Abstract
Pemphigus are organ-specific autoimmune diseases, where autoantibodies (mainly IgG) directed against epidermal targets (glycoproteins of the desmosomal core) are detected. Endemic pemphigus foliaceus or fogo selvagem (FS) is one of the variants of pemphigus foliaceus (PF) that shares the same clinical and immunopathological features of the classic nonendemic PF form, including pathogenic IgG (mainly IgG4) autoantibodies directed against the ectodomain of desmoglein 1 (Dsg1), that lead to acantholysis. Pathogenesis of FS is complex, involving genetic, environmental and immunological factors. HLADRB1 alleles DRB1*0404, *1402, *1406 or *0102 have been previously identified as risk factors for FS (relative risk > 14). Individuals exposed to hematophagous insects are more susceptible to develop the disease. Nonpathogenic anti-Dsg1 antibodies of the IgG1 subclass, directed against the extra-cellular 5 domain of Dsg1 are detected in patients in the preclinical stage of the disease, and also in healthy controls living in endemic areas. In counterpart, patients with FS show pathogenic anti-Dsg1 IgG4 auto-antibodies that bind the pathogenic extracellular 1 and 2 domains of Dsg 1, emphasizing the intramolecular epitope spreading hypothesis. A possible explanation for the development of the autoimmune process would be antigenic mimicry, initiated by environmental stimuli in those genetically predisposed individuals. Characterization of the pathogenesis of FS will allow the development of specific therapeutic targets, and the elucidation of other autoimmune processes.
Keywords: autoimmunity, pemphigus, desmoglein, immunoglobulin G, immunofluorescence
1. Introduction
Pemphigus comprises a group of autoimmune mucocutaneous blistering diseases and its hallmark is acantholysis, a consequence of loss of cell-cell adhesion due to the binding of autoantibodies (mainly IgG) against epithelial targets located within the desmosomal core, mostly desmoglein 1 (Dsg1) and desmoglein 3 (Dsg3). (1)
Main forms of the pemphigus group are pemphigus foliaceus (PF) and pemphigus vulgaris (PV). Pemphigus foliaceus, first reported by Cazenave in 1844, is characterized by superficial blisters and absence of mucous tissue involvement, as a result from the binding of IgG autoantibodies directed against Dsg1.(2-3) There is an endemic form of PF, also known as fogo selvagem (FS), that shares the clinical, histologic and most of the immunological features with the classic form, but with a unique epidemiological profile.(4-5)
1. Historical Aspects
FS was first reported in Brazil as a superficial mycosis known as tinea imbricata or “tokelau” in 1903.(6) The distribution of endemic sites varies over time, once the disease decays with urbanization, and new foci appear in areas of recent occupation.(5) One of the most affected regions in Southeastern Brazil during the first half of the 20th century was the State of Sao Paulo, where a Hospital (Adhemar de Barros) was built for treating those patients. (6) Mortality rates due to severe infection and cachexia in the pre-steroid era were high (close to 85-90%), and many patients were abandoned at hospitals by their relatives. (Figure 1)(3-4)
Figure 1.
Typical house of a fogo selvagem patient at an endemic region in central Brazil.
FS is present in the rural areas, and the geographic distribution follows the course of creeks and streams. To date, major affected Brazilian regions include the Midwest and the Southeastern States. (Figures 2,3)(7-8) However, new sites have been characterized in the Northern parts of Brazil.(9)
Figure 2.
Patient with a severe form of Fogo selvagem at the Pemphigus Hospital, São Paulo, Brazil, 1940.(6)
Figure 3.
Map of endemic FS sites in Brazil.
Other endemic foci of the disease have been observed in South America countries, such as Colombia, Ecuador, Peru, Paraguay, Venezuela and also in the African continent (Tunisia). (4, 10-12)
2. Clinical Features
The typical primary lesion of FS is a superficial blister that easily ruptures, with a positive Nikolsky sign in patients with active disease. Most affected sites include scalp and face, neck and upper trunk. UV exposure enhances or triggers skin lesions and the disease progresses in weeks or months. Fulminant forms of FS are rare, characterized by extensive bullae eruption over a period of 1-3 weeks. Clinical forms usually presents as follows:
2.1 Localized form
Also known as forme frustre, it is characterized by superficial blisters and vesicles, erosions and crusts are seen on seborrheic areas of the face and upper trunk. Round or oval keratotic plaques, with a yellow-brown surface may also be observed. Another cutaneous manifestation on seborrheic areas is characterized by erythemato-violaceus or hypepigmented papules and plaques, that resemble discoid lupus erythematous (Figure 4).
Figure 4.
Fogo selvagem, localized form.
The course of localized forms of FS may lead to spontaneous remission after months or a few years, or the initial lesions may generalize to the trunk and acral regions, evolving into generalized disease.(2, 4)
2.2 Generalized forms(2, 4)
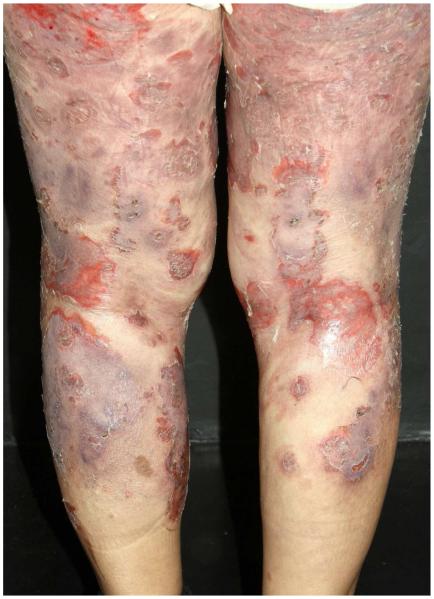
Bullous invasive FS: Acute and aggressive course, with widespread, blistering lesions. (Figure 5) At the onset of the blistering invasion, other signals or symptoms such as fever, arthralgia, and general malaise may appear.
Exfoliative erythroderma: Blisters on erythematous base erode, leaving a moist surface and originating a typical smell that resembles a rat’s nest. (Figure 6)
Keratotic: Disseminated, keratotic plaques and nodular lesions, similar to the ones present in chronic and localized forms of the disease. This form may be related to refractory FS.
Figure 5.
Fogo selvagem, bullous invasive form.
Figure 6.
Fogo selvagem, exfoliative erythroderma.
2.3 Other clinical forms of FS(4)
Hyperpigmented: Often seen in patients undergoing remission, it may be restricted to areas of previous lesions, or disseminated. Before the introduction of systemic treatment with corticosteroids, diffuse hyperpigmentation was an early indicator of spontaneous remission or cure. Patients on clinical remission would experience changes in their skin color, with marked skin darkening.
Pemphigus herpetiformis (PH): Characterized by vesicles or pustules in herpetiform arrangement, that may either precede or follow typical FS lesions. Laboratory profile reveals eosinophilic spongiosis, and usual immunoreactivity against either Dsg1 or Dsg3. (13-15)
Tinea-imbricata-like FS: Vesicles or blisters form circinate or annular patterns, and after rupturing, produce exfoliation resembling the superficial mycosis, tinea imbricata. (Figure 7) (2-5)
Umbilical pemphigus: Rare clinical presentation of FS, it is characterized by erosions or vegetating lesions on the umbilicus, resembling intestinal fistula or pyogenic granuloma. (16)
Figure 7.
Fogo selvagem, tinea-imbricata-like: Vesicles or blisters form circinate or annular patterns.
Complications
Prior to the steroid era, some complications such as growth retardation and dwarfism in children, and azoospermia in adults have been described.(6) Viral infections, such as warts and severe herpes simplex are observed in patients with severe forms of FS.(17) (Figure 8)
Figure 8.
Fogo selvagem and severe herpes simplex infection.
After the establishment systemic corticosteroids as the mainstay therapy for pemphigus, opportunistic infections (OI), although not rare, are seldom reported. Nocardia, Cytomegalovirus, Legionella and Listeria are the most frequent agents linked to pemphigus, but Pneumocystis, Sarcoptes and Strongyloides infections must be discarded before or during immunosuppressant therapy.(18)
3. Pathogenesis of Fogo Selvagem
Pathogenesis of FS is still an intriguing quest for investigators, once it involves a combination of environmental and genetic factors modulating the break of tolerance that leads to autoimmunity.
3A.Environmental factors
Since the first reports regarding the etiology of FS, the investigators have hypothesized possible environmental trigger(s), based on its geographic distribution occurring in rural surroundings, far away from the ocean and urbanization, familial cases and temporal clustering, and increased occurrence in young adults and children.(3, 6, 8, 19-20)
In Brazil, the geographical sites of FS show a dynamic course. The first reports in Brazil indicate a first peak in the Southeastern States of Brazil (São Paulo, Minas Gerais, and Paraná, first half of the 20th century)(3, 6, 20), and then a second peak in the Midwestern region (Goiás, Mato Grosso and Mato Grosso do Sul, second half of the 29th century). (19, 21) Interestingly, long-term studies demonstrate that when tracking down the original described endemic sites, the occurrence of FS decreased as the areas urbanized; moreover, most of the patients with active disease that enrolled the study were in remission, suggesting an environmental role for the disease maintenance.(8, 19, 22)
Some Native Brazilian settlements from Central Brazil, such as the Xavante and the Terena tribes have been the focus of our team, the Cooperative Group on Fogo Selvagem Research (CGFSR).(7, 23) First settlement to be evaluated started at Pimentel Barbosa Reservation circa 1990, where 10 out of 795 Xavante Indians were diagnosed as FS, and relevant genetic findings had started. (23) However, follow-up of this community were interrupted due to the remote location of the village.
The second Indian settlement that has been analyzed by our group since 1994 was the Terena tribe, from the Limao Verde Reservation in the State of Mato Grosso do Sul. This village showed all the ideal features for a long term study: high prevalence (3.2%) of FS, incidence of 1-4 new FS cases per year, low migration rates, an easier access from the urban centers, and the valuable collaboration from the native community and local research team.(7) (Figure 9)
Figure 9.
Researchers from the Cooperative Group on Fogo Selvagem Research at the Terena reservation in Limao Verde, MS, Brazil.
The potential role of a hematophagous trigger has been hypothesized since the first bursts of the disease during the past century. (3, 20) The CGFSR started a hospital-based epidemiological case-control study that revealed that Simulium (black fly) bites were 4.7 times more frequent in individuals developing FS than in control individuals.(24) Further studies detected that a predominant black fly species (Simulium nigrimanum) in the Terena reservation of Limao Verde, which is rarely seen in non-endemic areas of Brazil, reinforcing the potential role of environment in FS.(25)
In 2004, a case-control study was performed in the Terena village (Figure 4). Major findings of this project indicated that risk factors for individuals living in this particular endemic area would be the type of housing (thatched roofs, adobe walls) and exposure to hematophagous insect bites (Triatoma or Cimex). In the same study, FS patients showed high frequency of Simulium (87%), Triatoma (67%), and Cimex (60%) bites.(26)
Most of the geographical areas of FS overlap with those described in Chagas‘ disease, and leishmaniasis. (6) Therefore, the next step was to investigate the occurrence of anti-desmoglein 1 antibody in patients with cutaneous leishmaniasis, onchocerciasis, and Chagas disease, parasitic infestations mediated by the three groups of hematophagous vectors above mentioned. Non-pathogenic autoantibodies directed against Dsg1 were seen in Chagas disease (58%), leishmaniasis (43%), and onchocerciasis (81%), reinforcing the hypothesis of long-term exposure to hematophagous insects as a trigger for FS.(27)
It is possible that these vectors carry a molecule that triggers the anti-Dsg1 response through antigen mimicry or cross-reactivity. In counterpart, a recent report from our group detected absent reactivity against Trypanosoma cruzi, the agent of Chagas’ disease, in all FS sera from the Terena tribe at Limao Verde, suggesting that the vector rather than the parasite, participates in the pemphigus autoimmune response.(28)
Molecular studies utilizing recent technologies such sialotranscriptomes have been performed from salivary glands of adult females of Simulium nigrimanum and from Triatoma matogrossensis from endemic regions of FS.(29-30) Sialotranscriptomes provide an infinite platform for testing pemphigus patient sera against recombinant salivary proteins from hematophagous vectors, and also a relevant basis for future therapeutic targets.
A recent finding by our group evidenced that FS sera react against salivary proteins (LJM11) from Lutzomyia longipalpis (sand fly), one of the vectors of cutaneous leishmaniasis. Anti-Dsg1 monoclonal autoantibodies derived from FS patients also cross-react with LJM11, and there is production of anti-Dsg1 antibodies when murine models are immunized with this salivary protein. It is therefore hypothesized that insect bites release salivary proteins that initiate a pathogenic cross-reactive response in genetically prone individuals, leading to FS. (31)
3B. Genetics
The first Brazilian reports on FS identified familial occurrence of the disease among blood-related individuals.(6, 20) A huge study from Goiania, Brazil, that enrolled more than 2,800 individuals with the disease, revealed that 18% of the patients were blood relatives, and 93% of the familial cases were found in genetically related family members.
Further publications showed that the expression of HLADRB1-0404, 1402, or 1406 alleles is linked to FS, with a relative risk of 14. The hypervariable region of the DRB1 gene of these alleles at the level of residues 67-74 shares the same sequence: LLEQRRAA, which confers susceptibility to FS. (32)
3C.The autoimmune response in FS
• Desmoglein 1 as the main target auto-antigen in FS
The first evidence of desmoglein 1 as the target autoantigen in FS was reported by Eyre and Stanley, who demonstrated by immunoprecipitation that sera of patients with either classic forms of PF or FS recognize Dsg1, a 160kDa transmembrane calcium-dependent glycoprotein of the cadherin superfamily (33) (34-37)
Desmogleins are glycoproteins with an ectodomain that contains six putative calcium binding sites, a transmembrane region, and an intracellular domain that is linked to the keratinocyte cytoskeleton via desmosomal plaque proteins. (26, 38)
Dsg1 is not the exclusive target for IgG autoantibodies in FS. One of the reasons remains on the extensive homology between desmosomal cadherins and other members of this gene superfamily of adhesion moleclules, such as desmocollins, and E and P cadherins.(39) Dsg3, the major auto-antigen for PV is seldom recognized by FS patients (7%)(40). Our group identified cross-reactivity of FS sera and also of healthy controls from the endemic sites with E-cadherin and other desmosomal cadherins rather than Dsg1.(41)
• FS: Breaking the immune tolerance
IgG anti-Dsg1
Beutner and colleagues, in 1968, were the first investigators to detect intraepithelial IgG auto-antibodies in FS. (42) Almost two decades later, the CGFS published the first study on the pathogenicity of the IgG autoantibodies in FS, by reproducing the clinical and immunopathological findings of the disease in murine models.(43) Later on, the characterization of the IgG isotypes involved in FS showed that the autoimmune response was predominantly of the IgG4 subclass; those total IgG4, F(ab')2 and Fab' fragments of FS IgG proved to be pathogenic in the FS mouse model.(44-45)
One of the most striking findings of our group was the detection of anti-Dsg1 antibodies in normal controls that live in endemic areas. (46) Even more interesting was the observation that the percentage of ELISA-positive sera for IgG anti-Dsg1 among the normal control population is inversely related to the distance from the endemic FS focus. (46). Moreover, the predominant subclasses of FS patients and healthy controls from the endemic areas have a divergent profile: IgG4 is more frequent in FS patients with active disease, while IgG1 is seen in FS patients in remission, or in those individuals that are in the pre-clinical stage of the disease. (47)
Further studies utilizing molecular biology demonstrated that at the molecular level, FS immunopathogenesis presents as an epitope spreading model (Figure 5). Anti-Dsg1 antibodies from FS patients on remission and from healthy controls, recognize the non-pathogenic extracellular domain 5 (EC-5) of the molecule, whereas FS patients with active disease have a major reactivity against the pathogenic extracellular domains 1 and 2 (EC1-2) of Dsg1, when utilizing domain-swapped Dsg1. (48)
EC-5 domain remains the major portion of Dsg1 involved in the autoimmune response, and it is mainly recognized by non-pathogenic IgG1 autoantibodies, probably produced under chronic stimuli (e.g constant exposure to insect bites). However, intra-molecular spreading in genetic-prone individuals living in endemic sites may occur, inducing an IgG isotype switch (IgG1 to IgG4), and culminates with disease onset, leading to an EC1-2 oriented IgG4 response.(48)
IgG4 is also represents a novel classifier/predictor that identifies donors with immunologic features of FS and is highly sensitive [92% (95%CI: 82–95)] and specific [97% (95% CI: 89–100)]. In a FS-prone population, with a prevalence of 3% of the disease, it has a positive predictive value (PPV) of 49% and a negative predictive value (NPV) of 99.7%.(49)
IgM anti-Dsg1 response
In areas at high risk for FS, continuous environmental triggers that may share epitopes with Dsg1 are a strong stimulus to production of non-pathogenic anti-IgM and IgG. Interestingly, FS patients who migrate to urban areas show a marked decrease in the IgM anti-Dsg1 response, suggesting that environment does interfere with the immune response. IgM anti-Dsg1 autoantibodies recognize non-pathogenic EC5 of Dsg1, and are potential serological markers for FS, once they are mostly detected in healthy individuals from endemic areas for FS and also in FS patients.(50)
IgE anti-Dsg1 response
It is known that IgG4 and IgE antibodies are detected in individuals that are chronically exposed to allergens or to immunotherapy.(51) There is strong evidence to consider IgE anti-Dsg1 as another serological potential marker for FS, once there is significant difference of the IgE levels between FS and PF patients from the Northern hemisphere. (52)
T cell response
T cells obtained from patients with FS recognize epitopes on the ectodomain of Dsg1. The proliferation of FS T cells to Dsg1 is antigen-specific and restricted to HLA-DR. T cells are CD4 memory T cells, produce IL-4, IL-5, and IL-6, but not ⍰-IFN, suggesting a Th2-like cytokine profile.(53)
Acantholysis
Epidermal cell detachment in FS still remains to befully elucidated. There are some hypothesis to explain the loss of cell adhesion, such as: impairment of Dsg1 or Dsg3 adhesive function, binding of pemphigus auto-antibodies to the epidermis, either leading to alteration of the normal distribution of Dsg1 and Dsg3 (compensation theory), or triggering phosphorylation and activation of transmembrane signaling pathways (release of effector molecules-plasminogen activator).(54)
FS represents an autoimmune organ-specific disease with a multifaceted pathogenesis that involves immune dysfunction (break of tolerance and autoantibody production anti-Dsg1), genetic predisposition (HLADRB1) and environmental triggers (continuous exposure to hematophagous insect bites). Elucidating the key steps of such a complex dermatosis may be the basis for new therapeutic targets, and a model for other autoimmune conditions. (Figure 10)
Figure 10.
Pathogenesis of Fogo Selvagem.
Acknowledgments
VA: Conselho Nacional de Pesquisa, Brazil-CNPq (grant# 305592/2011-4)
LAD: National Institutes of Health, USA (grant #2RO1AR032599-31)
Footnotes
Conflict of interest: none declared.
References
- 1.Amagai M, Stanley JR. Desmoglein as a target in skin disease and beyond. J Invest Dermatol. 2012 Mar;132:776–84. doi: 10.1038/jid.2011.390. 3 Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, et al. Endemic pemphigus foliaceus (fogo selvagem). I. Clinical features and immunopathology. J Am Acad Dermatol. 1989 Apr;20(4):657–69. doi: 10.1016/s0190-9622(89)70079-7. [DOI] [PubMed] [Google Scholar]
- 3.Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, et al. Endemic pemphigus foliaceus (Fogo Selvagem): II. Current and historic epidemiologic studies. J Invest Dermatol. 1989 Jan;92(1):4–12. doi: 10.1111/1523-1747.ep13070394. [DOI] [PubMed] [Google Scholar]
- 4.Sampaio SA, Rivitti EA, Aoki V, Diaz LA. Brazilian pemphigus foliaceus, endemic pemphigus foliaceus, or fogo selvagem (wild fire) Dermatol Clin. 1994 Oct;12(4):765–76. [PubMed] [Google Scholar]
- 5.Aoki V, Sousa JX, Diaz LA, Research CGoFS Pathogenesis of endemic pemphigus foliaceus. Dermatol Clin. 2011 Jul;29(3):413–8. doi: 10.1016/j.det.2011.03.014. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira JP. 1940. Novas contribuições ao estudo do pênfigo foliáceo endêmico (fogo selvagem) no Estado de São Paulo.: Empresa Gráfica da Revista dos Tribunais;
- 7.Hans-Filho G, dos Santos V, Katayama JH, Aoki V, Rivitti EA, Sampaio SA, et al. An active focus of high prevalence of fogo selvagem on an Amerindian reservation in Brazil. Cooperative Group on Fogo Selvagem Research. J Invest Dermatol. 1996 Jul;107(1):68–75. doi: 10.1111/1523-1747.ep12298213. [DOI] [PubMed] [Google Scholar]
- 8.Empinotti JC, Diaz LA, Martins CR, Rivitti EA, Sampaio SA, Lombardi C, et al. Endemic pemphigus foliaceus in western Parana, Brazil (1976-1988). Cooperative Group for Fogo Selvagem Research. Br J Dermatol. 1990 Oct;123(4):431–7. doi: 10.1111/j.1365-2133.1990.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 9.Pires CA, Viana VB, Araújo FC, Müller SF, Oliveira MS, Carneiro FR. Evaluation of cases of pemphigus vulgaris and pemphigus foliaceus from a reference service in Pará state, Brazil. An Bras Dermatol. 2014 Jul;89(4):556–61. doi: 10.1590/abd1806-4841.20142679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortega-Loayza AG, Ramos W, Gutierrez EL, Jimenez G, Rojas I, Galarza C. Endemic pemphigus foliaceus in the Peruvian Amazon. Clin Exp Dermatol. 2013 Aug;38(6):594–600. doi: 10.1111/ced.12029. [DOI] [PubMed] [Google Scholar]
- 11.Robledo MA, Prada S, Jaramillo D, Leon W. South American pemphigus foliaceus: study of an epidemic in El Bagre and Nechi, Colombia 1982 to 1986. Br J Dermatol. 1988 Jun;118(6):737–44. doi: 10.1111/j.1365-2133.1988.tb02590.x. [DOI] [PubMed] [Google Scholar]
- 12.Abida O, Kallel-Sellami M, Joly P, Ben Ayed M, Zitouni M, Masmoudi A, et al. Anti-desmoglein 1 antibodies in healthy related and unrelated subjects and patients with pemphigus foliaceus in endemic and non-endemic areas from Tunisia. J Eur Acad Dermatol Venereol. 2009 Sep;23(9):1073–8. doi: 10.1111/j.1468-3083.2009.03265.x. [DOI] [PubMed] [Google Scholar]
- 13.Santi CG, Maruta CW, Aoki V, Sotto MN, Rivitti EA, Diaz LA. Pemphigus herpetiformis is a rare clinical expression of nonendemic pemphigus foliaceus, fogo selvagem, and pemphigus vulgaris. Cooperative Group on Fogo Selvagem Research. J Am Acad Dermatol. 1996 Jan;34(1):40–6. doi: 10.1016/s0190-9622(96)90832-4. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Takahata H, Teye K, Ishii N, Hashimoto T, Muto M. A case of pemphigus herpetiformis-like atypical pemphigus with IgG anti-desmocollin 3 antibodies. Br J Dermatol. 2014 May;:7. doi: 10.1111/bjd.13088. [DOI] [PubMed] [Google Scholar]
- 15.Matsukura S, Takahashi K, Hirokado M, Ikezawa Y, Nakamura K, Fukuda S, et al. Recalcitrant pemphigus herpetiformis with high titer of immunoglobulin G antibody to desmoglein 1 and positive IgG antibody to desmocollin 3, elevating thymus and activation-regulated chemokine. Int J Dermatol. 2014 Aug;53(8):1023–6. doi: 10.1111/j.1365-4632.2012.05725.x. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira Júnior JV, Maruta CW, Sousa JX, Santi CG, Valente NY, Ichimura LM, et al. Clinical and immunological profile of umbilical involvement in pemphigus vulgaris and pemphigus foliaceus. Clin Exp Dermatol. 2013 Jan;38(1):20–4. doi: 10.1111/j.1365-2230.2012.04468.x. [DOI] [PubMed] [Google Scholar]
- 17.Castro Martins R. In: Pênfigo foliáceo na infância. Takahashi Denise M., editor. Anais Brasileiros de Dermatologia; Brazil: 1986. [Google Scholar]
- 18.Leshem YA, Gdalevich M, Ziv M, David M, Hodak E, Mimouni D. Opportunistic infections in patients with pemphigus. J Am Acad Dermatol. 2014 Aug;71(2):284–92. doi: 10.1016/j.jaad.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Auad A, Castro RM, Fraga S, Furtado TA, Rossi DM, Rivitti EA, et al. The treatment of Brazilian pemphigus foliaceus (fogo selvagem) Int J Dermatol. 1970 Apr-Jun;9(2):130–6. doi: 10.1111/j.1365-4362.1970.tb04592.x. [DOI] [PubMed] [Google Scholar]
- 20.1942. Aranha-Campos, editor. Pênfigo foliáceo (fog selvagem): aspectos clínicos e epidemiológicos: Companhia Melhoramentos;
- 21.Hans-Filho G, Aoki V, Rivitti E, Eaton DP, Lin MS, Diaz LA. Endemic pemphigus foliaceus (fogo selvagem)--1998. The Cooperative Group on Fogo Selvagem Research. Clin Dermatol. 1999 Mar-Apr;17(2):225–35. doi: 10.1016/s0738-081x(99)00014-0. 1999. discussion 105-6. [DOI] [PubMed] [Google Scholar]
- 22.Empinotti JC, Aoki V, Filgueira A, Sampaio SA, Rivitti EA, Sanches JA, et al. Clinical and serological follow-up studies of endemic pemphigus foliaceus (fogo selvagem) in Western Parana, Brazil (2001-2002) Br J Dermatol. 2006 Aug;155(2):446–50. doi: 10.1111/j.1365-2133.2006.07302.x. [DOI] [PubMed] [Google Scholar]
- 23.Friedman H, Campbell I, Rocha-Alvarez R, Ferrari I, Coimbra CE, Moraes JR, et al. Endemic pemphigus foliaceus (fogo selvagem) in native Americans from Brazil. J Am Acad Dermatol. 1995 Jun;32(6):949–56. doi: 10.1016/0190-9622(95)91330-0. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi C, Borges PC, Chaul A, Sampaio SA, Rivitti EA, Friedman H, et al. Environmental risk factors in endemic pemphigus foliaceus (Fogo selvagem). "The Cooperative Group on Fogo Selvagem Research". J Invest Dermatol. 1992 Jun;98(6):847–50. doi: 10.1111/1523-1747.ep12456932. [DOI] [PubMed] [Google Scholar]
- 25.Eaton DP, Diaz LA, Hans-Filho G, Santos VD, Aoki V, Friedman H, et al. Comparison of black fly species (Diptera: Simuliidae) on an Amerindian reservation with a high prevalence of fogo selvagem to neighboring disease-free sites in the State of Mato Grosso do Sul, Brazil. The Cooperative Group on Fogo Selvagem Research. J Med Entomol. 1998 Mar;35(2):120–31. doi: 10.1093/jmedent/35.2.120. [DOI] [PubMed] [Google Scholar]
- 26.Aoki V, Millikan RC, Rivitti EA, Hans-Filho G, Eaton DP, Warren SJ, et al. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem) J Investig Dermatol Symp Proc. 2004 Jan;9(1):34–40. doi: 10.1111/j.1087-0024.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 27.Diaz LA, Arteaga LA, Hilario-Vargas J, Valenzuela JG, Li N, Warren S, et al. Anti-desmoglein-1 antibodies in onchocerciasis, leishmaniasis and chagas disease suggest a possible etiological link to fogo selvagern. J Invest Dermatol. 2004 Dec;123(6):1045–51. doi: 10.1111/j.0022-202X.2004.23438.x. [DOI] [PubMed] [Google Scholar]
- 28.Sousa JX, Diaz LA, Eaton DP, Hans-Filho G, Lanzani de Freitas E, Delgado L, et al. Profile of Trypanosoma cruzi Reactivity in a Population at High Risk for Endemic Pemphigus Foliaceus (Fogo Selvagem) Am J Trop Med Hyg. 2012 Jul; doi: 10.4269/ajtmh.2012.12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro JM, Valenzuela JG, Pham VM, Kleeman L, Barbian KD, Favreau AJ, et al. An insight into the sialotranscriptome of Simulium nigrimanum, a black fly associated with fogo selvagem in South America. Am J Trop Med Hyg. 2010 Jun;82(6):1060–75. doi: 10.4269/ajtmh.2010.09-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assumpcao TCF, Eaton DP, Pham VM, Francischetti IMB, Aoki V, Hans G, et al. An Insight into the Sialotranscriptome of Triatoma matogrossensis, a Kissing Bug Associated with Fogo Selvagem in South America. Am J Trop Med Hyg. 2012 Jun;86(6):1005–14. doi: 10.4269/ajtmh.2012.11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian Y, Jeong JS, Maldonado M, Valenzuela JG, Gomes R, Teixeira C, et al. Cutting Edge: Brazilian Pemphigus Foliaceus Anti-Desmoglein 1 Autoantibodies Cross-React with Sand Fly Salivary LJM11 Antigen. J Immunol. 2012 Aug 15;189(4):1535–9. doi: 10.4049/jimmunol.1200842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moraes ME, Fernandez-Vina M, Lazaro A, Diaz LA, Filho GH, Friedman H, et al. An epitope in the third hypervariable region of the DRB1 gene is involved in the susceptibility to endemic pemphigus foliaceus (fogo selvagem) in three different Brazilian populations. Tissue Antigens. 1997 Jan;49(1):35–40. doi: 10.1111/j.1399-0039.1997.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 33.Eyre RW, Stanley JR. Identification of pemphigus vulgaris antigen extracted from normal human epidermis and comparison with pemphigus foliaceus antigen. J Clin Invest. 1988 Mar;81(3):807–12. doi: 10.1172/JCI113387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998 Jan;110(1):76–8. doi: 10.1046/j.1523-1747.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T, Amagai M, Garrod DR, Nishikawa T. Immunofluorescence and immunoblot studies on the reactivity of pemphigus vulgaris and pemphigus foliaceus sera with desmoglein 3 and desmoglein 1. Epithelial Cell Biol. 1995;4(2):63–9. [PubMed] [Google Scholar]
- 36.Dmochowski M, Hashimoto T, Amagai M, Kudoh J, Shimizu N, Koch PJ, et al. The extracellular aminoterminal domain of bovine desmoglein 1 (Dsg1) is recognized only by certain pemphigus foliaceus sera, whereas its intracellular domain is recognized by both pemphigus vulgaris and pemphigus foliaceus sera. J Invest Dermatol. 1994 Aug;103(2):173–7. doi: 10.1111/1523-1747.ep12392664. [DOI] [PubMed] [Google Scholar]
- 37.Amagai M, Hashimoto T, Green KJ, Shimizu N, Nishikawa T. Antigen-specific immunoadsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995 Jun;104(6):895–901. doi: 10.1111/1523-1747.ep12606168. [DOI] [PubMed] [Google Scholar]
- 38.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–35. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 39.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007 Nov;127(11):2499–515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- 40.Hilario-Vargas J, Dasher DA, Li N, Aoki V, Hans-Filho G, dos Santos V, et al. Prevalence of anti-desmoglein-3 antibodies in endemic regions of Fogo selvagem in Brazil. J Invest Dermatol. 2006 Sep;126(9):2044–8. doi: 10.1038/sj.jid.5700388. [DOI] [PubMed] [Google Scholar]
- 41.Flores G, Culton DA, Prisayanh P, Qaqish BF, James K, Maldonado M, et al. IgG Autoantibody Response against Keratinocyte Cadherins in Endemic Pemphigus Foliaceus (Fogo Selvagem) J Invest Dermatol. 2012 Jul; doi: 10.1038/jid.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beutner EH, Prigenzi LS, Hale W, Leme Cde A, Bier OG. Immunofluorescent studies of autoantibodies to intercellular areas of epithelia in Brazilian pemphigus foliaceus. Proc Soc Exp Biol Med. 1968 Jan;127(1):81–6. doi: 10.3181/00379727-127-32626. [DOI] [PubMed] [Google Scholar]
- 43.Roscoe JT, Diaz L, Sampaio SA, Castro RM, Labib RS, Takahashi Y, et al. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. J Invest Dermatol. 1985 Dec;85(6):538–41. doi: 10.1111/1523-1747.ep12277362. [DOI] [PubMed] [Google Scholar]
- 44.Rock B, Labib RS, Diaz LA. Monovalent Fab' immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J Clin Invest. 1990 Jan;85(1):296–9. doi: 10.1172/JCI114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labib RS, Rock B, Martins CR, Diaz LA. Pemphigus foliaceus antigen: characterization of an immunoreactive tryptic fragment from BALB/c mouse epidermis recognized by all patients' sera and major autoantibody subclasses. Clin Immunol Immunopathol. 1990 Nov;57(2):317–29. doi: 10.1016/0090-1229(90)90045-r. [DOI] [PubMed] [Google Scholar]
- 46.Warren SJ, Lin MS, Giudice GJ, Hoffmann RG, Hans-Filho G, Aoki V, et al. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. Cooperative Group on Fogo Selvagem Research. N Engl J Med. 2000 Jul 6;343(1):23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- 47.Warren SJ, Arteaga LA, Rivitti EA, Aoki V, Hans-Filho G, Qaqish BF, et al. The role of subclass switching in the pathogenesis of endemic pemphigus foliaceus. J Invest Dermatol. 2003 Jan;120(1):104–8. doi: 10.1046/j.1523-1747.2003.12017.x. [DOI] [PubMed] [Google Scholar]
- 48.Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem) J Exp Med. 2003 Jun 2;197(11):1501–10. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qaqish BF, Prisayanh P, Qian Y, Andraca E, Li N, Aoki V, et al. Development of an IgG4-based predictor of endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2009 Jan;129(1):110–8. doi: 10.1038/jid.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaz LA, Prisayanh PS, Dasher DA, Li N, Evangelista F, Aoki V, et al. The IgM anti-desmoglein 1 response distinguishes Brazilian pemphigus foliaceus (fogo selvagem) from other forms of pemphigus. J Invest Dermatol. 2008 Mar;128(3):667–75. doi: 10.1038/sj.jid.5701121. [DOI] [PubMed] [Google Scholar]
- 51.Muller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005 Aug;5(4):343–7. doi: 10.1097/01.all.0000173783.42906.95. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y, Prisayanh P, Andraca E, Qaqish BF, Aoki V, Hans-Filhio G, et al. IgE, IgM, and IgG4 anti-desmoglein 1 autoantibody profile in endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2011 Apr;131(4):985–7. doi: 10.1038/jid.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin MS, Fu CL, Aoki V, Hans-Filho G, Rivitti EA, Moraes JR, et al. Desmoglein-1-specific T lymphocytes from patients with endemic pemphigus foliaceus (fogo selvagem) J Clin Invest. 2000 Jan;105(2):207–13. doi: 10.1172/JCI8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci U S A. 2006 Aug 22;103(34):12855–60. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]