Abstract
Cardiovascular disease (CVD) biomarkers of biological effect (BoBE), including hematologic biomarkers, serum lipid-related biomarkers, other serum BoBE, and one physiological biomarker, were evaluated in adult cigarette smokers (SMK), smokeless tobacco consumers (STC), and non-consumers of tobacco (NTC). Data from adult males and females in the US National Health and Nutrition Examination Survey and a single site, cross-sectional study of healthy US males were analyzed and compared. Within normal clinical reference ranges, statistically significant differences were observed consistently for fibrinogen, C-reactive protein (CRP), hematocrit, mean cell volume, mean cell hemoglobin, hemoglobin, white blood cells, monocytes, lymphocytes, and neutrophils in comparisons between SMK and NTC; for CRP, white blood cells, monocytes, and lymphocytes in comparisons between SMK and STC; and for folate in comparisons with STC and NTC. Results provide evidence for differences in CVD BoBE associated with the use of different tobacco products, and provide evidence of a risk continuum among tobacco products and support for the concept of tobacco harm reduction.
Keywords: BoBE, cigarettes, CVD, NHANES, smokeless tobacco
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide. Changes in specific biochemical and physiological biomarkers of biological effect (BoBE) that have been associated with the development of CVD include homocysteine, fibrinogen, C-reactive protein, serum lipids, white blood cell count, differential white cell counts, red blood cell count, hematocrit, hemoglobin, platelets, and ankle brachial index (ABI) (USDHHS, 2010).
Cigarette smoking is a modifiable risk factor for CVD and levels of several of these biomarkers have been reported to be different in cigarette smokers compared with non-smokers. In addition, some of these biomarkers have shown changes with altered cigarette consumption and smoking cessation (i.e. have a dose–response relationship) (Calapai et al., 2009; Frost-Pineda et al., 2011; Hatsukami et al., 2005, 2006; USDHHS, 2004, 2010).
The American Heart Association has stated that “[c]ompared with cigarette smoking, the [cardiovascular disease] risk associated with [smokeless tobacco] use is markedly lower” (Piano et al., 2010). In US and Swedish studies of smokeless tobacco consumers, data on CVD generally provide evidence that there are no significant differences in CVD BoBE in consumers of smokeless tobacco products compared with non-consumers of tobacco (Bolinder et al., 1997a,b; Eliasson et al., 1991, 1995; Ernster et al., 1990; Norberg et al., 2006; Siegel et al., 1992; Wallenfeldt et al., 2001; Wennmalm et al., 1990).
The tobacco harm reduction concept is based on the idea that tobacco consumption-related morbidity and mortality might be decreased without completely eliminating the consumption of tobacco products (Stratton et al., 2001). One approach toward achieving harm reduction is for cigarette smokers to migrate from cigarette smoking to consuming tobacco products that are less harmful. Consumption of non-combustible tobacco products is considered to be less hazardous than cigarette smoking (Nutt et al. 2014; Zeller et al., 2009).
Prognostic biomarkers of disease can be used to assess potential harm and the potential for harm reduction in consumers of various tobacco products. In order to better understand the effect of tobacco consumption (i.e. combustible and non-combustible products) on CVD BoBE, an evaluation and comparison of hematologic biomarkers, serum lipid-related biomarkers, other serum BoBE, and one physiological BoBE in cigarette smokers (SMK), smokeless tobacco consumers (STC), and non-consumers of tobacco (NTC) was performed. Three different data sets, including survey results from the US National Health Examination Survey (NHANES) and results from a single site, cross-sectional study conducted in the US, were evaluated and compared to identify any consistent similarities and/or differences among SMK, STC, and NTC.
Methods
Samples
Data sets 1 and 2: NHANES 1999–2008: NHANES is conducted by the US National Center for Health Statistics (NCHS) of the US Centers for Disease Control and Prevention and is designed to assess annually the health and nutritional status of adults and children in the US. Data are publicly available and are representative of the civilian, non-institutionalized US population. Detailed survey methodology has been published (CDC, 2010a). Self-reported tobacco consumption and selected biomarker data collected in the NHANES Mobile Examination Centers (MEC) from 1999 to 2008 were used. The categories for tobacco consumption (snuff, chewing tobacco, or cigarettes) or non-consumption were determined by an individual indicating on the MEC questionnaire that a particular tobacco category was consumed (or not consumed) in the last 5 d.
Data set 1 included male and female self-identified SMK, STC, and NTC aged 20 years and older from the NHANES 1999–2008. Self-reported snuff (from the survey: “such as Skoal, Skoal Bandits, or Copenhagen”) and chewing tobacco (from the survey: “such as Redman, Levi Garrett, or Beechnut”) consumers were combined into one STC category due to small sample sizes. The sample of exclusive consumers included 5040 SMK, 368 STC, and 16 443 NTC. The following were excluded from the sample for analysis: individuals reporting the consumption of multiple tobacco products or pipes, cigars, or nicotine replacement therapy (NRT) (n = 534); self-identified NTC with a serum cotinine value greater than 15 ng mL−1 (NCI, 1999) (n = 297); and individuals with missing tobacco consumption data, or if a response was refused or reported as “do not know” (n = 2011).
In order to compare findings more directly with Data set 3 (see below), Data set 2 was limited to males aged 26–49 years from the NHANES 1999–2008, including self-identified SMK, snuff only (from the survey: “such as Skoal, Skoal Bandits, or Copenhagen”) consumers (STC), and NTC. In this data set, the sample of exclusive consumers included 1440 SMK, 69 STC, and 2501 NTC. The followings were excluded: individuals reporting the consumption of multiple tobacco products, pipes, cigars, chewing tobacco, or NRT (n = 281); self-identified NTC with a serum cotinine value greater than 15 ng mL−1 (n = 51); and individuals with missing tobacco consumption data, or if a response was refused or reported as “do not know” (n = 392).
Data set 3: Cross-sectional study of male tobacco consumers and never consumers of tobacco: A single site, cross-sectional study was conducted between September 2008 and February 2009 in the US to evaluate several biomarkers of tobacco exposure and biological effect in exclusive SMK (n = 60), exclusive moist snuff consumers (MSC, n = 48), and NTC (n = 60) (ClinicalTrials.gov Identifier: NCT01692353). Details have been reported elsewhere (Campbell et al., 2015; Nordskog et al., 2015). Briefly, participants were generally healthy males, aged 26–49 years. SMK had smoked at least 15 cigarettes per day for at least 3 years prior to study screening, had limited lifetime usage of other tobacco products (i.e. <10 cigars, <10 pipes, and <10 packs/tins of smokeless tobacco, lifetime), and expired carbon monoxide levels (ECO) between 2 and 125 parts per million (ppm); MSC reported using at least two cans of moist snuff per week for at least 3 years prior to study screening, had limited lifetime usage of other tobacco products (i.e. <20 packs of cigarettes, <10 cigars, <10 pipes, and <10 packs/tins of any other smokeless tobacco, lifetime), and ECO ≤5 ppm; NTC had a limited lifetime usage of tobacco products (i.e. lifetime usage having not exceeded: 20 packs of cigarettes, 20 cans or packs of smokeless tobacco, 50 cigars, 50 pipes of tobacco) and ECO ≤5 ppm (Campbell et al., 2015).
Biomarkers for analysis
For all three data sets, the followings were evaluated:
Hematologic biomarkers, including white blood cells, neutrophils, lymphocytes, monocytes, and eosinophils, markers of inflammation; hemoglobin, platelets, red blood cells, mean platelet volume, red cell distribution width, mean cell hemoglobin, mean cell volume, and hematocrit, markers of hypercoagulation.
Serum lipid-related biomarkers, including total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), markers of CVD risk.
Serum BoBE, including C-reactive protein (CRP), a marker of inflammation; fibrinogen and homocysteine, markers of hypercoagulation; folate, a marker of vitamin absorption, and apolipoprotein B100, a marker of lipid metabolism.
A physiological biomarker, i.e. ankle brachial index (ABI), a marker of peripheral artery disease.
Statistical analysis of biomarkers
In Data sets 1 and 2 (NHANES 1999–2008), for each biomarker evaluated, the sample size varied based on the years for which the survey data were available and the proportion of the total sample for which the biomarker was measured. Details are available in the NHANES laboratory documentation (CDC, 2010b). All statistical methods were performed using the appropriate statistical weights and design parameters provided by NCHS. The survey procedures available in SAS® v.9.2 (SAS Institute Inc., Cary, NC) were used for the analyses. For comparing the biomarkers with the clinical reference ranges as well as between groups (i.e. SMK versus NTC, SMK versus STC, and STC versus NTC), the 25th, 50th, and 75th percentiles were calculated for all three data sets. To identify statistically significant differences between consumption groups, multiplicative factors to the geometric mean and corresponding 95% confidence intervals were compared. Multiplicative factors were computed by exponentiation of the (tobacco) exposure-specific regression coefficients from multiple linear regression models for the natural log-transformed mean biomarker values. A statistically significant difference between exposures was identified when the 95% confidence interval on a multiplicative factor did not include 1.00. The regression analyses included adjustments for body mass index (BMI, four categories), tobacco consumption category, age (six categories for Data set 1, four categories for Data sets 2 and 3), race/ethnicity (for Data sets 1 and 2), sex (for Data set 1), poverty index ratio (three categories, Data set 1), and survey year (for Data sets 1 and 2). Race/ethnicity (in Data sets 1 and 2) was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, and Other. The Hispanic category included those self-identified in NHANES as Mexican American and Other Hispanic.
Results
Sample characteristics
Characteristics of the NHANES 1999–2008 samples by tobacco category are presented in Tables 1 (Data set 1) and 2 (Data set 2). Characteristics of the cross-sectional study sample (Data set 3) are presented in Table 3. In Data sets 1 and 2, the sample sizes of STC were small relative to the SMK and NTC, representing less than 2% of the total sample in both data sets. Additionally in Data sets 1 and 2, STC were nearly 90% non-Hispanic White, compared with approximately 70% of SMK and NTC. A higher proportion of smokeless consumers trended towards increased BMI, with approximately 70% of the STC having BMI greater than 26.1 (i.e. considered overweight or obese) compared with 50% of SMK and 60% of NTC. In Data set 1, which included both males and females, STC were more than 90% males. Across the age categories, Data set 1 appeared similar by tobacco consumption category; Data set 2 showed a higher percentage of STC (limited to snuff only) in the 32–37 years age group, whereas the proportions of SMK and NTC were similar. Comparison of Data set 2 with Data set 1 indicated that males aged 26–49 years represented 29% of all SMK (1440/5040) and 15% of all NTC (2501/16 443); male STC (snuff only) aged 26–49 years represented 19% of all STC (69/368). The Data set 3 study population was largely homogenous by design (a result of the single US site and recruitment for targeted sample size overall) within each consumption category and within each age group. In Data set 3, both tobacco consumption groups were greater than 95% non-Hispanic White and NTC were 75% non-Hispanic White. Similar to the NHANES samples, in Data set 3, a larger percentage of MSC tended towards greater BMI (i.e. 79% of MSC, 65% of SMK, and 59% of NTC with BMI greater than 26.1).
Table 1. Characteristics of Data set 1a (NHANES 1999–2008) by tobacco consumption category.
| SMK | STCb | NTC | |
|---|---|---|---|
| Sample size | 5040 | 368 | 16 443 |
| Gender (%) | |||
| Male | 54.1 | 93.3 | 43.4 |
| Female | 45.9 | 6.7 | 56.6 |
| Race/ethnicity (%) | |||
| Non-Hispanic White | 71.2 | 88.4 | 71.8 |
| Hispanicc | 11.8 | 3.0 | 13.2 |
| Non-Hispanic Black | 12.1 | 5.9 | 10.0 |
| Other | 4.9 | 2.7 | 5.0 |
| Age (years) (%) | |||
| 20–29 | 25.6 | 15.8 | 16.0 |
| 30–39 | 23.3 | 28.2 | 18.1 |
| 40–49 | 23.9 | 23.8 | 20.6 |
| 50–59 | 16.1 | 15.7 | 17.5 |
| 60–69 | 7.6 | 7.4 | 12.6 |
| ≥70 | 3.5 | 9.1 | 15.2 |
| BMI (%) | |||
| ≤22.7 | 24.8 | 8.7 | 17.3 |
| 22.8–26.1 | 25.3 | 18.4 | 22.9 |
| 26.2–30.2 | 23.5 | 30.6 | 27.6 |
| ≥30.3 | 26.4 | 42.3 | 32.2 |
| PIR (%) | |||
| Low (≤1.7) | 42.9 | 29.3 | 29.0 |
| Medium (1.8–3.9) | 32.2 | 31.7 | 31.9 |
| High (>3.9) | 24.9 | 39.0 | 39.1 |
| Serum cotinine (ng mL−1)d | 196 (101, 296) | 292 (146, 465) | 0.04 (0.02, 0.10) |
SMK, cigarette smokers; NTC, non-consumers of tobacco; BMI, body mass index; PIR, poverty index ratio.
aSee text for details.
bSTC, smokeless tobacco consumers. Includes chewing tobacco and snuff.
cIncludes Mexican American and other Hispanic.
dMedian (25th and 75th percentiles).
Table 2. Characteristics of Data set 2a (NHANES 1999–2008) by tobacco consumption category.
| SMK | STCb | NTC | |
|---|---|---|---|
| Sample size | 1440 | 69 | 2501 |
| Race/ethnicity (%) | |||
| Non-Hispanic White | 66.1 | 89.3 | 67.2 |
| Non-Hispanic Black | 13.2 | 1.6 | 9.5 |
| Hispanicc | 15.0 | 6.8 | 18.0 |
| Other | 5.7 | 2.3 | 5.3 |
| Age, years (%) | |||
| 26–31 | 26.7 | 17.5 | 21.8 |
| 32–37 | 24.0 | 41.8 | 23.7 |
| 38–43 | 24.7 | 17.3 | 27.4 |
| 44–49 | 24.6 | 23.4 | 27.1 |
| BMI (%) | |||
| ≤22.7 | 21.5 | 5.5 | 10.4 |
| 22.8–26.1 | 27.0 | 22.4 | 25.1 |
| 26.2–30.2 | 29.2 | 29.0 | 34.0 |
| ≥30.3 | 22.3 | 43.1 | 30.5 |
| Serum cotinine (ng mL−1)d | 203 (93, 301) | 329 (176, 500) | 0.04 (0.02, 0.13) |
SMK, cigarette smokers; NTC, non-consumers of tobacco; BMI, body mass index. Results did not differ with poverty index ratio (PIR) in the model.
aSee text for details.
bSTC, smokeless tobacco consumers. Includes snuff.
cIncludes Mexican American and other Hispanic.
dMedian (25th and 75th percentiles).
Table 3. Characteristics of Data set 3a (cross-sectional study) by tobacco consumption category.
| SMK | MSC | NTC | |
|---|---|---|---|
| Sample size | 60 | 48 | 60 |
| Race/ethnicity (%) | |||
| Non-Hispanic White | 95 | 98 | 75 |
| Non-Hispanic Black | 3 | 2 | 10 |
| Hispanic | 0 | 0 | 8 |
| Other | 2 | 0 | 7 |
| Age, years (%) | |||
| 26–31 | 25 | 29 | 25 |
| 32–37 | 25 | 25 | 25 |
| 38–43 | 25 | 31 | 25 |
| 44–49 | 25 | 15 | 25 |
| BMI (%) | |||
| ≤22.7 | 15 | 4 | 13 |
| 22.8–26.1 | 20 | 17 | 28 |
| 26.2–30.2 | 42 | 31 | 37 |
| ≥30.3 | 23 | 48 | 22 |
| Serum cotinine (ng mL−1)b | 339 (261, 419) | 467 (292, 788) | 0.12 (0, 0.24) |
SMK, cigarette smokers; MSC, moist snuff consumers; NTC, non-consumers of tobacco; BMI, body mass index.
aSee text for details.
bMedian (25th and 75th percentiles).
BoBE
For all biomarkers evaluated in all three data sets, median values were within clinically normal reference ranges (Supplementary material, Tables S-1, S-2, S-3, and S-4). In all three data sets, however, statistically significant (i.e. 95% confidence intervals on multiplicative factors not including 1.00) differences in adjusted mean biomarker values between group comparisons (i.e. SMK versus NTC, SMK versus STC, and STC versus NTC) were observed (Table 4). No statistically significant differences were observed in Data sets 2 and 3 (i.e. the two data sets limited to males aged 26–49 years and snuff only) that were not also observed in Data set 1. In Data set 2, excluding survey participants who reported taking cholesterol-lowering drugs from the analysis (n = 56 SMK, n = 2 STC (snuff only), n = 124 NTC) did not alter the results (data not shown). Additionally in Data set 2, including poverty index ratio in the regression model did not alter the results (data not shown).
Table 4. Statistically significant differencesa between groups, NHANES 1999–2008 (Data sets 1 and 2b), and cross-sectional study (Data set 3b).
| SMK versus NTC |
SMK versus STCc |
STC versus NTC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Markerd | Data set 1 | Data set 2 | Data set 3 | Data set 1 | Data set 2 | Data set 3 | Data set 1 | Data set 2 | Data set 3 |
| Total Cholesterol | ↑ | ||||||||
| HDL-C | ↓ | ↓ | ↓ | ||||||
| Triglycerides | ↑ | ↑ | ↑ | ↑ | ↓ | ||||
| Homocysteine | ↑ | ↑ | ↑ | ↑ | |||||
| Fibrinogen | ↑ | ↑ | ↑ | ↑ | ↑ | ||||
| C-reactive protein | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| Hematocrit | ↑ | ↑ | ↑ | ↑ | |||||
| Platelets | ↑ | ||||||||
| Red cell distribution width | ↑ | ||||||||
| Mean cell volume | ↑ | ↑ | ↑ | ↑ | ↑ | ||||
| Mean cell hemoglobin | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| Hemoglobin | ↑ | ↑ | ↑ | ↑ | |||||
| White blood cells | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Eosinophils | ↑ | ↑ | ↑ | ||||||
| Monocytes | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| Lymphocytes | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| Neutrophils | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| Apolipoprotein B100 | ↑ | ||||||||
| Folate | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| Ankle brachial index | ↓ | ↓ | ↓ | ↓ | ↓ | ||||
SMK, cigarette smokers; NTC, non-consumers of tobacco; HDL-C, high-density lipoprotein cholesterol.
aBased on multiplicative factors and corresponding 95% confidence intervals that did not include 1.00.
bSee text for details regarding Data sets 1, 2, and 3.
cSTC, smokeless tobacco consumers. See text for details of products included.
dAll markers listed were assessed in all three data sets; blank cells indicate that no statistically significant differences were found.
SMK versus NTC
Statistically significantly higher levels of fibrinogen (<10%), CRP (30 to >100%), hematocrit (<5%), mean cell volume (<5%), mean cell hemoglobin (<5%), hemoglobin (<5%), white blood cells (∼20%), monocytes (10–15%), lymphocytes (20%), and neutrophils (20–49%) were observed consistently across all three data sets in comparisons of SMK versus NTC (Table 4, Figure 1, and Supplementary Tables S-5–S-7); statistically significantly lower levels folate (15–20%) and ABI measures (3–5%) were also observed consistently across the three data sets in SMK versus NTC. HDL level was statistically significantly lower (5–11%) in two of the three data sets (Data sets 1 and 3), and not statistically significantly lower (2%) in Data set 2. Eosinophil counts were statistically significantly higher (30–32%) in two of the three data sets (Data sets 1 and 2), and not statistically significantly different in Data set 3. Total cholesterol (1%), platelets (3%), and red cell distribution width (1%) were statistically significantly higher in SMK versus NTC in Data set 1, but not in the other two data sets.
Figure 1.
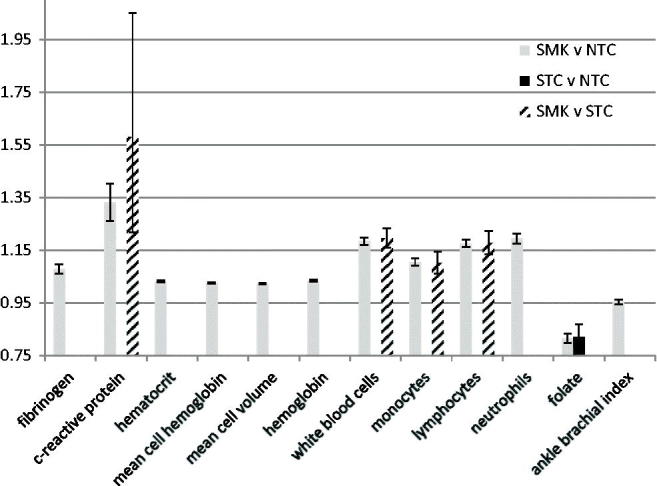
Biomarkers of biological effect with statistically significant differences between tobacco consumption groups in all three data sets. Results are fold-differences (i.e. multiplicative factors to the geometric mean) and 95% confidence intervals between exposure groups from Data set 1. Statistically significant = 95% confidence interval did not include 1.00. SMK, cigarette smokers; STC, smokeless tobacco consumers; NTC, non-consumers of tobacco.
SMK versus STC
Statistically significantly higher levels of CRP (60–90%), white blood cells (15–25%), monocytes (10–15%), and lymphocytes (20%) were observed consistently across all three data sets (Table 4, Figure 1, and Supplementary Tables S-5–S-7). Statistically significantly higher levels of triglycerides (12–13%), fibrinogen (7–8%), mean cell volume (2–4%), mean cell hemoglobin (3–4%) were observed in two of the three data sets, and statistically significantly lower measures of ABI were observed in two of the three data sets. A statistically significantly lower level of HDL was observed in Data set 1 only for the SMK versus STC comparison.
STC versus NTC
Statistically significantly lower levels of folate (10–20%) were observed consistently across the three data sets in comparisons of STC and NTC (Table 4, Figure 1, and Supplementary Tables S-5–S-7). Other statistically significant differences in biomarker measures, including triglycerides, homocysteine, mean cell hemoglobin, white blood cells, and neutrophils were observed in one of the data sets but not the other two.
Discussion
The results of this analysis and comparison of 1999–2008 US NHANES survey data and a single site, US cross-sectional study were consistent with previous reports of differences in CVD BoBE (i.e. white blood cells, hematocrit, fibrinogen, and CRP) in cigarette smokers compared with non-consumers of tobacco (Frost-Pineda et al., 2011; USDHHS, 2004, 2010). Additionally, evidence for consistent differences in mean cell volume and hemoglobin, markers of hypercoagluation, and monocytes, lymphocytes, and neutrophils, markers of inflammation, among SMK compared with NTC, not identified in previous studies, has been provided.
In comparisons of SMK and STC, this evaluation found consistent differences in white blood cell counts across the three data sets, similar to previous study findings (Eliasson et al., 1991). Differences in CRP, monocytes, and lymphocytes (all markers of inflammation) were also observed here in comparisons of SMK and STC, which have not been reported previously. Previous studies have reported differences in fibrinogen, hemoglobin, and platelets, in comparisons of smokers and smokeless tobacco users (Eliasson et al., 1991, 1995; Wennmalm et al., 1990). In the current evaluation, differences in fibrinogen were observed in two of the three data sets, differences in hemoglobin were observed in one of the three data sets, and differences in platelets were not observed in any of the three data sets.
Similar to previous studies, comparisons of STC and NTC in the current evaluation indicated no differences in white blood cells, hematocrit, hemoglobin, platelets, fibrinogen, apolipoprotein B, total cholesterol, HDL, LDL, triglycerides, and CRP (Bolinder et al., 1997a,b; Eliasson et al., 1991, 1995; Ernster et al., 1990; Norberg et al., 2006; Siegel et al., 1992; Wallenfeldt et al., 2001; Wennmalm et al., 1990). It is notable that in the current evaluation, lower triglyceride levels in STC versus NTC were observed in one data set, with no differences observed in the other two data sets. Previously, Wallenfeldt et al. (2001) reported higher levels of triglycerides in a Swedish sample of smokeless tobacco consumers compared with non-consumers of tobacco, and Norberg et al. (2006) reported increased risk for elevated triglycerides in Swedish smokeless tobacco consumers. Inconsistencies might be attributed to differing sample demographics (e.g. US versus Swedish populations). Finally, this evaluation indicated consistent decreased levels of folate in STC compared with NTC in the three data sets, a finding that was not identified in previous studies.
As noted, the only consistent statistically significant difference in STC compared with NTC was decreased serum folate, also observed in SMK compared with NTC. No studies of folate levels in STC were identified in the scientific literature, although previous studies have indicated lower levels of folate in smokers compared with non-smokers (Mansoor et al., 2011; Mouhamed et al., 2011; Okumura et al., 2011). Some, but not all, epidemiology studies have indicated that dietary folate may reduce the risk of CVD and that low blood folate is associated with increased risk of CVD (Okumura et al., 2011; Silaste, 2003). However, low folate is associated with elevated homocysteine levels, and high homocysteine is associated with higher risk of CVD (Okumura et al., 2011; Silaste, 2003). It is not clear whether low folate or elevated homocysteine is more predictive of CVD risk. In this analysis, consistent changes in homocysteine were not observed across the three data sets. However, increases in homocysteine were observed in two of the three data sets (Data sets 1 and 2) in comparisons of SMK versus NTC, and in one of the three data sets comparing STC with NTC (Data set 1). Low folate in tobacco consumers is possibly due to differences in dietary habits, with tobacco consumers eating fewer fruits and vegetables (Chao et al., 2002; Giraud et al., 1995; Henley et al., 2005; Okumura et al., 2011).
Comparison of data from a single site cross-sectional study with data from NHANES (Data sets 1 and 2) is a strength of this evaluation. NHANES is a well-established biomonitoring program in the US. NHANES data provide a large sample, which is designed to be representative of the US population, and individual level data are available to account for potential confounders such as age, race/ethnicity, and BMI.
A limitation of the use of NHANES data as Data sets 1 and 2 for comparison with the single site cross-sectional study is the definition of tobacco consumer groups. First, consumer groups in Data sets 1 and 2 were based on self-reporting of tobacco consumption (or non-consumption) in the previous 5 d before the questionnaire was administered in the MEC. Thus, usage in these groups, unlike in Data set 3, might not have been representative of exclusive long-term tobacco consumption or non-consumption. Although poly-tobacco users were excluded from the SMK and STC groups, former consumers of alternate tobacco products could have been included, and in the non-consumption groups, recent tobacco quitters might have been included. Additionally, information about smokeless tobacco consumption duration was not available in the NHANES MEC questionnaire (i.e. age of initiation of smokeless tobacco consumption was not asked). If durations of use were different between the SMK and STC, differences in BoE might be attributed to these differences in durations of exposure. Given the proportion of STC in Data sets 1 and 2 are consistent with previous estimates of lifetime current smokeless tobacco consumers in the United States (Agaku et al., 2014), it is likely that STC in Data sets 1 and 2 consist mostly, if not wholly, of lifetime smokeless tobacco consumers. Additionally, consistent findings across the three data sets indicate that the durations of use was not likely to be a discriminating factor.
An additional distinction between participants in Data sets 1 and 2 and the single site cross-sectional study (Data set 3) is that participants in the cross-sectional study were, by design, relatively healthy, whereas, by design, NHANES (Data sets 1 and 2) includes a sample representative of the general US population, and therefore, may be less healthy. Although the intention here was to determine consistency between NHANES and the single site cross-sectional study, changes seen in both Data sets 1 and 2, but not in Data set 3 (e.g. increased triglycerides in SMK versus NTC and SMK versus STC), are likely to be relevant.
Previously reported results from a comparison of biomarkers of exposure in cigarette smokers, smokeless tobacco consumers, and non-consumers of tobacco using NHANES 1999–2008 (Naufal et al., 2011) indicated that, in general, smokeless tobacco consumers had lower blood and urine concentrations of certain tobacco exposure-related constituents (e.g. volatile organic compounds, polycyclic aromatic hydrocarbons, metals, and acrylamide) compared with cigarette smokers, and concentrations in smokeless tobacco consumers were not different than those of non-consumers of tobacco. For constituents measured in common, similar results were observed in the single site US cross-sectional study discussed here (Campbell et al., 2015). These data provide an additional metric of consistency, in the context of comparing NHANES data with the single site cross-sectional study. They are additionally meaningful relative to the importance of reducing exposure as a first step towards potentially reducing risk (Stratton et al., 2001).
Although “to date, there are no data on how changes in smoking related biomarkers predict risk of disease” (USDHHS, 2010), the results of this evaluation provide support for the notion of tobacco harm reduction and the existence of a risk continuum among tobacco products. The findings from this assessment have indicated that biological and physiological biomarkers related to CVD were, for the most part, reduced in comparisons of STC and NTC with SMK and not different in STC compared with NTC. These results are consistent with previous evaluations of similar biomarkers in tobacco consumers, as well as with the evidence from epidemiology studies that CVD risk is increased in cigarette smokers, and in comparison with smokeless tobacco consumers. That is, although no tobacco product has been shown to be safe and without risks, the health risks associated with cigarettes are significantly greater than those associated with the use of smoke-free tobacco and nicotine products.
Acknowledgements
The authors gratefully acknowledge Dr. Summer N. Hanna, Mr. David L. Heavner, Dr. Leanne R. Campbell, Dr. Ziad S. Naufal, Dr. Gaddamanugu L. Prasad, Mr. Thomas J. Steichen, and Dr. Eugenia H. Theophilus.
SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. ®indicates USA registration.
Declaration of interest
All authors are current or former employees of R.J. Reynolds Tobacco Company and/or RAI Services Company.
References
- Agaku IT, King BA, Husten CG, et al. Tobacco product use among adults—United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63:542–7. [PMC free article] [PubMed] [Google Scholar]
- Bolinder G, Norén A, de Faire U, Wahren J. Smokeless tobacco use and atherosclerosis: an ultrasonographic investigation of carotid intima media thickness in healthy middle-aged men. Atherosclerosis. 1997a;132:95–103. doi: 10.1016/s0021-9150(97)00075-0. [DOI] [PubMed] [Google Scholar]
- Bolinder G, Norén A, Wahren J, de Faire U. Long-term use of smokeless tobacco and physical performance in middle-aged men. Eur J Clin Invest. 1997b;27:427–33. doi: 10.1046/j.1365-2362.1997.1290677.x. [DOI] [PubMed] [Google Scholar]
- Calapai G, Caputi AP, Mannucci C, et al. Cardiovascular biomarkers in groups of established smokers after a decade of smoking. Basic Clin Pharmacol Toxicol. 2009;104:322–8. doi: 10.1111/j.1742-7843.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- Campbell LR, Brown BG, Jones BA. Study of cardiovascular disease biomarkers among tobacco consumers, Part 1: Biomarkers of exposure. Inhal Toxicol. 2015 doi: 10.3109/08958378.2015.1013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data. 2010a http://www.cdc.gov/nchs/nhanes.htm Available from: [Last accessed 3 Aug 2012]
- Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). 2010b. 2010b http://www.cdc.gov/nchs/nhanes.htm Available from: [Last accessed 3 Aug 2012]
- Chao A, Thun MJ, Henley SJ, et al. Cigarette smoking, use of other tobacco products and stomach cancer mortality in US adults: the Cancer Prevention Study II. Int J Cancer. 2002;101:380–9. doi: 10.1002/ijc.10614. [DOI] [PubMed] [Google Scholar]
- Eliasson M, Lundblad D, Hägg E. Cardiovascular risk factors in young snuff-users and cigarette smokers. J Intern Med. 1991;230:17–22. doi: 10.1111/j.1365-2796.1991.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Eliasson M, Asplund K, Evrin PE, Lundblad D. Relationship of cigarette smoking and snuff dipping to plasma fibrinogen, fibrinolytic variables and serum insulin. The Northern Sweden MONICA Study. Atherosclerosis. 1995;113:41–53. doi: 10.1016/0021-9150(94)05425-i. [DOI] [PubMed] [Google Scholar]
- Ernster VL, Grady DG, Greene JC, et al. Smokeless tobacco use and health effects among baseball players. JAMA. 1990;264:218–24. [PubMed] [Google Scholar]
- Frost-Pineda K, Liang Q, Liu J, et al. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob Res. 2011;13:182–93. doi: 10.1093/ntr/ntq235. [DOI] [PubMed] [Google Scholar]
- Giraud DW, Martin HD, Driskell JA. Plasma and dietary vitamin C and E levels of tobacco chewers, smokers, and nonusers. J Am Diet Assoc. 1995;95:798–800. doi: 10.1016/S0002-8223(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Allen S, et al. Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest. 2005;128:2528–37. doi: 10.1378/chest.128.4.2528. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Rennard SI, et al. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine Tob Res. 2006;8:600–22. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes Contr. 2005;16:347–58. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- Mansoor MA, Hervig T, Stakkestad JA, et al. Serum folate is significantly correlated with plasma cysteine concentrations in healthy industry workers. Ann Nutr Metab. 2011;58:68–73. doi: 10.1159/000325537. [DOI] [PubMed] [Google Scholar]
- Mouhamed DH, Ezzaher A, Neffati F, et al. Effect of cigarette smoking on plasma homocysteine concentrations. Clin Chem Lab Med. 2011;49:479–83. doi: 10.1515/CCLM.2011.062. [DOI] [PubMed] [Google Scholar]
- Naufal ZS, Marano KM, Kathman SJ, Wilson CL. Differential exposure biomarker levels among cigarette smokers and smokeless tobacco consumers in the National Health and Nutrition Examination Survey 1999–2008. Biomarkers. 2011;16:222–35. doi: 10.3109/1354750X.2010.546013. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) Health effects of exposure to environmental tobacco smoke. In: Smoking and tobacco control monograph no. 10. 1999 http://cancercontrol.cancer.gov/tcrb/monographs/10/ Available from: [Last accessed 3 Aug 2012]
- Norberg M, Stenlund H, Lindahl B, et al. Contribution of Swedish moist snuff to the metabolic syndrome: a wolf in sheep's clothing? Scand J Public Health. 2006;34:576–83. doi: 10.1080/14034940600665143. [DOI] [PubMed] [Google Scholar]
- Nordskog BK, Brown BG, Marano KM. Study of cardiovascular disease biomarkers among tobacco consumers. Part 2: biomarkers of biological effect. Inhal Toxicol. 2015 doi: 10.3109/08958378.2015.1013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Phillips LD, Balfour D, et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res. 2014;20:218–25. doi: 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- Okumura K, Tsukamoto H. Folate in smokers. Clin Chim Acta. 2011;412:521–6. doi: 10.1016/j.cca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Piano MR, Benowitz NL, Fitzgerald GA, et al. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment. A policy statement from the American Heart Association. Circulation. 2010;122:1520–44. doi: 10.1161/CIR.0b013e3181f432c3. American Heart Association Council on Cardiovascular Nursing. [DOI] [PubMed] [Google Scholar]
- Siegel D, Benowitz N, Ernster VL, et al. Smokeless tobacco, cardiovascular risk factors, and nicotine and cotinine levels in professional baseball players. Am J Public Health. 1992;82:417–21. doi: 10.2105/ajph.82.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silaste ML. Dietary effects on antioxidants, oxidised LDL and homocysteine. Dissertation Oulu University. 2003 http://herkules.oulu.fi/isbn9514270703/ Available from: [Last accessed 3 Aug 2012]
- Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction – executive summary. Tob Control. 2001;10:189–95. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services (USDHHS) The health consequences of smoking: a report of the surgeon general. 2004 http://www.surgeongeneral.gov/library/reports/index.html Available from: [Last accessed 3 Aug 2012]
- US Department of Health and Human Services (USDHHS)) How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease. 2010 http://www.ncbi.nlm.nih.gov/books/NBK53017/ Available from: [Last accessed 11 Nov 2014] [PubMed]
- Wallenfeldt K, Hulthe J, Bokemark L, et al. Carotid and femoral atherosclerosis, cardiovascular risk factors and C-reactive protein in relation to smokeless tobacco use or smoking in 58-year-old men. J Intern Med. 2001;250:492–501. doi: 10.1046/j.1365-2796.2001.00917.x. [DOI] [PubMed] [Google Scholar]
- Wennmalm A, Benthin G, Granström EF, et al. Relation between tobacco consumption and urinary excretion of thromboxane A2 and prostacyclin metabolites in 756 randomly sampled young men. In: Sameulsson B, Paoletti R, editors. Advances in prostaglandin, thromboxane, and leukotriene research. Vol. 21. New York: Raven Press Ltd; 1990. p. 615. [PubMed] [Google Scholar]
- Zeller M, Hatsukami D. The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–32. doi: 10.1136/tc.2008.027318. Strategic Dialogue on Tobacco Harm Reduction Group. [DOI] [PMC free article] [PubMed] [Google Scholar]


