Abstract
Background:
The large and giant skull base meningiomas are challenging lesions, and the involvement of crucial neurovascular structures needs the surgical removal as the primordial treatment. The authors report on a series of patients with large and giant skull base meningiomas who were treated with the goal of radical removal.
Methods:
A retrospective study including 49 patients with large and giant skull base meningiomas was carried out. Tumors presenting 3 cm or larger were included.
Results:
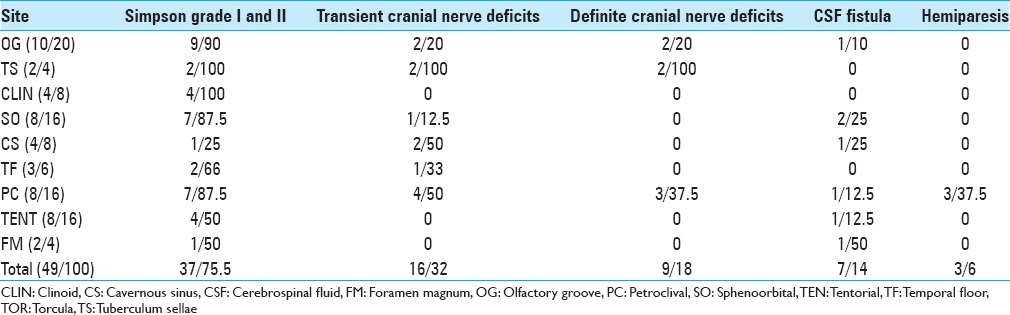
The meningiomas in the sample included the following types: 10 olfactory groove, 8 sphenoorbital, 8 petroclival, 8 tentorial, 4 clinoidal, 4 cavernous sinus, 3 temporal floor, 2 tuberculum sellae and 2 foramen magnum. The average age was 53 years, the mean follow-up period was 52 months, Simpson Grades I and II were obtained in 75.5%. The overall mortality was 5%. Transient cranial nerve deficits occurred in 32% with definite cranial nerve lesion in 18%. Cerebrospinal fluid leak occurred in 14%.
Conclusions:
The surgical treatment is a mandatory option for large and giant skull base meningiomas. The radical removal is achievable and should be considered an alternative with a good outcome and an acceptable morbidity for such challenge lesions.
Keywords: Meningioma, Simpson grade, skull base, surgical treatment
INTRODUCTION
The skull base meningiomas are difficult lesions to treat when insinuated among cranial nerves and vital neurovascular structures. Nowadays, different protocols are applied to deal with such tumors, including simple observation, partial resection, radiosurgery as primary or adjuvant therapy, and aggressive surgical removal. Nevertheless, large and giant meningiomas usually are surgical lesions because of the mass effect and involvement of the brain stem, cranial nerves, dural sinuses or vascular structures. The authors present the experience of a surgery-oriented center in the treatment of such large tumors.
MATERIALS AND METHODS
A retrospective study that included patients operated on between 2004 and 2014 was performed. The inclusion criteria were large and giant meningiomas of the anterior, middle and posterior skull base, operated on by the first author (CES). The meningiomas with 3 cm or larger, measured in one of the three axis, were included. The data related to the surgical interventions were reviewed, and special attention was given to the Simpson grade, previous radiation treatment, and the site of the meningioma. We reviewed the medical records, operative reports, radiological exams and follow-up information of the cases. All patients underwent surgery with the intent of the most extensive radical removal possible including dura and bone invasions.
The patients underwent several skull base approaches according to the site of the meningioma. Olfactory groove (OG) lesions were removed through supraorbital, cranioorbital and supraorbital bifrontal approaches; sphenoorbital meningiomas (SOM) were removed via the cranioorbital zygomatic (COZ) approach; temporal floor (TF) meningiomas were operated on through the zygomatic and COZ approaches; cavernous sinus (CS) lesions were treated via the COZ approach; petroclival (PC) meningiomas were removed through the posterior petrosal approach; and torcular and tentorial (TEN) meningiomas were removed through the suboccipital and transmastoid retrosigmoid approaches.
RESULTS
Between 2004 and 2014, 49 large and giant meningiomas were operated on. The group was composed of the following lesions: 10 OG, 8 SO, 8 PC, 8 TEN, 4 clinoidal, 4 CS, 3 TF, 2 tuberculum sellae and 2 foramen magnum. The group was composed of 38 females and 11 males with an average age of 53 years (range: 31–83). The mean follow-up period was 52 months (range: 06–120 months). Simpson Grades I and II were obtained following 75.5% of the surgeries; 45% Simpson Grade I and 30.5% Simpson Grade II. The meningiomas that had previously been irradiated composed 16% of the cases. The meningiomas were larger than 4 cm in 45%. Subsequent recurrence after aggressive removal (Simpson Grade I) occurred in 4% of the cases within the evaluated period.
The overall mortality was 5%, and such patients presented giant meningiomas (larger than 4 cm). Transient cranial nerve deficits occurred in 32% of the cases, and complete recoveries were observed within 3 months of follow-up. The definite cranial nerve deficit was 18%. Cerebrospinal fluid (CSF) leak occurred in 14%, hemiparesis was 6% and infection was 2%.
Table 1 presents the findings of the series.
Table 1.
Characteristics of the patients with large and giant meningiomas
DISCUSSION
The OG meningiomas were treated through the supraorbital approach, the cranioorbital approach, which is a variation of the COZ approach that includes only the cranioorbital flap, and through the supraorbital bifrontal approach. The approaches were selected according to the extension of the tumors into the surrounding neurovascular structures and lead to a large exposure of the tumors and aggressive removal of the dural and bone involvement. Four cases of the series were recurrent OG meningiomas, with the common feature of bone involvement of the tumors that was not removed during the first surgery in other centers. This feature has also been observed in other series of recurrent OG meningiomas and represents the most relevant finding for the failure of the surgical control of the disease.[15,21] In cases which the dura and hyperostotic bone involvement of the anterior fossa were evidenced in preoperative images, aggressive removal of all disease were performed, and the anterior skull base was reconstructed using pericranial flaps and fascia lata, achieving Simpson Grade 1 in all cases, but one[20] [Figures 1 and 2]. Such patient was a comatose patient with a giant recurrent tumor, previously operated on in another department, and a Simpson Grade III was done, with long postoperative Intensive Care Unit period. In 1 patient who underwent surgery in our department, a giant meningioma was removed with the dural and bone involvement of the anterior fossa, and a small recurrence occurred 6 years after surgery in the most anterior part of the anterior fossa near the falx. We performed a second radical removal of this small recurrence and only the dura was removed because there was no evidence of bone involvement. This case likely represents a regional multicentricity of the meningioma, since the recurrence occurred more anteriorly, and the follow-up of this case was altered to shorter intervals in order to observe any other recurrence.
Figure 1.
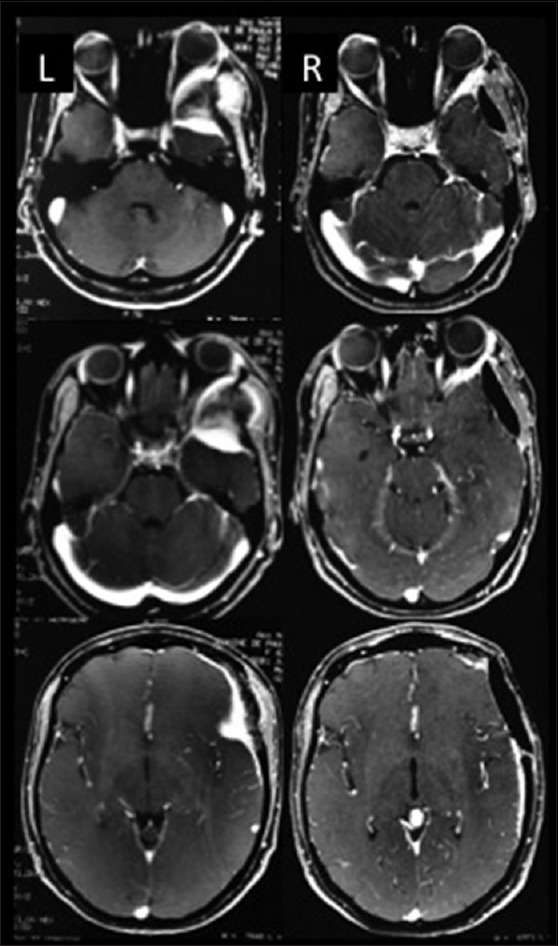
Illustrative case – Left sphenoorbital meningioma. L (left): Preoperative images, R (right): Postoperative images
Figure 2.
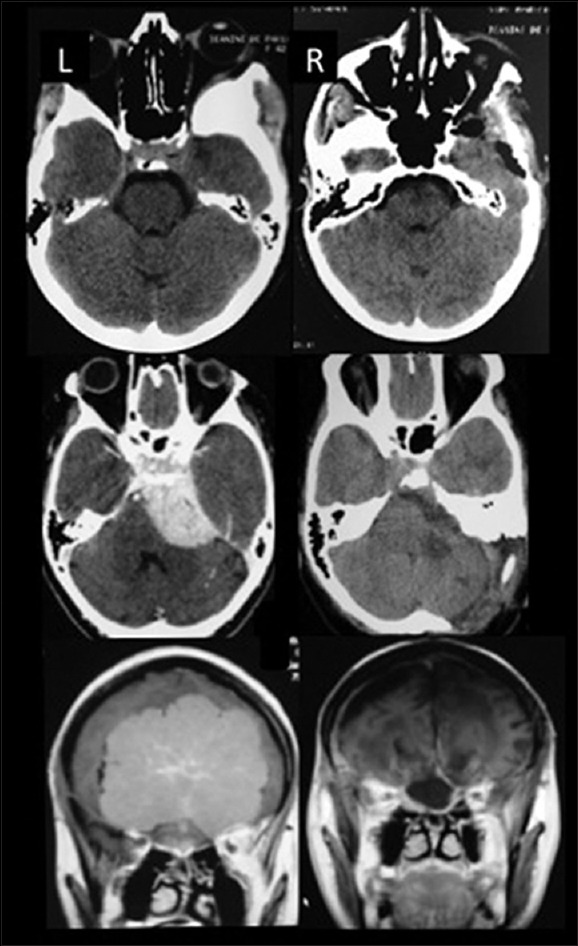
Illustrative cases – Upper: Computed tomography (CT) scan sphenoorbital meningioma, Middle: CT scan petroclival meningioma, Lower: Magnetic resonance imaging olfactory groove meningioma. L (left): Preoperative images, R (right): Postoperative images. Observe the aggressive bone removal in all three cases
Sphenoorbital meningiomas are challenging lesions in terms of radical removal because orbital and CS structures are involved in many cases. Patients with large and giant SOM usually develop exophthalmos, visual disturbances and eye pain, and magnetic resonance imagings (MRIs) and computed tomography reveal bone invasion of the sphenoid wing and orbit.[23,30] The first surgical approach for SOM should include orbital bone removal even of cases of very minor hyperostotic changes because hyperostosis indicates tumor bone invasion and a higher rate of recurrence[21,25,28] [Figures 1 and 2]. In five cases of SOM, Simpson Grade I was achieved, in two cases a Simpson Grade II was possible. Four cases were recurrent tumors, operated on in other departments and three cases received Simpson Grade I resection. In only one case, a giant recurrent SOM operated on 7 times in another centers, a partial decompression was done. Such patient presented CSF leak and infection during the postoperative period. COZ and cranioorbital approaches were utilized in all cases of SOM and included radical resections of the dura and bone involvement. SOM that involve the medial portion of the sphenoid wing represent more challenging lesions due to the involvement of the neurovascular structures, but there is an arachnoidal plane between the neurovascular structures and the medial portion of the sphenoid wing meningioma.[5,30] Using microsurgical techniques via the arachnoidal plane allows for the removal of such meningiomas with low morbidity rates.[5] Two cases presented CSF leak, which required fascia lata reconstruction of the dura mater under the temporal lobe. 1 patient presented transient third nerve palsy with complete recover in 60 days.
The clinoidal meningiomas included were all Al-Mefty's type II. These meningiomas arise from the superior and/or lateral portion of the anterior clinoid, with arachnoidal membrane of the carotid artery between the tumor and arterial adventitia.[5] All cases received Simpson Grade I resection with no morbidity.
In hard, consistent tumors of the CS, radical resection of the dura and bone is impossible to achieve in many cases.[13,26,27] In such tumors, gross total removal (GTR) (Simpson Grade III) is reasonable and is a successful surgical treatment. Considering that the mean age of the group was 53 years old, the life expectancy for the majority of the cases was high. In our opinion, the concerns related to reducing the transoperative risks and maintaining quality of life led to incorrect decisions in terms of the best surgical options for these patients. It is important to consider that radically treated meningiomas can result in high recurrence-free rates at 5 and 20 years of follow-up.[1,17] Despite of CS meningiomas were considered “no man's land” for so many time, during the first surgery they were removed as radically as possible. Two CS meningiomas, with a very hard consistency, underwent GTR (Simpson Grade III). The third CS meningioma was a soft tumor and was removed via peeling of the middle fossa and the lateral wall of the CS, which was considered to be a Simpson Grade II resection. This patient presented with transient cranial nerve III and IV palsies and total recovery in 3 months. A hard tumor, with encasement of the internal carotid artery (ICA), received a debulking and partial removal, in the extracavernous portion (Simpson Grade IV). At our department, ICA preservation is a goal, even if it means leaving a residual tumor adherent to the artery.
Three patients with TF meningiomas were included and two received Simpson Grade I resection. One case was a recurrence of a giant TF tumor with infratemporal fossa invasion that followed a Simpson Grade III removal and conformational radiotherapy in another department. The tumor was totally removed through the zygomatic approach with aggressive bone resection of the TF (Simpson Grade I). Seven years later, the patient presented with a new small recurrence in the lateral wall of the CS and medial portion of the TF, which was removed through the COZ approach and achieved a Simpson Grade III with a transient sixth nerve palsy recovered in 3 months. This case illustrates the importance of the first surgery for the local control of meningiomas. The absence of the arachnoidal plane and associated postirradiation disturbances make radical removal of such meningiomas without definite morbidity impossible. We have closely followed this patient over the last 3 years and are worried about the next recurrence of this irradiated meningioma, which is at risk for malignant progression.[4,9] In the second case, the MRI presented necrosis and a cystic portion invading the temporal lobe, and a Simpson Grade I resection was performed. Histological analysis diagnosed a malignant meningioma and conformational radiotherapy was carried out. Close following with MRI each 6 months in the first 2 years have been done without any recurrence. The third case, a 87-year-old patient with invasive TF and petrous bone meningioma, receives a Simpson Grade III removal without any postoperative complication.
Petroclival meningiomas are the most challenging tumors because of their neurovascular involvements. In the present series, they were the only topography with major motor impairments. Large and giant meningiomas in the PC region mean brain stem and cranial nerves compression. The same concept of the arterial preservation is applied to the basilar artery and perforators in PC lesions. In 3 patients, with giant PC tumors and one previously irradiated, contralateral hemiparesis were observed in the postoperative follow-up. Two giant recurrent PC meningiomas have previously been operated on with partial resections. The rescue surgery achieved a Simpson Grade II, but the previously irradiated case, mentioned above, developed a venous infarction of the antero-lateral portion of the pons and presented with definite hemiparesis and VI and VII nerve palsies. In 1 patient, presenting bilateral six nerves palsy, a complete recover of the palsies were obtained after a Simpson Grade II removal of a soft meningioma. Here, as in the CS topography, the consistency of the tumor is crucial for the success of the surgical removal. In the present series, a more aggressive surgery Simpson Grade II, was possible only in soft tumors. The posterior petrosal approach was fundamental for the aggressive removal obtained in seven of the eight cases included in the present series. Such approach allows to control and exposure from the third to 12 cranial nerves, basilar artery and branches, and the brain stem structures.[7,3,14,25] For large and giant PC meningiomas, when the goal is the complete removal, the posterior petrosal approach, preserving the labyrinthine structures, leads to a wide and safe exposure[7] [Figure 2].
The giant TEN and torcular meningiomas are difficult lesions because of the venous relations of the tumors. In one giant torcular meningioma that had been partially removed and previously irradiated presented with a total occlusion of the torcula (TOR) on magnetic resonance venography. The radical “en bloc” removal of the TOR and TEN involvements led to a massive posterior fossa venous infarct, and patient died in the postoperative period. The TEN meningiomas related to the lower surface of the temporal lobe need to a complete temporal venous drainage study. The venous configuration and preservation is very important to avoid venous infarction in the postoperative period.[25] Simpson Grades I and II were obtained in 50% of the cases.
The Simpson paper highlighted the importance of radical removal during the surgical treatment of meningiomas in the prevention of tumor recurrence and different series observed similar results.[1,16,17,19,29] Nowadays, there is a trend to be less radical during the removal of skull base meningiomas due to concerns related to the quality of life of the patient and the goal of reducing the morbidity of the surgical procedure.[22,23,30] Stereotactic radiosurgery has been used as primary or adjuvant therapy in many cases of skull base meningiomas, and the recurrence of such cases result in lesions that are clearly difficult to manage.[4,9] In skull base meningiomas, such aggressive resection is not possible in many cases due to the surrounding vital neurovascular structures. Despite this, there are numerous series that have considered the application of modern skull base techniques, fluorescent markers, transoperative imaging, and neuronavigational systems that have reported high rates of complete removal of skull base meningiomas and low morbidity rates.[2,3,5,6,7,8,10,11,12]
The correct application of the Simpson concept to skull base meningiomas is relevant. Some authors have described GTR and suggested that such patients are radically treated in terms of the risk of recurrence. In some series, patients with skull base meningiomas included in GTR groups are Simpson Grade III, and their recurrence rates are obviously higher than those of patients for whom true total removal is achieved. In this study, true total removal includes the resection of all dura and bone involvement, a Simpson Grade I, and it was achieved in 45%.
The efforts to achieve the most radical resections possible in the large and giant meningiomas were reinforced by the ages of the patients. Younger patients should be treated while considering that longer follow-up periods are favorable for recurrence in any modality of treatment for meningiomas. The surgical modality should offer the best chance for local control and thus should be a radical removal whenever it is possible. The chance of surgical control of the disease in recurrence is lower, but when there is a possibility of achieving extensive resection with lower morbidity, we also believe that this is the best surgical option.[7,13,14,26,27]
The mean follow-up period of the patients was 52 months. In one OG meningioma, a small recurrence was diagnosed 6 years after a supposed Simpson Grade I resection, and one TF meningioma recurred for the 2nd time 7 years after a radical removal. These findings are consonant with those on the literature, which suggests the importance of long follow-up periods for benign meningiomas and supports the concept that Simpson Grades I and II are related to long periods of local tumor control.[1,16,17] An extended follow-up period is important to evaluate the success rates of all modalities of treatment that are proposed for the management of such tumors. Short follow-ups periods tend to overestimate the treatment effect in the control of the disease.[1,16,17,29]
Currently, we follow patients with benign meningiomas and consider the Ki-67 index, hormonal receptors and molecular cytogenetics to predict their recurrence.[8,18,22,24] In cases in which recurrences are diagnosed, we consider surgical removal as the first treatment option. When surgical removal is not possible, the cytogenetic profile is unfavorable, and growth of the meningioma is documented, we consider radiosurgery. In our department, radiosurgery is avoided as the first option for benign meningiomas and as immediate adjuvant therapy even in cases of less radical surgeries. Depending on the molecular findings and the proliferating index, in favorable cases, such tumors remain stable and with no recurrence over long periods.[1,17] In cases that have undergone radical removal, local control is even better, which justifies the avoidance of submitting patients to irradiation and following the cases over years.[1,7,14,21] In atypical meningiomas (WHO II), if resection achieves Simpson Grade I or II, a close follow-up with MRI each 3 months in the 1st year and each 6 months during the 2nd year is recommended. If recurrence is evidenced, surgical removal followed by radiosurgery is the choice of the department. In malignant meningiomas, as radical resection as possible is followed by radiosurgery.
CONCLUSIONS
Radical removal in large and giant skull base meningiomas is achievable and should be considered an option with good outcomes and acceptable morbidity. The authors recommend extensive dura and bone removal in the surgical treatment of such lesions whenever possible to obtain higher rates of local control. An extended follow-up period, cytogenetic analysis, and proliferating index are crucial to evaluate the local control rates and subsequent therapies.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/1/113/159489
Contributor Information
Carlos Eduardo da Silva, Email: dasilvacebr@yahoo.com.br.
Paulo Eduardo Peixoto de Freitas, Email: pefreitas@terra.com.br.
REFERENCES
- 1.Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51–6. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mefty O, Anand VK. Zygomatic approach to skull-base lesions. J Neurosurg. 1990;73:668–73. doi: 10.3171/jns.1990.73.5.0668. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mefty O, Fox JL, Smith RR. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22:510–7. doi: 10.1227/00006123-198803000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mefty O, Topsakal C, Pravdenkova S, Sawyer JR, Harrison MJ. Radiation-induced meningiomas: Clinical, pathological, cytokinetic, and cytogenetic characteristics. J Neurosurg. 2004;100:1002–13. doi: 10.3171/jns.2004.100.6.1002. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990;73:840–9. doi: 10.3171/jns.1990.73.6.0840. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mefty O. Supraorbital-pterional approach to skull base lesions. Neurosurgery. 1987;21:474–7. doi: 10.1227/00006123-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O. True petroclival meningiomas: Results of surgical management. J Neurosurg. 2014;120:40–51. doi: 10.3171/2013.8.JNS13535. [DOI] [PubMed] [Google Scholar]
- 8.Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.2.FOCUS1116. [DOI] [PubMed] [Google Scholar]
- 9.Couldwell WT, Cole CD, Al-Mefty O. Patterns of skull base meningioma progression after failed radiosurgery. J Neurosurg. 2007;106:30–5. doi: 10.3171/jns.2007.106.1.30. [DOI] [PubMed] [Google Scholar]
- 10.da Silva CE, da Silva JL, da Silva VD. Skull base meningiomas and cranial nerves contrast using sodium fluorescein: A new application of an old tool. J Neurol Surg B. 2014;75:255–60. doi: 10.1055/s-0034-1372466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva CE, da Silva JL, da Silva VD. Use of sodium fluorescein in skull base tumors. Surg Neurol Int. 2010;1:70. doi: 10.4103/2152-7806.72247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva CE, da Silva VD, da Silva JL. Sodium fluorescein in skull base meningiomas: A technical note. Clin Neurol Neurosurg. 2014;120:32–5. doi: 10.1016/j.clineuro.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 13.DeMonte F, Smith HK, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245–51. doi: 10.3171/jns.1994.81.2.0245. [DOI] [PubMed] [Google Scholar]
- 14.Erkmen K, Pravdenkova S, Al-Mefty O. Surgical management of petroclival meningiomas: Factors determining the choice of approach. Neurosurg Focus. 2005;19:E7. doi: 10.3171/foc.2005.19.2.8. [DOI] [PubMed] [Google Scholar]
- 15.Feiz-Erfan I, Han PP, Spetzler RF, Horn EM, Klopfenstein JD, Porter RW, et al. The radical transbasal approach for resection of anterior and midline skull base lesions. J Neurosurg. 2005;103:485–90. doi: 10.3171/jns.2005.103.3.0485. [DOI] [PubMed] [Google Scholar]
- 16.Ildan F, Erman T, Göçer AI, Tuna M, Bagdatoglu H, Cetinalp E, et al. Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: A multivariate analysis in the midterm follow-up. Skull Base. 2007;17:157–71. doi: 10.1055/s-2007-970554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jääskeläinen J. Seemingly complete removal of histologically benign intracranial meningioma: Late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461–9. doi: 10.1016/0090-3019(86)90259-4. [DOI] [PubMed] [Google Scholar]
- 18.Ketter R, Henn W, Niedermayer I, Steilen-Gimbel H, König J, Zang KD, et al. Predictive value of progression-associated chromosomal aberrations for the prognosis of meningiomas: A retrospective study of 198 cases. J Neurosurg. 2001;95:601–7. doi: 10.3171/jns.2001.95.4.0601. [DOI] [PubMed] [Google Scholar]
- 19.Kinjo T, al-Mefty O, Kanaan I. Grade zero removal of supratentorial convexity meningiomas. Neurosurgery. 1993;33:394–9. doi: 10.1227/00006123-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Liu JK, Niazi Z, Couldwell WT. Reconstruction of the skull base after tumor resection: An overview of methods. Neurosurg Focus. 2002;12:e9. doi: 10.3171/foc.2002.12.5.10. [DOI] [PubMed] [Google Scholar]
- 21.Obeid F, Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53:534–42. doi: 10.1227/01.neu.0000079484.19821.4a. [DOI] [PubMed] [Google Scholar]
- 22.Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: Integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012;117:121–8. doi: 10.3171/2012.3.JNS111945. [DOI] [PubMed] [Google Scholar]
- 23.Oya S, Sade B, Lee JH. Sphenoorbital meningioma: Surgical technique and outcome. J Neurosurg. 2011;114:1241–9. doi: 10.3171/2010.10.JNS101128. [DOI] [PubMed] [Google Scholar]
- 24.Ragel BT, Jensen RL. Molecular genetics of meningiomas. Neurosurg Focus. 2005;19:E9. doi: 10.3171/foc.2005.19.5.10. [DOI] [PubMed] [Google Scholar]
- 25.Sakata K, Al-Mefty O, Yamamoto I. Venous consideration in petrosal approach: Microsurgical anatomy of the temporal bridging vein. Neurosurgery. 2000;47:153–60. doi: 10.1097/00006123-200007000-00032. [DOI] [PubMed] [Google Scholar]
- 26.Sekhar LN, Burgess J, Akin O. Anatomical study of the cavernous sinus emphasizing operative approaches and related vascular and neural reconstruction. Neurosurgery. 1987;21:806–16. doi: 10.1227/00006123-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Sekhar LN, Sen CN, Jho HD, Janecka IP. Surgical treatment of intracavernous neoplasms: A four-year experience. Neurosurgery. 1989;24:18–30. doi: 10.1227/00006123-198901000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Silva CE, Freitas PE, Romero AD, Pereyra TM, Fonseca VF, Martins WA, et al. Orbital meningiomas. J Bras Neurocir. 2010;21:31–8. [Google Scholar]
- 29.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sughrue ME, Rutkowski MJ, Chen CJ, Shangari G, Kane AJ, Parsa AT, et al. Modern surgical outcomes following surgery for sphenoid wing meningiomas. J Neurosurg. 2013;119:86–93. doi: 10.3171/2012.12.JNS11539. [DOI] [PubMed] [Google Scholar]


