Abstract
Background
In 2006, bevacizumab, a targeted therapy agent was combined with FOLFIRI for the firstline treatment of patients with unresectable metastatic colorectal cancer.
Methods/Results
A study on a homogenous series of 111 patients from the Brittany and Pays de la Loire areas who received bevacizumab-FOLFIRI as first-line treatment in 2006 showed the following results: 51 responses, 29 stabilisations, 21 progressions and 10 cases of toxicity prior to assessment. Median overall survival (OS) was 25.1 months and median progression-free survival was 10.2 months. Surgery secondary to treatment tripled median OS which reached 59.2 months in resected patients versus 18.8 months in unresected patients. Comparison of patients aged more or less than 70 years showed no differences in terms of benefits or risks.
Conclusion
Bevacizumab-FOLFIRI could be administered as part of a routine care protocol to elderly patients previously evaluated by a geriatric assessment and validated by a multidisciplinary staff.
Keywords: Elderly patients, Bevacizumab, Metastatic colorectal cancer, Cohort, Daily practice, Liver surgical resection
Résumé
En 2006, bevacizumab-FOLFIRI représente la thérapie ciblée administrable dès la première ligne chez les patients porteurs d’un cancer colorectal métastatique non opérable. Une série homogène de 111 patients colligés en région Bretagne et Pays de la Loire ayant reçu du bevacizumab- FOLFIRI en première ligne en 2006 révèle les résultats suivants: 51 réponses, 29 stabilités, 21 progressions et 10 toxicités avant évaluation. La médiane de survie globale (OS) est de 25,1 mois et la médiane de survie sans progression (PFS) de 10,2 mois. Dans le cas d’une chirurgie secondaire, l’OS médian triple de 18,8 mois chez les patients non réséqués versus 59,2 mois ceux réséqués. En comparant les sujets âgés de plus et de moins de 70 ans, aucune différence n’a été mise en évidence en termes de bénéfice ou de risque. Bevacizumab-FOLFIRI pourrait être administré en pratique courante chez les personnes âgées sous couvert d’une évaluation gériatrique et d’une approche multidisciplinaire.
Mots clés: Sujet âgé, Bevacizumab, Cancer colorectal métastatique, Cohorte, Pratique courante, Résection hépatique
Introduction
Management of patients with metastatic colorectal cancer (mCRC) is evolving continuously. Use of conventional chemotherapy and targeted agent combinations has become the standard treatment and option of choice for these patients. Treatment options are essentially based on multicenter, international, phase III studies whose main objectives include progression-free survival (PFS) and even overall survival (OS) [1–7]. Bevacizumab, cetuximab and, more recently, panitumumab were successively studied for this purpose. A regimen combining bevacizumab with irinotecan-based chemotherapy is more effective than a protocol without bevacizumab [4], which led to the granting of the first label for bevacizumab in 2005. However, the combination tested was IFL (Irinotecan Fluorouracil Leucovorin) protocol, which was found to be dramatically toxic [7–9]. Given a lack of data on the FOLFOX protocol, the French teams resorted to use the FOLFIRI protocol, reputed less toxic than the IFL protocol. As the American teams had access to the results of the Goldberg study demonstrating the superiority of the FOLFOX protocol compared with the IFL protocol, they started combining bevacizumab with FOLFOX right away [7,10–12]. The German teams use the weekly AIO regimen (irinotecan 80 mg/m2, leucovorin 500 mg/m2, followed by 5-FU 2600 mg/m2 administered continuously for a period of 22 hours), but the results of this regimen have not been compared with those of the bevacizumab—FOLFIRI combination.
The inclusion criteria in prospective studies exclude generally elderly patients more than 75 years old. However, large numbers of elderly patients are treated on a daily practice with targeted agent therapy. It therefore became necessary to obtain information on patients receiving routine care and not included in clinical trials. The available cohort studies predominantly included North-American patients. In the routine care series, the reference chemotherapy in the United States was found to be FOLFOX and XELOX (capecitabine and oxaliplatin) or capecitabine administered as monotherapy rather than FOLFIRI [11,12].
Studies published on patients falling in the oncogeriatrics category and receiving bevacizumab treatment generally concern patients over age of 65 years [13–15]. Seventyfive years and above seems to be a better age for oncogeriatric considerations. Studies of patients over 75 years and even over 80 years are recently published [16]. Moreover, the importance of studying response rates, PFS and OS in routine care cohorts is now being associated with assessment of side effects and, particularly, the safety monitoring of the combinations (pharmacovigilance review). Given that management has become global, it seems important to determine how many patients underwent liver metastasis resection secondary to treatment. However, in most cases, this mainly consists in collecting information on the healthcare centres of the patient, including any potential medical-surgical management and multiple-line therapy instituted (successive chemotherapy lines used following disease progression depending on the patient’s general condition).
Many studies and the options available following firstline treatment have made cohort studies necessary. Cohort studies by no means replace phase III studies, but allow confirmation of response rates, of PFS and of OS with full knowledge of the treatments administered. Furthermore, they provide pharmacovigilance information about the products and also allow post-label monitoring of the products in routine clinical practice.
In the framework of its principal missions, the Observatory of Cancer of the Brittany and Pays de Loire areas (Observatoire dédié au Cancer des Régions Bretagne Pays de la Loire — France) decided to study these treatment situations and to constitute the most exhaustive cohorts possible. The Centre was first created in 2003 through the common wills of the Brittany and Pays de Loire Area Hospitalization Agencies (ARH), two physicians and one pharmacist. The initiative was rapidly accepted and supported by the Regional Anti-Cancer Networks of both regions and by physicians and pharmacists of public hospitals (University Hospitals [CHU], General Hospitals [CHG] and Cancer Centers [CRLCC]) and private hospitals in the Grand-Ouest area. When Regional Health Agencies (ARS) were created, the structure was validated and linked to the Observatory of Drugs, Medical Devices and Therapeutic Innovation (OMEDIT) of each region while remaining an inter-regional structure. As soon as bevacizumab was labelled for mCRC, a cohort named AVASTIN OUEST was initiated. The concept whereby subgroups of patients would be studied such as those falling into the oncogeriatrics category and those eligible for resection secondary to treatment was already topics to be studied.
Materials and methods
Patients
AVASTIN OUEST is a multicentric, retrospective observational study. All patients over the age of 18 years having received bevacizumab—FOLFIRI in one of the two regions for the first-line treatment of unresectable mCRC outside of a study in 2006 were included in the cohort. Data collected from medical records of patients included date of diagnosis of cancer, date of diagnosis of metastases, age, gender, potential comorbidities, use or not of adjuvant chemotherapy for locally advanced disease, initial unresectability of metastases, type of conventional chemotherapy regimen combined with bevacizumab, presence of grade III/IV toxicities, presence of adverse events having led to treatment discontinuation, best response rate obtained (as assessed by the multidisciplinary team in charge of the patient and reported in the source file), secondary surgery of initially unresectable metastases, time to progression and OS. A comparison of subgroups of patients under and over the age of 70 years was planned.
The study received a favourable opinion from the CCTIRS (Advisory Committee for the Treatment of Information obtained from Research in Health matters) on 8 September 2011 and from the CNIL (French Data Protection Authority) on 10 October 2012. A letter of non-opposition to participation in the study describing the study and its objectives and guaranteeing the patient’s personal details that would remain anonymous was sent to all the patients who were still alive.
Study design
The dosage of bevacizumab combined with FOLFIRI for the first-line treatment of mCRC was 5 mg/kg every two weeks. The analyses were performed on the intent-to-treat population. The combination was administered until disease progression, presence of major toxicity, secondary surgery or death.
Efficacy
The purpose of the AVASTIN OUESTstudy was to assess the efficacy and safety of bevacizumab administered in combination with FOLFIRI for the first-line treatment of unresectable mCRC. The primary objective of the study was OS. The secondary objectives were objective response (OR), PFS, liver resection (or other surgery) and safety.
OS was defined as the interval between start of therapy to death or last follow-up visit. OR was defined as complete or partial response observed by imagery according to RECIST 1.0 criteria and corresponded to the best response to treatment for the duration of the treatment period. PFS was defined as the time between the start of treatment and an event (relapse, progression, death).
Safety
The adverse events were graded according to the National Cancer Institute’s CTCAE version 4. Only events graded 3 or above were recorded.
Statistical analysis
Presentation of results
The data are presented as percentages for qualitative variables (OR, toxicity), as means and standard deviations, medians and extremes for quantitative variables. Survival rate (time to onset of death or progression) are summarized using Kaplan Meier survival curves. The date of origin is the date of inclusion. Medians for OS and PFS estimated using the Kaplan Meier procedure were expressed with a 95% confidence interval.
Comparisons
The results were compared with historical data. The times to onset of death and disease progression were therefore analysed using a log-rank test. All the combinations of qualitative variables (such as toxicities, responses to different treatments) were analysed using a χ2 test or the Fisher test. All the tests were two-tailed tests and considered exact at the 5% level.
Sample size justification or power analysis
The purpose of the AVASTIN OUEST study was to show that daily practice results are equivalent to those reported in the literature. The study was an exhaustive observational study. Therefore, no single endpoint was assessed, but a detailed overview of all the descriptive analyses was provided. The assessments were therefore performed for all the patients so the population sample was representative and power of the tests satisfactory. All the patients in the Brittany and Pays de la Loire areas eligible for inclusion and not opposed to participating were included in the study treated in Public (University and General hospitals), Private hospitals and Cancer Centers. The AVASTIN OUEST cohort included patients treated for mCRC in 2006.
Results
Description of the population
One hundred and eleven patients were included in the study, treated from 1 January 2006 to 31 December 2006 and the cut-off date was 1 August 2012 (public hospitals: 47%; private centers: 31.5% and cancer centers: 21.5%). All the patients with metastases were initially considered unresectable by the multidisciplinary teams managing them. They were treated with the bevacizumab—FOLFIRI protocol. The sex ratio was 44 women (40%) for 67 men (60%). The mean age of the patients was 60.7 years (+/-9.6 years) and the median age was 61 years [20;83]. Ninety-one patients were 70 or younger than 70 and 20 were older than 70 years (18%), including 5 who were older than 75 (4.5%). Of the 20 patients older than 70, 14 were men and 6 were women; their mean age was 74.2 years (+/-3.2 years) and their median age, 73 years [71;83] (Fig. 1). Seventy-two patients (65%) presented with synchronous metastases, 34 presented with locally advanced disease at diagnosis and then presented metachronous metastases at the inclusion of the study (31%) and data about metastases (syn- or metachronous) were missing for 5 patients. Resection of the primary tumour was carried out during the overall time of management in 88 cases (80%). Forty-one patients were given prior adjuvant chemotherapy (for locally advanced cancer) and 3 patients were given neo-adjuvant chemotherapy.
Fig. 1.
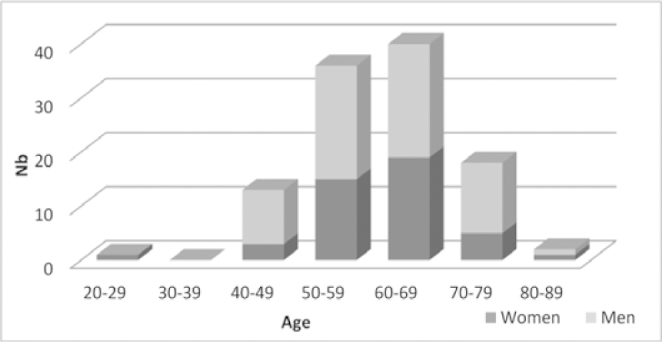
Distribution of population by age and sex
Treatments
The median of number of cycles of bevacizumab—FOLFIRI was 12 [1;47] and the mean number of cycles received was 12 (+/-7.8). Seventy-one patients, i.e., 64% of the cohort, were given a further line of treatment after receiving the bevacizumab—FOLFIRI combination (Table 1). The second-line treatments were either chemotherapies: oxaliplatin-based (24 patients), irinotecan-based (3), another agent (1) or targeted therapies: bevacizumab (combined with other drugs in 7 patients and used as monotherapy in 4 patients) or cetuximab (combined with other drugs in 30 patients and used as monotherapy in 2 patients).
Table 1.
Description of successive lines received beyond the first line.
| Second line (n = 71) 64% | Third line (n = 35) 32% | Fourth line (n = 20) 18% | Fifth line (n = 7) 6% | |
|---|---|---|---|---|
| Cetuximab irinotecan | 20 | 7 | 4 | 1 |
| FOLFOX | 19 | 8 | 1 | 2 |
| Cetuximab FOLFIRI | 8 | 1 | ||
| XELOX | 5 | 1 | ||
| Bevacizumab monotherapy | 4 | |||
| Bevacizumab XELOX | 3 | 1 | ||
| FOLFIRI | 3 | 4 | ||
| Bevacizumab FOLFOX | 2 | 2 | 1 | |
| Bevacizumab LV5FU2 | 2 | 1 | 2 | |
| Cetuximab FOLFOX | 2 | 1 | ||
| Cetuximab monotherapy | 2 | 2 | ||
| Capécitabine | 1 | 4 | ||
| Bevacizumab FOLFIRI | 2 | 1 | ||
| Bevacizumab XELIRI | 1 | |||
| Bevacizumab capécitabine | 1 | |||
| Panitumumab FOLFIRI | 1 | 1 | ||
| Panitumumab | 1 | 3 | ||
| Mitomycine C | 1 | 1 | ||
| Capécitabine - mitomycine C | 3 | |||
| Clinical trial | 2 | 1 |
Patients receiving third-line treatment (35 patients, i.e., 32%) received the following chemotherapies: oxaliplatin (9 patients), irinotecan (4) or another agent (2), or the following targeted therapies: bevacizumab (combined with other drugs in 7 patients or reintroduced in 20% of the patients receiving third-line treatment), cetuximab (combined with other drugs in 8 patients and used as monotherapy in 3 patients) or panitumumab (combined with other drugs in 1 patient and used as monotherapy in 1 patient).
Only 20 patients (18%) were given fourth-line treatment which included capecitabine (n=7), oxaliplatin (1), an inclusion in a clinical trial investigating a new drug (1), a bevacizumab protocol (combined with other drugs in 2 patients, or reintroduced in 10% of the third-line patients), cetuximab (n=5) or panitumumab (n=4).
Regarding fifth-line of treatment or more (7 patients), protocol distribution was very heterogeneous (Table 1).
Efficacy
Overall survival
OS median were respectively 25.1 months (95%CI: [20.6; 29.2]) for the whole cohort, 26.5 months (95%CI: [21.5; 31.1]) for the patients under the age of 70 and 19.1 months (95%CI: [9; 29.9]) for the patients over the age of 70 (p=0.062) (Fig. 2).
Fig. 2.
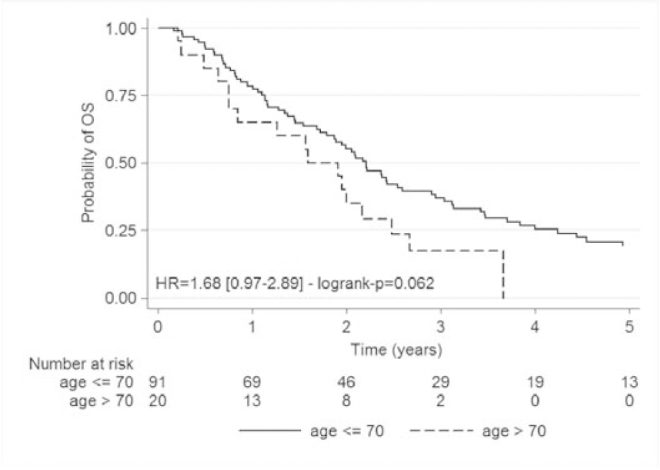
Kaplan Meier overall survival analysis for less than 70 years patients and for over 70 years
For the 30 patients having undergone secondary resection of metastases, the OS median was 59.2 months (95%CI: [41.0; 78.6]) versus 18.8 months (95%CI: [13.7; 23.4]) in the 80 unresected patients (p<10−7) (1 patient was not evaluable) (Fig. 3).
Fig. 3.
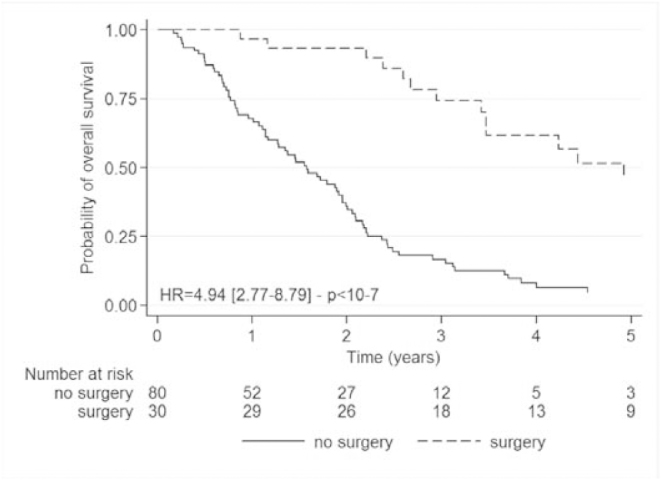
Kaplan Meier overall survival analysis according to metastases resection
Objective responses
A review of OR rates, stability, progression and treatment discontinuation for toxicity of the global intention-to-treat population as a function of age is provided in Table 2. Assessment of response by imaging is generally carried out every 2 to 3 months depending on the practices of the centres. In 9 patients, the best response to treatment was observed at about 1 month of treatment. The best response was observed between 2 to 4 months of treatment in 58 patients and between 5 to 7 months of treatment in 29 patients. In 9 patients, the best response was observed between 8 to 10 months of treatment. Three patients were still responding to treatment at 15 months (2 patients) and at 18 months (1 patient).
Table 2.
Distribution of best response obtained during bevacizumab FOLFIRI and of secondary chirurgical resections depending on age and in intention to treat.
| Response | Global population | Patients < 70 | Patients > 70 |
|---|---|---|---|
| n = 91 | n = 20 | ||
| Complete response | 3 (3%) | 3 (3%) | |
| Partial response | 48 (43%) | 39 (43%) | 9 (45%) |
| Stable disease | 29 (26%) | 25 (27%) | 4 (20%) |
| Progression | 21 (20%) | 17 (19%) | 4 (20%) |
| Toxicity | 10 (8%) | 7 (7%) | 3 (15%) |
| Resection | 30 | 27 | 3 |
| Liver resection | 24 | 21 | 3 |
In the general (in intent to treat) population, an OR was observed in 46% of patients (3% of complete responses and 43% of partial responses); stabilization was observed in 26% of patients and progression in 20%. In 8% of cases, response rates were uninterpretable (the patients rapidly presented with treatment-related toxicity and no imaging was carried out before treatment was discontinued).
Objective response in the intention-to-treat population was 46% (3% of complete responses and 43% of partial responses) for the patients younger than 70 and 45% (no complete responses and 45% of partial responses) for the patients older than 70.
Liver resections
Thirty patients (27%) were qualified for metastasis resection, including 24 for liver metastases. Among the 24 patients having undergone surgery for liver metastases, half of the patients were not given any second-line therapy (only 2 patients had died at the date of last contact) and the other half was given second-line therapy.
Distribution per age based on the 70-year-old limit showed the following results for secondary surgery:
Patients older than 70 (n=20): 3 of the 20 patients (15%) underwent surgery for liver metastases. Of these, two received no post-operative or second-line chemotherapy and only one patient was given FOLFIRI.
Patients younger than 70 (n=91): a total of 27 surgery were performed (30%), including 21 liver resections. None of the patients were given adjuvant therapy. Ten of the patients received no second-line therapy. Eleven of the patients were given second-line chemotherapy including oxaliplatin (n=4) or cetuximab (n=5) or bevacizumab (n=2).
Progression-free survival
PFS medians were 10.2 months (95%CI: [8.8; 11.5]) for the overall population, 10.6 months (95%CI: [8.8; 11.5]) for the patients under the age of 70 and 10.1 months (95%CI: [5.0; 17.6]) for the patients over the age of 70 (p=0.828), which is non-significant (7 patients were not evaluable) (Fig. 4).
Fig. 4.
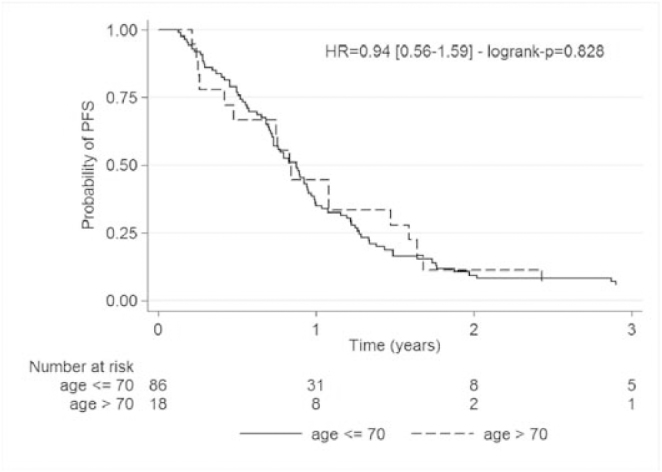
Kaplan Meier progression free survival analysis for less than 70 years patients and for over 70 years
Safety
Eighteen patients (16%) had one or several grade III/IV bevacizumab—FOLFIRI treatment-related adverse events (Table 3): 6 patients presented with haematological disorders, 4 patients with gastrointestinal disorders and 5 patients with thromboembolic events. One case of asthenia was reported as well as one case of anaphylactic shock, one case of mucitis, one case of proteinuria and one case of hand-foot syndrome
Table 3.
Treatment-related grade III/IV toxicities for 18 patients (16%).
| Grade III/IV toxicities | Global population | Patients < 70 | Patients > 70 |
|---|---|---|---|
| Hematological | 6 | 6 | |
| Thromboembolic event | 5 | 5 | |
| Gastrointestinal | 4 | 4 | |
| Asthenia | 1 | 1 | |
| Ischemic stroke | 1 | 1 | |
| Anaphylactic shock | 1 | 1 | |
| Mucositis | 1 | 1 | |
| Proteinuria | 1 | 1 | |
| Hand-foot syndrome | 1 | 1 |
Grade III/IV toxicity depending on the age was as follows:
One patient over the age of 70 presented with anaphylactic shock.
Seventeen patients under the age of 70 presented with haematological (n=6), thromboembolic (n=5) or gastrointestinal disorders (n=4).
Treatment was discontinued in 13 of the 111 patients (12%) because of adverse reactions as follows (Table 4):
Thromboembolic events (n=5), including one case of pulmonary embolism and one case of left middle cerebral artery stroke.
Haematological disorders (n=3), including two cases of prolonged neutropenia and one case of thrombocytopenia.
Gastrointestinal disorders (n=3).
Proteinuria (n=1).
Missing data (n=1).
Table 4.
Distribution of events related to treatment disruption.
| Total population | Patients < 70 years | Patients > 70 years | |
|---|---|---|---|
| Medical decision | 31 (28%) | 28 (31%) | 3 (15%) |
| • Surgery | 21 | 19 | 2 |
| • Best treatment response expected | 3 | 3 | 0 |
| • Therapeutic break | 4 | 4 | 0 |
| • Deterioration of general state | 2 | 1 | 1 |
| • Therapeutic abstention | 1 | 1 | 0 |
| End of treatment | 33 (30%) | 27 (30%) | 6 (30%) |
| Progression | 29 (26%) | 23 (25%) | 6 (30%) |
| Toxicity | 14 (12.5%) | 11 (12%) | 3 (15%) |
| Patient choice | 4 (3.5%) | 2 (2%) | 2 (10%) |
Furthermore, toxicity led to treatment discontinuation in 12% (n=11) of patients under the age of 70 years and in 15% (n=3) of patients over the age of 70. The same applies to the causes of treatment discontinuation as a function of age summarized in Table 4.
No significant differences were observed in the distribution of causes of discontinuation: treatment was discontinued in 30% of cases because the end of treatment had been reached, in 28% of cases following a medical decision in 26% of cases due to disease progression, in 12.5% of cases due to toxicity and in 3.5% of cases based on the patients’ decision.
Discussion
This cohort of 111 patients included all the patients receiving first-line treatment in our areas in 2006 and who were not included in a clinical trial. Our study AVASTIN OUEST provides information on the profile of patients receiving routine care compared with findings published following phase III clinical trials. The patients received first-line treatment with bevacizumab—FOLFIRI after bevacizumab was labelled for mCRC. The cohort provides information on routine practices in a population pool representative of slightly more than 10% of the French population in 2006. The study not only investigated which first-line treatment was used for mCRC, but it also assessed the whole multidisciplinary healthcare process (successive lines of treatment received after first-line therapy and implementation of secondary surgery). The cohort is a true portrayal of management in the two regions because all the private and public hospitals and clinics of the areas (27 hospitals altogether) participated in the study. No first-line prescription of the bevacizumab FOLFOX combination was found. This situation using FOLFOX would not have been considered justified then because the thesaurus in force considered that bevacizumab could only be combined with irinotecan-based chemotherapy at the time (2006).
The second particularity of our study is that it was conducted under the auspices of the Regional Health Agencies and independently of any pharmaceutical companies. The study was developed by the Scientific Committee of the Observatory of Cancer of the Brittany and Pays de la Loire regions, including administrative supervision representatives, physicians from public and private hospitals and pharmacists, and which is coordinated by a pharmacist. The study federated healthcare professions (physicians and pharmacists of the Brittany and Pays de la Loire areas) and led to the development of further multidisciplinary projects extending well-beyond gastrointestinal oncology [17; H Bourgeois et al. Poster JFHOD 2012, H Bourgeois et al. SABCS 2012 Poster A416, F Grudé et al. ASCO GI 2012 Poster A 88601, F Grudé et al. ASCO 2011 Poster A80324, JP Metges et al. Poster ESMO 2010].
The purpose of the AVASTIN OUEST study was to assess the efficacy and safety of bevacizumab administered in combination with FOLFIRI for the first-line treatment of unresectable mCRC. The cohort was exhaustive of all the patients treated with bevacizumab and FOLFIRI in 2006 in the Brittany and Pays de la Loire areas, only one patient opposed to participating was withdrawn from the study. In addition to enabling a census to be taken of all the prescriptions of bevacizumab for the indication, the study also permitted all the side effects reported by the care-giving teams to be noted and verification of the causes of treatment discontinuation, including when they were related to side effects. Patients were given multiagent chemotherapy including conventional chemotherapy and a targeted therapy agent. The rate of treatment-related withdrawal was 12%. The causes of withdrawal were comparable to those identified for bevacizumab (thromboembolic events, proteinuria, and so on) and for FOLFIRI (haematological and gastrointestinal toxicity).
The population aged older than 70 years accounted for 18% of the patients, including 4.5% who were older than 75 years. The Folprecht study, published in 2008, is a meta-analysis of published phase III studies investigating the prescription of irinotecan-based chemotherapy without a targeted therapy agent [18]. Its purpose was to demonstrate that there is no relationship between the efficacy of FOLFIRI treatment and the age of patients. The study comprised a total of 997 patients including a group of 220 patients who were older than 70 years (22%) and 64 patients older than 75 years (6.4%). Therefore, our study providing information on bevacizumab combined with FOLFIRI concerns a similar proportion of patients, i.e., 18% of patients over the age of 70 years and 4.8% of patients over the age of 75. However, in our study the patients underwent no prior pre-selection contrary to the Folprecht study where the patients were all included in phase III studies (risk of selection bias). Our study results are therefore probably a more faithful reflection of daily practice in the whole of France The Folprecht study found no age-related differences for response rate which was confirmed in our study (OR: 46% in patients under 70 years versus 45% in patients older than 70).
The Folprecht study [18], however, did not broach the possibility of secondary resection of metastases. Yet, 24% of the patients in our cohort were eligible for metastasis resection. This figure is high because rates of only 10% are found in the literature [7,9,19]. However, the vast majority of resectable patients were under 70 years old. It is probable that secondary resection of metastases in these initially unresectable patients is beneficial because survival medians were significantly increased in these patients (59.2 months versus 18.8 months).
Another strong point of our study is that we are perfectly well-informed about all the treatments received by the patients after first-line therapy. The cohort study was started in 2006 when all the patients received their first-line of treatment and they were followed up until death. Regarding further lines of treatment, 64% of patients were given secondline therapy. These figures are comparable with those of the literature. With conventional chemotherapy agents, Tournigand reported that 74% of patients received second-line therapy with FOLFOX following first-line therapy with FOLFIRI, in accordance with the protocol [20]. In the COIN study, 50% of patients were given second-line therapy [21]. In the phase III studies testing bevacizumab with IFL or FOLFOX, the proportions of patients given second-line therapy were 50 % [4] and 46% [7], respectively.
In the BRITE cohort, 81% of patients were given second-line treatment [11]. In this registry study, similarly to the first BEAT trial, a wide variety of conventional chemotherapy protocols were associated with bevacizumab (FOLFOX, XELOX, capecitabine and FOLFIRI). In the BEAT cohort, FOLFOX and FOLFIRI accounted for respectively 29% and 26% of the combinations [22]. In the BRITE cohort, most patients were treated with the FOLFOX protocol (56%), with only 14% of patients being treated with the FOLFIRI protocol. In this study, a subgroup of patients continued to be treated with bevacizumab after having received first-line therapy. At the time, however, there were no data in the literature supporting such a prescription. In our study, we also found cases of continued use of bevacizumab and/or of further use in other lines combined with a different protocol to the FOLFIRI protocol when the first-line response had been good. However, only a very small proportion of patients received bevacizumab in further lines of treatment (11 patients/71) in second-line therapy, 7/35 in third-line therapy, 2/20 in forth-line therapy and 3/7 in fifth-line therapy). These figures cannot be used for statistical analysis of the concept of maintenance of anti-VEGF therapy over several lines. Two phase III studies, the preliminary results of which directly (study TML with bevacizumab) or indirectly (study Velour with Aflibercept after bevacizumab) address the issue of whether anti-angiogenic agents should continue to be used for first-line and second-line therapy have just been published [12,23].
Our study also included patients having later received anti-EGFR (Epidermal Growth Factor Receptor) treatment. In 2006, anti-EGFR treatment was not yet indicated for use in patients who had not been pre-treated. Since then, cetuximab (combined with irinotecan-based chemotherapy or with FOLFOX) and panitumumab (combined with FOLFOX) have been approved for first-line use in patients with the wild-type Kras gene [6,24,25]. During the ASCO 2013 congress (Chicago) and the ECCO/ESMO 2013 congress in Amsterdam, the FIRE 3 study comparing the target therapy agents bevacizumab and cetuximab combined with FOLFIRI for the first-line treatment of Kras wild type mCRC was presented twice [19]. The principal objective of the study was to determine OR rates, which were found to be non-significant in the ITT population but significant in evaluable patients, with the cetuximab protocol showing better OS. New data regarding the evaluation of Ras status and not only usual Kras status were presented and tended to show a more important benefit regarding PFS and OS for the Super wild type patients treated the cetuximab arm than the previous data with usual Kras status. However, concerning the main objective (OR rate), the percentage of the two groups was not statistically different. The results of other studies such as the CALGB/SWOG 80405 study will become available in 2014.
Management of patients with unresectable mCRC has evolved over the past few years with more and more treatment options becoming available. The new Ras status is probably a very important information regarding the best first-line or next line to propose [25]. Recently, the CORRECT study demonstrated the value of regorafenib [26] in patients with good general health (OMS 0–1) and in whom conventional chemotherapy or targeted agent therapy has failed. The study results, presented on several occasions at the start of 2012, are not expressed in terms of OR but as a statistical increase in PFS and OS of 15 days.
The question of the best line of conduct as a function of the number of lines received and the need to find objective parameters to make the best possible decision on a case-by-case basis is currently the subject of a study (the Palliachim routine care study), coordinated by Françoise Grudé and Hugues Bourgeois as part of the activity of the Observatory of Cancer of the Brittany and Pays de la Loire areas. The first phase of the study prospectively validated clinical and laboratory parameter scores based on general condition, the number of metastatic sites, albumin and LDH levels in a population of 300 patients followed on an outpatient basis. Three groups of patients with different median survival times were identified using the score system, i.e., patients with a good score (0–3) presenting a median survival time of 301 days 95CI [209–348], patients with an intermediate score (4–7) presenting a median survival time of 78 days 95CI [71–113] and patients with a poor score (8–10) presenting a median survival time of 35 days 95CI [14–56]. The score can thus be used by clinicians as a decision-support tool. It may be used to detect patients at high risk of presenting a major toxic reaction and/or those patients with a very short-term prognosis and potentially nothing to gain from receiving a further line of treatment. The results for colorectal cancer reported at the JFHOD 2012 (French-speaking Days of Digestive, Hepato-Gastroenterology and Oncology) demonstrated the potential value of the score for daily practice [H Bourgeois et al. Poster JFHOD 2012].
All in all, the study demonstrated the everyday reality of first-line use of bevacizumab and FOLFIRI in a cohort of homogeneous patients and the absence of major unexpected side effects or of effects not described in the literature. Nonetheless, the reporting of serious adverse events to the Regional Pharmacovigilance Centres (CRPV) remains absolutely indispensible. In this framework, the implementation of a structure termed QSP (Quality, Safety, Pharmacovigilance) bringing together the Regional Pharmacovigilance Centres of Angers, Brest, Nantes and Rennes and the Cancer Monitoring Centre encouraged this practice in all the public and private hospitals and clinics in the Brittany and Pays de la Loire areas. The federative initiative led to a 34% increase in 6 months (2010–2011) of reporting of bevacizumabrelated adverse events in the National Pharmacovigilance base. These figures might mean that serious adverse events may be under-reported at the national level [27].
Our study, because it grouped together pubic university and non-university hospitals as well as private hospitals and clinics, provides a good overview of intermediate care practices. The study confirms that better OS is observed in patients under the age of 70 compared with older patients, but with no statistical difference. The study presents strong arguments for the oncogeriatric assessment of drugs which would enable patients over the age of 70 to benefit from innovative therapies similarly to younger subjects seeing that they do not seem to suffer more than younger subjects from toxicity. This study is the first of a series of studies initiated by the Observatory of Cancer of the Brittany and Pays de la Loire areas in the framework of research on the good use of drugs in elderly patients, i.e., one of our most important challenges given demographics today and the profiles of patients receiving routine care.
Conflict of interest J.-P. Metges: participation in the AMGEN and SANOFI conferences (on colorectal cancer). Others authors: no conflict of interest to declare for the last two years.
Nota Bene J.-P. Metges: National coordination of the Sanofi-Aventis study “VELOUR” (performance of Aflibercept in treating second-line metastatic colorectal cancer).
Open Access : This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
This article is published with Open Access at link.springer.com
References
- 1.Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 3.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Pectasides D, Papaxoinis G, Kalogeras K, et al. XELIRI- Bevacizumab versus FOLFIRI-Bevacizumab as first line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology group phase III Trial with collateral biomarker analysis. BMC Cancer. 2012;12:271. doi: 10.1186/1471-2407-12-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 7.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 8.Delaunoit T, Goldberg RM, Sargent DJ, et al. Mortality associated with daily bolus 5-fluorouracil/leucovorin administered in combination with either irinotecan or oxaliplatin: results from Intergroup Trial N9741. Cancer. 2004;101:2170–2176. doi: 10.1002/cncr.20594. [DOI] [PubMed] [Google Scholar]
- 9.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26(33):5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 14.Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol. 2009;27:199–205. doi: 10.1200/JCO.2008.17.7931. [DOI] [PubMed] [Google Scholar]
- 15.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 16.Mohile SG, Hardt M, Tew W, et al. Toxicity of bevacizumab in combination with chemotherapy in older patients. Oncologist. 2013;18:408–414. doi: 10.1634/theoncologist.2012-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grudé F, Bessard R, Bourgeois H, et al. Optimizing good use and costs of anticancer drugs: A French inter regional study of the Observatory of Cancer. Bull Cancer. 2013;100(3):271–282. doi: 10.1684/bdc.2013.1715. [DOI] [PubMed] [Google Scholar]
- 18.Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann V, Fischer von Weikersthal L, Decker T, et al. (2013) Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS wild-type metastatic colorectal cancer: German AIO study KRK-0306 (FIRE3). J Clin Oncol 31 (suppl; abstr LBA3506) [DOI] [PubMed]
- 20.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer-a GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 21.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT Study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 23.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 24.Amado RG, Wolf M, Peeters M, et al. Wild-type kras is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 25.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 26.Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebocontrolled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 27.Ruellan AL, Bellissant E, Bourgeois H, et al. Collaboration entre l’observatoire dédié au cancer et CRPV: premiers résultats sur la pharmacovigilance des anticancéreux. 2012. [Google Scholar]


