cohort studies [cited 2013 Mar 10]. Available from: http://dcc.cochrane.org/beoordelingsformulieren-en-andere-downloads.
- 11.Pelizzo G, Nakib G, Alfei A, Lasci A, Cena H, Locatelli D, Mosconi M, Zappoli F, Calcaterra V. Fetal neural tube defects in pregnant women previously submitted to bariatric surgery: more attention to a new emerging entity. Prenat Diagn 2013;33:196–7. [DOI] [PubMed] [Google Scholar]
- 12.Pelizzo G, Calcaterra V, Fusillo M, Nakib G, Ierullo AM, Alfei A, Spinillo A, Stronati M, Cena H. Malnutrition in pregnancy following bariatric surgery: three clinical cases of fetal neural defects. Nutr J 2014;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bebber FE, Rizzolli J, Casagrande DS, Rodrigues MT, Padoin AV, Mottin CC, Repetto G. Pregnancy after bariatric surgery: 39 pregnancies follow-up in a multidisciplinary team. Obes Surg 2011;21:1546–51. [DOI] [PubMed] [Google Scholar]
- 14.Dao T, Kuhn J, Ehmer D, Fisher T, McCarty T. Pregnancy outcomes after gastric-bypass surgery. Am J Surg 2006;192:762–6. [DOI] [PubMed] [Google Scholar]
- 15.Nomura RMY, Dias MCG, Igai AMK, Paiva LV, Zugaib M. Anemia during pregnancy after silastic ring Roux-en-Y gastric bypass: influence of time to conception. Obes Surg 2011;21:479–84. [DOI] [PubMed] [Google Scholar]
- 16.Gadgil MD, Chang HY, Richards TM, Gudzune KA, Huizinga MM, Clark JM, Bennett WL. Laboratory testing for and diagnosis of nutritional deficiencies in pregnancy before and after bariatric surgery. J Womens Health (Larchmt) 2014;23:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon JB, Dixon ME, O'Brien PE. Birth outcomes in obese women after laparoscopic adjustable gastric banding. Obstet Gynecol 2005;106:965–72. [DOI] [PubMed] [Google Scholar]
- 18.Faintuch J, Dias MCG, De Souza Fazio E, de Oliveira FC, Nomura RM, Zugaib M, Cecconello I. Pregnancy nutritional indices and birth weight after roux-en-Y gastric bypass. Obes Surg 2009;19:583–9. [DOI] [PubMed] [Google Scholar]
- 19.Jans G, Guelinckx I, Voets W, Galjaard S, Van Haard PM, Vansant GM, Devlieger R. Vitamin K1 monitoring in pregnancies after bariatric surgery: a prospective cohort study. Surg Obes Relat Dis 2014;10:885–90. [DOI] [PubMed] [Google Scholar]
- 20.Devlieger R, Guelinckx I, Jans G, Voets W, Vanholsbeke C, Vansant G. Micronutrient levels and supplement intake in pregnancy after bariatric surgery: a prospective cohort study. PLoS ONE 2014;9:e114192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bersani I, Carolis MPD, Salvi S, Zecca E, Romagnoli C, De Carolis S. Maternal-neonatal vitamin K deficiency secondary to maternal biliopancreatic diversion. Blood Coagul Fibrinolysis 2011;22:334–6. [DOI] [PubMed] [Google Scholar]
- 22.Eerdekens A, Debeer A, Van Hoey G, De Borger C, Sachar V, Guelinckx I, Devlieger R, Hanssens M, Vanhole C. Maternal bariatric surgery: adverse outcomes in neonates. Eur J Pediatr 2010;169:191–6. [DOI] [PubMed] [Google Scholar]
- 23.Van Mieghem T, Van Schoubroeck D, Depiere M, Debeer A, Hanssens M. Fetal cerebral hemorrhage caused by vitamin K deficiency after complicated bariatric surgery. Obstet Gynecol 2008;112:434–6. [DOI] [PubMed] [Google Scholar]
- 24.Cools M, Duval EL, Jespers A. Adverse neonatal outcome after maternal biliopancreatic diversion operation: report of 9 cases. Eur J Pediatr 2006;165:199–202. [DOI] [PubMed] [Google Scholar]
- 25.Gilchrist H, Taranath DA, Gole GA. Ocular malformation in a newborn secondary to maternal hypovitaminosis A. J AAPOS 2010;14:274–6. [DOI] [PubMed] [Google Scholar]
- 26.Huerta S, Rogers LM, Li Z, Heber D, Liu C, Livingston EH. Vitamin a deficiency in a newborn resulting from maternal hypovitaminosis A after biliopancreatic diversion for the treatment of morbid obesity. Am J Clin Nutr 2002;76:426–9. [DOI] [PubMed] [Google Scholar]
- 27.Smets KJ, Barlow T, Vanhaesebrouck P. Maternal vitamin A deficiency and neonatal microphthalmia: complications of biliopancreatic diversion? Eur J Pediatr 2006;165:502–4. [DOI] [PubMed] [Google Scholar]
- 28.Cabano R, Mannarino S, Bollani L, Stronati M. Neonatal aortic dilatation associated with vitamin A deficiency and its subsequent remission after supplementation therapy. Acta Paediatr 2012;101:e530. [DOI] [PubMed] [Google Scholar]
- 29.Campbell CD, Ganesh J, Ficicioglu C. Two newborns with nutritional vitamin B12 deficiency: challenges in newborn screening for vitamin B12 deficiency. Haematologica 2005;90(12, Suppl):ECR45. [PubMed] [Google Scholar]
- 30.Celiker MY, Chawla A. Congenital B12 deficiency following maternal gastric bypass. J Perinatol 2009;29:640–2. [DOI] [PubMed] [Google Scholar]
- 31.Wardinsky TD, Montes RG, Friederich RL, Broadhurst RB, Sinnhuber V, Bartholomew D. Vitamin B12 deficiency associated with low breast-milk vitamin B12 concentration in an infant following maternal gastric bypass surgery. Arch Pediatr Adolesc Med 1995;149:1281–4. [DOI] [PubMed] [Google Scholar]
- 32.Grange DK, Finlay JL. Nutritional vitamin B(12) deficiency in a breastfed infant following maternal gastric bypass. Pediatr Hematol Oncol 1994;11:311–8. [DOI] [PubMed] [Google Scholar]
- 33.Gurewitsch ED, Smith-Levitin M, Mack J. Pregnancy following gastric bypass surgery for morbid obesity. Obstet Gynecol 1996;88:658–61. [DOI] [PubMed] [Google Scholar]
- 34.Moliterno JA, DiLuna ML, Sood S, Roberts KE, Duncan CC. Gastric bypass: a risk factor for neural tube defects? Case report. J Neurosurg Pediatr 2008;1:406–9. [DOI] [PubMed] [Google Scholar]
- 35.Iavazzo C, Ntziora F, Rousos I, Paschalinopoulos D. Complications in pregnancy after bariatric surgery. Arch Gynecol Obstet 2010;282:225–7. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Lobo V, Landy HJ, Matsumoto C, Fishbein TM. Pregnancy in an intestinal transplant recipient. Obstet Gynecol 2012;120:497–500. [DOI] [PubMed] [Google Scholar]
- 37.Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, Suttorp M, Hilton L, Santry HP, Morton JM, Livingston EH, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA 2008;300:2286–96. [DOI] [PubMed] [Google Scholar]
- 38.Johansson K, Cnattingius S, Näslund I, Roos N, Lagerros Y, Granath F, Stephansson O, Neovius M. Outcomes of pregnancy after bariatric surgery. N Engl J Med 2015;372:814–24. [DOI] [PubMed] [Google Scholar]
- 39.Center for Evidence-Based Management. What are the levels of evidence? [cited 2014 Aug 11]. Available from: http://www.cebma.org/frequently-asked-questions/what-are-the-levels-of-evidence/.
- 40.Dean SV, Lassi ZS, Imam AM, Bhutta ZA. Preconception care: nutritional risks and interventions. Reprod Health 2014;11(Suppl 3):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittgrove AC, Jester L, Wittgrove P, Clark GW. Pregnancy following gastric bypass for morbid obesity. Obes Surg 1998;8:461–4; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 42.American Congress of Obstetricians and Gynecologists. ACOG practice bulletin no. 105: bariatric surgery and pregnancy. Obstet Gynecol 2009;113:1405–13. [DOI] [PubMed] [Google Scholar]
- 43.McArdle HJ, Gambling L, Kennedy C. Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proc Nutr Soc 2014;73:9–15. [DOI] [PubMed] [Google Scholar]
- 44.Sen S, Iyer C, Meydani SN. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol 2014;34:105–11. [DOI] [PubMed] [Google Scholar]
- 45.Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am 2009;56:1105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 2008;9:140–50. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol 2008;198:611–9. [DOI] [PubMed] [Google Scholar]
- 48.Guelinckx I, Devlieger R, Donceel P, Bel S, Pauwels S, Bogaerts A, Thijs I, Schurmans K, Deschilder P, Vansant G. Lifestyle after bariatric surgery: a multicenter, prospective cohort study in pregnant women. Obes Surg 2012;22:1456–64. [DOI] [PubMed] [Google Scholar]
- 49.Mathus-Vliegen EM. Long-term health and psychosocial outcomes from surgically induced weight loss: results obtained in patients not attending protocolled follow-up visits. Int J Obes (Lond) 2007;31:299–307. [DOI] [PubMed] [Google Scholar]
- 50.AURORA. Bariatric surgery registration in women of reproductive age: information platform and database [cited 2014 Aug 8]. Available from: www.aurorastudy.org/en.