Abstract
An age-stratified, cross-sectional study was conducted in the US among healthy adult male cigarette smokers, moist snuff consumers, and non-tobacco consumers to evaluate cardiovascular biomarkers of biological effect (BoBE). Physiological assessments included flow-mediated dilation, ankle-brachial index, carotid intima-media thickness and expired carbon monoxide. Approximately one-half of the measured serum BoBE showed statistically significant differences; IL-12(p70), sICAM-1 and IL-8 were the BoBE that best differentiated among the three groups. A significant difference in ABI was observed between the cigarette smokers and non-tobacco consumer groups. Significant group and age effect differences in select biomarkers were identified.
Keywords: Cigarette, CVD, BoBE, moist snuff, tobacco
Introduction
Attention has been focused on cigarette smoking cessation strategies aimed at helping individuals to quit smoking and, ultimately, to reduce the prevalence of cigarette smoking-related diseases. The success of these tobacco abstinence programs tends to be limited. An alternative to cigarette smoking cessation for adults who choose to continue to use tobacco products includes the migration to smokeless or non-burning tobacco products like moist snuff, snus and/or dissolvable tobacco products.
According to several published reports (Eliasson et al., 1991; Hergens et al., 2005; Huhtasaari et al., 1992; Piano et al., 2010; Siegel et al., 1992; Zeller et al., 2009), scientific evidence supports the use of non-burning tobacco products as a less harmful alternative to cigarette smoking in relation to cardiovascular disease (CVD) and other cigarette smoking-related diseases. Despite the potential risk reductions from transitioning to non-burning or smokeless tobacco consumption, few studies have directly compared biomarkers of biological effect (BoBE) among smokers, moist snuff consumers and non-consumers of tobacco. The identification of relevant BoBE is important in order to understand how BoBE: (i) are related to tobacco consumption associated disease, including progression or regression of disease; (ii) change in tobacco consumers over time and (iii) may be different in consumers of different types of tobacco products (i.e. combustible versus non-combustible). Until recently, researchers have conducted relatively small studies measuring BoBE in smokers compared to non-consumers of tobacco. BoBE that have shown consistent differences between cigarette smokers and non-smokers include fibrinogen (Bazzano et al., 2003; Eliasson et al., 1995; Kannel et al., 1987; Yarnell et al., 2000) and white blood cell (WBC) count (Calapai et al., 2009b; Roethig et al., 2010; Yarnell et al., 2000). Other BoBE-like intracellular adhesion molecule-1 (ICAM-1) (Levitzky et al., 2008; Nguyen et al., 2010) and high-density lipoprotein cholesterol (HDL) (Calapai et al., 2009a; Eliasson et al., 1995; Lowe et al., 2009; Roethig et al., 2008) typically show differences between smokers and non-consumers of tobacco, but not always. Recently, Frost-Pineda et al. (2011) published a large-scale cross-sectional study consisting of 3585 adult smokers and identified 21 BoBE that differ between smokers and non-consumers of tobacco. Marano et al. (2015) identified similar and consistent differences in certain BoBE between cigarette smokers, smokeless tobacco users and non-consumers of tobacco in analysis of data from the National Health and Nutrition Examination Survey (NHANES), a large US government-supported database (Centers for Disease Control and Prevention, 2011). Fewer studies have examined BoBE in consumers of smokeless tobacco products than in cigarette smokers, although the available studies indicate BoBE are similar in smokeless tobacco consumers and in non-consumers of tobacco.
This article presents the results of the examination of several “traditional” CVD BoBE (serum biomarkers and physiological measures), in three exclusive use groups [cigarette smokers (SMK), moist snuff consumers (MSC) and non-consumers of tobacco (NTC)]. Results of this study provide a foundation for understanding how consumption of different tobacco products (combustible versus non-combustible) affects CVD BoBE (i.e. proteins, lipids and cellular components) measured in serum and urine.
Methods
Study design and participants
Details of study design, participants and conduct have been reported elsewhere (Campbell et al., 2015). Briefly, this study was a single site, age-stratified, cross-sectional study design conducted between September 2008 and February 2009 and managed by Celerion (formerly MDS Pharma Services, Lincoln, Nebraska) (ClinicalTrials.gov; identifier: NCT01692353). The study was approved by the MDS Pharma Services Institutional Review Board and was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki and applicable sections of the US Code of Federal Regulations: 21 CFR. All subjects signed informed consent prior to any study procedures being performed and were compensated for their time and participation. Study participants were healthy males, aged 26–49 years, and recruited into one of three exclusive use groups (i.e. SMK, n = 60; MSC, n = 48; NTC, n = 60) (Campbell et al., 2015). SMK had smoked at least 15 cigarettes per day with mainstream smoke “tar” yields >6.0 mg for at least 3 years prior to the study screening and had expired carbon monoxide (ECO) levels between 10 and 100 parts per million (ppm). During the 6 months prior to study enrollment, SMK were required to have exclusively smoked cigarettes and not to have used any other types of tobacco. MSC reported using at least two cans of moist snuff per week for at least 3 years prior to study screening and had ECO levels ≤5 ppm. During the 6 months prior to study enrollment, MSC were required to use moist snuff exclusively and not to have used any other form of tobacco or nicotine replacement therapy (NRT). MSC had limited lifetime usage of other types of tobacco. NTC had limited lifetime usage of tobacco and had never used NRT and had ECO levels ≤5 ppm.
Study conduct
On Day 1, eligible participants were admitted, confined overnight and discharged approximately at noon on the following day (Day 2). On Day 1, participants observed a 45-min tobacco abstention period followed by use of a single UB tobacco product, referred to as a “challenge” (Campbell et al., 2015). Fifteen minutes post-challenge, urine and blood were collected, and ECO and ankle brachial index (ABI) were measured. At 30-min post-challenge, flow-mediated dilation (FMD) was measured (Campbell et al., 2015). On the morning of Day 2 following an overnight tobacco abstention and fast, blood and spot urine samples were collected, and ECO, ABI, FMD and carotid intima-media thickness (CIMT) were measured.
Serum BoBE
BoBE were analyzed from fasting blood samples collected the morning of Day 2. Interleukin (IL)-1β, IL-6, IL-8, IL-12 (p40), IL-12 (p70), soluble ICAM-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble E-selectin (sSELE), soluble P-selectin (sSELP), enothelin-1 (ET-1), tissue necrosis factor-α (TNF-α), monocyte chemotactic protein-1 (MCP-1), soluble CD40 ligand (sCD40L), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), high-sensitivity C-reactive protein (hsCRP), interferon-γ (IFN-γ), Regulated upon Activation, Normal T-cell Expressed and Secreted (CCL5/RANTES), matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-3 (MMP-3), matrix metalloproteinase-9 (MMP-9) and von Willebrand factor (vWF) were measured at Rules Based Medicine (Austin, Texas) by ELISA or xMAP® multiplex bead-based technology (Luminex, Austin, Texas). Total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), HDL-cholesterol (HDL-C), very LDL-cholesterol (VLDL-C), apolipoprotein A1 (Apo A1), apolipoprotein A2 (Apo A2), apolipoprotein B100 (Apo B100), apolipoprotein(a) [Lp(a)], oxidized LDL (ox-LDL), folate, fibrinogen and tissue inhibitor of matrix metalloproteinase-1 (TIMP1) were measured at Pacific Biomarkers Inc. (Seattle, Washington). α1-Antitrypsin (AAT) was measured at ARUP Laboratories (Salt Lake City, Utah). Standard clinical blood hematology measures were analyzed at Celerion.
Urine BoBE
Urinary BoBE were analyzed from spot urines taken on Days 1 and 2 of the study. Isoprostanes iPF2α-III and iPF2α-VI were analyzed at Celerion and 11-dehydro-thromboxane B2 (TXB2) was measured at Analytisch-biologisches Forschungslabor GmbH (ABF) (Munich, Germany), both using liquid chromatography-tandem mass spectrometry (LC-MS/MS) techniques. Urine BoBE were normalized to urine creatinine levels (Campbell et al., 2015).
CVD-related physiological assessments
FMD provides a measure of endothelial dysfunction. FMD measurements were performed on the non-dominant arm using a high-resolution Doppler imaging machine and software to measure and analyze the changes in the diameter of the brachial artery in response to a rapid increase of flow stimulus (post-occlusion). The exact location of an acceptable brachial artery image and forearm cuff position for each subject was measured and recorded relative to a line drawn through the antecubital fossa. FMD was calculated by the software as the difference between the maximum post-occlusive (forearm cuff, 300 mmHg) diameter and the average baseline diameter, relative to the average baseline diameter [expressed as a percentage (%FMD)]. Pre- and post-occlusive brachial artery blood velocity (Doppler) was recorded as confirmation of hyperemia. FMD precision [coefficient of variation (CV)] was determined in a subset of 33 subjects (11 from each of the three groups) randomly selected (temporally across the study) and invited to participate in one additional Day 2 fasting determination for CV calculations. FMD was measured on both Day 1 and 2.
ABI was measured to assess peripheral artery disease as described by Smith et al. (2005). It was calculated as the ratio of the systolic blood pressure (SBP) at the ankle divided by the SBP at the brachial artery of the arm. The SBP was measured at six locations including the brachial artery of both arms, and the dorsalis pedis and posterior tibial arteries of both ankles. The greater of the two pressures at the arms (denominator) and the greatest of the four pressures at the ankles (numerator) were used in determining the ABI (Redberg et al., 2003; Smith et al., 2005). ABI was measured on both Day 1 and 2.
To measure CIMT, a high-resolution B-mode ultrasound was used to assess the thickness of the intima-media region of the carotid artery as described elsewhere (Redberg et al., 2003). CIMT was measured for six angles (left side from 90,120, 150°; right side from 210, 240, 270°), after the subject had rested supine for 10 min. The composite CIMT mean value was calculated for each subject. Thus, a total of six intima-media thickness values were reported. Data for near wall thickness was incomplete due to difficulties associated with measuring near wall thickness. The same two sonographers were used to independently assess CIMT based on the average from the other angles to arrive at the composite value. CIMT was measured on Day 2 only. ECO levels (ppm) were measured using the Micro IV Smokerlyzer® Breath Carbon Monoxide Monitor (Bedfont Scientific Ltd, Haddonfield, NJ).
Statistical analyses
A description of statistical analyses has been presented elsewhere (Campbell et al., 2015). Briefly, analysis of variance (ANOVA) model using least squares means was used in to compare urine and blood biomarkers among the three groups (i.e. SMK, MSC and NTC). Group, age stratum (i.e. 26–31, 32–37, 38–43 and 44–49 years), and the interaction between group and age were fixed effects in the model (Campbell et al., 2015). Additionally, principal component analysis (PCA) was performed on serum BoBE having significant group differences (p < 0.05) identified by ANOVA. The analysis was performed at Rules Based Medicine. Analytes that did not contribute significantly to the PCA were removed from the analysis. The case-wise scores from the top principal components were then plotted onto proximity maps. A proximity map shows individual scores with the most similar pattern of analytes being nearest to each other, and those with opposite levels of analytes being furthest away.
Results
Hematologic biomarkers
All hematology results were within the normal references ranges for males in the age range of this study (Rush University Medical Center, 2011). However, six hematologic biomarkers were significantly different for at least one pairwise group comparison (Table 1). SMK had statistically significantly higher levels of hemoglobin, and hematocrit relative to NTC. The mean corpuscular hemoglobin (MCH) and mean corpuscular volume (MCV) were elevated in SMK relative to both MSC and NTC. WBC count was significantly higher in SMK compared to both MSC and NTC groups. In addition, SMK had significantly higher percentages of neutrophils compared to NTC.
Table 1. Hematologic biomarkers.
| LS meansa |
Group comparison p values |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Age (years) | SMK | MSC | NTC | SMK versus MSC | SMK versus NTC | MSC versus NTC |
| Hemoglobin (g dL−1) | All ages | 15.91 | 15.53 | 15.35 | 0.1137 | 0.0027 | 0.9126 |
| Hematocrit (%) | All ages | 46.10 | 45.25 | 44.50 | 0.2409 | 0.0012 | 0.3678 |
| Platelet count (thou μL−1) | All ages | 251.93 | 256.59 | 244.95 | 1.0000 | 1.0000 | 0.7050 |
| RBC count (mil μL−1) | All ages | 5.06 | 5.08 | 5.06 | 1.0000 | 1.0000 | 1.0000 |
| RDW (%) | All ages | 12.81 | 12.85 | 12.77 | 1.0000 | 1.0000 | 1.0000 |
| MCH (pg) | All ages | 31.51 | 30.59 | 30.38 | 0.0007 | <0.0001 | 1.0000 |
| MCHC (g dL−1)b | 26–31 | 34.49 | 34.32 | 34.24 | 1.0000 | 0.5217 | 1.0000 |
| 32–37 | 34.59 | 34.61 | 34.84 | 1.0000 | 0.5565 | 0.7221 | |
| 38–43 | 34.40 | 34.49 | 34.48 | 1.0000 | 1.0000 | 1.0000 | |
| 44–49 | 34.55 | 33.83 | 34.44 | 0.0072 | 1.0000 | 0.0282 | |
| All ages | 34.51 | 34.31 | 34.50 | 0.1635 | 1.0000 | 0.1965 | |
| MCV (fL) | All ages | 91.30 | 89.16 | 88.05 | 0.0036 | <0.0001 | 0.2728 |
| MPV (fL) | All ages | 8.43 | 8.14 | 8.23 | 0.2931 | 0.6435 | 1.0000 |
| WBC count (thou μL−1) | All ages | 8.48 | 7.53 | 6.75 | 0.0139 | <0.0001 | 0.0591 |
| Basophils (%) | All ages | 0.44 | 0.45 | 0.52 | 1.0000 | 0.1383 | 0.3567 |
| Eosinophils (%) | All ages | 2.07 | 2.16 | 2.86 | 1.0000 | 0.0606 | 0.1767 |
| Lymphocytes (%) | All ages | 27.80 | 28.19 | 30.19 | 1.0000 | 0.1797 | 0.4422 |
| Monocytes (%) | All ages | 6.81 | 6.74 | 7.53 | 1.0000 | 0.1410 | 0.1383 |
| Neutrophils (%) | All ages | 62.89 | 62.45 | 58.90 | 1.0000 | 0.0252 | 0.0906 |
aLeast square means.
bAge main effect (p < 0.05).
Serum lipid-related biomarkers
Results for lipids, lipoproteins and apolipoproteins are shown in Table 2. Apolipoprotein A concentrations were the only serum lipid-related biomarker identified with significant changes. Apo A-1 serum concentration was significantly lower in both SMK and MSC compared to NTC, while Apo A-2 was significantly lower in SMK compared to NTC. For lipids that have significant age effects, a small number of cohort comparisons were found to be significant for individual age groups (e.g. cholesterol in the MSC–NTC comparison for the 44–49 years age group).
Table 2. Serum lipid biomarkers.
| LS meansa |
Group comparison p values |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Age (years) | SMK | MSC | NTC | SMK versus MSC | SMK versus NTC | MSC versus NTC |
| Total cholesterol (mg dL−1)b | 26–31 | 185.53 | 182.71 | 178.87 | 1.0000 | 1.0000 | 1.000 |
| 32–37 | 199.93 | 194.33 | 214.00 | 1.0000 | 0.7515 | 0.3915 | |
| 38–43 | 202.27 | 199.40 | 206.07 | 1.0000 | 1.0000 | 1.0000 | |
| 44–49 | 188.20 | 208.29 | 222.60 | 0.5727 | 0.0162 | 1.0000 | |
| All ages | 193.98 | 196.18 | 205.38 | 1.0000 | 0.1902 | 0.5022 | |
| LDL-C (mg dL−1)b | 26–31 | 117.93 | 112.64 | 108.80 | 1.0000 | 1.0000 | 1.0000 |
| 32–37 | 126.67 | 110.82 | 134.20 | 0.5475 | 1.0000 | 0.1500 | |
| 38–43 | 126.13 | 125.40 | 130.87 | 1.0000 | 1.0000 | 1.0000 | |
| 44–49 | 120.13 | 136.29 | 150.60 | 0.7152 | 0.0174 | 0.8874 | |
| All ages | 122.72 | 121.29 | 131.12 | 1.0000 | 0.3744 | 0.3027 | |
| HDL-C (mg dL−1) | All ages | 41.93 | 42.30 | 46.83 | 1.0000 | 0.0858 | 0.1866 |
| VLDL-C (mg dL−1) | All ages | 29.42 | 31.71 | 27.42 | 1.0000 | 1.0000 | 0.3654 |
| ox-LDL (U L−1) | All ages | 76.42 | 78.93 | 77.40 | 1.0000 | 1.0000 | 1.0000 |
| Lp(a) (mg dL−1) | All ages | 24.98 | 21.48 | 30.41 | 1.0000 | 1.0000 | 1.0000 |
| Triglycerides (mg dL−1)b | 26–31 | 156.47 | 156.57 | 125.40 | 1.0000 | 0.7338 | 0.7536 |
| 32–37 | 153.33 | 222.17 | 157.87 | 0.0474 | 1.0000 | 0.072 | |
| 38–43 | 158.40 | 160.33 | 146.00 | 1.0000 | 1.0000 | 1.0000 | |
| 44–49 | 120.00 | 120.14 | 118.07 | 1.0000 | 1.0000 | 1.0000 | |
| All ages | 147.05 | 164.80 | 136.83 | 0.6645 | 1.0000 | 0.1647 | |
| Apo A1 (mg dL−1)b | 26–31 | 110.47 | 113.07 | 124.87 | 1.0000 | 0.2310 | 0.4617 |
| 32–37 | 124.33 | 118.92 | 138.87 | 1.0000 | 0.2229 | 0.0639 | |
| 38–43 | 127.93 | 123.93 | 132.87 | 1.0000 | 1.0000 | 0.8133 | |
| 44–49 | 126.33 | 130.43 | 139.60 | 1.0000 | 0.3090 | 1.0000 | |
| All ages | 122.27 | 121.59 | 134.05 | 1.0000 | 0.0123 | 0.0156 | |
| Apo A2 (mg dL−1) | All ages | 37.73 | 38.42 | 41.27 | 1.0000 | 0.0054 | 0.0600 |
| Apo B100 (mg dL−1) | All ages | 92.40 | 93.05 | 94.20 | 1.0000 | 1.0000 | 1.0000 |
aLeast square means. bAge main effect (p < 0.05).
Serum BoBE
All biomarker values fell within normal reference ranges for males in the age range of this study (Rush University Medical Center, 2011) (Table 3). Significant group by age interactions were observed for sICAM-1, PDGF, ADMA and L-NMMA. Two biomarkers [AAT and IL-12(p70)] were statistically significantly greater in SMK compared to MSC and NTC. Statistically significantly higher levels of sICAM-1 were detected in SMK compared to NTC except for the 44–49 years age group. IL-8 and MCP-1 were elevated in both tobacco groups compared to NTC. Four biomarkers were significantly different in only one of the three pairwise comparisons (TIMP1, sVCAM, VEGF and vWF). Some statistically significant differences were observed in group comparisons within individual age categories of ADMA, although the main cohort comparisons were all non-significant. Significant age main effects were observed for several biomarkers (data not shown).
Table 3. Serum BoBE.
| LS meansa |
Group comparison p values |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Age (years) | SMK | MSC | NTC | SMK versus MSC | SMK versus NTC | MSC versus NTC |
| AAT (mg dL−1) | All ages | 132.98 | 119.59 | 120.70 | <0.0001 | <0.0001 | 1.0000 |
| Folate (nmol L−1) | All ages | 24.15 | 25.27 | 28.84 | 1.0000 | 0.0054 | 0.0840 |
| TIMP1 (ng mL−1) | All ages | 82.88 | 77.88 | 73.39 | 0.1032 | <0.0001 | 0.0942 |
| Fibrinogen (mg dL−1) | All ages | 386.38 | 359.02 | 360.37 | 0.1095 | 0.0918 | 1.0000 |
| IL-8 (pg mL−1) | All ages | 15.10 | 13.18 | 10.69 | 0.0924 | <0.0001 | 0.0165 |
| IL-12(p40) (ng mL−1) | All ages | 0.35 | 0.39 | 0.38 | 0.9201 | 1.0000 | 1.0000 |
| IL-12(p70) (pg mL−1) | All ages | 60.53 | 50.26 | 49.02 | <0.0001 | <0.0001 | 1.0000 |
| sICAM-1 (ng mL−1)b | 26–31 | 149.87 | 128.00 | 109.67 | 0.0921 | 0.0003 | 0.2082 |
| 32–37 | 162.53 | 124.58 | 109.87 | 0.0012 | <0.0001 | 0.4830 | |
| 38–43 | 143.00 | 126.93 | 105.93 | 0.3147 | 0.0006 | 0.1038 | |
| 44–49 | 124.80 | 99.57 | 115.80 | 0.1281 | 1.0000 | 0.5721 | |
| All ages | 145.05 | 119.77 | 110.32 | <0.0001 | <0.0001 | 0.2385 | |
| sVCAM (ng mL−1) | All ages | 580.35 | 567.78 | 513.12 | 1.0000 | 0.0123 | 0.0933 |
| sSELE (ng mL−1)b | All ages | 47.33 | 46.96 | 44.47 | 1.0000 | 1.0000 | 1.0000 |
| sSELP (ng mL−1) | All ages | 64.33 | 62.54 | 55.46 | 1.0000 | 0.0738 | 0.2919 |
| MCP-1 (pg mL−1) | All ages | 397.48 | 356.93 | 286.97 | 0.3696 | <0.0001 | 0.0249 |
| sCD40L (ng mL−1) | All ages | 0.72 | 0.76 | 0.70 | 1.0000 | 1.0000 | 1.0000 |
| VEGF (pg mL−1)b | 26–31 | 594.27 | 589.14 | 445.37 | 1.0000 | 0.4203 | 0.4842 |
| 32–37 | 626.80 | 552.75 | 256.87 | 1.0000 | 0.0009 | 0.0180 | |
| 38–43 | 462.87 | 601.60 | 499.00 | 0.5040 | 1.0000 | 0.9216 | |
| 44–49 | 522.67 | 644.29 | 582.80 | 1.0000 | 1.0000 | 1.0000 | |
| All ages | 551.65 | 596.94 | 446.10 | 1.0000 | 0.1101 | 0.0189 | |
| PDGF (pg mL−1) | 26–31 | 1799.5 | 3909.3 | 3942.2 | 0.0002 | 0.0001 | 0.9532 |
| 32–37 | 2963.3 | 3204.2 | 1821.7 | 0.6803 | 0.0396 | 0.0190 | |
| 38–43 | 3155.3 | 3304.9 | 2689.3 | 0.7861 | 0.3982 | 0.2649 | |
| 44–49 | 2769.3 | 3504.3 | 2457.8 | 0.2882 | 0.5720 | 0.1312 | |
| All ages | 2671.9 | 3480.7 | 2727.8 | 0.0228 | 1.0000 | 0.0387 | |
| hsCRP (μg mL−1) | All ages | 3.06 | 2.71 | 1.79 | 1.0000 | 0.1413 | 0.5583 |
| RANTES (ng mL−1) | All ages | 16.05 | 17.26 | 15.50 | 1.0000 | 1.0000 | 0.8643 |
| MMP-3 (ng mL−1) | All ages | 5.24 | 5.24 | 4.96 | 1.0000 | 1.0000 | 1.0000 |
| vWF (μg mL−1) | All ages | 27.18 | 22.95 | 19.68 | 0.0627 | <0.0001 | 0.2220 |
| Arg (μg mL−1) | All ages | 13.46 | 12.21 | 12.80 | 0.2289 | 0.9126 | 1.0000 |
| ADMA (μg mL−1)b | 26–31 | 0.27 | 0.17 | 0.25 | <0.0001 | 0.2805 | 0.0005 |
| 32–37 | 0.16 | 0.19 | 0.22 | 0.3652 | 0.0068 | 0.0954 | |
| 38–43 | 0.15 | 0.20 | 0.18 | 0.0498 | 0.2928 | 0.3583 | |
| 44–49 | 0.23 | 0.18 | 0.18 | 0.0895 | 0.0442 | 0.9284 | |
| All ages | 0.20 | 0.18 | 0.21 | 0.2199 | 1.0000 | 0.1070 | |
| SDMA (μg mL−1) | All ages | 0.43 | 0.42 | 0.49 | 1.0000 | 0.2094 | 0.1314 |
| L-NMMA (μg mL−1)b | 26–31 | 0.064 | 0.044 | 0.053 | <0.0001 | 0.0003 | 0.0066 |
| 32–37 | 0.048 | 0.047 | 0.051 | 0.7508 | 0.4169 | 0.2793 | |
| 38–43 | 0.046 | 0.048 | 0.047 | 0.5076 | 0.7162 | 0.7646 | |
| 44–49 | 0.055 | 0.047 | 0.049 | 0.0538 | 0.0809 | 0.5886 | |
| All ages | 0.053 | 0.047 | 0.050 | 0.0003 | 0.1011 | 0.1443 | |
| Hcy (μg mL−1) | All ages | 0.07 | 0.07 | 0.06 | 1.0000 | 0.6363 | 1.0000 |
| Cit (μg mL−1)b | 26–31 | 4.54 | 5.50 | 4.92 | 0.1980 | 1.0000 | 0.8070 |
| 32–37 | 6.02 | 6.27 | 5.18 | 1.0000 | 0.3069 | 0.1407 | |
| 38–43 | 5.47 | 4.95 | 5.78 | 0.9180 | 1.0000 | 0.3150 | |
| 44–49 | 6.13 | 5.79 | 5.88 | 1.0000 | 1.0000 | 1.0000 | |
| All ages | 6.13 | 5.63 | 5.44 | 1.0000 | 1.0000 | 1.0000 | |
| Met (μg mL−1) | 3.79 | 3.78 | 4.27 | 1.0000 | 0.0624 | 0.0858 | |
IL-1β, IL-6, TNFα, INF-γ, MMP-2 and MMP-9 were not analyzed because values were below the limit of detection.
aLeast square means. bAge main effect (p < 0.05).
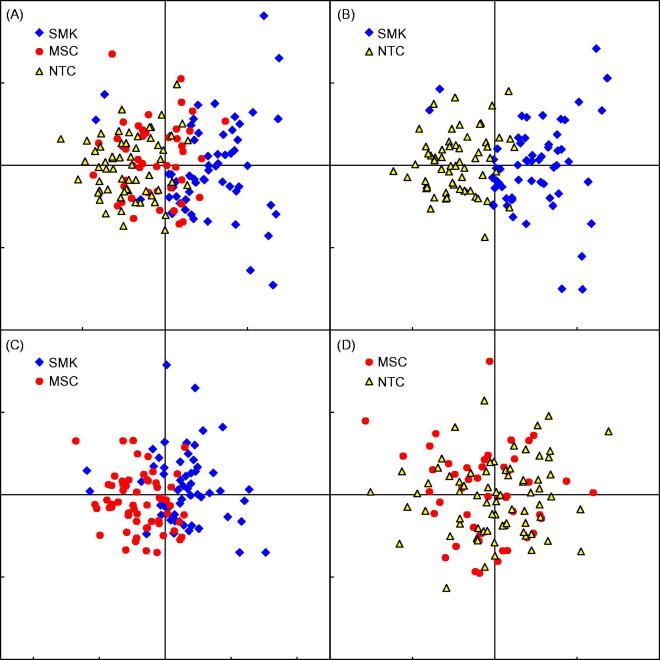
In order to determine how well individual members of each group could be differentiated based on the values from identified analytes, a PCA was performed on analytes that showed a significance group effect in the ANOVA. Analytes were sequentially removed from the PCA model if the resulting verification plot of case-wise scores showed poor separation of the groups. The components for BoBE that best differentiated the three groups in this study were IL-12p70, sICAM-1 and IL-8. The case-wise scores of these components are shown in Figure 1. Samples with the most similar overall pattern of analyte levels are located near each other, while samples with different patterns are farther apart. Figure 1(A) shows these top three analyte components plotted for all groups. Figures 1(B–D) represent each of the three group comparisons. Panels displaying SMK and NTC together (Figure 1B) as well as SMK and MSC (Figure 1C) resulted in group-specific clustering, whereas no separation was observed between the MSC and NTC (Figure 1D).
Figure 1.
PCA. The case-wise scores of the top two principal components were plotted onto proximity maps. The top candidate analytes for BoBE that best differentiated the three study groups in this study were IL-12p70, ICAM-1 and IL-8. The concept of differentiation, or separation, in this analysis means that these biomarkers correlate and vary in such a way that identifies SMK from non-smokers (NTC and MSC) at the individual level, as opposed to an aggregate or mean level. (A) The top three biomarkers plotted for all three groups, showing a moderate separation for SMK and little separation of MSC and NTC overall. (B) A clearer separation of SMK to NTC with few individual exceptions. (C) Comparatively greater amount of overlap between SMK and MSC, while in (D), no separation is observed between MSC and NTC.
Urine BoBE
iPF2α-III and TXB2 levels were statistically significantly elevated in SMK compared to MSC and NTC on Days 1 and 2 (Table 4). iPF2α-VI was statistically significantly elevated in SMK compared to MSC on Day 1 and elevated compared to MSC and NTC on Day 2. No statistically significant differences were observed between MSC and NTC for these biomarkers.
Table 4. Urine BoBE.
| LS meansa |
Group comparison p values |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Age (years) | SMK | MSC | NTC | SMK versus MSC | SMK versus NTC | MSC versus NTC |
| Day 1 | |||||||
| iPF2α-III (pg mg−1 CRE) | All ages | 447.61 | 207.74 | 215.29 | <0.0001 | <0.0001 | 1.0000 |
| iPF2α-VI (pg mg−1 CRE) | All ages | 3257.27 | 2403.48 | 2745.55 | 0.0423 | 0.1761 | 0.9771 |
| TXB2 (ng mg−1 CRE) | All ages | 0.45 | 0.31 | 0.29 | <0.0001 | <0.0001 | 1.0000 |
| Day 2 | |||||||
| iPF2α-III (pg mg−1 CRE) | All ages | 407.01 | 201.80 | 191.68 | <0.0001 | <0.0001 | 1.0000 |
| iPF2α-VI (pg mg−1 CRE) | All ages | 2760.76 | 2050.14 | 2267.35 | 0.0006 | 0.0159 | 0.7455 |
| TXB2 (ng mg−1 CRE) | All ages | 0.66 | 0.48 | 0.39 | 0.0002 | <0.0001 | 0.1137 |
aLeast square means. CRE, creatinine.
Physiological biomarkers of effect
FMD and ABI were measured on Day 1 (post-“challenge”) and Day 2 (post-tobacco abstention/fasting) (Table 5). ABI was statistically significantly higher in SMK compared to NTC on Day 1, but this effect was not observed on Day 2. No statistically significant differences were observed in FMD and CIMT assessments between groups; however, both nominal and statistically significant increases in CIMT were noted as age increased (data not shown). ECO was statistically significantly higher in SMK compared to the other two groups on both days (Table 5).
Table 5. Physiological assessments of biological effect.
| LS meansa |
Cohort comparison p values |
||||||
|---|---|---|---|---|---|---|---|
| Assessment | Age (Years) | SMK | MSC | NTC | SMK versus MSC | SMK versus NTC | MSC versus NTC |
| Day 1 | |||||||
| FMD (%) | All ages | 8.59 | 6.57 | 8.64 | 0.4134 | 1.0000 | 0.3895 |
| ABI | All ages | 1.12 | 1.14 | 1.15 | 0.3252 | 0.0056 | 0.5830 |
| ECO (ppm) | All ages | 34.32 | 1.63 | 1.67 | <0.0001 | <0.0001 | 1.0000 |
| Day 2 | |||||||
| FMD (%) | All ages | 10.19 | 9.97 | 8.26 | 1.0000 | 0.5935 | 0.8773 |
| ABI | All ages | 1.16 | 1.15 | 1.17 | 1.0000 | 1.0000 | 0.5853 |
| ECO (ppm) | All ages | 12.87 | 2.12 | 1.75 | <0.0001 | <0.0001 | 1.0000 |
| CIMT (mm)b | 26–31 | 0.56 | 0.58 | 0.56 | 1.0000 | 1.0000 | 1.0000 |
| 32–37 | 0.61 | 0.63 | 0.61 | 1.0000 | 1.0000 | 1.0000 | |
| 38–43 | 0.65 | 0.66 | 0.63 | 1.0000 | 1.0000 | 0.6399 | |
| 44–49 | 0.73 | 0.63 | 0.69 | 0.0174 | 0.3822 | 0.3501 | |
| All ages | 0.64 | 0.63 | 0.62 | 1.0000 | 0.6381 | 1.0000 | |
aLeast square means. bAge main effect (p < 0.05).
Discussion
The primary purpose of this study was to identify CVD BoBE that differ among SMK, MSC and NTC. Several BoBE, most with previously identified roles in CVD disease pathogenesis, were measured and compared among three groups. In addition, three physiological BoBE were assessed.
The cell adhesion molecules sICAM-1 and VCAM-1 have been reported to be elevated in smokers compared to NCT in some reports (Winkelmann et al., 2001) but not others (Takeuchi et al., 2002). In this study, sICAM-1 was elevated in SMK compared to both MSC (age group 32–37 years) and NTC (age groups 26–31, 32–37 and 38–43 years); VCAM-1 was increased in SMK compared to NTC. Combined with the observation that both MCP-1 and IL-8 were elevated in both SMK and MSC compared to NTC, these results suggest that a tobacco exposure-related response at the endothelial level is potentially increasing the recruitment and migration of blood leukocytes into the intimal region of the artery. Similar observations have been made in in vitro studies with regards to elevated MCP-1 and IL-8 (Giunzioni et al., 2014). Results of the physiological endpoint measurements in the three groups only indicated a significant decrease in ABI on Day 1 in SMK compared to NTC. No other major differences at the vascular endothelium were observed.
Based on the literature, fibrinogen was expected to be elevated in smokers compared with NCT (Bazzano et al., 2003; Frost-Pineda et al., 2011), yet similar between smokeless tobacco consumers and NCT (Eliasson et al., 1995; Huhtasaari et al., 1992). However, in this study, no significant differences in serum fibrinogen levels were observed. This may be due to the small sample size and/or to the fact that all participants were generally healthy.
Several biomarkers associated with CVD or previously shown to be affected by smoking (IL-6, IL-1β, INF-γ, MMP-2 and MMP-9) (de Maat and Kluft, 2002; Unverdorben et al., 2009), and TNFα (Petrescu et al., 2010) were present at levels below the LOD or were detectable in only a small percentage of serum samples. It is unclear why these biomarkers were not detectable in this study, although differences in study design (e.g. inclusion criteria) may be a factor.
Endothelial dysfunction was further estimated by measuring serum vWF and, more rigorously, by measuring FMD and ABI. Several studies have identified vWF as a biomarker of endothelial dysfunction (Blann et al., 1998; Mannucci, 1998). In this study, SMK had significant elevations in vWF compared to NTC, suggesting increased endothelial dysfunction in SMK. Frost-Pineda et al. (2011) reported similar findings. TBX2, an inactive metabolite of thromboxane A2, has been shown to be involved in platelet activation and aggregation and was elevated in SMK compared to MSC and NTC in this study, suggesting potential additional endothelial effects. Statistically significant increases in the excretion rates of TXB2 in smokers compared to NCT have previously been reported (Barrow et al., 1989). However, these physiological measures and platelet count (no measure of platelet activation) do not support any measureable effects in endothelial dysfunction. The oldest age group in this study consisted of males between 44 and 49 years, and it is unclear whether we would have observed additional differences in participants >49 years of age. Typically, physiological measures of endothelial function and artery abnormalities are not performed on younger individuals, as little prognostic value is discernible.
Results from the current analysis indicated no significant differences in FMD among SMK, MSC and NTC both following “challenge” (Day 1) as well as following overnight tobacco abstention (Day 2). As noted, FMD mean results on Day 1 were 8.6, 6.6 and 8.6% for SMK, MSC and NTC, respectively. On Day 2, FMD mean results were 10.2, 10.0 and 8.3% for SMK, MSC and NTC, respectively. These values are within the normal range of FMD values reported in the literature. As reported in previously published studies, mean baseline FMD values for NTC ranged between 6.5 and 16.1% and between 1.4 and 12.3% for SMK (Celermajer et al., 1993; Esen et al., 2004; Heffernan et al., 2010; Hidaka et al., 2010; Karatzi et al., 2007a; Neunteufl et al., 2002; Ozaki et al., 2010; Poredos et al., 1999; Siasos et al., 2008, 2009; Thorne et al., 1998; Wiesmann et al., 2004; Yoshida et al., 2010; Yufu et al., 2007, 2009). Based on two available studies, mean baseline FMD values in smokeless tobacco consumers were reported to be 3.4–4.1% (Granberry et al., 2003; Rohani & Agewall, 2004). Different from the this study’s findings, previous study results have typically indicated significantly lower mean baseline FMD values in smokers compared with NCT (Celermajer et al., 1993; Heffernan et al., 2010; Hidaka et al., 2010; Esen et al., 2004; Ozaki et al., 2010; Poredos et al., 1999; Thorne et al., 1998; Wiesmann et al., 2004; Yufu et al., 2007, 2009) and in MSC compared with NCT (Granberry et al., 2003). Results from Karatzi et al. (2007a) indicated no difference in FMD between smokers and NCT. Celermajer et al. (1993) reported a dose-dependent decrease in FMD with increasing pack-years of cigarette smoking. In experimental studies, significant declines in FMD in smokers and non-tobacco consumers following cigarette smoking (Ciftci et al., 2009; Esen et al., 2004; Karatzi et al., 2007a,b; Neunteufl et al., 2002; Papamichael et al., 2004; Siasos et al., 2008, 2009) and in MSC following oral snuff consumption (Rohani & Agewall, 2004) have been reported. One study reported no significant change in FMD after smoking (Poredos et al., 1999). Noting differences in the study designs of these previous publications in comparison to the present findings may help to explain differences in the observed FMD results. Study design differences included the evaluation of non-US populations (e.g. Japanese, Greek, Turkish), differing sample sizes, differing age groups, the inclusion of both females and males, and potentially poor exposure classification (i.e. non-exclusive tobacco consumer groups). Additionally, differences between studies in the timing of FMD measurements relative to exposure should be considered. However, taking this into consideration, FMD values at baseline for tobacco consumers were higher than expected.
Total cholesterol and triglycerides have generally been reported in the scientific literature as elevated in smokers relative to NCT (Craig et al., 1989; Frost-Pineda et al., 2011; Unverdorben et al., 2009); however, several publications have reported no significant differences between these groups (Calapai et al., 2009a; Eliasson et al., 1995; Lowe et al., 2009). A few reports have measured lipid levels in MSC (Bolinder et al., 1994; Eliasson et al., 1991, 1995; Norberg et al., 2006; Siegel et al., 1992; Tucker, 1989). Contrary to some previous reports (Craig et al., 1989; Frost-Pineda et al., 2011; Unverdorben et al., 2009), statistically significant differences in total cholesterol or in the Apo B containing lipoproteins or triglycerides was not observed between groups. Lack of differences may be explained by the inclusion criteria used for participation in this study, as all individuals were generally healthy and free of clinically significant health problems. However, differences were observed in the apolipoproteins A-1 and A-2. SMK had decreased levels of Apo A-1 compared to both MSC and NTC. For Apo A-2I, SMK levels were significantly lower than NTC. These observations are consistent with previous reports (Craig et al., 1989; Frost-Pineda et al., 2011; Sanderson et al., 1995), which generally show a decrease in apolipoproteins A-1 and A-2.
Several of the hematology biomarkers relating to the transport of oxygen were elevated in SMK compared to the two non-smoking groups. This is consistent with red blood cells (RBC) compensating for the presence of carbon monoxide generated during pyrolysis of the cigarette. The absolute number of RBCs was similar in all three groups; however, the volume of the RBCs in SMK was increased possibly to compensate for an increased amount of hemoglobin necessary to transport adequate levels of oxygen or due to oxidative stress. WBC alterations were expected, as this has been reported by other investigators for smokers compared to NCT (Calapai et al., 2009b; Frost-Pineda et al., 2011; Sanderson et al., 1995) and for smokers who have reduced cigarette consumption (O’Callaghan et al., 2005). Statistically significant increases in WBC (SMK > MSC > NTC) were observed in this study, and most of the WBC subtypes were elevated in SMK compared to NTC. Neutrophils were primarily responsible for the elevation of WBC in the MSC compared to NTC.
Isoprostanes are prostaglandin-like compounds produced in vivo by non-enzymatic free radical-induced peroxidation of arachidonic acid (Morrow et al., 1990). iPF2α-III has been reported to be elevated in healthy SMK as compared to non-smokers (Reilly et al., 1996). Factors such as diet, alcohol and exercise can affect isoprostane levels (Rokach et al., 1997); therefore, isoprostanes are not tobacco-specific. However, the elevation of iPF2α-III and iPF2α-VI is suggestive of increased peroxidation in SMK compared to the non-smoking groups. Interestingly, MSC isoprostane levels were similar to NTC, indicating that the increased peroxidation of arachidonic acid is specific for combustible tobacco products.
This is the first study that has attempted to differentiate dissimilar tobacco-use groups based on CVD BoBE. Using a stepwise elimination procedure to remove biomarkers that contributed minimally to a principal component analysis, we identified three biomarkers that provided the best differentiation between groups: IL-12(p70), sICAM-1 and IL-8. IL-12(p70) has been shown to stimulate both the innate and adaptive immune system (Gee et al., 2009). Elevation of sICAM-1 has been implicated in both leukocyte adhesion and migration (Haverslag et al., 2008). More recently, the role of sICAM-1 in signal transduction, resulting in the recruitment of inflammatory cells to sites of inflammation/injury, has been described (Liu et al., 2012). IL-8 has been shown to be a major mediator involved in the inflammatory response (Apostolakis et al., 2009). IL-8 functions primarily as a chemokine responsible for the recruitment of neutrophils to sites of inflammation. This is consistent with the levels of IL-8 and neutrophils reported in this study.
The biological functions associated with IL-12(p70), sICAM-1 and IL-8 are associated well with physiological responses described by both smoking and CVD. Smoking is known to cause a chronic inflammatory response in the lungs resulting in local and systemic measures of inflammation (Arnson et al., 2010). The state of systemic inflammation in the MSC appears to be attenuated compared to SMK based on the inflammation markers observed in this study. Several reports have demonstrated that risk of CVD in consumers of moist snuff is minimal (Eliasson et al., 1991; Huhtasaari et al., 1992; Siegel et al., 1992). However, the American Heart Association has stated that there is evidence that long-term smokeless tobacco consumption may be associated with increased risk of cardiovascular mortality, specifically myocardial infarction and stroke, although the risk is lower than for cigarette smoking (Piano et al., 2010).
The PCA analysis clearly identifies a separation between SMK and both MSC and NTC suggesting that IL-12(p70), sICAM-1 and IL-8, representing inflammation and immunity, are elevated in SMK compared to the other two groups. Further research assessing long-term cessation or migration may identify IL-12(p70), sICAM-1 and IL-8 as screening metrics to assess CVD risk and for monitoring smoking abstinence compliance.
Subjects included this study were healthy males ranging in ages from 26 to 49 years and excluded female for two reasons. First, the potential female recruitment pool for the MSC group was low. Second, complications in data interpretation related to between-gender and within-gender differences (especially during menstrual cycle) in biomarkers of inflammation such as the ILs and C-reactive protein (Jilma et al., 1997). Additional studies designed specifically between-gender and within-gender differences are recommended but may be difficult to conduct due to the low recruitment pool for female MSC.
Conclusions
While this study focused on “traditional” CVD BoBE, ongoing investigations using transcriptomics and metabolomics should assist in identifying novel BoBE related to smoking and CVD. Ideally, a systems biology approach will provide the greatest likelihood that novel BoBE will be identified. However, a true systems biology approach is currently impractical for several obvious reasons, such as clinical study expenses, executional logistics. Employing practical and currently available technology, this study has identified several BoBE that are dissimilar between consumers of combustible and non-combustible tobacco products and NCT. Additionally, these study results provide evidence that for the biomarkers measured, the risk profile of MSC is skewed towards that of NTC, with several biomarkers overlapping. Continued biomarker research will enhance our understanding of the pathogenesis of disease and assist in monitoring the regression of tobacco-related diseases, like CVD, as smokers migrate to smokeless or other novel non-combustible tobacco products.
Acknowledgements
The authors would like to express gratitude to Drs Paul Harp, Serban Moldoveanu and Thomas Griggs for their critical review of this article. We would also like to thank David Heavner, Thomas Steichen and Celerion for their participation in and contributions to this study.
Declaration of interest
All authors are current or former employees of R.J. Reynolds Tobacco Company and RAI Services Company and declare no competing interest.
References
- Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res. 2009;84:353–60. doi: 10.1093/cvr/cvp241. [DOI] [PubMed] [Google Scholar]
- Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Barrow SE, Ward PS, Sleightholm MA, et al. Cigarette smoking: profiles of thromboxane- and prostacyclin-derived products in human urine. Biochim Biophys Acta. 1989;993:121–7. doi: 10.1016/0304-4165(89)90151-7. [DOI] [PubMed] [Google Scholar]
- Bazzano LA, He J, Muntner P, et al. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138:891–7. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- Blann A, Bignell A, McCollum C. von Willebrand factor, fibrinogen and other plasma proteins as determinants of plasma viscosity. Atherosclerosis. 1998;139:317–22. doi: 10.1016/s0021-9150(98)00090-2. [DOI] [PubMed] [Google Scholar]
- Bolinder G, Alfredsson L, Englund A, de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am J Public Health. 1994;84:399–404. doi: 10.2105/ajph.84.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LR, Brown BG, Jones BA, Marano KM, Borgerding MF. Study of cardiovascular disease biomarkers among tobacco consumers, Part 1: Biomarkers of exposure. Inhal Toxicol. 2015 doi: 10.3109/08958378.2015.1013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G, Caputi AP, Mannucci C, et al. A cross-sectional investigation of biomarkers of risk after a decade of smoking. Inhal Toxicol. 2009a;21:1138–43. doi: 10.3109/08958370902798455. [DOI] [PubMed] [Google Scholar]
- Calapai G, Caputi AP, Mannucci C, et al. Cardiovascular biomarkers in groups of established smokers after a decade of smoking. Basic Clin Pharmacol Toxicol. 2009b;104:322–8. doi: 10.1111/j.1742-7843.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–55. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention. 2011 http://www.cdc.gov/nchs/nhanes/about_nhanes.htm Available from: [Last accessed: 12 Oct 2012]
- Ciftci O, Gullu H, Caliskan M, et al. Mentholated cigarette smoking and brachial artery, carotid artery, and aortic vascular function. Turk Kardiyol Dern Ars. 2009;37:234–40. [PubMed] [Google Scholar]
- Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ. 1989;298:784–8. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maat MP, Kluft C. The association between inflammation markers, coronary artery disease and smoking. Vascul Pharmacol. 2002;39:137–9. doi: 10.1016/s1537-1891(02)00301-4. [DOI] [PubMed] [Google Scholar]
- Eliasson M, Asplund K, Evrin PE, Lundblad D. Relationship of cigarette smoking and snuff dipping to plasma fibrinogen, fibrinolytic variables and serum insulin. The Northern Sweden MONICA Study. Atherosclerosis. 1995;113:41–53. doi: 10.1016/0021-9150(94)05425-i. [DOI] [PubMed] [Google Scholar]
- Eliasson M, Lundblad D, Hagg E. Cardiovascular risk factors in young snuff-users and cigarette smokers. J Intern Med. 1991;230:17–22. doi: 10.1111/j.1365-2796.1991.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Esen AM, Barutcu I, Acar M, et al. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J. 2004;68:1123–6. doi: 10.1253/circj.68.1123. [DOI] [PubMed] [Google Scholar]
- Frost-Pineda K, Liang Q, Liu J, et al. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob Res. 2011;13:182–93. doi: 10.1093/ntr/ntq235. [DOI] [PubMed] [Google Scholar]
- Gee K, Guzzo C, Che Mat NF, et al. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- Giunzioni I, Bonomo A, Bishop E, et al. Cigarette smoke condensate affects monocyte interaction with endothelium. Atherosclerosis. 2014;234:383–90. doi: 10.1016/j.atherosclerosis.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Granberry MC, Smith ES, III, Troillett RD, Eidt JF. Forearm endothelial response in smokeless tobacco users compared with cigarette smokers and nonusers of tobacco. Pharmacotherapy. 2003;23:974–8. doi: 10.1592/phco.23.8.974.32871. [DOI] [PubMed] [Google Scholar]
- Haverslag R, Pasterkamp G, Hoefer IE. Targeting adhesion molecules in cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets. 2008;8:252–60. doi: 10.2174/187152908786786188. [DOI] [PubMed] [Google Scholar]
- Heffernan KS, Karas RH, Patvardhan EA, Kuvin JT. Endothelium-dependent vasodilation is associated with exercise capacity in smokers and non-smokers. Vasc Med. 2010;15:119–25. doi: 10.1177/1358863X09358750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergens MP, Ahlbom A, Andersson T, Pershagen G. Swedish moist snuff and myocardial infarction among men. Epidemiology. 2005;16:12–6. doi: 10.1097/01.ede.0000147108.92895.ba. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Hata T, Soga J, et al. Increased leukocyte rho kinase (ROCK) activity and endothelial dysfunction in cigarette smokers. Hypertens Res. 2010;33:354–9. doi: 10.1038/hr.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtasaari F, Asplund K, Lundberg V, et al. Tobacco and myocardial infarction: is snuff less dangerous than cigarettes? BMJ. 1992;305:1252–6. doi: 10.1136/bmj.305.6864.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilma B, Dirnberger E, Löscher I, et al. Menstrual cycle-associated changes in blood levels of interleukin-6, alpha1 acid glycoprotein, and C-reactive protein. J Lab Clin Med. 1997;130:69–75. doi: 10.1016/s0022-2143(97)90060-3. [DOI] [PubMed] [Google Scholar]
- Kannel WB, D’Agostino RB, Belanger AJ. Fibrinogen, cigarette smoking, and risk of cardiovascular disease: insights from the Framingham Study. Am Heart J. 1987;113:1006–10. doi: 10.1016/0002-8703(87)90063-9. [DOI] [PubMed] [Google Scholar]
- Karatzi K, Papamichael C, Karatzis E, et al. Acute smoke-induced endothelial dysfunction is more prolonged in smokers than in non-smokers. Int J Cardiol. 2007a;120:404–6. doi: 10.1016/j.ijcard.2006.07.200. [DOI] [PubMed] [Google Scholar]
- Karatzi K, Papamichael C, Karatzis E, et al. Acute smoking induces endothelial dysfunction in healthy smokers. Is this reversible by red wine’s antioxidant constituents? J Am Coll Nutr. 2007b;26:10–5. doi: 10.1080/07315724.2007.10719580. [DOI] [PubMed] [Google Scholar]
- Levitzky YS, Guo CY, Rong J, et al. Relation of smoking status to a panel of inflammatory markers: the framingham offspring. Atherosclerosis. 2008;201:217–24. doi: 10.1016/j.atherosclerosis.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Place AT, Chen Z, et al. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood. 2012;120:1942–52. doi: 10.1182/blood-2011-12-397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe FJ, Gregg EO, McEwan M. Evaluation of biomarkers of exposure and potential harm in smokers, former smokers and never-smokers. Clin Chem Lab Med. 2009;47:311–20. doi: 10.1515/CCLM.2009.069. [DOI] [PubMed] [Google Scholar]
- Mannucci PM. von Willebrand factor: a marker of endothelial damage? Arterioscler Thromb Vasc Biol. 1998;18:1359–62. doi: 10.1161/01.atv.18.9.1359. [DOI] [PubMed] [Google Scholar]
- Marano KM, Kathman SJ, Jones BA, Nordskog BK, Brown BG, Borgerding MF. Study of cardiovascular disease biomarkers among tobacco consumers, Part 3: Evaluation and comparison with the US National Health and Nutrition Examination Survey. Inhal Toxicol. 2015 doi: 10.3109/08958378.2015.1009196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunteufl T, Heher S, Kostner K, et al. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J Am Coll Cardiol. 2002;39:251–6. doi: 10.1016/s0735-1097(01)01732-6. [DOI] [PubMed] [Google Scholar]
- Nguyen QM, Srinivasan SR, Xu JH, et al. Distribution and cardiovascular risk correlates of plasma soluble intercellular adhesion molecule-1 levels in asymptomatic young adults from a biracial community: the Bogalusa Heart Study. Ann Epidemiol. 2010;20:53–9. doi: 10.1016/j.annepidem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Norberg M, Stenlund H, Lindahl B, et al. Contribution of Swedish moist snuff to the metabolic syndrome: a wolf in sheep’s clothing? Scand J Public Health. 2006;34:576–83. doi: 10.1080/14034940600665143. [DOI] [PubMed] [Google Scholar]
- O’Callaghan PA, Fitzgerald A, Fogarty J, et al. New and old cardiovascular risk factors: C-reactive protein, homocysteine, cysteine and von Willebrand factor increase risk, especially in smokers. Eur J Cardiovasc Prev Rehabil. 2005;12:542–7. doi: 10.1097/00149831-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Hori T, Ishibashi T, et al. Effects of chronic cigarette smoking on endothelial function in young men. J Cardiol. 2010;56:307–13. doi: 10.1016/j.jjcc.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Papamichael CM, Aznaouridis KA, Stamatelopoulos KS, et al. Endothelial dysfunction and type of cigarette smoked: the impact of ‘light’ versus regular cigarette smoking. Vasc Med. 2004;9:103–5. doi: 10.1191/1358863x04vm529oa. [DOI] [PubMed] [Google Scholar]
- Petrescu F, Voican SC, Silosi I. Tumor necrosis factor-alpha serum levels in healthy smokers and nonsmokers. Int J Chron Obstruct Pulmon Dis. 2010;5:217–22. doi: 10.2147/copd.s8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MR, Benowitz NL, Fitzgerald GA, et al. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122:1520–44. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- Poredos P, Orehek M, Tratnik E. Smoking is associated with dose-related increase of intima-media thickness and endothelial dysfunction. Angiology. 1999;50:201–8. doi: 10.1177/000331979905000304. [DOI] [PubMed] [Google Scholar]
- Redberg RF, Vogel RA, Criqui MH, et al. 34th Bethesda Conference: task force #3–what is the spectrum of current and emerging techniques for the noninvasive measurement of atherosclerosis? J Am Coll Cardiol. 2003;41:1886–98. doi: 10.1016/s0735-1097(03)00360-7. [DOI] [PubMed] [Google Scholar]
- Reilly M, Delanty N, Lawson JA, Fitzgerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- Roethig HJ, Feng S, Liang Q, et al. A 12-month, randomized, controlled study to evaluate exposure and cardiovascular risk factors in adult smokers switching from conventional cigarettes to a second-generation electrically heated cigarette smoking system. J Clin Pharmacol. 2008;48:580–91. doi: 10.1177/0091270008315316. [DOI] [PubMed] [Google Scholar]
- Roethig HJ, Koval T, Muhammad-Kah R, et al. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regul Toxicol Pharmacol. 2010;57:333–7. doi: 10.1016/j.yrtph.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Rohani M, Agewall S. Oral snuff impairs endothelial function in healthy snuff users. J Intern Med. 2004;255:379–83. doi: 10.1046/j.1365-2796.2003.01279.x. [DOI] [PubMed] [Google Scholar]
- Rokach J, Khanapure SP, Hwang SW, et al. The isoprostanes: a perspective. Prostaglandins. 1997;54:823–51. doi: 10.1016/s0090-6980(97)00183-4. [DOI] [PubMed] [Google Scholar]
- Rush University Medical Center . Rush Medical Laboratory user’s guide. Chicago, IL: Rush University Medical Center; 2011. http://rml.rush.edu/webapps/rml/RMLRanges.jsp [Google Scholar]
- Sanderson KJ, van Rij AM, Wade CR, Sutherland WH. Lipid peroxidation of circulating low density lipoproteins with age, smoking and in peripheral vascular disease. Atherosclerosis. 1995;118:45–51. doi: 10.1016/0021-9150(95)05591-j. [DOI] [PubMed] [Google Scholar]
- Siasos G, Tousoulis D, Vlachopoulos C, et al. Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int J Cardiol. 2008;126:394–9. doi: 10.1016/j.ijcard.2007.04.057. [DOI] [PubMed] [Google Scholar]
- Siasos G, Tousoulis D, Vlachopoulos C, et al. The impact of oral L-arginine supplementation on acute smoking-induced endothelial injury and arterial performance. Am J Hypertens. 2009;22:586–92. doi: 10.1038/ajh.2009.57. [DOI] [PubMed] [Google Scholar]
- Siegel D, Benowitz N, Ernster VL, et al. Smokeless tobacco, cardiovascular risk factors, and nicotine and cotinine levels in professional baseball players. Am J Public Health. 1992;82:417–21. doi: 10.2105/ajph.82.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Jr, Hirsch AT, Haskal ZJ, et al. ACC/AHA Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2005;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Kawamura T, Kanai A, et al. The effect of cigarette smoking on soluble adhesion molecules in middle-aged patients with type 2 diabetes mellitus. Diabet Med. 2002;19:57–64. doi: 10.1046/j.1464-5491.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- Thorne S, Mullen MJ, Clarkson P, et al. Early endothelial dysfunction in adults at risk from atherosclerosis: different responses to L-arginine. J Am Coll Cardiol. 1998;32:110–6. doi: 10.1016/s0735-1097(98)00211-3. [DOI] [PubMed] [Google Scholar]
- Tucker LA. Use of smokeless tobacco, cigarette smoking, and hypercholesterolemia. Am J Public Health. 1989;79:1048–50. doi: 10.2105/ajph.79.8.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverdorben M, von Holt K, Winkelmann BR. Smoking and atherosclerotic cardiovascular disease: part II: role of cigarette smoking in cardiovascular disease development. Biomark Med. 2009;3:617–53. doi: 10.2217/bmm.09.51. [DOI] [PubMed] [Google Scholar]
- Wiesmann F, Petersen SE, Leeson PM, et al. Global impairment of brachial, carotid, and aortic vascular function in young smokers: direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2056–64. doi: 10.1016/j.jacc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Winkelmann BR, Boehm BO, Nauck M, et al. Cigarette smoking is independently associated with markers of endothelial dysfunction and hyperinsulinaemia in nondiabetic individuals with coronary artery disease. Curr Med Res Opin. 2001;17:132–41. [PubMed] [Google Scholar]
- Yarnell JW, Sweetnam PM, Rumley A, Lowe GD. Lifestyle and hemostatic risk factors for ischemic heart disease: the Caerphilly Study. Arterioscler Thromb Vasc Biol. 2000;20:271–9. doi: 10.1161/01.atv.20.1.271. [DOI] [PubMed] [Google Scholar]
- Yoshida O, Kondo T, Kureishi-Bando Y, et al. Pitavastatin, an HMG-CoA reductase inhibitor, ameliorates endothelial function in chronic smokers. Circ J. 2010;74:195–202. doi: 10.1253/circj.cj-09-0345. [DOI] [PubMed] [Google Scholar]
- Yufu K, Takahashi N, Hara M, et al. Measurement of the brachial-ankle pulse wave velocity and flow-mediated dilatation in young, healthy smokers. Hypertens Res. 2007;30:607–12. doi: 10.1291/hypres.30.607. [DOI] [PubMed] [Google Scholar]
- Yufu K, Takahashi N, Okada N, et al. Influence of systolic blood pressure and cigarette smoking on endothelial function in young healthy people. Circ J. 2009;73:174–8. doi: 10.1253/circj.cj-08-0467. [DOI] [PubMed] [Google Scholar]
- Zeller M, Hatsukami D. Strategic Dialogue on Tobacco Harm Reduction Group The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–32. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]